Abstract.
Fear of adverse events (AEs) negatively affects compliance to mass drug administration (MDA) for lymphatic filariasis (LF) elimination program. Systemic AEs are believed to occur because of killing of microfilariae, whereas localized soft tissue reactions might be due to the death of adult worms following therapy. Most AEs are mild and self-limited. However, localized AEs are sometimes more significant and of concern to participants. Here, we describe localized AEs that were noted during a large community study that evaluated the safety of a triple-drug regimen (ivermectin, diethylcarbamazine, and albendazole) for the treatment of LF in India. We have also discussed the importance of timely detection and careful management of AEs for preserving community confidence in MDA.
The WHO has adopted annual mass drug administration (MDA) with single-dose diethylcarbamazine (DEC) and albendazole as the principal strategy for lymphatic filariasis (LF) elimination in areas where onchocerciasis is not coendemic.1 Yearly MDA for 5 years with 65% compliance is recommended for successful elimination. Fear of adverse events (AEs) and rumors about such events can significantly decrease MDA compliance and efficacy.2 Recently, a triple-drug regimen (ivermectin plus DEC and albendazole, IDA) has been reported to be more effective than DEC plus albendazole (DA) for clearing microfilaremia (Mf).3,4 We conducted a study in an LF-endemic area in South India to evaluate the safety, efficacy, and effectiveness of IDA and DA when these medications were provided as MDA in communities.5,6 Here, we describe a series of localized AEs that occurred during this study and discuss their significance.
An open-label, block-randomized, controlled trial was carried out in six villages in Yadgir district, Karnataka, India, as part of a five-country multicenter study. Details regarding that study have been recently published.6 The study villages were endemic for bancroftian filariasis. Although the area had received MDA for 13 years, compliance had been suboptimal. Enrolled participants were tested with Alere™ Filariasis Test Strip (FTS) for filarial antigenemia. Persons with positive antigen tests had night blood testing for Mf (60-µL-thick smear). Participants were administered (irrespective of infection status) either DA or IDA according to their village of residence. Participants were actively assessed for AEs by medical teams for 2 days, and passive follow-up was continued for another 5 days. Follow-up studies to assess the efficacy of these treatments in infected individuals and the impact of MDA on LF prevalence in the study villages will be reported separately. The study was approved by the Institute Human Ethics Committee of the ICMR-Vector Control Research Centre in Puducherry, India, and the trial was registered with the Clinical Trial Registry India-CTRI/2016/10/007399.5
Baseline antigenemia and Mf prevalence in the study area were 25.3% and 6.3%, respectively. A total of 9,060 participants were provided either DA or IDA, and of these, 98.7% of participants were evaluated in their homes by medical teams during active follow-up on day 1 and 2 after treatment. The overall AE rate in the 7 days post-treatment was 7.1%, and the vast majority of these AEs were mild systemic events, such as headache, fever, and myalgia.6 Eight participants (0.08%) experienced localized AEs in the week following treatment.
CASE DESCRIPTIONS
Transient lymphedema.
