Abstract.
Pneumonia is one of the leading causes of death in children under 5 years worldwide. In resource-limited settings, WHO recommendations state that pneumonia can be presumptively diagnosed through the presence of cough and/or difficult breathing and a respiratory rate (RR) that is higher than age-specific cutoffs. As a new diagnostic aid the children’s automated respiration monitor (ChARM) can automatically measure and classify RR in children under 5 years, but the effect of its chest attachment on the RR has not been studied. The aim of this study was to understand if misclassification of the true RR occurred by ChARM attachment. Two hundred eighty-seven children at a health center in South Ethiopia were screened for eligibility, with 188 children aged 2–59 months enrolled in the study. The RR was measured manually before and 1, 3, and 5 minutes after ChARM attachment. The proportion of children with fast or normal RR classification at baseline and the change between RR classifications over time were analyzed. Eight (4.9%; 95% CI 2.1, 9.4) of 163 children changed RR classification from normal to fast between the baseline RR count and the 1 minute RR count. Results from this study suggest that ChARM has a minor influence on the RR of children immediately after attachment, in most cases without clinical importance.
INTRODUCTION
Pneumonia is the leading cause of death after the neonatal period among children under 5 years of age globally, amounting to an estimated 880,000 deaths each year.1 Two-thirds of all pneumonia-caused deaths in children are centered in only 15 countries in sub-Saharan Africa and Asia.2 In Ethiopia, pneumonia is the top cause of under-five mortality, being responsible for almost 18% of all deaths and approximately 3.9 million new pneumonia cases per year.3,4 Mortality from pneumonia in children mainly follows from inadequate prevention from risk factors such as malnutrition, delayed presentation of symptoms, inappropriate care and treatment or a presumption that the symptoms are caused by other illnesses, such as malaria, in endemic areas.5,6
As per WHO Integrated Management of Neonatal and Child Illness (IMNCI) and Integrated Community Case Management recommendations and tools, the diagnosis of pneumonia in low-resource settings is based on assessing if the respiratory rate (RR), the number of respiratory cycles in 60 seconds, is higher than the normal parameters for a child of that age with cough and/or difficult breathing. A child aged 0 to < 2 months, 2 to < 12 months, and 12 to 59 months has fast breathing if the RR is over 60, 50, and 40 breaths per minute or more, respectively.7–9
Currently, the standard practice for first-level health workers and community health workers is to count RR by observing chest movements during 60 seconds, typically using an acute respiratory infection timer, which is a simple small device that beeps after 60 seconds from being started. However, this approach can be difficult and highly subjective for all levels of providers, as children breathe irregularly and faster than adults, the child may not be calm and still for a full minute, and it is difficult to define what is and is not a breath.10 Although smartphone applications based on the observed RR have been introduced with some promise of improved accuracy and efficiency,11 misclassification of the RR using this standard approach is still common, subsequently leading to misdiagnosis of children with pneumonia and inappropriate treatment.6,12,13
Several new RR diagnostic support aids are under development from the private and public sector, but no ultimate aid has so far been found that can enhance the accuracy and effectiveness of diagnosing pneumonia in low-resource settings.10,14,15 Within the Acute Respiratory Infection Diagnostic Aid project, United Nations Children’s Fund (UNICEF) aimed to contribute to the development of automated RR counting aids that health workers in low-resource settings, both in the community and at health facilities, could use.10,16,17 Although challenges regarding the accuracy and usability of such devices clearly exist, they might provide a level of objectivity to RR measurements. The first counting aid to be considered within the project was the children’s automated respiration monitor (ChARM), which comprises an accelerometer-based RR counter with a belt to attach the device to the child.
The device dimensions are 65 × 65 × 28 mm (without belt), it weighs 95 g (including belt), and it has a battery life of 2,000 measurements over 2 years of usage. After the child has been properly positioned and is calm, the device is attached to the belly of the child and the age group to which the child belongs to is selected, and the device counts the RR and automatically classifies the RR as normal or fast according to the WHO recommended thresholds. The ChARM has been approved for all the necessary regulatory safety compliances for a medical device (CE class IIa, IEC 60601-1 Ed3.1, IEC 60601-1-2 Ed 3, and IEC 60601-1-11 Ed1).18
Developed in 2015, the ChARM had only been subject to one presented evaluation to date. Providing a first preliminary study of the accuracy of the ChARM, Shah et al.19 found that there were no statistically significant difference between the ChARM and the mean manual RR count of one IMNCI-trained health worker and two IMNCI-trained physicians in Kenya, suggesting that ChARM could serve as an effective diagnostic aid for identifying children with fast breathing. More performance data are forthcoming; however, the results seem to be comparable with that of other counting aids such as mobile applications11 and timers10 as well as the performance of community health workers counting RR when diagnosing pneumonia in low-income settings12,20,21 and within the range of ± 2 breaths per minute from the actual RR.19
While ChARM has not been subject to rigorous external evaluation, it is accurate and consistent in its readings according to the early results described previously and by the manufacturer.22 Yet, the RR of a child can be influenced by a number of physiological and contextual factors, and studies have shown that contextual factors such as noise or other disturbances, for example, the touch of a stethoscope elevates the RR.13,23,24 There is a clear lack of evidence to what extent the touch or attachment of devices such as ChARM themselves lead to an elevated RR. Even though the ChARM is noninvasive, the attachment itself could disturb the child, subsequently leading to a rise in RR and potential unintentional misclassification of the RR by the device. Because this is critical for the performance and clinical applicability of ChARM it merits further investigation.
