Abstract
Context:
Rosai–Dorfman disease, also known as Sinus histiocytosis with massive lymphadenopathy, is a benign proliferative disorder of histiocytes. It typically affects lymph nodes; however, extranodal disease is being increasingly reported. The latter entity poses exceptional diagnostic challenge clinico-radiologically by forming mass lesions. Fine needle aspiration cytology (FNAC) is the investigation of choice to avoid unnecessary surgery as the majority are self-limiting.
Aims:
The objective is to assess the utility of FNAC in the diagnosis of Rosai–Dorfman disease and to highlight the diagnostic difficulties.
Material and Methods:
The cytology features of 12 cases of Rosai–Dorfman disease were analyzed and correlated with histopathology and immunohistochemistry.
Results:
The present study included six nodal and six extranodal Rosai–Dorfman disease. The cytology smears showed a variable number of large histiocytes exhibiting characteristic emperipolesis. Ten cases diagnosed as Rosai–Dorfman disease on cytology were confirmed on histopathology. The presence of granulomas, atypical histiocytes, insignificant emperipolesis, and eosinophil infiltration were the challenges we faced.
Conclusions:
FNAC, a simple and cost-effective method with its unique cytology features is the first line of investigation in the diagnosis of Rosai–Dorfman disease.
Keywords: Emperipolesis, eosinophils, granulomas, Rosai–Dorfman Disease, Sinus Histiocytosis with massive lymphadenopathy
INTRODUCTION
Rosai–Dorfman Disease (RDD) is a rare nonneoplastic proliferation of atypical histiocytes of unknown etiology. Bilateral massive and painless cervical lymphadenopathy is classical. However, it affects other lymph nodes and many extranodal locations.[1] This heterogeneous presentation poses a diagnostic challenge as the clinical and radiological appearances simulate nonneoplastic and neoplastic lesions including malignancy. Despite its alarming presentation, RDD is self-limited and simple excision is curative. It has distinct morphology, and fine-needle aspiration cytology (FNAC) plays a significant role in the primary diagnosis by typical cytology features.
We are presenting twelve cases of nodal and extranodal RDD, the largest number of cytology series described in the medical literature, highlighting the challenges encountered. Atypical presentation and unusual morphology led to diagnostic difficulty in a few cases.
MATERIAL AND METHODS
The cytology features of 12 cases of RDD were studied for 10 years from January 2009 to December 2018. After clinical examination of the patients, FNAC was performed with 22 G needle, and the aspiration smears were fixed in isopropyl alcohol and stained with Hematoxylin and Eosin. May–Grunwald–Giemsa stain was used for air-dried smears. The surgical specimens were fixed and processed using routine methods. The cytomorphological features were analyzed and correlated with histopathology and immunohistochemistry (IHC) wherever available.
RESULTS
Out of twelve cases of RDD, six were nodal and six extranodal. The patients' age ranged from 7 to 60 years, and there were seven females and five males. Tables 1 and 2 show the clinical features of RDD.
Table 1.
Clinical features of Nodal RDD
| Case number | Age in years | Sex | Lymph nodes involved | Duration | Other symptoms | Investigations |
|---|---|---|---|---|---|---|
| 1 | 7 | Male child | Bilateral cervical and left inguinal | 3 years | Recurrent fever URTI |
Elevated ESR Hypergammaglobulinemia US abdomen: NAD |
| 2 | 60 | Female | Right and left cervical | 1 month | Asymptomatic | Elevated ESR |
| 3 | 10 | Male child | Right submandibular | 10 months | Fever | Elevated ESR |
| 4 | 45 | Male | Bilateral cervical | 1 year | Asymptomatic | Elevated ESR |
| 5 | 42 | Male | Bilateral inguinal and left cervical | 1 month | Weight loss | ESR, RA factor, CRP, Alkaline phosphatase- Elevated |
| 6 | 45 | Male | Bilateral cervical | 4 months | Asymptomatic | Elevated ESR |
Table 2.
