Abstract.
The infection dynamics of Opisthorchis viverrini metacercariae was analyzed in cyprinid fish from endemic areas in Mukdahan Province, Thailand, and Khammouane Province, Lao PDR. The fish were collected during the cool-dry (November–February), hot-dry (March–May), and rainy (June–October) seasons in 2017. They were examined by the digestion method, and the infection status was statistically analyzed by study area, season, and fish size. The prevalence (no. of fish positive/no. of fish examined × 100) and metacercarial intensities (no. of metacercariae detected/no. of fish positive) of O. viverrini in both study areas depended on season, being high in the cool-dry season and varying in the hot-dry and rainy seasons. In Mukdahan Province, the average prevalence was 18.3% (range 11.0–46.7%, n = 420) and the intensity was 4.07 ± 5.86 cysts/fish (mean ± SD), whereas in Khammouane Province, the prevalence was 51.9% (range 9.1–70.6%, n = 673) and the intensity was 6.67 ± 12.88 cysts/fish. Among the cyprinid fish species examined, the infection was associated with fish body size and predominantly found in Hampala dispar (86.5%), Cyclocheilichthys armatus (73.2%), and Puntius brevis (42.7%). The distribution of O. viverrini metacercariae in fish was skewed, with most of the fish having a low worm burden with an average of four to six cysts/fish. The findings that seasonality, sampling locality, fish size, and species of fish play roles in the risk of O. viverrini infection imply that these host and environmental factors are important for the transmission dynamics and control of O. viverrini.
INTRODUCTION
Opisthorchis viverrini is a pathogenic fish-borne zoonotic trematode that is distributed across continental Southeast Asia, including, Thailand, Lao PDR, Vietnam, Myanmar, and Cambodia.1–3 At least 10 million people in endemic areas are infected with O. viverrini who are at the risk of developing hepatobiliary disease, including cholangiocarcinoma (CCA), due to the infection.4,5 Opisthorchis viverrini and also Clonorchis sinensis, an East Asian species, are contracted via the consumption of undercooked fish. They are both known to be carcinogenic parasites causing CCA in humans.6,7
Freshwater fish, especially cyprinids highly infected with O. viverrini metacercariae, act as the possible infection sources in definitive hosts, including humans, in endemic areas of opisthorchiasis. Among 13 provinces in northeast Thailand, the metacercariae were found in six different species of cyprinid fish, that is, Cyclocheilichthys armatus, Puntius orphoides, Hampala dispar, Henicorhynchus siamensis, Osteochilus hasselti, and Puntioplites proctozysron.8 In Lao PDR, O. viverrini metacercariae have been found in numerous species of cyprinid fish (> 20 species) from endemic areas, particularly Vientiane, Champasak, Savannakhet, Luang Prabang, Khammouane, and Saravane provinces.9–12 In addition to the diversity of fish species, seasonality influences the transmission dynamics of O. viverrini in cyprinid fish, as reported more than 20 years ago in Thailand13,14 and recently in Lao PDR.12 Because of climate and environmental changes, more current data are needed to gain more understanding on the patterns of infection dynamics of O. viverrini in different endemic areas.
To determine the current transmission pattern of O. viverrini from their fish intermediate hosts to the final host, we examined O. viverrini infection in cyprinid fish in two known endemic localities, Mukdahan Province in Thailand and Khammouane Province in Lao PDR, by monitoring seasonal patterns of prevalence, intensity of infection, species diversity of the fish intermediate hosts, and size-related infection of metacercariae in these hosts.
MATERIALS AND METHODS
Study areas and fish sample collection.
Freshwater fish were sampled from two geographical localities in the Nam Don River, Thakhek district, Khammouane Province, Lao PDR (17°30′0″N, 104°46′0″E), and the Huai Khi Lek Reservoir, Nikhom Kham Soi district, Mukdahan Province, Thailand (16°21′33.90″N, 104°34′7.92″E). Two surveyed areas are well-known endemic localities in two countries and separated each other by about 128-km distance.8,12 Cyprinid fish present in the study areas were wild and naturally bred species and were classified as low-value fish. Fish were caught from the freshwater bodies in the study areas using fishing net trapping overnight and were harvested in the next morning. The fish samples were collected seasonally during the cool-dry (November–February), hot-dry (March–May), and rainy (June–October) seasons in 2017 from the two study areas. After the catch, the fish samples were kept in ice and transported to the laboratory of the Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, for analysis.
Screening for O. viverrini metacercariae in fish.
The fish samples were sorted into separate species according to their morphological characteristics.15 For screening purpose, the designated fish species were pooled (5–50 fish) for the detection and enumeration of metacercariae using the standard pepsin digestion method as described previously.14 The fish samples were homogenized using an electrical blender and digested with pepsin, examined, and the O. viverrini metacercariae were counted under a stereomicroscope and identified by morphological criteria.16,17 If the pooled samples were positive for O. viverrini metacercariae, the remaining fish of the same species were subsequently selected for individual examination for metacercarial burden by the same method. A similar protocol to that used by Phan et al.18 was adopted to recover, identify, and count the metacercariae individually by a stereo and a compound microscope.16 The data obtained were used to calculate the prevalence and intensity of O. viverrini infection. The prevalence of infection (no. of fish positive/no. of fish examined × 100) and the metacercarial intensities (no. of metacercariae detected/no. of fish positive) were calculated.