Hypothesizing that the ChARM attachment could cause a misclassification of the RR, the aim of this study was to understand if the attachment of the ChARM had an impact on the classifications of fast or normal breathing in children aged between 2 and 59 months in a controlled setting.
MATERIALS AND METHODS
Study design, setting, and population.
This was a descriptive study conducted in a health center setting in the Southern Nations, Nationalities, and Peoples’ Region (SNNPR) in Ethiopia. After assessing health centers in the region based on logistical feasibility and a suitable number of eligible children, the Millionium Health Centre in Hawassa was purposively chosen.
All children aged 0–5 years seeking routine care from the Millionium Health Centre were potential participants in the study. Hence, all children presenting at the health facility during weekdays from the March 6, 2018 to March 23, 2018 were systematically screened for eligibility. Young infants (0 to < 2 months) were excluded from the evaluation because of the anticipated difficulty in measuring RR in that age group for an extended period of time. Children who were 2–59 months of age accompanied by a parent or guardian who was 18 years or older, not too agitated to be assessed by a research nurse, did not present any IMNCI general danger signs (active convulsions/fits, unconscious/lethargic, and not breastfeeding/not drinking or vomiting everything), IMNCI pink referral signs for severe disease (stridor, severe dehydration, severe persistent diarrhea, very severe febrile disease, severe complicated measles, mastoiditis, complicated severe malnutrition, and severe anemia), chest indrawing, or device manufacturer safety exclusion criteria (wearing supportive device at the area of chest/belly or skin not intact in chest/belly) were eligible to participate in the study. Written informed consent was obtained from the parent or guardian before enrolment in the study.
Outcomes.
The primary outcome was the proportion of children aged 2–59 months whose RR classification of normal or fast breathing changed, as per WHO recommendations, when measured without and with the ChARM attached.
Secondary outcomes were the proportion of unsuccessful attempts in obtaining a manual RR and an automated ChARM RR count, respectively, as well as the reasons for these being unsuccessful. The failed attempts were categorized based on whether the attempt was made with the manual device (expert counter lost count, child became agitated, the RR reading could not be obtained during examination period, or other) or with ChARM (device fault, child became agitated, the ChARM reading could not be obtained during examination period, or other). The time it took from start to finish for a health facility worker to attain a ChARM reading, from when the device was strapped to the child until a reading could be obtained, was also documented.
Procedures.
A standard process for data collection was developed together with the personnel at the health center in accordance with the patient flow plans, ensuring that the normal clinical management of the child was not compromised with and adhered to the country and health facility triage and clinical management algorithms, including IMNCI.
The eligibility of potential participants was ascertained through a screening process. The screening results were documented on a paper-based standardized screening checklist and later entered into a digital form on tablets. First, the child had their medical history taken and physical examination performed by a research nurse to verify that the child did not have any of the exclusion criteria. If the child could not be assessed for general danger signs and IMNCI pink referral signs for severe disease or chest indrawing due to the child being agitated for a prolonged period of time (3–5 minutes), the screening process ended and the child was not included. If the child presented none of the exclusion criteria, the research nurse continued the screening procedure to first confirm that the age of child’s parent or guardian was 18 years and greater, that the age of the child was not less than 2 or greater than 59 months, and at last conducted a quick assessment of the child to ensure the child did not have any device manufacturer safety exclusion criteria. The research nurse and the expert counter were selected based on academic qualifications, essential registration to work in a health facility in Ethiopia and professional experience in the care of children and IMNCI.
If the child passed the screening process, the parent or guardian of the child was invited to undergo the informed consent process. In this process, a paper-based information and consent form was given to the parent or guardian or verbally presented by the research nurse. At the end of the consent process, the parent or guardian and the research nurse signed or printed their thumbprint on two copies of the consent document. A copy was retained by the research team and one was given to the parent or guardian who also kept the information form.
If informed consent was obtained, an expert counter (a nurse trained in counting the RR) validated the screening checklist to make sure that the child was eligible. If the validation confirmed eligibility and informed consent was obtained, the subject was enrolled in the study and the RR classification evaluation began.