Clinical features of Extranodal RDD
| Case number | Age in years | Sex | Site | Duration in months | Clinical diagnosis | Investigations |
|---|---|---|---|---|---|---|
| 7 | 27 | Female | Swelling left thigh | 2 | Neurofibroma | Normal ESR |
| 8 | 45 | Female | Lumps both breasts | 1 | Fibroadenomas | Mammosonogram: Fibroadenomas |
| 9 | 55 | Female | Lump left breast and swelling left axilla | 1 | Carcinoma Breast with metastasis | Ultrasound: Hepatomegaly Increased ESR |
| 10 | 45 | Female | Recurrent lumps both breasts and swelling anterior abdominal wall | 8 | Mastitis/Carcinoma | Mammography: BIRADS V |
| 11 | 55 | Female | Mass lesion Nasopharynx | 2 | Carcinoma | CT and MRI: Carcinoma |
| 12 | 49 | Female | Lump right breast and right Epitrochlear lymph node |
3 | Carcinoma | Mammosonogram: Carcinoma |
All the six patients of nodal RDD were presented with cervical lymphadenitis, while two patients showed significant inguinal lymph node enlargement. The details of interesting cases are described below.
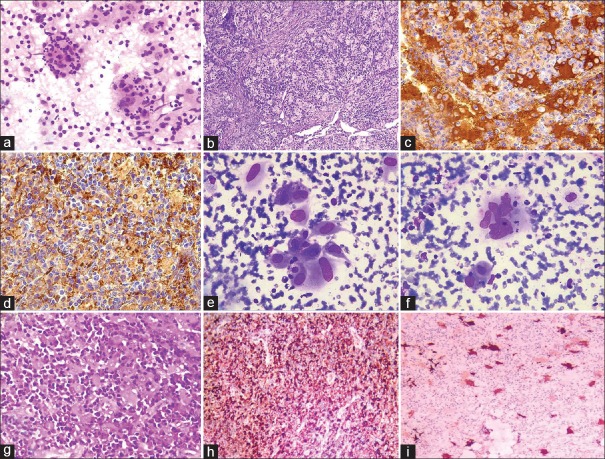
Case 1: Cytology smears were highly cellular and comprised of histiocytes and lymphocytes. The histiocytes were large with abundant eosinophilic cytoplasm exhibiting emperipolesis of lymphocytes. In addition, few histiocytes showed engulfed fragmented neutrophils, which is an unusual feature [Figure 1a–d].
Figure 1.
Case 1 a-d. (a) Cytology smear with large histiocytes exhibiting emperipolesis and fragmented neutrophils. (H and E, ×400). (b) Histopathology showing dilated sinuses filled with histiocytes exhibiting emperipolesis. (H and E, ×200). (c) S100 positivity. (×400). (d) CD 68 positivity. (×200). Case 11 e-i, (e and f) Cytology smears showing atypical histiocytes with sparse emperipolesis. (MGG, ×400). (g) Histopathology showing sheets of atypical histiocytes with dense inflammatory infiltrate. (H and E, ×400). (h) CD68 positivity. (×100). (i) S100 positivity. (×200)
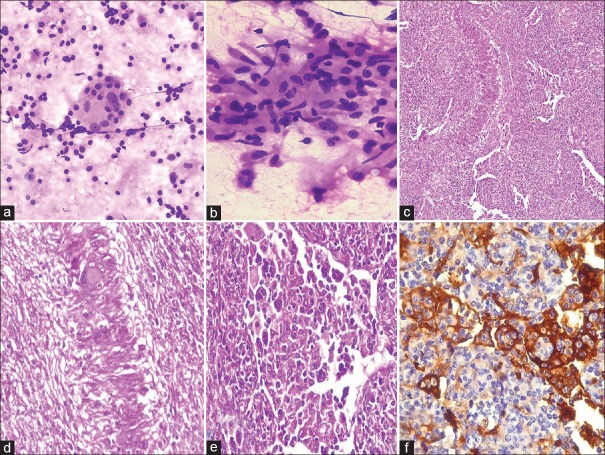
Case 5: Another patient was a 42-year-old male with swellings in both groins of 1 month duration. Clinical examination showed 4 × 3 cm and 3 × 2 cm bilateral inguinal lymph nodes. Hematological investigations were abnormal with raised erythrocyte sedimentation rate (ESR) and C reactive protein and hypergammaglobulinemia. Serology was negative for HBV, HCV, and HIV. Ultrasound abdomen revealed borderline hepatomegaly with mild splenomegaly and subtly altered echotexture of both kidneys. Liver function tests were found abnormal with elevated alkaline phosphatase. Rheumatoid factor levels were likewise elevated. FNAC of inguinal lymph node showed both medium and large-sized histiocytes displaying emperipolesis of lymphocytes and plasma cells. In addition, there were many clusters of epithelioid cells and Langhans giant cells. The cytology picture was interpreted as RDD with granulomatous inflammation. Histopathology revealed loss of lymph nodal architecture with moderately dilated sinuses, which contain histiocytes with emperipolesis. The presence of ill-defined granulomas of histiocytes, epithelioid cells, and giant cells with focal palisading pattern raised the suspicion of tuberculosis. Acid-fast staining on smears and sections, chest X-ray, and quantiferon gold test were negative excluding this possibility. IHC done with CD 68 and S100 showed positivity. A final diagnosis of RDD with associated granulomatous inflammation was made [Figure 2].