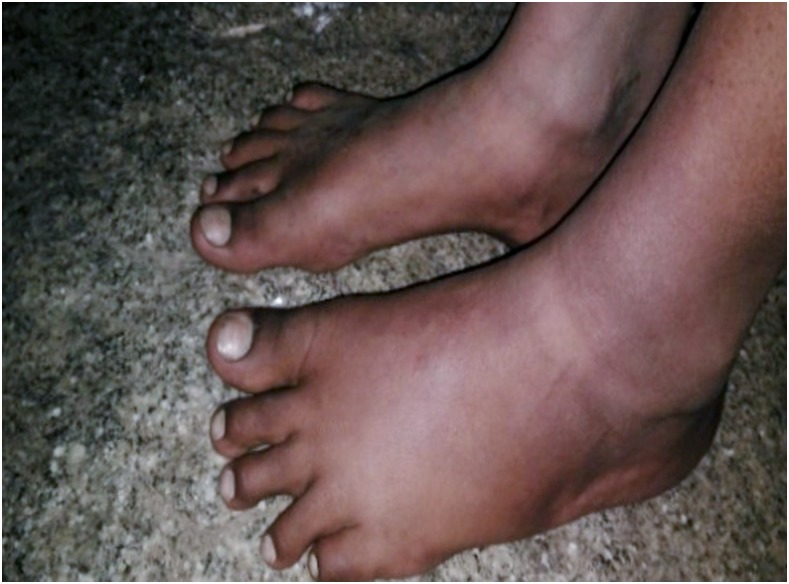
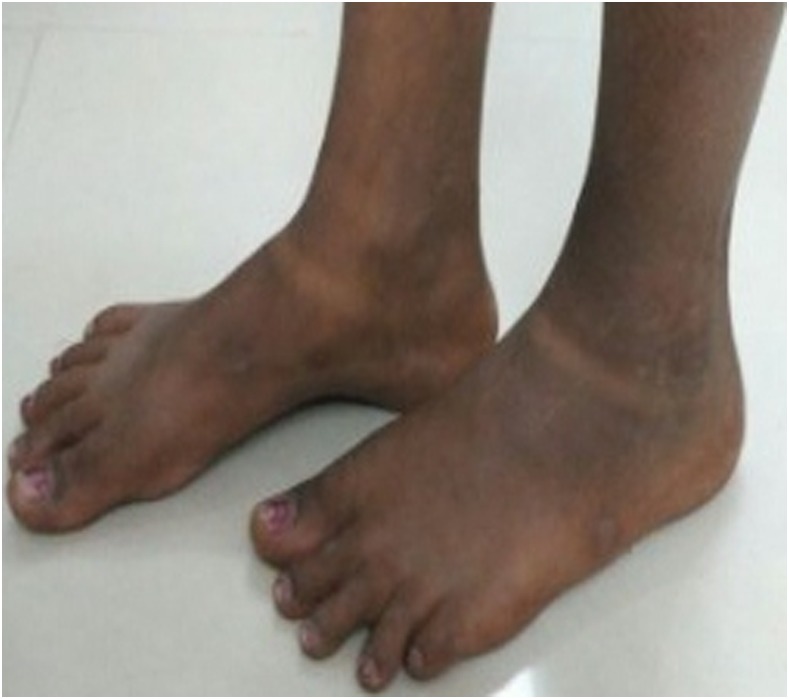
A 13-year-old girl (FTS positive, Mf negative) developed swelling and pain in her left inguinal region 4 days after treatment with DA. Two days later, she noticed swelling of her left ankle and the dorsal aspect of her left foot, and pitting edema was present (Figure 1). Tender left-sided inguinal lymphadenopathy was still present at that time. There was no history of trauma. She had no redness or pain over the leg and no other symptoms. She was reassured, advised to elevate her left leg at night, and instructed on exercises to improve lymphatic drainage. The child was also treated with diclofenac/ranitidine for 1 week. She was observed by our medical team periodically at home, and her family members were reassured. Her lymphadenitis subsided after one additional week and her edema resolved fully after 1 month (Figure 2). The girl was revaluated 1 year later. Her leg and inguinal area were normal; her filarial antigen test remained positive, but her Mf test was negative. She was re-treated with IDA at that time and followed up for 1 month. No AEs were observed.
Figure 1.
A 13-year-old girl developed edema of her left ankle and the dorsum of her left foot approximately 1 week after treatment with diethylcarbamazine plus albendazole. This figure appears in color at www.ajtmh.org.
Figure 2.
Resolution of the left foot and ankle edema one month after treatment. This figure appears in color at www.ajtmh.org.
Scrotal swelling.
Details for participants who developed testicular swelling are provided in Table 1.
Table 1.