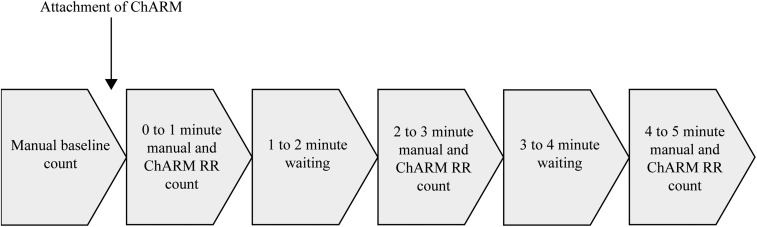
The assessment was time bound (Figure 1) and no repeat RR readings were taken. If a reading could not be obtained within the assessment period, it was recorded as an unsuccessful attempt and the assessment continued if possible. After confirming the enrolment of the child, the child was positioned comfortably on the parent or guardian’s lap or on the examination couch (with child’s chest and belly fully exposed). The expert counter conducted a manual baseline RR count, and a research officer recorded the baseline RR count and RR classification on a digital data collection form. Directly after obtaining a manual baseline RR count, the expert counter attached the ChARM according to the device instructions. After the attachment, the expert counter conducted a 1-minute manual RR count and the research officer conducted a 1-minute ChARM RR count. This was repeated for the 3- and 5-minute counts, respectively. If obtained, the research officer recorded the RR count and classification on the digital data collection form. The research officer also recorded the unsuccessful attempts to obtain an RR count with the reason for it. To estimate how long a ChARM RR count takes in practice, the research officer started a stopwatch when the expert counter took the ChARM from the nearby table and stopped the clock when the first RR reading was obtained. This was not repeated for subsequent measurements.
Figure 1.
Timeline for the respiratory rate (RR) classification assessment.
Rigorous quality assurance measurements were undertaken. All data collection forms were entered into the data collection software CommCare (version 2.38.1, Dimagi, Cambridge, MA) for each enrolled participant, linked by use of a unique identification code and stored on password-protected tablets and uploaded to a CommCare server immediately after entry (providing mobile network access) or on a daily basis when the research team returned to base. A standard operating procedures document with roles and responsibilities of all research team members was developed before the start of the study. The research nurse and expert counter participated in a 1-day training on counting RR in children and using the ChARM correctly and were examined on counting the RR on training videos, where they provided results that showed they were standardized to ± 2 breaths per minute from the actual RR in the videos. Furthermore, a 1-day pretest of the data collection tools and processes was conducted at the health center. All data collected were subject to validation by a data manager to ensure accurate, valid, and complete data in the correct format (text, number, and predefined options). In addition, 10% of all the data were entered twice by the data manager to make sure that the data were accurate. At the end of each day of the data collection period, the research officer checked all data collection forms and followed up any incomplete or inaccurate data submissions.
Statistical analysis.
The study was powered for the primary outcome, that is, to measure the proportion of children aged 2–59 months whose RR classification changed, as per WHO recommendations, when measured with the ChARM attached, using the sample size formula for a proportion (or prevalence) survey with a specified level of confidence and precision; n = (1.96)2(p [1−p]/e2), where p is the expected population proportion and e is the desired precision (half desired CI width). Because of the very limited knowledge of the expected true proportion of classifications that change from normal to fast or vice versa after ChARM attachment, the expected true proportion was estimated to be 75%, drawing on past experiences of the research team. The desired precision was set at 7.5%, allowing for a more feasible sample size and confidence level to 95%. This resulted in a necessary sample size of 129 subjects with the 95% CI limits 67.5–82.5% (these limits equal prevalence ± precision). The number was inflated to 154 to account for potential losses to follow-up.
The primary outcome was analyzed through classifying the RR before (at baseline) and after the ChARM attachment (at 1, 3, and 5 minutes) based on the manual RR count as described earlier. This was to ensure that the only variable parameter was the attachment of the ChARM. A CI for the proportions of the number of children classified as having a normal and fast RR, respectively, between each time point was constructed through the Clopper–Pearson method.25 All quantitative data were analyzed in R 3.4 statistical software (R Foundation for Statistical Computing, Vienna, Austria).26
Ethical considerations.
The ChARM being used in this evaluation has European conformity medical device approval. The device has already been tested in the field in Kenya.19 No adverse events associated with the ChARM have been reported. A device programming error was reported and resolved before the study started, and the updated ChARM was used in this study. Importantly, the ChARM is not invasive and does not cause the child undue pain or discomfort. The device does not elicit pain or other noxious stimuli when near or in contact with the child’s body. The study protocol was submitted to and approved by the SNNPR Health Bureau (ref: 9026-19/16635).
RESULTS
Description of the population.