Measurement of seasonal prevalence and intensity of infection.
Based on the data of screening for O. viverrini metacercariae, the infected species were processed for determination of the prevalence and intensity of infection for each seasonal sample. Infected species of fish were subjected to individual extraction for metacercarial burden. Body length and width of fish were measured and recorded.
Statistical analysis.
Chi-square and Mann–Whitney U tests were used to compare the prevalence and intensity of O. viverrini metacercariae by fish species, season, and surveyed area. The intensity of O. viverrini metacercariae in three species of fish over three seasons was log-transformed. The associations between prevalence and season were verified by chi-square test, and Friedman's test was used for evaluating the trends between intensity and season. Associations between O. viverrini infection and related factors were evaluated using binary logistic regression analyses of odds ratios and their 95% CIs. Data for the intensity of infection with O. viverrini metacercariae at different seasons and among fish species were analyzed using the nonparametric Friedman test. Statistical tests were performed using SPSS Statistics V24.0 (IBM Corporation, Armonk, NY). The statistical tests were considered significant when P < 0.05.
RESULTS
Species diversity of cyprinid fish harboring O. viverrini metacercariae.
In total, ten species of cyprinid fish were sampled from Khammouane Province, Lao PDR, comprising C. armatus, H. dispar, Puntius brevis, H. siamensis, Labiobarbus leptocheilus, Labiobarbus siamensis, Discherodontus ashmeadi, O. hasselti, Crossocheilus reticulatus, and Systomus rubripinnis (Table 1). The mean abundance (in terms of metacercariae/fish) of O. viverrini was highest in C. armatus, followed by H. dispar and P. brevis. In Mukdahan Province, Thailand, six species of cyprinid fish were sampled comprising C. armatus, H. dispar, P. brevis, L. siamensis, Barbonymus schwanenfeldii, and S. rubripinnis. Two fish species carried O. viverrini metacercariae. The order of worm burden in these species was highest in C. armatus, followed by H. dispar and then P. brevis, and except for P. brevis, they were consistently infected during all seasons (Table 1).
Table 1.
Screening of cyprinid fish sampled from Khammouane Province, Lao PDR, and Mukdahan Province, Thailand, for the presence of Opisthorchis viverrini MTC
| Season | Fish species | Khammouane, Lao PDR | Mukdahan, Thailand | ||
|---|---|---|---|---|---|
| No. of fish | MTC/fish | No. of fish | MTC/fish | ||
| Cool-dry | C. armatus | 15 | 18 | 10 | 13.4 |
| H. dispar | 15 | 7 | 5 | 0.4 | |
| P. brevis | 20 | 7 | 10 | 0 | |
| L. leptocheilus | 20 | 0.15 | – | – | |
| L. siamensis | 20 | 0.55 | 10 | 0 | |
| H. siamensis | 20 | 0.45 | – | – | |
| D. ashmeadi | 20 | 0.75 | – | – | |
| O. hasselti | 20 | 0.05 | – | – | |
| B. schwanenfeldii | – | – | 3 | 0 | |
| Hot-dry | C. armatus | 5 | 7 | 61 | 2.1 |
| H. dispar | 11 | 0.27 | 26 | 0.11 | |
| P. brevis | 20 | 5.05 | 78 | 0 | |
| L. leptocheilus | 20 | 0 | – | – | |
| L. siamensis | 20 | 0.1 | – | – | |
| H. siamensis | 20 | 0 | – | – | |
| D. ashmeadi | 15 | 0 | – | – | |
| O. hasselti | 19 | 0 | – | – | |
| C. reticulatus | 9 | 0 | – | – | |
| B. schwanenfeldii | – | – | 1 | 0 | |
| Rainy | C. armatus | 17 | 0.17 | 50 | 0.2 |
| H. dispar | 1 | 0 | 10 | 0.3 | |
| P. brevis | 37 | 8.13 | 3 | 0 | |
| L. siamensis | 50 | 0 | – | – | |
| D. ashmeadi | 5 | 0.2 | – | – | |
| O. hasselti | 16 | 0 | – | – | |
| C. reticulatus | 30 | 0.73 | – | – | |
| S. rubripinnis | 50 | 1.06 | 22 | 0 | |
B. schwanenfeldii = Barbonymus schwanenfeldii; C. armatus = Cyclocheilichthys armatus; C. reticulatus = Crossocheilus reticulatus; D. ashmeadi = Discherodontus ashmeadi; H. dispar = Hampala dispar; H. siamensis = Henicorhynchus siamensis; L. leptocheilus = Labiobarbus leptocheilus; L. siamensis = Labiobarbus siamensis; MTC = metacercariae; O. hasselti = Osteochilus hasselti; P. brevis = Puntius brevis; S. rubripinnis = Systomus rubripinnis.
