Abstract.
Angiostrongylus cantonensis is a zoonotic, parasitic nematode causing angiostrongyliasis or rat lungworm disease. Clinical diagnosis in humans is currently confirmed by detection of parasite DNA in cerebrospinal fluid. This study estimated human exposure to A. cantonensis in volunteer participants solicitated via public venues on east Hawai’i Island using blood-based tests. Antibodies were screened in sera by crude antigen ELISA, followed by a 31-kDa dot-blot test developed and validated in Thailand. Human participants (n = 435) donated blood samples and completed a questionnaire to self-report relevant symptomology or clinical diagnosis. Among symptoms reported by participants diagnosed by licensed clinicians, headaches, high eosinophil counts, stiff neck, fatigue, and joint pain were most severe during the initial 3 months of infection. ELISA results revealed 22% of the serum samples as positive, 46% as equivocal, and 32% as negative. A subset of 186 samples was tested by dot blot, with 30% testing positive and 70% testing negative. A significantly higher mean ELISA value was found among recently (2014–2015) clinically diagnosed participants as than among those with a diagnosis before 2010 (P = 0.027). All dot-blot positives were also ELISA positive and were significantly associated with higher ELISA values compared with dot-blot negatives (P = 0.0001). These results suggest that an ELISA using crude antigen isolated from adult A. cantonensis from Hawai’i may be an effective initial screening method for estimating exposure to A. cantonensis in Hawai’i and likewise suggest that dot-blot tests using the 31-kDa antigen exhibit efficacy as a diagnostic for exposure.
INTRODUCTION
The nematode Angiostrongylus cantonensis is a rat lungworm, a zoonotic pathogen that in Hawai’i, as in many other tropical locations, is the main cause of human eosinophilic meningitis and ocular angiostrongyliasis,1 known colloquially as rat lungworm disease. Initially discovered in China in 1935,2 the nematode is found in at least 30 countries, including many Pacific islands and the continental United States (at the time of this writing, Louisiana, Texas, Florida, Tennessee, and Oklahoma).3,4 The definitive host is the rat, which acquires the third-stage (L3) larvae by eating an infected mollusk host; after being ingested by the rat, the L3 larvae migrate to the central nervous system (CNS), where they become subadults (L5). From the CNS, the nematodes move to the pulmonary arteries where they mature sexually, mate, and lay eggs. First-stage larvae (L1) hatch from eggs and migrate up the bronchial tree, are swallowed, and pass through the host’s digestive system and feces. The cycle in the rat, from initial infection with L3 to excretion of L1, takes approximately 6 to 8 weeks.5 Slugs or snails acquire L1 larvae by ingesting rat feces, and the parasite develops from the L1 to the L3 larval stage in these intermediate mollusk hosts. Humans are accidental hosts and become infected by ingesting L3 larvae via intermediate or paratenic hosts on contaminated food or in water or other liquid. The L3 larvae, once ingested, have historically been presumed to then migrate to the CNS or to the eye chamber (in the case of ocular angiostrongyliasis), where the parasite remains until its death.6 There have, however, been a number of reports of adult worms found in the hearts and lungs of humans on autopsy, demonstrating that larvae are able to exit the CNS in humans.7–11
Definitive diagnosis of angiostrongyliasis is made by identifying A. cantonensis nematodes in the cerebrospinal fluid (CSF) of patients or by detection of parasitic DNA in the CSF12; both require lumbar puncture. However, diagnosis can also be made by serological detection of A. cantonensis antigens or specific antibodies. Glycoproteins of approximately 31 kDa isolated from adult A. cantonensis worms have been shown to be diagnostic for human infection in Thailand with high sensitivity and specificity.13
Eamsobhana et al.14 found an ELISA using a 31-kDa antigen to be 100% sensitive and 100% specific in sera from human A. cantonensis infections. This finding was supported by a separate study,15 where nine diagnostic laboratories in Thailand were each provided with an identical 31-kDa ELISA kit to screen sera from a range of human parasitic infections, including angiostrongyliasis, gnathostomiasis, and filariasis. In all nine independent laboratories, the 31-kDa kit15 was shown to have 100% sensitivity and 100% specificity.