Figure 2.
Case 5. (a) Cytology smears showing emperipolesis, (H and E, ×200) and (b) Epithelioid cells (H and E, ×400). (c) Histopathology showing histiocytes in dilated sinuses exhibiting emperipolesis. (H and E, ×100). (d) Palisaded granulomas. (H and E, ×200). (e) Emperipolesis. (H and E, ×400). (f) S100 positivity. (×400)
All six patients with extranodal RDD were adult females with ages between 27 and 55 years. Four cases involved the breast, a single case of soft tissue, and another nasopharynx. Diagnosis was unsuspected in all because of unusual locations.
In case 7, FNAC from 4 × 3 cm firm and fixed mass on the left thigh conceded moderate cellularity, composed of lymphocytes and few histiocytes and very occasional histiocytes exhibiting emperipolesis of lymphocytes. Biopsy followed by immunohistochemistry with S100 and CD68 confirmed the cytological diagnosis of RDD.
Case 8 presented with multiple lumps in both breasts, which were clinically and radiologically diagnosed as fibroadenomas. FNAC of both breast lumps showed the picture of RDD and follow-up of the patient revealed complete resolution.
Similarly, in case 9, clinically suspected malignancy has turned out to be RDD on cytology, which was confirmed by IHC.
Case 11 was a 55-year-old female presented with difficulty in swallowing and loss of hearing for 2 months. Clinical examination showed 2 × 2 cm ill-defined swelling on the right side of the neck. CT scan of head and neck revealed a diffuse mass lesion in nasopharynx extending into tonsillar fossa, soft palate, and posterior pharyngeal wall and the impression was malignancy. The moderately cellular cytology smears were composed of large histiocytes with abundant vacuolated cytoplasm. Many cells were atypical with irregular nuclei and nucleoli. Bi and multinucleate forms, bizarre cells, and few mitoses were seen. Very occasional cells exhibited emperipolesis of plasma cells and neutrophils. Few eosinophils were also present. A diagnosis of atypical histocytic lesion was made. Histopathology showed fragmented tissue composed of sheets of histiocytes exhibiting atypia. Prominent inflammatory component in the form of lymphocytes, eosinophils, and plasma cells was seen. Considering the presence of histiocytes, eosinophils, and few doubtful nuclear grooves, Langerhans cell histiocytosis (LCH) was suspected and IHC was done. On IHC, S100 and CD68 were positive while CD1a and CK were negative. Hence, a diagnosis of RDD was made [Figure 1e–i].
The cytological features of 12 cases were analyzed and the findings are in Table 3.
Table 3.