The number of O. viverrini–infected fish species showed significant seasonal variation in Khammouane Province (χ2 = 67.72, P < 0.001), but such variation was not found in Mukdahan Province (Table 2). In Khammouane Province, eight of 10 species of cyprinid fish (80%) harbored O. viverrini metacercariae. More species of fish were found to be infected with O. viverrini metacercariae in the cool-dry season (100%), and fewer species of fish were infected in the hot-dry (44.4%) and rainy seasons (75%). In Mukdahan Province, Thailand, a similar number of O. viverrini–infected fish species were found over the three seasons. The number of infected species was between 40% and 50% in all seasons (Table 2).
Table 2.
Species of cyprinid fish harboring Opisthorchis viverrini metacercariae by season in Khammouane Province, Lao PDR, and Mukdahan Province, Thailand
| Country | No. of positive species/no. of total species (%) | ||
|---|---|---|---|
| Cool-dry | Hot-dry | Rainy | |
| Khammouane, Lao PDR* | 8/8 (100) | 4/9 (44.4) | 5/8 (75) |
| Mukdahan, Thailand† | 2/5 (40) | 2/4 (50) | 2/4 (50) |
* χ2 = 6.17, P = 0.04.
† χ2 = 0.12, P > 0.05.
Seasonal prevalence and intensity of O. viverrini metacercariae.
Based on the aforementioned screening, three cyprinid fish species comprising C. armatus, H. dispar, and P. brevis were selected for long-term seasonality studies. In total, 1,093 fish were available for analyses, and of these, 673 and 420 fish were from the Nam Don River in Khammouane Province, Lao PDR, and the Huai Khi Lek Reservoir in Mukdahan Province, Thailand, respectively.
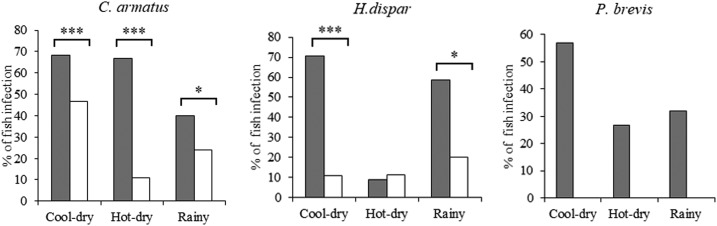
In Khammouane Province, the order of prevalence of O. viverrini metacercariae was highest in H. dispar, followed by C. armatus and then P. brevis. In Mukdahan Province, the prevalence by order was C. armatus and H. dispar (Figure 1).
Figure 1.
Seasonal prevalence of Opisthorchis viverrini metacercarial infection among three cyprinid fish species (*P < 0.05, **P < 0.01, and ***P < 0.001) in Lao PDR (black bars) and Thailand (white bars).
In Khammouane Province, the prevalence of O. viverrini metacercariae in three cyprinid fish species (C. armatus, H. dispar, and P. brevis) varied with season with relatively high prevalence in the cool-dry and hot-dry seasons and the lowest prevalence in the rainy season (χ2 = 26.17, P < 0.001) (Figure 1). In Mukdahan Province, a similar seasonal prevalence pattern was detected in C. armatus (χ2 = 24.62, P < 0.001), but no trend was observed for H. dispar (Figure 1). The prevalence of O. viverrini in each species of fish in Khammouane Province was significantly higher than those in Mukdahan Province for all seasons (P < 0.001). The overall prevalence rate of O. viverrini in the three species of fish over three seasons in Khammouane Province was 51.9% (349/673) and in Mukdahan Province was 18.3% (77/420) (χ2 = 72.32, P < 0.001).
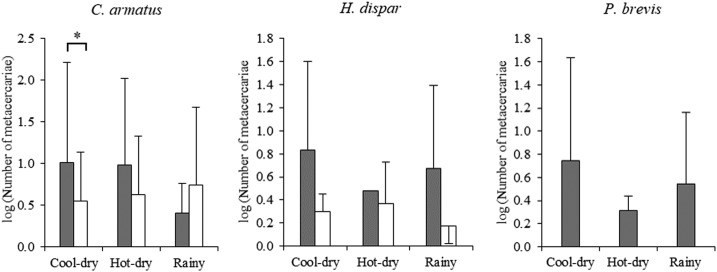
The intensities of O. viverrini metacercarial infection (metacercariae/fish) varied with season when the three species of fish were combined in Khammouane Province (Friedman test, χ22 = 13.3, P = 0.001) and for comparison among fish species (χ22 = 18.2, P < 0.001). In Mukdahan Province, there was no apparent trend in intensity with season for C. armatus and H. dispar (Figure 2). The intensity of O. viverrini in C. armatus and H. dispar from Khammouane Province was greater than those in Mukdahan Province in each season, and significant differences were found in the cool-dry season (P < 0.05). Puntius brevis was not infected during all seasons in Mukdahan Province so that comparison with this species from Khammouane Province was not possible. The overall intensity of O. viverrini in three species of fish over three seasons in Khammouane Province was 6.67 ± 12.88 and in Mukdahan Province was 4.07 ± 5.86.
Figure 2.
Intensity of Opisthorchis viverrini metacercariae among three cyprinid fish species (*P < 0.05, **P < 0.01, and ***P < 0.001) in Lao PDR (black bars) and Thailand (white bars).