A lateral flow assay using the 31-kDa antigen was used to test 97 serum samples from clinically diagnosed subjects with parasitic infections and from 35 negative controls.16 Although one of four of the serum samples from hookworm-infected subjects was positive, this subject was later shown to have had a concomitant or previous infection with A. cantonensis.16 This assay for A. cantonensis showed 100% sensitivity, 98.72% specificity, 95% positive predictive value, and 100% negative predictive value.16 With crude antigen, cross-reactivity was observed in sera from cases of gnathostomiasis, toxocariasis, filariasis, and paragonimiasis, but results could be useful for initial screening in regions where nontarget parasite prevalence is low.17
The 31-kDa–based test was developed and validated in Thailand.14–17 Genetic studies show little difference between Thai and Hawaiian strains of A. cantonensis (Nei’s genetic distance estimated at 0.03).18 We therefore expect the Thailand 31-kDa–based test to effectively detect antibodies specific for A. cantonensis in our Hawai’i study population, thereby serving as an indicator of human exposure to this parasite. To test these hypotheses, we initially screened serum samples with an ELISA using crude antigen from A. cantonensis collected in Hawai’i. We then performed dot blots on a selective subset of samples using the 31-kDa proteins isolated from Thailand A. cantonensis as antigen and tested a further subset by real-time polymerase chain reaction (PCR) for parasite DNA in the blood. Participants in this study completed a questionnaire to correlate symptomology with test results.
MATERIALS AND METHODS
Study design.
This study was conducted using convenience sampling coupled with self-reported responses to a questionnaire. Volunteer participants were solicited via radio ads and notices posted in local newspapers and public areas on the eastside of Hawai’i Island. Participants completed consent forms and questionnaires with assistance from study personnel and licensed staff of the Puna Community Medical Center, Pahoa, Hawai’i. No preference for participants of known A. cantonensis infection status was given.
Ethical considerations.
This study was reviewed and approved by the University of Hawai’i Institutional Review Board. Written, informed consent was obtained from adult participants and guardians of participants aged 17 years and younger by study staff.
Sample collection.
Blood samples were collected as per standard protocol by the licensed staff of Clinical Laboratories of Hawai’i from July to December 2015. A total of 435 blood samples were collected from human volunteer participants between June 11 and September 16, 2015. Serum samples were collected in serum separator tubes (SSTs) and blood was collected in heparinized collection vials for plasma isolation. Serum separator tubes were centrifuged at 1,889 × g for 10 minutes and stored at 4°C until transport to the research laboratory. Whole blood in heparinized tubes was stored at 4°C until transport. All samples were transported in an insulated cooler with ice packs. All samples were de-identified and labeled with numerical coding sans personal data.
Sample processing.
A sample of 100 µL heparinized whole blood was transferred to a tube containing 100 µL DNA lysis buffer (0.1 M Tris–hydrochloric acid [HCl], 0.1 M ethylenediaminetetraacidic acid [EDTA], 2% sodium dodecyl sulfate [SDS]), gently mixed by manual inversion, and stored at −80°C until DNA extraction was performed. Heparinized blood samples were centrifuged for 5 minutes at 3,000 × g. Plasma was transferred to sterile screw-capped tubes and stored at −80°C until testing. Sera from samples collected in SSTs were transferred to individual sterile screw-capped aliquot tubes and stored at −80°C until testing.
Controls.
Among the participants were 15 individuals who reported recent clinical diagnosis of angiostrongyliasis by PCR of CSF. Sera from these individuals were used for positive controls. Sera from individuals newly arrived in Hawai’i from the U.S. mainland who reported no exposure to A. cantonensis were used for negative controls.
Preparation of crude antigen.