Cytology features of RDD
| Case number | Cellularity | Predominant population of cells | Emperipolesis | Type of engulfed cells | Additional features |
|---|---|---|---|---|---|
| 1 | High | Histiocytes and lymphocytes |
Frequent | Lymphocytes | Epithelioid cells Fragmented neutrophils |
| 2 | High | Lymphocytes | Frequent | Lymphocytes | Nil |
| 3 | High | Lymphocytes | Moderate | Lymphocytes | Nil |
| 4 | High | Histiocytes and lymphocytes |
Moderate | Lymphocytes | Fibrous tissue |
| Plasma cells | |||||
| 5 | High | Histiocytes and lymphocytes |
Moderate | Lymphocytes | Epithelioid cell clusters |
| Plasma cells | Langhans type giant cells | ||||
| RBC | |||||
| 6 | High | Histiocytes | Frequent | Lymphocytes | Nil |
| 7 | Moderate | Lymphocytes Very few histiocytes | Occasional | Lymphocytes | Lymphoglandular bodies Fibrofatty tissue |
| 8 | High | Lymphocytes Histiocytes |
Frequent | Plasma cells | Many plasma cells with Russel bodies |
| 9 | High | Histiocytes Lymphocytes |
Frequent | Plasma cells | Cytoplasmic vacuoles |
| 10 | High | Lymphocytes Histiocytes |
Frequent | Plasma cells | Nil |
| 11 | Moderate | Histiocytes | Occasional | Plasma cells | Atypia |
| Nucleoli | |||||
| Vacuoles | |||||
| 12 | Moderate | Lymphocytes | Sparse | Lymphocytes | Fibrosis |
Ten out of twelve cases were diagnosed on cytology as RDD. A single case (11) with atypical histiocytes and inconspicuous emperipolesis was diagnosed as a histiocytic lesion. Another one (12) with moderate cellularity and sparse emperipolesis was diagnosed as lymphoproliferative disorder [Figure 3].
Figure 3.
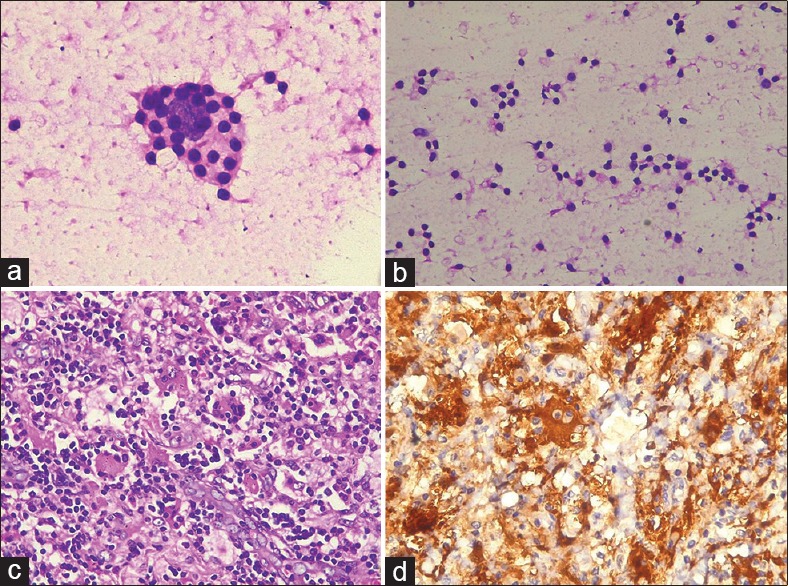
Case 12. H and E stained cytology smears. (a) Very occasional emperipolesis.(×400) (b) Background lymphocytes. (×200). (c) Histopathology. Histiocytes with emperipolesis (×400). (d) S100 positivity (×400)
Ten cases were followed by histopathology, of which IHC was done for 7 cases and the details are summarized in Table 4.
Table 4.