Size-related prevalence and intensity of O. viverrini metacercarial infection.
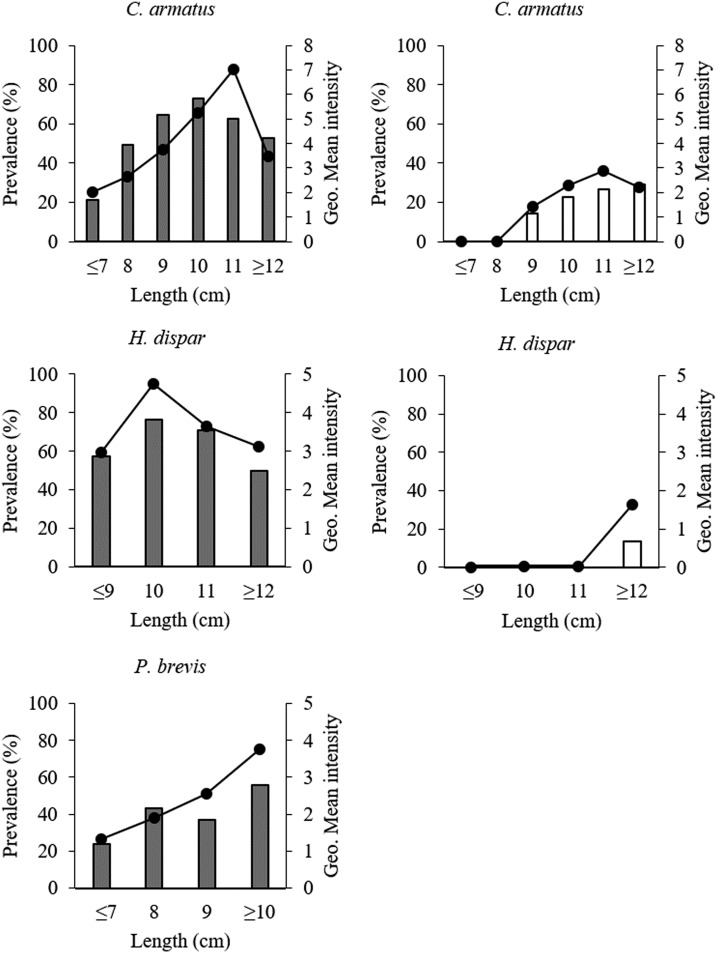
The profiles of prevalence and intensity of infection with O. viverrini showed increasing trends with fish body length (χ2 = 18.09, P < 0.001). Peak prevalence for C. armatus occurred at 10- and 11-cm body length of fish in Khammouane and Mukdahan Province, respectively (Figure 3). The intensity of metacercarial infection (cysts/fish) slightly increased with fish size but did not reach statistical significance (P > 0.05) and peaked at 11-cm body length in C. armatus in both Khammouane and Mukdahan Province. In the case of H. dispar, the peak prevalence and intensity profiles occurred in the 10-cm body length group, but such a pattern was not clear in Mukdahan Province because of an inadequate fish sample size. In the case of P. brevis from Khammouane Province, the prevalence and intensity of O. viverrini increased with fish size (P < 0.001) and peaked at 12-cm length.
Figure 3.
Prevalence and intensity of Opisthorchis viverrini infection in different size classes of Cyclocheilichthys armatus, Hampala dispar, and Puntius brevis in Khammouane Province, Lao PDR (black bars), and Mukdahan Province, Thailand (white bars). Bar graph shows the prevalence (%) of infected fish and line graph shows the geometric mean intensity of metacercaria in size class.
Frequency distribution of metacercariae.
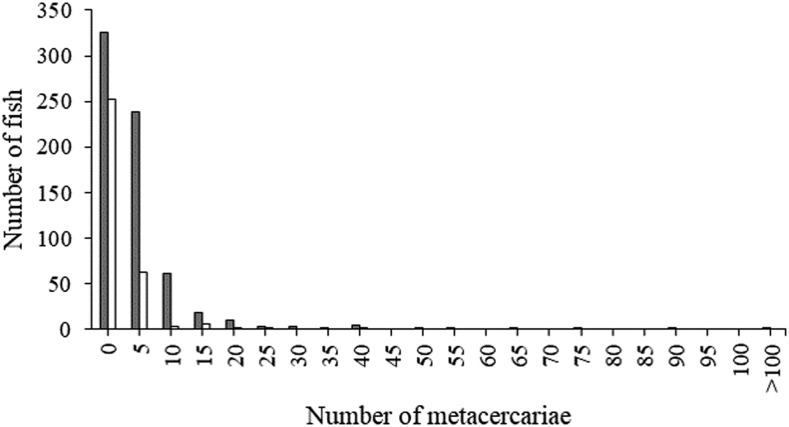
The frequency distributions of metacercariae when the three cyprinid fish species were combined with seasons were skewed in both study areas. Most fish (71.2%) examined harbored a few metacercariae (one to five cysts/fish), and a small percentage of fish (13.4%) had a high metacercarial burden (> 15 cysts/fish) (Figure 4). The overall average intensity was 6.67 ± 12.88 for Khammouane Province and 4.07 ± 5.86 for Mukdahan Province. When individual fish species were considered, a similar skewed distribution pattern was observed (data not shown), and the average metacercarial burdens in fish from Khammouane Province were 8.40 ± 16.41, 6.03 ± 7.61, and 4.37 ± 8.39 for C. armatus, H. dispar, and P. brevis, respectively. In Mukdahan Province, the average burden was 4.30 ± 6.06 and 2.00 ± 1.53 for C. armatus and H. dispar, respectively.