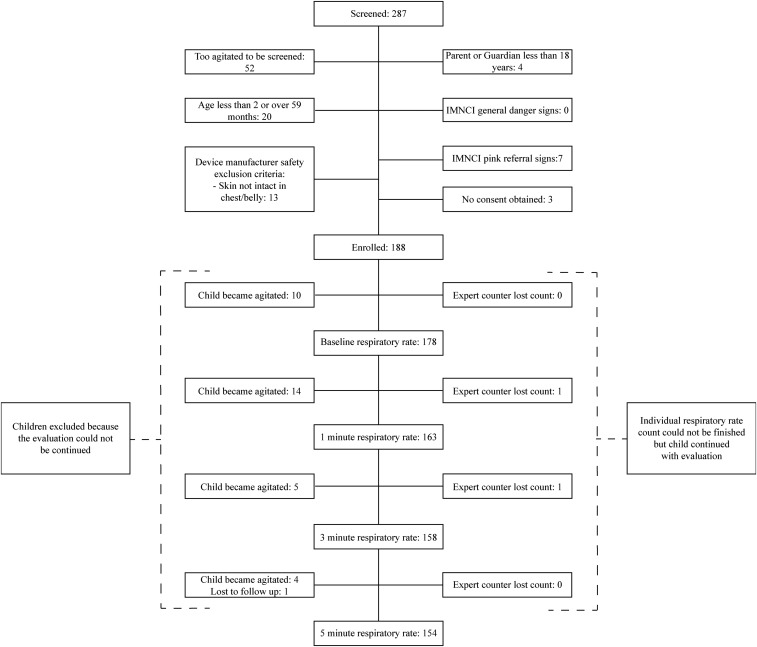
From March 6, 2018 to March 23, 2018, all 287 children aged 0–5 years who presented at the health center were screened for eligibility. Of those, 52 children were too agitated to be screened for inclusion, whereas 47 were subject to other exclusion criteria. Subsequently, 188 children were deemed eligible according to study criteria and were enrolled. At every stage of the evaluation, a number of children were excluded leading to a constant decline in study participants who were able to start and finish all RR assessments, with the foremost reason being that the child became too agitated to allow for continuation of the assessment (Figure 2).
Figure 2.
Study flowchart. IMNCI = Integrated Management of Newborn and Child Illnesses.
The characteristics of the enrolled participants are described in Table 1 together with the measurement of the time duration of the first ChARM count. As the classification of RR is specific for different age groups of children, Table 1 illustrates that the number of the enrolled subjects are balanced with regard to gender and that 30% of the children were in the younger age group. The mean time for the expert counter to conduct the first ChARM assessment was 135 seconds or 2.25 minutes (minimum 83 seconds and maximum 204 seconds).
Table 1.
Descriptive characteristics of the enrolled children
| Characteristic | N | % | Mean (SD) | Median |
|---|---|---|---|---|
| Age (all, months) | 188 | 100 | 24.9 (16.6) | 20.0 |
| Age (2 to < 12 months) | 56 | 29.8 | 7.7 (2.5) | 8 |
| Age (12–59 months) | 132 | 70.2 | 32.2 (14.4) | 30 |
| Gender: male | 97 | 48.4 | – | – |
| Gender: female | 91 | 51.6 | – | – |
The primary outcome, the proportion of children aged 2–59 months whose RR classification changed when measured with ChARM, is presented in Table 2, which describes the proportions of children whose RR classification changed from one time point to another. Overall, there were few children who changed RR classification between time points during the evaluation with the proportion having the same RR classification between all time points being very similar. However, eight (4.9%; 95% CI 2.1, 9.4) of 163 children changed RR classification from normal to fast between the baseline RR count and the 1-minute RR count. This change does not appear between other time points, and there seem to be an immediate change back from fast to normal RR classification between the 1 and 3 minutes time-point. For the whole duration of the evaluation, from baseline to the 5-minute RR count, six children (3.9%; 95% CI 1.4, 8.3) changed from normal to fast RR classification, whereas two children (1.3%; 95% CI 0.2, 4.6) changed from fast to normal RR classification.
Table 2.
Respiratory rate classification over time (measured by expert counter)
| Time points | |||||
|---|---|---|---|---|---|
| Baseline → 1 minute, N = 163 | 1 minute → 3 minutes, N = 157 | 3 minutes → 5 minutes, N = 153 | Baseline → 3 minutes, N = 158 | Baseline → 5 minutes, N = 154 | |
| Classification at time points | % (95% CI) [N] | % (95% CI) [N] | % (95% CI) [N] | % (95% CI) [N] | % (95% CI) [N] |
| Normal–normal | 70.6 (62.9–77.4) [115] | 70.1 (62.3–77.1) [110] | 72.5 (64.8–79.4) [111] | 70.6 (63.8–78.4) [113] | 72.1 (64.3–79.0) [111] |
| Normal–Fast | 4.9 (2.1–9.4) [8] | 0.6 (0.0–3.5) [1] | 0.7 (0.0–3.6) [1] | 3.8 (1.4–8.1) [6] | 3.9 (1.4–8.3) [6] |
| Fast–normal | 0.0 (0.0–2.2) [0] | 2.5 (0.7–6.4) [4] | 0.7 (0.0–3.6) [1] | 1.3 (0.2–4.5) [2] | 1.3 (0.2–4.6) [2] |
| Fast–fast | 24.5 (18.1–31.9) [40] | 26.8 (20.0–34.4) [42] | 26.1 (19.4–33.9) [40] | 23.4 (17.1–30.8) [37] | 22.7 (16.4–30.2) [35] |
The difference in total number of RR counts between Table 2 and Figure 2 is due to the fact that the expert counter lost count of the RR at two separate instances, once at the 1-minute time point and once at the 3-minute time point. However, this did not lead to the exclusion of all of the child´s measurements; hence, because Table 2 accounts for the classification change between two time points, these instances lead to one lesser observation for the change between the 1–3 and 3–5 time points, respectively.