Cytohistopathological correlation and Immunohistochemistry findings
| Case Number | Cytological diagnosis | Histopathology | Immunohistochemistry |
|---|---|---|---|
| 1 | RDD | RDD | S100, CD68 Positive |
| 2 | RDD | RDD | Not done |
| 3 | RDD | RDD | Not done |
| 4 | RDD | RDD | Not done |
| 5 | RDD with Granulomatous inflammation | RDD with Granulomatous inflammation | S100, CD68 Positive |
| 6 | RDD | Not done | - |
| 7 | RDD | RDD | S100, CD68 Positive CD1a Negative |
| 8 | RDD | Not done | - |
| 9 | RDD | RDD | S100, CD68 Positive |
| 10 | RDD | RDD | S100, CD68 Positive |
| 11 | Histiocytic lesion | LCH | S100, CD68 Positive CD1a and CK Negative |
| 12 | Lymphoproliferative disorder | RDD | S100, CD68 Positive |
DISCUSSION
RDD, now a well-defined clinicopathological entity, was first described in 1965 by a pathologist Destombes.[2] Later in 1969, it was delineated as a distinct disease, sinus histiocytosis with massive lymphadenopathy by Rosai and Dorfman.[3] Since the original description, there have been several case reports and studies describing the etiopathogenesis and the morphological spectrum of this rare condition.[2,3] There were about 1000 case descriptions in the medical literature till now.[4] The exact etiology of this self-limited disease is not known and probably multifactorial. Human Herpesvirus 6, Epstein-Barr virus, and polyomavirus were implicated in its causation, but were not proved.[5] Another factor supposed to be responsible is immune dysregulation. As per the recent data, RDD is due to an exaggerated immune response of the hematolymphoid system to infection, leading to monocyte colony-stimulating factor-mediated immune activation and cell proliferation.[6,7]
The polyclonal nature of proliferating histiocytes, also known as Rosai–Dorfman cells, indicates that they are reactive rather than neoplastic.[8] However, current studies identified mutations in BRAF, KRAS, NRAS, and PIK3CA genes of RAS-MAPK pathway and proposed that a subset of RDD may be clonal.[4,9] These cells differ in morphology and immune phenotype from the normal histiocytes. They are large with abundant eosinophilic cytoplasm and nuclei may show atypia. Histiocytes are known for their phagocytic property, whereas emperipolesis is the characteristic of RDD. Emperipolesis as defined by Humble is an active penetration of one cell by another where it remains intact.[10] It is nondestructive phagocytosis of inflammatory cells, often lymphocytes and also plasma cells, neutrophils, and RBC. Emperipolesis, otherwise lymphocytophagocytosis is required to diagnose RDD, the absence of which makes the diagnosis difficult.[5] However, it is not the diagnostic feature as is seen in normal marrow and in several hematological and nonhematological malignancies such as leukemia, lymphoma, and neuroblastoma, making it a nonspecific finding.[10] The immune profile of histiocytes in RDD shows the qualities of both histiocytes and Langerhans cells. Like histiocytes, they express CD68 and Lysozyme, but unlike them, they are strong S100 positive.[6,11] All these findings suggest that atypical histiocytes are activated macrophages obtained from circulating monocytes. These monocytes migrate to sinuses of lymph nodes and transform to RDD histiocytes possibly mediated by cytokines.[12]
Cytology has an important role in the diagnosis of RDD, as it has characteristic cytological features ensuring an accurate diagnosis. The cellularity is usually high with plenty of large histiocytes both with and without emperipolesis and also lymphocytes. The presence of plasma cells and neutrophils is a frequent finding.[13,14] In the present study, aspirates from the lymph nodes yielded a predominant population of lymphoid cells except in three cases, where there were an equal number of lymphocytes and histiocytes. Whereas four extranodal RDD predominantly showed histiocytes and two cases showed lymphocytes. Except for three, FNAC of nine cases yielded high cellularity. Emperipolesis, the hallmark of RDD is considerable in nine cases. In lymph nodal RDD, the dominant cells engulfed were lymphocytes, whereas, in extranodal RDD, they were plasma cells.
The differential diagnoses to be considered on cytology of nodal RDD are reactive lymphadenitis, sinus histiocytosis, granulomatous inflammation, and LCH.[13,14] Emperipolesis can be mistaken for overlapping lymphocyte and dendritic cell clusters in a reactive lymph node. However, the smooth contour of histiocytes and plasma cells inside are useful clues for the unequivocal identification of emperipolesis in our experience. Prominent histiocytosis as seen in sinus histiocytosis and granulomatous inflammation differ from RDD by smaller size, absence of emperipolesis, and S100 negativity.