Figure 4.
Frequency distribution of metacercaria in Lao PDR (black bars) and Thailand (white bars) with the mean and SD of 6.67 ± 12.88 and 4.09 ± 5.82, respectively. The metacercarial distributions significantly deviated from normal distribution in both localities (P < 0.05).
Risk factors for O. viverrini infections in cyprinid fish.
A univariate logistic regression model (Table 3) revealed that the risk of infection with O. viverrini was higher in Khammouane Province than in Mukdahan Province. The cool-dry season was associated with a higher risk of infection than other seasons. For fish species, C. armatus was significantly associated with O. viverrini infection. Furthermore, fish with a body length greater than 10 cm were likely to have a higher intensity of infection with O. viverrini than smaller fish of less than 10-cm length.
Table 3.
Binary logistic regression model for the relationships between different variables and Opisthorchis viverrini infection in fish
| Factor | Odd ratio | P-value | 95% CI |
|---|---|---|---|
| Country | |||
| Thailand | Reference | – | – |
| Laos | 6.21 | 0.00* | 4.07–9.49 |
| Season | |||
| Rainy | Reference | – | – |
| Cool-dry | 2.76 | 0.00* | 2.06–3.70 |
| Hot-dry | 0.54 | 0.00* | 0.37–0.79 |
| Species | |||
| Puntius brevis | Reference | – | – |
| Cyclocheilichthys armatus | 1.55 | 0.01* | 1.08–2.23 |
| Hampala dispar | 1.02 | 0.91 | 0.63–1.65 |
| Body size | |||
| < 10 cm | Reference | – | – |
| > 10 cm | 1.50 | 0.00* | 1.17–1.94 |
* Statistical significance (P < 0.05).
DISCUSSION
Transmission of O. viverrini in endemic areas in Southeast Asia is well documented, mainly in humans.1,10,19 Reports on infections in cyprinid fish intermediate hosts acting as a source of infection for humans and reservoir animals, indicating active transmission, are available in some geographical localities.12–14,20 In our study, O. viverrini metacercariae were detected in 10 fish species in Khammouane Province, Lao PDR, and two species in Mukdahan Province, Thailand, similar to the previous records.8,12 Indeed, a high diversity of cyprinid fish has been reported to serve as the intermediate host of O. viverrini in Southeast Asia; as many as 47 species have been recorded from Lao PDR and 46 from Thailand, with many species being shared in both countries.21 Among the cyprinid fish, the most common genera infected with O. viverrini metacercariae are Cyclocheilichthys, Puntius, and Hampala in both Lao PDR and Thailand.10,12,13,22 In our study, O. viverrini metacercariae were found throughout the year in these genera, namely in the species, C. armatus, H. dispar, and P. brevis. The different infection rates observed in the reservoirs/dams in Khammouane Province (51.9%) were greater than in Mukdahan Province (18.3%), which is dependent on local transmission potential.
Previous studies have reported that O. viverrini metacercariae are found to have a greater prevalence in reservoirs than in rivers.8,23 In addition to habitat ecology, this difference may be due to multiple factors, such as fish biodiversity in the Mekong Basin, prevalence in humans and transmission in the snail first intermediate hosts.8,24 Previous surveys have reported a higher human infection prevalence (81.1%) in Khammouane Province25 than in Mukdahan Province (29.5%) (Thai Departments of Prevention and Control of Disease).8
With respect to the species diversity of fish intermediate host, noncyprinid fish such as Channa limbata have been reported to serve as an intermediate host for O. viverrini in Vietnam26 and also for another species of the liver flukes, Opisthorchis lobatus, in Lao PDR.27 So far, there has been no similar evidence reported in Thailand, and thus, noncyprinid fish were not examined in the current study. Moreover, our recent population genetic study suggests that species of cyprinid fish make little contribution to genetic diversity of O. viverrini in Thailand and Lao PDR.28 Thus, the reason for O. viverrini occurring predominantly in certain species of fish reminds to be determined.