The Hawai’i strain of A. cantonensis was used to prepare crude antigen. Adult worms were collected in the course of previous animal studies. At the end of the studies, rats were humanely euthanized and adult A. cantonensis were dissected from the hearts and lungs. The live nematodes were rinsed three times in 0.01 M phosphate buffer saline (PBS) (Life Technologies, Grand Island, NY) containing 1 × protease inhibitor (Biochem Cocktail Set V EDTA-free; Thermo Scientific, Waltham, MA). Rinsed, clean nematodes were then placed in 200 µL PBS/protease inhibitor and stored at −80°C. Crude A. cantonensis antigen preparation was completed with some modification as described in Eamsobhana et al.14 Briefly, 10 male and 10 female adult A. cantonensis worms in PBS/protease inhibitor were exposed to three freeze/thaw cycles and homogenized with 400 µL PBS/protease inhibitor. This preparation was sonicated (Microson Ultrasonic Disrupter, Misonix, Inc., Farmingdale, NY) on ice four times using 3-second pulses and stored overnight at 4°C. Crude antigen was centrifuged at 2,400 × g for 15 minutes, and the supernatant was collected. A Bradford assay kit (Bio-Rad Labs Inc., Hercules, CA) was used to quantify proteins recovered.
Indirect ELISA.
ELISA was based on the methods described in Eamsobhana et al.19 with the following modifications: optimal concentration of antigen and serum (positive control) was determined by checkerboard titration. A concentration of 1:200 for primary antibody was derived by titration of positive and negative serum controls with a range of 1:100 to 1:1,000. Serum and plasma were tested, and no differences in reactivity were noted; serum was used for the remainder of the study. Each plate included two positive controls, two negative controls, and multiple carbonate buffer controls (without antigen). Crude A. cantonensis antigen was diluted with 0.05 M carbonate buffer pH 9.6 to a final concentration of 0.5 µg/mL. All washing steps were conducted on a 405 Select TS microplate washer (BioTek Inc., Winooski, VT). After sensitization, plates were washed four times with 300 µL PBS–0.05% Tween 20 (PBS-T) (pH 7.4) with a 2-minute pause after every other wash. Plates were then blocked with 125 µL 5% bovine serum albumin PBS-Tween (PBS-T) for at least 2 hours at room temperature with gentle shaking. Blocking solution was removed, and plates were washed twice with PBS-T with no pause in between. Serum (100 µL) diluted to 1:200 with PBS was added to appropriate wells and incubated for 2 hours at 37°C with shaking. All samples were run in triplicate. After incubation with primary antibody, plates were washed six times with 300 µL PBS-T, with a 2-minute pause after every other wash. A 1:1,000 dilution of horseradish peroxidase (HRP)-conjugated goat antihuman IgG Fc (Immunology Consultant Laboratory Inc., Portland, OR) (100 µL) was added, and plates were incubated for 1 hour at 37°C with gentle shaking. Plates were then washed six times with PBS-T, pausing every other wash. TMB-solubilized substrate solution (TMBOne® 3, 3′, 5, 5′, tetramethylbenzidine; Promega Corporation, Fitchburg, WI) for HRP was added according to the manufacturer’s protocols. Reaction was stopped with 1 N HCl after 15 seconds. Quantification was determined on an iMark microplate absorbance reader (BioRad) at 450 nm.
Dot-blot ELISA.
Nitrocellulose membrane blots with 0.2 µg of 31 kDa isolate of A. cantonensis were provided by P. E., Mahidol University, Bangkok, Thailand. Procedures were followed as previously described.17 Briefly, each membrane was placed in a sterile 12-well cell culture plate well with lid (CytoOne, USA Scientific, Ocala, FL) and immersed in 1 mL blocking solution (5% nonfat dry milk in PBS-T) with gentle shaking for 1 hour at room temperature. Membranes were washed three times with PBS-T and shaken gently for 5 minutes. Membranes were then incubated for 1 hour at 37°C with serum samples diluted 1:200 in 1 mL 1% nonfat dry milk in PBS and washed as described previously. Washed membranes were immersed in HRP-conjugated goat antihuman IgG Fc diluted 1:1,000 with 1% nonfat dry milk in PBS and incubated for 1 hour at 37°C. Membranes were again washed as earlier and blotted dry in between washes. Blots were then transferred to new trays and incubated at room temperature with 500 µL chloronapthol substrate (ThermoScientific) with gentle shaking until color appeared (∼30 minutes). Reactions were stopped by washing twice with dH2O. Blots were dried and scanned (V500 Photo; Epson, Epson America, Inc., Long Beach, CA) at 1,200 dpi.