Dilated lymph node sinuses packed with histiocytes showing emperipolesis are the classical histopathology of RDD. However, emperipolesis is better appreciable on cytology. A feature of diagnostic importance on histology is halo surrounding the engulfed cells due to fixation artefact.[1,15]
Extranodal RDD is not uncommon and constitutes up to 40% of RDD. It appears either alone or with lymphadenopathy. In contrast to nodal RDD, which is frequent in young males, extranodal is common in adults with a striking female preponderance.[11] We observed similar findings in our study. It can occur anywhere in the body, the most common sites in the orient being skin and soft tissue. Other sites of involvement are head and neck, eye and ocular adnexa, bone, brain, breast, etc., Extranodal RDD mimics neoplasm by forming mass lesion and radiological findings are usually inconclusive.[16,17,18] The cyto-histopathology features are similar to that of nodal RDD except for fibrosis and less conspicuous emperipolesis. The diagnosis of extranodal RDD is challenging and needs a high degree of suspicion. The differential diagnoses depend on the location and vary from inflammatory lesions to carcinoma.
RDD involving upper aerodigestive tract is uncommon, and few cases reported were young. Diagnosis of RDD at this unusual location is challenging for its close mimicry of malignancy.[19] We encountered such a rare case in an adult female with a diffuse mass lesion involving nasopharynx, tonsillar fossa, soft palate, and posterior pharyngeal wall. The atypical histiocytes with nucleoli and insignificant emperipolesis limited our diagnosis to a histiocyte disorder on cytology. Histopathology showed sheets of atypical histiocytes with inflammatory infiltrate in the form of plasma cells and eosinophils. Owing to the presence of histiocytes and eosinophils, we initially suspected LCH. Of the panel of immune markers, CD1a and CK were negative, and CD 68 and S100 were strongly positive confirming the diagnosis as RDD. RDD has to differentiate from the clonal and potentially fatal LCH. The combination of histiocytes and eosinophils is a strong indication of LCH, which is typified by irregular nuclear contours, nuclear grooves, CD1a positivity, and Birbeck granules on electron microscopy. The presence of eosinophil infiltration is considered as a feature against RDD and is used to differentiate RDD from LCH. However, the present case suggests that prominent eosinophil infiltration can occur in RDD, and its presence should not deny the diagnosis.[20,21] Atypical histiocytes with convoluted nuclei, bizarre forms, and multinucleation as seen in our case may be observed occasionally in RDD and are not a sign of malignancy.[22] Emperipolesis, the hallmark of RDD is a dynamic process and evolves with time, and the amount of emperipolesis depends upon the stage of the lesion, which explains its absence in the present case.[12]
Emperipolesis is a nonphagocytic engulfment of hematopoietic cells, where a cell enters a histiocyte stays for some time and then exits. We noted in our study, a single case of RDD in a child displaying fragmented neutrophils in histiocytes. Similar findings were observed by Kusutani et al., and they considered them as apoptotic bodies. They hypothesized that the engulfed neutrophils undergo apoptosis inside the cytoplasm of histiocyte because of their short survival period.[23] This patient had recurrent attacks of upper respiratory infection since childhood suggesting an immune deficiency needing further workup.
Case five was an exceptional combination of RDD with granulomatous inflammation, which was conspicuous even on cytology. Histopathology differed from the classical RDD by lack of dilated sinuses and by the presence of numerous granulomas some of which were in the form of palisades. Immunohistochemistry showed strong S100 positivity in the histiocytes showing emperipolesis. Even though emperipolesis itself is not pathognomonic of RDD, the presence of S100 positive histiocytes exhibiting emperipolesis is diagnostic.[20] Probably, this case was the first to report such a combination of RDD and granulomatous inflammation.[24,25]
Breast is an uncommon site for RDD with only 34 cases described in the medical literature so far.[26] The clinical presentation is variable with single or multiple mass lesions in one or both breasts. The disease may be confined to breast alone or is part of a multifocal disease with multiorgan involvement. Within the breast, lesions occur either in deep dermis and subcutis or in the parenchyma.[26,27] Similar diverse presentation was observed in our study as well. The radiological findings of RDD of the breast are noncontributory and simulate primary tumors of the breast including malignancy. The cytology and histopathology are essentially similar to that of nodal RDD, except for more fibrosis and less emperipolesis, which makes the diagnosis a real challenge to the pathologist.[26,27]
The prognosis of RDD is excellent with spontaneous remission. However, few cases may recur or have a prolonged course or may even be fatal. Fatal cases are those, which occur either in vital organs or those associated with severe immune dysfunction.