Details of cases with scrotal swelling
| Case No. | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age (years) | 24 | 26 | 24 |
| Drug regimen | Double-drug therapy (DA) | Triple-drug therapy (IDA) | Triple-drug therapy (IDA) |
| FTS test | Strong positive | Strong positive | Strong positive |
| Mf test | 4 per 60 µL | 1 per 60 µL | 7 per 60 µL |
| Clinical features | Pain and swelling of the right testicle on day 2 after treatment. Participant was anxious and stressed | Pain and swelling of the right testicle 5 days after treatment | Fever, headache, body ache, pain, and swelling of the right testicle on day 2 after treatment |
| Duration (days) | 7 | 7 | 10 |
| Treatment | Reassurance and treatment with diclofenac and ranitidine | Reassurance and treatment with diclofenac and ranitidine | Reassurance and treatment with paracetamol, diclofenac, and ranitidine |
| Follow-up after 1 year | |||
| FTS test | Strong positive | Strong positive | Strong positive |
| Mf test | Negative | Negative | Negative |
FTS = Filariasis Test Strips; Mf = microfilaremia.
Skin nodules/subcutaneous swellings.
Case 1.
A 25-year-old woman noticed a small nodule in the right arm above the elbow 7 days after DA treatment. Her FTS test was positive but Mf was absent. Swelling was nonprogressive but painful. She reported her problem to the field medical team 12 days after treatment. Two nodules measuring 1 cm in diameter were present on the outer aspect of the right arm. There was no axillary lymphadenopathy. She did not have other AEs. She was treated with diclofenac/ranitidine for 1 week. Swelling resolved after 2 weeks. One year later, her arm was normal; she was still FTS positive and Mf negative.
Case 2.
A 30-year-old woman developed fever, nausea, vomiting, and abdominal pain the day after IDA treatment. Her FTS test was strongly positive, and she had 36 Mf per 60 µL. She had a tender subcutaneous nodule on the left arm, 2 cm below the deltoid tuberosity. She was treated with paracetamol, diclofenac, ranitidine, and ondansetron. She became afebrile the next day and vomiting also subsided. Diclofenac and ranitidine were continued for another 5 days. She was followed up by a medical team at her home regularly, and swelling disappeared after 2 weeks. She refused testing and re-treatment when she was visited 1 year later.
Case 3.
A 14-year-old girl developed fever the night after IDA treatment. Her FTS test was strongly positive and she had 4 Mf per 60 µL. She noted tender swelling over her right forearm 2 days later. There was no lymphadenitis. She was treated with paracetamol, diclofenac, and ranitidine. Pain and fever resolved after 3 days. Diclofenac and ranitidine were continued for another 3 days. The swelling resolved over a period of 1 week. One year later, her FTS result was still positive but her Mf test was negative.
Lymphadenitis.
Case 1.
A 35-year-old woman developed pain and swelling in her left axilla with a tender 1-cm palpable lymph node 2 days after treatment with IDA. Her FTS test was positive, but her Mf test was negative. On day 4 after treatment, she noted tenderness and 3- by 2-cm area of induration over the medial aspect of her left forearm, 3 cm below the elbow. She was reassured and treated with diclofenac and ranitidine for 1 week, and both the swellings resolved. Her arm was normal and her FTS test was negative 1 year after IDA treatment.
Case 2.
Inguinal lymphadenitis was present in the girl with leg edema, as aforementioned.
DISCUSSION
Systemic reactions (fever/headache/myalgia) and localized reactions (skin nodules/breast and scrotal swellings) have been reported following the treatment of filariasis with DEC or with other drugs.7–11 Systemic AEs are believed to be caused by host responses to dying Mf and localized reactions, by host responses to dead/dying adult filarial worms.7,12
Limb swelling following MDA similar to our case has been reported previously.8–10 However, clinical details, treatment provided, and long-term outcome were not reported. Two studies documented reversal of lymphatic pathology following single-dose treatment with DEC plus albendazole in children infected with filarial worms.13,14 None of the participants in those studies developed new onset lymphedema or soft tissue reactions. Scrotal pain and swelling are well recognized but uncommon AEs following LF treatment. This appears to be especially common after prolonged treatment with high doses of albendazole.15,16 Prior studies/reviews have also documented the occurrence of post-treatment subcutaneous nodules and lymphangitis.12,17
Managing AEs in village communities poses unique challenges. People often consider AEs to be the direct effect of drugs, and it can be difficult to convince them that the AEs result from death of filarial worms. Rumors can quickly spread and decrease compliance with MDA. Community trust was a challenge for our study, as the region had already received MDA for many years with poor compliance and villagers had many misconceptions. Relatives of participants who experienced AEs reacted harshly, and sometimes their neighbors refused to participate. Often, illiterate villagers abused the staff and threatened violence. There is no shortcut for establishing trust in a community. However, our close cooperation with local health officials/village leaders and careful management of AEs improved the situation over time.