The prevalence of O. viverrini in cyprinid fish from both study localities was highest in the cool-dry, followed by rainy and then by the hot-dry seasons, which agrees with previous studies by Vichasri et al.13 and Sithithaworn et al.14 and to an extent to that of Manivong et al.12 The underlying mechanism driven by seasonality is not fully known, but it is believed that the physical availability of rainwater may facilitate the movement of feces contaminated with eggs into bodies of water, allowing the eggs to be ingested by snail intermediate hosts as the first stage of their life cycle. Although rainwater and irrigated water increase the chance of eggs being passively transported to a suitable habitat for the Bithynia snail intermediate hosts, other local factors, such as temperature, play an important role in snail success29 as well as fish infection.30 Other hydrological conditions also support disease transmission, such as low water levels and a high prevalence of infection in the snail intermediate hosts in the cool-dry season.24 The occurrence of these seasonal transmission patterns of O. viverrini in fish has been used in the design of control options, which are then optimal in the cool-dry season.31
Our study is the first attempt to examine the correlation between prevalence and intensity of O. viverrini infection with the age (body size) of cyprinid fish. In Khammouane Province, the infection with O. viverrini metacercariae was highest in C. armatus, P. brevis, and H. dispar that were 10 cm in body length. In Mukdahan Province, peak infection in both C. armatus and H. dispar was found in fish of 12-cm body length. Based on data from aquaculture fish, the age of fish samples here should be less than a year,32 and this corresponds with the infection pattern in snails.33,24 Experimental infection of O. viverrini cercariae in Barbonymus gonionotus showed that the ability of O. viverrini to infect fish increased with increasing age and size of fish.30 The reason for a reduced infection in larger fish size (i.e., > 12 cm) is not clear but could be due to parasite-induced host mortality by removing large, heavily infected fish from the population.34
The skewed (aggregated) distribution patterns of O. viverrini metacercariae in fish from Khammouane and Mukdahan provinces are typical for helminth parasites,35 including previous records in humans.36 The average burdens of a few metacercariae per fish clearly indicate that low repeated dose or trickle infection patterns occur in humans and animal reservoir hosts. Therefore, moderate or heavy infections in human and animal hosts are likely to be a consequence of cumulative and or long-term exposure to infection, which is probably rare as most humans have light infections.37,38
Recently, Pitaksakulrat et al.28 used microsatellite makers for population genetic studies with different species of fish hosts in Thailand and Lao PDR. The result showed O. viverrini was separated into two clusters with one cluster from C. armatus, H. siamensis, and B. gonionotus from Lao PDR and the other cluster from P. brevis from Lao PDR. Only a single genotype was found from C. apogon and H. dispar in Thailand. Although no information is available regarding the infectivity of the different O. viverrini genotypes in cyprinid fish, there is evidence that O. viverrini from Nam Ngum, Lao PDR, has a greater infectivity and inflicts greater mortality in cyprinid fish than from Sakon Nakhon, Thailand.39 The genotype-specific biological differences also extended to reproductive potential and peak cercarial release in Bithynia snails.40,41 The gene flow and circulation of O. viverrini is thus governed by multiple factors related to species of intermediate and definitive host and environmental conditions.
Based on the long-term study design that included seasonal variability, our results revealed that there was a greater diversity of fish species acting as intermediate hosts of O. viverrini in Khammouane Province, Lao PDR, than those in Mukdahan Province, Thailand. The infection prevalence and intensity in fish showed a strong seasonal pattern with peak prevalence occurring in the cool-dry season in both study localities. Both prevalence and intensity of O. viverrini metacercariae were positively associated with the age (body size) of fish. The frequency distribution of O. viverrini metacercariae in fish was skewed, with most of the fish having a low worm burden, with an average burden of four to six cysts/fish. Analysis of risk of infection confirmed that four major factors were operating, namely, seasonality, sampling locality, fish body size, and species of cyprinid. The level of infection in fish may be associated with the net force of transmission locally and correlated with reported human infection in an endemic community.
Although our study design incorporated potential variability that may bias the results, there are several weak points that require consideration. First, our fish sample was incomplete because some species were not available in the seasonal samples. Second, large size ranges of fish were not available for metacercarial analysis. Third, more study sites in different river wetlands are needed to determine the transmission dynamic patterns over a wider geographical area.
In conclusion, this study provides new data on the infection dynamics of O. viverrini metacercaria in cyprinid fish in two endemic localities in Lao PDR and Thailand. The prevalence and intensity of O. viverrini metacercaria varied with season, being high in the cool-dry season and variable in the hot-dry and rainy seasons. The average prevalence and intensity of metacercarial infection was greater in Khammouane Province, Lao PDR, than in Mukdahan province, Thailand. Among the 10 species of cyprinid fish examined, the infection was associated with the fish body size and predominantly found in three species, H. dispar, C. armatus, and P. brevis. The distribution of O. viverrini metacercariae in fish was skewed, with most of the fish having a low worm burden with an average of four to six cysts/fish. The findings that seasonality, sampling locality, fish size, and species of fish play roles in the risk of O. viverrini infection indicate that these host and environmental factors are important for the transmission dynamics and control of O. viverrini.
Acknowledgment:
We acknowledge the support of the Cholangiocarcinoma Screening and Care Program (CASCAP), Khon Kaen University, Thailand.