CONCLUSION
In conclusion, Rosai–Dorfman Disease is a rare and distinctive disorder with typical clinical appearance and distinctive cytological, histopathological, and immunohistochemical profile. They produce tumor-like swellings, which may be confused for neoplasms and even malignancy. Preoperative recognition of this entity sometimes is challenging and requires awareness and a high degree of suspicion. The present study signifies the vital role of FNAC in diagnosing RDD with its unique cytological features, thereby avoiding unnecessary surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
To Patients and to Dr Manjula.
REFERENCES
- 1.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: A pseudolymphomatous benign disorder. Analysis of 34 cases. Cancer. 1972;30:1174–88. doi: 10.1002/1097-0142(197211)30:5<1174::aid-cncr2820300507>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Destombes P. Adenitis with lipid excess, in children or young adults, seen in the Antilles and in Mali. (4 cases) Bull Soc Pathol Exot Filiales. 1965;58:1169–75. [PubMed] [Google Scholar]
- 3.Rosai J. Sinus histiocytosis with massive lymphadenopathy: A newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63–70. [PubMed] [Google Scholar]
- 4.Abla O, Jacobsen E, Picarsic J, Krenova Z, Jaffe R, Emile JF, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131:2877–90. doi: 10.1182/blood-2018-03-839753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalia S, Sagatys E, Sokol L, Kubal T. Rosai-Dorfman disease: Tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322–7. doi: 10.1177/107327481402100408. [DOI] [PubMed] [Google Scholar]
- 6.Cai Y, Shi Z, Bai Y. Review of Rosai-Dorfman disease: New insights into the pathogenesis of this rare disorder. Acta Haematol. 2017;138:14–23. doi: 10.1159/000475588. [DOI] [PubMed] [Google Scholar]
- 7.Yoon AJ, Parisien M, Feldman F, Lee FY. Extranodal Rosai-Dorfman disease of bone, subcutaneous tissue and paranasal sinus mucosa with a review of its pathogenesis. Skeletal Radiol. 2005;34:653–7. doi: 10.1007/s00256-005-0953-4. [DOI] [PubMed] [Google Scholar]
- 8.Paulli M, Bergamaschi G, Tonon L, Viglio A, Rosso R, Facchetti F, et al. Evidence for a polyclonal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) Br J Haematol. 1995;91:415–8. doi: 10.1111/j.1365-2141.1995.tb05313.x. [DOI] [PubMed] [Google Scholar]
- 9.Shanmugam V, Margolskee E, Kluk M, Giorgadze T, Orazi A. Rosai–Dorfman disease harboring an activating KRAS K117N missense mutation. Head Neck Pathol. 2016;10:394–9. doi: 10.1007/s12105-016-0709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rastogi V, Sharma R, Misra SR, Yadav L, Sharma V. Emperipolesis–A review. J Clin Diagn Res. 2014;8:ZM01–2. doi: 10.7860/JCDR/2014/10361.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen RN, Buckley PJ, Rosai J. Immunophenotypic characterization of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) Semin Diagn Pathol. 1990;7:74–82. [PubMed] [Google Scholar]
- 12.Iyer VK, Handa KK, Sharma MC. Variable extent of emperipolesis in the evolution of Rosai Dorfman disease: Diagnostic and pathogenetic implications. J Cytol. 2009;26:111–16. doi: 10.4103/0970-9371.59398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar B, Karki S, Paudyal P. Diagnosis of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) by fine needle aspiration cytology. Diagn Cytopathol. 2008;36:691–5. doi: 10.1002/dc.20904. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande V, Verma K. Fine needle aspiration (FNA) cytology of Rosai Dorfman disease. Cytopathology. 1998;9:329–35. doi: 10.1046/j.1365-2303.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- 15.Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) Review of the entity Semin Diagn Pathol. 1990;7:19–73. [PubMed] [Google Scholar]