Community preparation with various IEC (information/education/communication) activities by our dedicated medical social workers and formation of community advisory boards comprising local leaders/village representatives/health staff to oversee study activities were helpful in gaining community trust. We provided a highly visible and well-equipped rapid response medical team with a hotline mobile telephone to respond to AE reports. Study staff were acquainted with the local culture, and care was taken to avoid panic and not to overreact to adversity. We preferred to manage AEs in participants’ home whenever possible rather than moving them to the local health center/hospital. Immediate management of AEs, good counseling, repeated home visits by our staff, and engagement of community leaders were crucial for ensuring participants’ confidence. Frequent and appropriate briefing of mass media (including social media) can help to curtail the spread of rumors regarding AEs.
The WHO has published guidelines addressing practical aspects of prevention/detection/management of serious AEs that occur following preventive chemotherapy programs (including MDA).18 Tanzania has adopted a modified version of these guidelines according to conditions specific to their country.19 As India is considering the use of IDA for LF elimination in specific situations, this is the right time for developing a preventive chemotherapy safety surveillance component in the program that will improve compliance and prevent misconceptions regarding MDA.
Acknowledgments:
We thank the Indian Council of Medical Research (ICMR) for supporting this study. We thank the project staff involved in participant enrollment, testing, and clinical care of the patients. We gratefully acknowledge the support of the National Vector Borne Disease Control Program (NVBDCP) in Delhi and in Karnataka state for this study. We thank the district VBDCP officer Suryaprakash M for his support in carrying out this work. We express our immense gratitude to village/community leaders Sharanappa, Parvath Reddy, Gopal Reddy, Sabanna, and Santosh for their enthusiasm in IEC activities in their respective villages. We also gratefully acknowledge the cooperation by primary health center (PHC) medical officers, other PHC staff, anganwadi teachers, and ASHA (Accredited Social Health Activist) workers.
REFERENCES
- 1.WHO , 2018. Global programme to eliminate lymphatic filariasis: progress report 2017. Wkly Epidemiol Rec 44: 589–604. [Google Scholar]
- 2.Krentel A, Fischer PU, Weil GJ, 2013. A review of factors that influence individual compliance with mass drug administration for elimination of lymphatic filariasis. PLoS Negl Trop Dis 7: e2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomsen EK, et al. 2016. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin Infect Dis 62: 334–341. [DOI] [PubMed] [Google Scholar]
- 4.King CL, et al. 2018. A trial of a triple-drug treatment for lymphatic filariasis. N Engl J Med 379: 1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CTRI/2016/10/007399 Triple drug study for filariasis elimination. A community based study to compare the safety, efficacy and acceptability of a triple drug regimen (ivermectin, diethylcarbamazine and albendazole) with a two drug regimen (diethylcarbamazine and albendazole) for lymphatic filaraisis elimination program. Available at: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=15852&EncHid=&userName=triple%20drug%20community%20study%20for%20filariasis.