REFERENCES
- 1.Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B, 2012. The current status of opisthorchiasis and clonorchiasis in the Mekong basin. Parasitol Int 61: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aung WP, et al. 2017. First report and molecular identification of Opisthorchis viverrini infection in human communities from Lower Myanmar. PLoS One 12: e0177130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suwannatrai A, Saichua P, Haswell M, 2018. Epidemiology of Opisthorchis viverrini infection. Adv Parasitol 101: 41–67. [DOI] [PubMed] [Google Scholar]
- 4.Andrews RH, Sithithaworn P, Petney TN, 2018. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol 24: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khuntikeo N, et al. 2015. Cohort profile: cholangiocarcinoma screening and care program (CASCAP). BMC Cancer 9: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IARC , 2011. IARC Monographs on the Evaluation of Carcinogen Risks to Humans. Lyon, France: International Agency Research On Cancer. [Google Scholar]
- 7.Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C, 2014. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci 21: 301–308. [DOI] [PubMed] [Google Scholar]
- 8.Pinlaor S, et al. 2013. Distribution and abundance of Opisthorchis viverrini metacercariae in cyprinid fish in northeastern Thailand. Korean J Parasitol 51: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholz T, Ditrich O, Giboda M, 1990. Larval stages of medically important flukes (Trematoda) from Vientiane Province, Laos. Part I. Metacercariae. Ann Parasitol Hum Comp 65: 238–243. [DOI] [PubMed] [Google Scholar]
- 10.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S, 2008. Fishborne trematode metacercariae detected in freshwater fish from Vientiane municipality and Savannakhet Province, Lao PDR. Korean J Parasitol 46: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisiengmay S, 2013. Fishborne trematode metacercariae in Luang Prabang, Khammouane, and Saravane Province, Lao PDR. Korean J Parasitol 51: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manivong K, Komalamisra C, Waikagul J, Radomyos P, 2009. Opisthorchis viverrini metacercariae in cyprinoid fish from three rivers in Khammouane Province, Lao PDR. J Trop Med Parasitol 32: 23–29. [Google Scholar]
- 13.Vichasri S, Viyanant V, Upatham ES, 1982. Opisthorchis viverrini: intensity and rates of infection in cyprinoid fish from an endemic focus in northeast Thailand. Southeast Asian J Trop Med Public Health 13: 138–141. [PubMed] [Google Scholar]
- 14.Sithithaworn P, Pipitgool V, Srisawangwong T, Elkins DB, Haswell-Elkins MR, 1997. Seasonal variation of Opisthorchis viverrini infection in cyprinoid fish in north-east Thailand: implications for parasite control and food safety. Bull World Health Organ 75: 125–131. [PMC free article] [PubMed] [Google Scholar]
- 15.Kottelat M, 2013. The fishes of the inland waters of southeast Asia: a catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bull Zool 27: 1–663. [Google Scholar]
- 16.Srisawangwong T, Sithithaworn P, Tesana S, 1997. Metacercariae isolated from cyprinoid fishes in Khon Kaen district by digestion technic. Southeast Asian J Trop Med Public Health 28: 224–226. [PubMed] [Google Scholar]
- 17.Kaewkes S, 2003. Taxonomy and biology of liver flukes. Acta Trop 88: 177–186. [DOI] [PubMed] [Google Scholar]
- 18.Phan VT, Ersbøll AK, Bui TQ, Nguyen HT, Murrell D, Dalsgaard A, 2010. Fish-borne zoonotic trematodes in cultured and wild-caught freshwater fish from the Red River Delta, Vietnam. Vector Borne Zoonotic Dis 10: 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai JY, et al. 2005. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane municipality and Saravane Province in Laos. J Helminthol 79: 283–289. [DOI] [PubMed] [Google Scholar]
- 20.Dao TT, Bui TV, Abatih EN, Gabriël S, Nguyen TT, Huynh QH, Nguyen CV, Dorny P, 2016. Opisthorchis viverrini infections and associated risk factors in a lowland area of Binh Dinh Province, Central Vietnam. Acta Trop 157: 151–157. [DOI] [PubMed] [Google Scholar]
- 21.Petney TN, Andrews RH, Saijuntha W, Tesana S, Prasopdee S, Kiatsopit N, Sithithaworn P, 2018. Taxonomy, ecology and population genetics of Opisthorchis viverrini and its intermediate hosts. Adv Parasitol 101: 1–39. [DOI] [PubMed] [Google Scholar]
- 22.Waikagul J, 1998. Opisthorchis viverrini metacercaria in Thai freshwater fish. Southeast Asian J Trop Med Public Health 29: 324–326. [PubMed] [Google Scholar]
- 23.Ong X, Wang Y, Sithithaworn P, Grundy-Warr C, Pitaksakulrat O, 2016. Dam influences on liver fluke transmission: fish infection and human fish consumption behavior. Ann Am Assoc Geogr 106: 755–772. [Google Scholar]