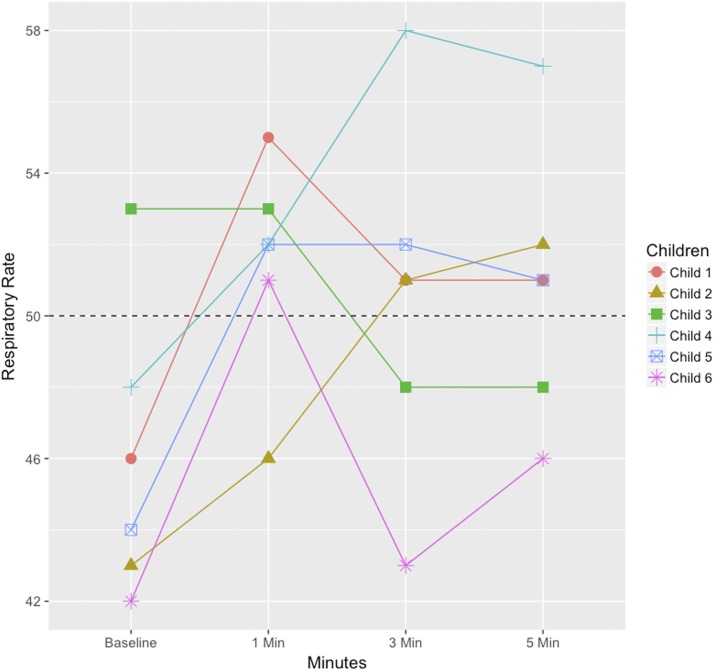
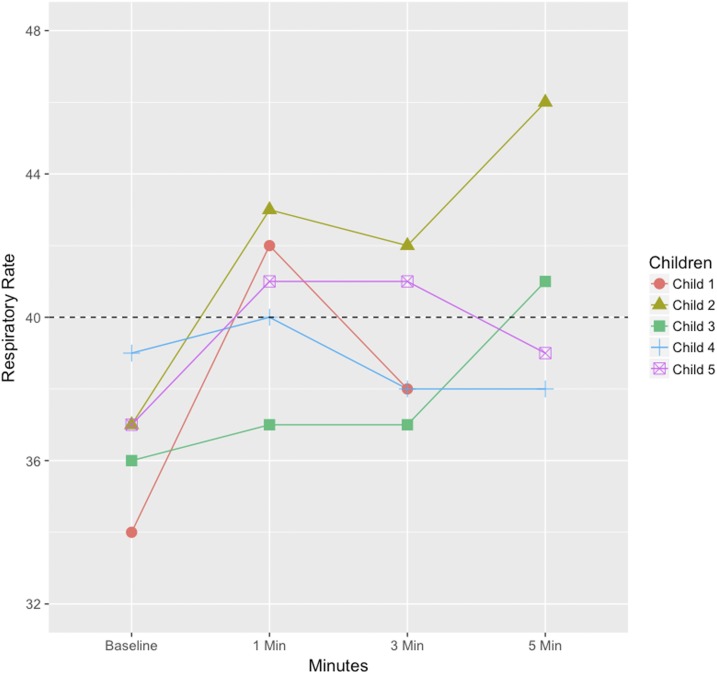
Figures 3 and 4 illustrate the specific RR counts of the children (in total 11, six children in age group 2 to < 12 months and five children in age group 12–59 months) who changed RR classification during the evaluation, to understand in greater detail the pattern of RR changes. For the age group 2 to < 12 months, four children changed from normal to fast RR when the ChARM was attached; of those, three children had a fast RR for the whole evaluation, whereas one child changed back to a normal RR. One child changed from fast to normal RR and vice versa in between the 3- and 5-minute RR count. Similarly, in the age group 12–59 months, four children changed RR classification from normal to fast when the ChARM was attached; however, two of those changed back to a normal RR from the 1- to 3-minute time point and one changed back to a normal RR between the 3- and 5-minute time point. In addition, one child changed from normal to fast RR between the 3- and 5-minute time point.
Figure 3.
Respiratory rate (RR) of children aged 2 to < 12 months whose RR classification changed during the evaluation (measured by expert counter). The dashed line is the cutoff value for the age group. This figure appears in color at www.ajtmh.org.
Figure 4.
Respiratory rate (RR) of children aged 12–59 months whose RR classification changed during the evaluation (measured by expert counter). The dashed line is the cutoff value for the age group. This figure appears in color at www.ajtmh.org.
DISCUSSION
This descriptive study conducted in a health center setting aimed to investigate if attachment of an accelerometer-based RR counter on children under 5 years can cause a misclassification of the RR in a child. The results from this study suggest that the ChARM had a small influence on the RR classification in children between 2 and 59 months after attachment that seem to stabilize after 3–5 minutes.