DNA extraction.
DNA extractions were performed on 58 dot-blot–positive samples using the Qiagen DNeasy Animal Blood and Tissue Kit for nonnucleated blood with slight modifications from the manufacturer’s suggested protocol. Whole blood in lysis buffer (0.1 M Tris-HCl pH 8.0, 0.1 M sodium EDTA, 2% SDS) was thawed completely, and 200 µL was combined with 20 µL proteinase K followed by overnight digestion at 55°C with gentle shaking. Extractions were otherwise completed according to the standard protocol.
Polymerase chain reaction.
Samples were subjected to PCR on a StepOnePlus RealTime PCR system (Life Technologies) using a Custom TaqMan Gene Expression Assay (Life Technologies, assay ID A139RIC) as described.20 Cycling conditions, primers (AcanITS1F1 and AcanITS1R1), and probe (AcanITS1P1) were those described by Qvarnstrom et al.21 Polymerase chain reactions were carried out in 20 μL total volume and included 0.25 μM probe, 0.9 μM forward and reverse primers, and 1X TaqMan Environmental Master Mix 2.0 (Life Technologies). A positive plasmid control and a negative control containing no DNA were included in all reactions. All PCR reactions were replicated for verification.
Indirect ELISA data analysis.
Positive controls were chosen by selecting the samples with the highest mean absorbance from recently clinically diagnosed participants. Negative control samples originated from two newly arrived volunteers from Nevada who had never been to the Hawaiian Islands or elsewhere in the tropics and whose sera showed consistently low absorbance values. ELISA value (%EV) was created to compare absorbance values as a percentage of positive and negative controls according to the following formula: %EV = (Sample Mean Absorbance − Negative Control Mean Absorbance)/(Positive Control Mean Absorbance − Negative Control Mean Absorbance) × 100. A two-sample t-test was performed in Minitab 18 to compare mean %EV between more recent clinically diagnosed participants (2014–2015) and those clinically diagnosed earlier (before 2010).
The consistency of ELISA absorbance data was evaluated by measuring coefficient of variability (%CV).22 An inter-assay %CV of the mean absorbance of each sample in triplicate was set to 15% and an intra-assay %CV of the mean absorbance of all samples on the microplate was set to 10%. A %CV value greater than the respective threshold was not considered consistent and that data were discarded. Inter-assay %CV was calculated as follows: (SD of plate means/Mean of Plate Means) × 100. Intra-assay %CV was calculated as follows: (SD of triplicate absorbance values/Mean of absorbance values in triplicate) × 100.
Dot-blot data analysis.
A positive result was determined by dot blot if a defined round circle could be seen by the naked eye in the photos taken immediately after enzymatic development. To be considered positive, circles had to appear at the vicinity of the small prick created during the absorption of the 31-kDa antigen and be of a size and density consistent with positive control results. If a dot was not seen on a blot, if an irregular shaped splotch was seen on the blot, or if the defined dot was only seen under altered contrast of the photo, the sample was considered negative for rat lungworm exposure. Negative results were numerically assigned a value of 0. If the dot was not visible by eye but appeared faint by enhancement, then it was assigned a value of 1. Weak positives were assigned a value of 2; these were seen by the naked eye without enhancement but were clearly not as dark as strong positives. Strong positives were assigned a value of three and were easily observed as a dark circle. For data analysis, we conservatively considered zero and one to be negative, whereas two and three were considered positive. To examine differences in ELISA values between participants with positive versus negative dot-blot test results, a nonparametric Wilcoxon rank-sum test was performed in R.23
Questionnaire data analysis.
Using results from the survey questionnaire (Supplemental File 1), ELISAs, and dot blots, two multivariable logistic regression models were generated to determine whether demographic (residence, type of water supply, gender, age, education level, and ethnicity) and food preparation (see Question 3 A-I in Supplemental File 1) variables were significantly associated with positive versus negative ELISA and dot-blot test results. Separate models were performed for ELISA and dot-blot test results.