- 24.Namsanor J, Sithithaworn P, Kopolrat K, Kiatsopit N, Pitaksakulrat O, Tesana S, Andrews RH, Petney TN, 2015. Seasonal transmission of Opisthorchis viverrini sensu lato and a lecithodendriid trematode species in Bithynia siamensis goniomphalos snails in northeast Thailand. Am J Trop Med Hyg 93: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai JY, et al. 2009. High prevalence of Haplorchis taichui, Phaneropsolus molenkampi, and other helminth infections among people in Khammouane Province, Lao PDR. Korean J Parasitol 47: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dao TH, Nguyen TG, Victor B, Gabriël S, Dorny P, 2014. Opisthorchis viverrini-like liver fluke in birds from Vietnam: morphological variability and rDNA/mtDNA sequence confirmation. J Helminthol 88: 441–446. [DOI] [PubMed] [Google Scholar]
- 27.Thaenkham U, Nuamtanong S, Vonghachack Y, Yoonuan T, Sanguankiat S, Dekumyoy P, Prommasack B, Kobayashi J, Waikagul J, 2011. Discovery of Opisthorchis lobatus (Trematoda: Opisthorchiidae): a new record of small liver flukes in the greater Mekong sub-region. J Parasitol 97: 1152–1158. [DOI] [PubMed] [Google Scholar]
- 28.Pitaksakulrat O, et al. 2017. Preliminary genetic evidence of two different populations of Opisthorchis viverrini in Lao PDR. Parasitol Res 116: 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasopdee S, Kulsantiwong J, Piratae S, Khampoosa P, Thammasiri C, Suwannatrai A, Laha T, Grams R, Loukas A, Tesana S, 2015. Temperature dependence of Opisthorchis viverrini infection in first intermediate host snail, Bithynia siamensis goniomphalos. Acta Trop 141: 112–117. [DOI] [PubMed] [Google Scholar]
- 30.Donthaisong C, Arunsan P, Suwannatrai K, Prasopdee S, Kulsantiwong J, Wongmaneeprateep S, Suwannatrai A, Tesana S, 2014. Experimental infection of Opisthorchis viverrini cercariae to the cyprinid fish, Barbonymus gonionotus. Acta Trop 136: 118–122. [DOI] [PubMed] [Google Scholar]
- 31.Hinz E, Saowakontha S, Pipitgool V, 1994. Opisthorchiasis control in northeast Thailand: proposal for a new approach. Appl Parasitol 35: 118–124. [PubMed] [Google Scholar]
- 32.Pitaksakulrat O, Sithithaworn P, Laoprom N, Laha T, Petney TN, Andrews RH, 2013. A cross-sectional study on the potential transmission of the carcinogenic liver fluke Opisthorchis viverrini and other fishborne zoonotic trematodes by aquaculture fish. Foodborne Pathog Dis 10: 35–41. [DOI] [PubMed] [Google Scholar]
- 33.Petney T, Sithithaworn P, Andrews R, Kiatsopit N, Tesana S, Grundy-Warr C, Ziegler A, 2012. The ecology of the Bithynia first intermediate hosts of Opisthorchis viverrini. Parasitol Int 61: 38–45. [DOI] [PubMed] [Google Scholar]
- 34.Poulin R, 2000. Variation in the intraspecific relationship between fish length and intensity of parasitic infection: biological and statistical causes. J Fish Biol 56: 123–137. [Google Scholar]
- 35.Anderson RM, May RM, 1985. Helminth infections of humans: mathematical models, population dynamics, and control. Adv Parasitol 24: 1–101. [DOI] [PubMed] [Google Scholar]
- 36.Sithithaworn P, Tesana S, Pipitgool V, Kaewkes S, Pairojkul C, Sripa B, Paupairoj A, Thaiklar K, 1991. Relationship between faecal egg count and worm burden of Opisthorchis viverrini in human autopsy cases. Parasitology 102: 277–281. [DOI] [PubMed] [Google Scholar]
- 37.Upatham ES, Viyanant V, Kurathong S, Rojborwonwitaya J, Brockelman WY, Ardsungnoen S, Lee P, Vajrasthira S, 1984. Relationship between prevalence and intensity of Opisthorchis viverrini infection, and clinical symptoms and signs in a rural community in north-east Thailand. Bull World Health Organ 62: 451–461. [PMC free article] [PubMed] [Google Scholar]
- 38.Saijuntha W, Duenngai K, Tangkawattana S, Petney TN, Andrews RH, Sithithaworn P, 2018. Recent advances in the diagnosis and detection of Opisthorchis viverrini sensu lato in human and intermediate hosts for use in control and elimination programs. Adv Parasitol 101: 177–214. [DOI] [PubMed] [Google Scholar]
- 39.Kopolrat K, Sithithaworn P, Kiatsopit N, Pitaksakulrat O, Tesana S, Andrews RH, Petney TN, 2016. Comparison of infectivity, metacercarial burden and host mortality induced by Opisthorchis viverrini sensu lato cercariae from Lao PDR compared with Thailand in cyprinid fish, Barbonymus gonionotus. Trans R Soc Trop Med Hyg 110: 46–54. [DOI] [PubMed] [Google Scholar]
- 40.Kiatsopit N, Sithithaworn P, Kopolrat K, Namsanor J, Andrews RH, Petney TN, 2016. Trematode diversity in the freshwater snail Bithynia siamensis goniomphalos sensu lato from Thailand and Lao PDR. J Helminthol 90: 312–320. [DOI] [PubMed] [Google Scholar]
- 41.Laoprom N, Kiatsopit N, Sithithaworn P, Kopolrat K, Namsanor J, Andrews RH, Petney TN, 2016. Cercarial emergence patterns for Opisthorchis viverrini sensu lato infecting Bithynia siamensis goniomphalos from Sakon Nakhon Province, Thailand. Parasitol Res 115: 3313–3321. [DOI] [PubMed] [Google Scholar]