To our knowledge, no previous studies have been conducted to evaluate how the ChARM attachment affects the RR in children. The study in Kenya by Shah et al.19 did not evaluate this feature of the performance of the ChARM. However, aspects of measuring RR in children in general and other devices that count RR specifically have been studied and are explored in the following paragraphs.
Difficulty of obtaining an RR in children.
In our study, only 154 out of the 188 enrolled subjects were assessed for all the four time points which occurred within approximately 6 minutes; almost all (32 out of 34 of the exclusions) were due to the child being too agitated to either start or continue the RR assessment. A study on the RR variability in children noted a similar rate of failure to attain an RR measurement,27 but many studies simply only refer to children being uncooperative as a reason for RR not easily being measured.28–30 It is possible that agitation is one of the reasons why RR is not measured. Moreover, the actual time it takes to conduct a ChARM RR assessment (in this study, mean time was 135 seconds) is longer if RR is counted manually, which could be one reason for children becoming agitated. In our study, we cannot conclude whether a child became agitated because of the ChARM attachment and continued use or if it was due to the state of the child or some other factor. Because there were a similar proportion of children who became too agitated to take part in the baseline count as the 1-minute count (5–6%), it does not seem that the ChARM attachment agitates the child, but it further emphasizes the need to ensure that the child is calm before starting any respiratory assessment, which holds true also for manual measurements. A change in study design, whereby a child is first observed for the set time of the assessment and then the actual assessment begin, could have shined more light on the issue. It is nevertheless clear that an uncooperative or agitated child severely hampers the possibility of conducting a successful RR assessment and, thus, limits the usability of RR as a vital clinical marker for pneumonia in children regardless if an RR counting aid such as ChARM is used or not.
Respiratory rate fluctuations over time in children.
With approximately 5% of the children changing RR classification from normal to fast between the baseline RR count and the 1-minute RR count (Table 2), with four out of eight children continuing to have fast breathing throughout the evaluation as illustrated by Figures 3 and 4, our results suggest that the attachment of an accelerometer-based RR counter influence the RR in children to a small degree and that the effect seems to stabilize over time. In other studies, albeit not statistically significant, the RR has been shown to initially decline and to be irregular in similar settings when documenting RR without any interference.13,31 Regrettably, the design of our study did not permit us to estimate or conclude how much of the change in RR of a child that was due to the attachment of the ChARM versus natural RR fluctuations over time. However, the RR in children has been shown to be subject to a great variability with nearly 50% of children having a variation of 14 breaths per minute over 1 hour in a similar setting.27 It might be so that the potential small effect on the RR shown in our study stems from the natural variability in RR. More so, as described in Figure 2, the main reason for not taking part in subsequent RR measurements was that the child became agitated. Because the children who were agitated were excluded, the participating subjects at the 3- and 5-minute RR measurement is likely biased toward being calmer than the population at baseline and 1 minute and, therefore, less prone to a change in RR which in turn could affect the RR classifications at the time points leading to an artificial trend.
Impact of medical devices on RR.
That the context might affect the RR assessment has been suggested at least since 183632; also in modern clinical practice, there is a widespread notion of “white coat pneumonia” whereby the presence of a health worker and the disturbance of measuring the RR is assumed to increase the RR in children. Measuring the RR with a stethoscope or electronically through chest wall sensors have been shown to elevate the RR in comparison with observation, but it is still debated if this is due to the increased sensitivity of the stethoscope or chest wall monitors to identify breaths compared with observing or if it indeed is because of the disturbance of the child.23,33 In addition, the difference does not seem to appear in children younger than 2 months.34 A large systematic review of observational studies by Fleming et al.35 showed that there were no significant differences in the RR of children if it was measured manually or in some automated way. For electronic RR measurement, there are two broad groups of devices, either basic chest wall sensors or invasive devices used in advanced hospital care.36 Because the device investigated in this study, ChARM, does not fall into either of those categories, it is difficult to try to compare the results from previous studies conducted with the results from this study. Still, our findings support the overall conclusion present in the literature that the attachment of a noninvasive medical device will elevate the RR in some children but to a limited degree, the implications of this limited potential influence on antibiotic treatment, and possible overuse of antibiotics must be evaluated further.
Strengths and limitations.
Acknowledging the limited literature on ChARM attachment, the strength of this study is that the phenomenon is investigated at a study site which is almost the exact context in which the device aims to be used in. Importantly, no previous study has examined the effects of ChARM attachment, giving insights that could assist with developing a ChARM classification algorithm that takes this contextual effect into account. Moreover, the sample size is sufficient to allow for sound statistical analysis to examine the influence of the ChARM on the RR.