- 16.Mantilla JG, Goldberg-Stein S, Wang Y. Extranodal Rosai-Dorfman disease: Clinicopathologic series of 10 patients with radiologic correlation and review of the literature. Am J Clin Pathol. 2016;145:211–21. doi: 10.1093/ajcp/aqv029. [DOI] [PubMed] [Google Scholar]
- 17.Komaragiri M, Sparber LS, Santos-Zabala ML, Dardik M, Chamberlain RS. Extranodal Rosai–Dorfman disease: A rare soft tissue neoplasm masquerading as a sarcoma. World J Surg Oncol. 2013;11:63. doi: 10.1186/1477-7819-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morkowski JJ, Nguyen CV, Lin P, Farr M, Abraham SC, Gilcrease MZ, et al. Rosai-Dorfman disease confined to the breast. Ann Diagn Pathol. 2010;14:81–7. doi: 10.1016/j.anndiagpath.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Ramadass T, Thulasi PD, Geetha N, Narayanan N, Ayyaswamy G, Swapna S. Extranodal manifestation of Rosai Dorfman Disease of the nasopharynx. Indian J Otolaryngol Head Neck Surg. 2007;59:178–81. doi: 10.1007/s12070-007-0054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cangelosi JJ, Prieto VG, Ivan D. Cutaneous Rosai-Dorfman disease with increased number of eosinophils: Coincidence or histologic variant.? Arch Pathol Lab Med. 2011;135:1597–600. doi: 10.5858/arpa.2010-0554-CR. [DOI] [PubMed] [Google Scholar]
- 21.Efared B, Mazti A, Chaibou B, Atsame-Ebang G, Sidibé IS, Tahiri L, et al. Bone pathologic fracture revealing an unusual association: Coexistence of Langerhans cell histiocytosis with Rosai-Dorfman disease. BMC Clin Pathol. 2017;17:5. doi: 10.1186/s12907-017-0044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GarzaGuajardo R, GarcíaLabastida LE, RodríguezSánchez IP, GómezMacías GS, DelgadoEnciso I, Sánchez Chaparro MM, et al. Cytological diagnosis of RosaiDorfman disease: A case report and revision of the literature. Biomed Rep. 2017;6:27–31. doi: 10.3892/br.2016.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusutani N, Tamiya H, Tsuruta D, Mizuno N, Sowa J, Kaida M, et al. Apoptosis of neutrophils resulting after emperipolesis in cutaneous Rosai–Dorfman disease: A new ultrastructural finding. J Cutan Pathol. 2011;38:529–31. doi: 10.1111/j.1600-0560.2011.01678.x. [DOI] [PubMed] [Google Scholar]
- 24.Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1–2. doi: 10.1016/j.jctube.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunes Rosado FG, Stratton CW, Mosse CA. Clinicopathologic correlation of epidemiologic and histopathologic features of pediatric bacterial lymphadenitis. Arch Pathol Lab Med. 2011;135:1490–3. doi: 10.5858/arpa.2010-0581-OA. [DOI] [PubMed] [Google Scholar]
- 26.Dai D, Cai Q, Vohra NA, Wong J, Therien ZP, Hewan-Lowe K, et al. Rosai-Dorfman disease presenting as a breast mass. Arch Pathol Clin Res. 2019;3:08–14. [Google Scholar]
- 27.Green I, Dorfman RF, Rosai J. Breast involvement by extranodal Rosai-Dorfman disease: Report of seven cases. Am J Surg Pathol. 1997;21:664–8. doi: 10.1097/00000478-199706000-00006. [DOI] [PubMed] [Google Scholar]