- 6.Weil GJ, et al. DOLF IDA Safety Study Group , 2019. The safety of double- and triple-drug community mass drug administration for lymphatic filariasis: a multicenter, open-label, cluster-randomized study. PLoS Med 16: e1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budge PJ, Herbert C, Andersen BJ, Weil GJ, 2018. Adverse events following single dose treatment of lymphatic filariasis: observations from a review of the literature. PLoS Negl Trop Dis 12: e0006454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO , 2003. Report on active surveillance for adverse events following the use of drug co-administrations in the global program to eliminate lymphatic filariasis. Wkly Epidemiol Rec 36: 315–317. [PubMed] [Google Scholar]
- 9.Babu BV, Rath K, Kerketta AS, Swain BK, Mishra S, Kar SK, 2006. Adverse reactions following mass drug administration during the Programme to Eliminate Lymphatic Filariasis in Orissa State, India. Trans R Soc Trop Med Hyg 100: 464–469. [DOI] [PubMed] [Google Scholar]
- 10.Lima AW, Medeiros Z, Santos ZC, Costa GM, Braga C, 2012. Adverse reactions following mass drug administration with diethylcarbamazine in lymphatic filariasis endemic areas in the Northeast of Brazil. Rev Soc Bras Med 45: 745–750. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin SI, Radday J, Michel MC, Addiss DG, Beach MJ, Lammie PJ, Lammie J, Rheingans R, Lafontant J, 2003. Frequency, severity, and costs of adverse reactions following mass treatment for lymphatic filariasis using diethylcarbamazine and albendazole in Leogane, Haiti, 2000. Am J Trop Med Hyg 68: 568–573. [DOI] [PubMed] [Google Scholar]
- 12.Ottesen EA, 1985. Efficacy of diethylcarbamazine in eradicating infection with lymphatic-dwelling filariae in humans. Rev Infect Dis 7: 341–356. [DOI] [PubMed] [Google Scholar]
- 13.Shenoy RK, Suma TK, Kumaraswami V, Rahmah N, Dhananjayan G, Padma S, 2009. Antifilarial drugs, in the doses employed in mass drug administrations by the global programme to eliminate lymphatic filariasis, reverse lymphatic pathology in children with Brugia malayi infection. Ann Trop Med Parasitol 103: 235–247. [DOI] [PubMed] [Google Scholar]
- 14.Kar SK, Dwibedi B, Das BK, Agrawala BK, Ramachandran CP, Horton J, 2017. Lymphatic pathology in asymptomatic and symptomatic children with Wuchereria bancrofti infection in children from Odisha, India and its reversal with DEC and albendazole treatment. PLoS Negl Trop Dis 11: e0005631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussein O, El Setouhy M, Ahmed ES, Kandil AM, Ramzy RM, Helmy H, Weil GJ, 2004. Duplex doppler sonographic assessment of the effects of diethylcarbamazine and albendazole therapy on adult filarial worms and adjacent host tissues in bancroftian filariasis. Am J Trop Med Hyg 71: 471–477. [PubMed] [Google Scholar]
- 16.Jayakody RL, de Silva CSS, Weerasooriya WMT, 1993. Treatment of bancroftian filariasis with albendazole: evaluation of efficacy and adverse reactions. Trop Biomed 10: 19–24. [Google Scholar]
- 17.Shenoy RK, George LM, John A, 1998. Treatment of microfilaraemia in asymptomatic brugian filaraisis: the efficacy and safety of the combination of single doses of ivermectin and diethylcarbamazine. Ann Trop Med Parasitol 92: 579–585. [DOI] [PubMed] [Google Scholar]
- 18.WHO , 2011. Assuring Safety of Preventive Chemotherapy Interventions for the Control of Neglected Tropical Diseases: Practical Advice for National Programme Managers on the Prevention, Detection and Management of Serious Adverse Events. Available at: http://apps.who.int/iris/bitstream/handle/10665/44683/9789241502191_eng.pdf. Accessed July 16, 2019. [Google Scholar]
- 19.Guidelines for assuring safety of preventive chemotherapy. National Programme for Control of Neglected Tropical Diseases (NPCNTD) and Tanzania Food and Drugs Authority (TFDA), Ministry of Health and Social Welfare, the United Republic of Tanzania; Available at: http://www.who.int/neglected_diseases/tz_guidelines_assuring_safety_preventive_chemotherapy.pdf. Accessed July 16, 2019. [Google Scholar]