There is no gold standard for measuring RR, but it is usually preferred that a panel of two to four expert pediatricians serves as the most correct proxy for the correct RR measurement.29 In this study, we used only one manual counter to determine the RR, serving as a base for the classification of the RR and subsequent analysis. This is a key limitation, but by analyzing only the manual count and not the ChARM RR count, we could be assured that we were only analyzing the RR difference and not the difference in measurement of the RR between the manual and the ChARM RR count. We tried to minimize the measurement error of the manual count through recruiting a well-experienced nurse to act as the counter and conducting extensive training and testing on how to correctly perform an RR measurement. Recognizing that the measurement error is not avoidable still, we choose in our analysis of the RR to account only for the RR classification and not analyze the RR itself over time. This means that for the measurement error to have any effect on the analysis, it must either be large or the RR of the child must be very close to the age-specific cutoff.
Another limitation of this study was that the duration within which the evaluation was conducted was only 5 minutes and the study population excluded children between 0 and 2 months; hence, we do not know how ChARM attachment would affect the RR beyond 5 minutes or children below two months, who might be more affected by the attachment than older children. On the other hand, this enabled us to reach a larger sample size feasibly, and we deem it unlikely that ChARM influences the RR of a child beyond a short period of time because the change in RR mainly occurred within the first minute after the ChARM was attached. Last, although attaching ChARM does not seem to impact the RR of a child to any large degree, it is important to stress that the actual accuracy of ChARM RR counts is uncertain at this stage. This must be examined in detail before any kind of endorsement of the device can be made.
CONCLUSION
The results from this study suggest that the Children´s Automated Respiration Monitor has a minor influence on the RR immediately after the attachment that seem to stabilize after 3–5 minutes in children aged 2–59 months, in most cases without clinical importance. Altogether, this strengthens the Children´s Automated Respiration Monitor devices’ suitability for clinical usage in low-resource settings.
Acknowledgments:
We extend our deep gratitude to the Regional Health Bureau in the Southern Nations, Nationalities, and Peoples’ Region, the staff at the Millionium Health Centre, Malaria Consortium Ethiopia, and the caregivers and children who took part in this study.
REFERENCES
- 1.World Health Organization , 2018. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Geneva, Switzerland: WHO. [Google Scholar]
- 2.Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta A, O’Brien KL, Campbell H, Black RE, 2013. Global burden of childhood pneumonia and diarrhoea. Lancet 381: 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H, 2008. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 86: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE, 2015. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385: 430–440. [DOI] [PubMed] [Google Scholar]
- 5.UNICEF, World Health Organization , 2013. The Integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea (GAPPD). Available at: https://apps.who.int/iris/bitstream/handle/10665/79200/9789241505239_eng.pdf. Accessed May 12, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukanga D, Babirye R, Peterson S, Pariyo GW, Ojiambo G, Tibenderana JK, Nsubuga P, Kallander K, 2011. Can lay community health workers be trained to use diagnostics to distinguish and treat malaria and pneumonia in children? Lessons from rural Uganda. Trop Med Int Health 16: 1234–1242. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization , 2014. Revised WHO Classification and Treatment of Childhood Pneumonia at Health Facilities. Available at: https://apps.who.int/iris/bitstream/handle/10665/137319/9789241507813_eng.pdf. Accessed May 16, 2019. [PubMed] [Google Scholar]
- 8.World Health Organization, UNICEF , 2011. Integrated Management of Childhood Illness: Caring for Newborns and Children in the Community. Available at: https://apps.who.int/iris/handle/10665/44398. Accessed May 16, 2019. [Google Scholar]
- 9.World Health Organization , 2014. Integrated Management of Childhood Illness (IMCI) Chart Booklet. Available at: https://apps.who.int/iris/bitstream/handle/10665/104772/9789241506823_Chartbook_eng.pdf?sequence=16. Accessed May 16, 2019. [Google Scholar]
- 10.UNICEF Supply Division , 2013. Pneumonia Diagnostics: Current Outlook and Perspectives. Available at: https://www.unicef.org/supply/files/Pneumonia_Diagnostics_Aid_Devices_and_Prespective.pdf. Accessed May 8, 2019. [Google Scholar]
- 11.Karlen W, Gan H, Chiu M, Dunsmuir D, Zhou G, Dumont GA, Ansermino JM, 2014. Improving the accuracy and efficiency of respiratory rate measurements in children using mobile devices. PLoS One 9: e99266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Källander K, Tomson G, Nsabagasani X, Sabiiti JN, Pariyo G, Peterson S, 2006. Can community health workers and caretakers recognise pneumonia in children? Experiences from western Uganda. Trans R Soc Trop Med Hyg 100: 956–963. [DOI] [PubMed] [Google Scholar]
- 13.Muro F, Mtove G, Mosha N, Wangai H, Harrison N, Hildenwall H, Schellenberg D, Todd J, Olomi R, Reyburn H, 2015. Effect of context on respiratory rate measurement in identifying non-severe pneumonia in African children. Trop Med Int Health 20: 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Källander K, Mark Y, Qazi S, 2014. Universal access to pneumonia prevention and care: a call for action. Lancet Respir Med 2: 950–952. [DOI] [PubMed] [Google Scholar]
- 15.Baker K, et al. 2019. Performance of four respiratory rate counters to support community health workers to detect the symptoms of pneumonia in children in low resource settings: a prospective, multicentre, hospital-based, single-blinded, comparative trial. EClinicalMedicine 12: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UNICEF Supply Division , 2014. Target Product Profile: Acute Respiratory Infection Diagnostic Aid (ARIDA). Available at: https://www.unicef.org/supply/files/Pneumonia_Diagnostics_Aid_Device_TPP_Introduction.pdf. Accessed May 10, 2019. [Google Scholar]
- 17.UNICEF , 2015. Request for Expression of Interest, Acute Respiratory Infection Diagnostic Aid (ARIDA). Available at: https://www.unicef.org/supply/files/2015_09_29_ARIDA_REOI_.pdf. Accessed May 10, 2019. [Google Scholar]
- 18.Philips , 2017. ChARM Instructions for Use. Available at: https://images.philips.com/is/content/PhilipsConsumer/Campaigns/CA20160908_Global_documents/CA20170829_CO_001-AAA-en_AA-ChARM_ Instructions_For_Use.pdf. Accessed May 16, 2019. [Google Scholar]
- 19.Shah R, Dadlani P, Mwangi I, Swedberg E, Afeworki A, Kibria G, Bunning N, Gigi E, Amskoye E, 2016. ChARM (Children’s Automated Respiratory Monitor)–An Innovative Easy to Use Pneumonia Screening Tool for Low Resource Settings. Abstract LB-5155, 65th Annual Meeting of American Society of Tropical Medicine and Hygiene. November 13–17, 2016, Atlanta, GA.
- 20.Cardemil CV, Gilroy KE, Callaghan-Koru JA, Nsona H, Bryce J, 2012. Comparison of methods for assessing quality of care for community case management of sick children: an application with community health workers in Malawi. Am J Trop Med Hyg 87: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalyango JN, Rutebemberwa E, Alfven T, Ssali S, Peterson S, Karamagi C, 2012. Performance of community health workers under integrated community case management of childhood illnesses in eastern Uganda. Malar J 11: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philips , 2017. ChARM. Available at: https://www.philips.com/a-w/about/sustainability/charm-monitor.html. Accessed May 16, 2019. [Google Scholar]
- 23.Berman S, Simoes EA, 1991. Respiratory rate and pneumonia in infancy. Arch Dis Child 66: 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusconi F, Castagneto M, Gagliardi L, Leo G, Pellegatta A, Porta N, Razon S, Braga M, 1994. Reference values for respiratory rate in the first 3 years of life. Pediatrics 94: 350–355. [PubMed] [Google Scholar]
- 25.Clopper CJ, Pearson ES, 1934. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26: 404–413. [Google Scholar]
- 26.R Foundation for Statistical Computing , 2017. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 27.Simoes EA, Roark R, Berman S, Esler LL, Murphy J, 1991. Respiratory rate: measurement of variability over time and accuracy at different counting periods. Arch Dis Child 66: 1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daw W, King’shott R, Elphick H, 2017. Variation in respiratory rate measurements in children. Arch Dis Child 102: A168. [Google Scholar]
- 29.Muro F, Mosha N, Hildenwall H, Mtei F, Harrison N, Schellenberg D, Olomi R, Reyburn H, Todd J, 2017. Variability of respiratory rate measurements in children suspected with non-severe pneumonia in north-east Tanzania. Trop Med Int Health 22: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gadomski AM, Khallaf N, Ansary SE, Black RE, 1993. Assessment of respiratory rate and chest indrawing in children with ARI by primary care physicians in Egypt. Bull World Health Organ 71: 523–527. [PMC free article] [PubMed] [Google Scholar]
- 31.Lanaspa M, Valim C, Acacio S, Almendinger K, Ahmad R, Wiegand R, Bassat Q, 2014. High reliability in respiratory rate assessment in children with respiratory symptomatology in a rural area in Mozambique. J Trop Pediatr 60: 93–98. [DOI] [PubMed] [Google Scholar]
- 32.Todd RB, 1836. Cyclopaedia of Anatomy and Physiology, Vol. 1 London, United Kindom: Sherwood, Gilbert and Piper. [Google Scholar]
- 33.Rusconi F, Castagneto M, Gagliardi L, Leo G, Pellegatta A, Porta N, Razon S, Braga M, 1994. Reference values for respiratory rate in the first 3 years of life. Pediatrics 94: 350–354. [PubMed] [Google Scholar]
- 34.Singhi S, Bhalla AK, Bhandari A, Narang A, 2003. Counting respiratory rate in infants under 2 months: comparison between observation and auscultation. Ann Trop Paediatr 23: 135–138. [DOI] [PubMed] [Google Scholar]
- 35.Fleming S, Thompson M, Stevens R, Heneghan C, Plüddemann A, Maconochie I, Tarassenko L, Mant D, 2011. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 377: 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folke M, Cernerud L, Ekström M, Hök B, 2003. Critical review of non-invasive respiratory monitoring in medical care. Med Biol Eng Comput 41: 377–383. [DOI] [PubMed] [Google Scholar]