Repeated measures of self-reported symptoms (nausea, headache, meningitis, high eosinophil counts, stiff neck, skin sensitivity, muscle soreness, fatigue, joint pain, fever, mental clarity, memory, speech, sexual dysfunction, vision, balance, bladder dysfunction, bowel dysfunction, and others) and their level of severity (mildest to most severe, with higher scores indicating more severity) were collected for each participant reporting clinical diagnosis early (< 3 months) and late (> 3 months) in infection. Three months was selected as a reasonable time post-symptom onset to distinguish early versus late, as Hawai’i Department of Health (HDOH) states that “symptoms usually last between 2 and 8 weeks; symptoms have been reported to last for longer periods of time” https://health.hawaii.gov/docd/disease_listing/rat-lungworm-angiostrongyliasis/#signs_symptoms. Normality of the severity score for each symptom was evaluated using Shapiro-Wilk tests performed in SPSS (version 25.0).24 Because symptom severity scores collected early and late in infection were not normally distributed, nonparametric Wilcoxon signed-rank tests were conducted to examine whether median change in each symptom’s severity was significantly different from zero. In addition, boxplots were generated to visually assess changes in symptom severity within and after 3 months of infection. Statistical significance was taken at the P < 0.05 level.
RESULTS
Demographics.
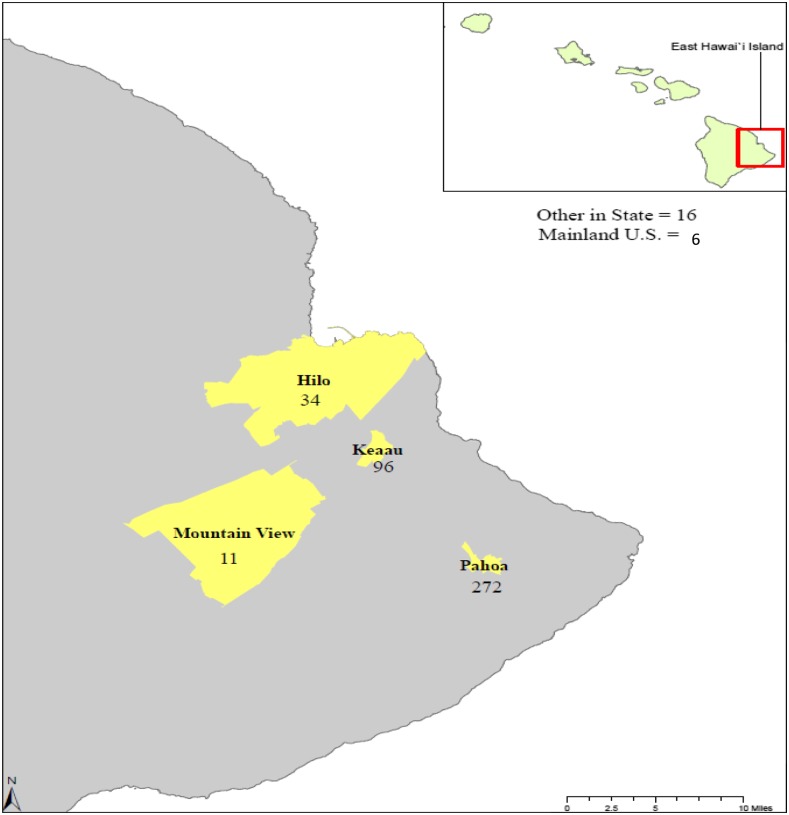
A total of 435 volunteers participated in this study, providing blood samples and responding to a questionnaire. Study location and participants’ reported places of residence are provided in Figure 1. The majority (272/435, 62.5%) are residents of Pahoa, HI, where sample collection took place. Eastern Hawai’i Island is rural, and most participants (83%) reported living in a rural environment versus city or suburban environment. There is unregulated, widespread use of rainwater catchment in east Hawai’i Island; nearly 78% of participants reported the use of rainwater catchment as a source of household water, as compared with 21% reporting a municipal water source. Most participants were female (56%), Caucasian (79%), and reported some level of college education (62%), with a mean age of 56 years (range: 6–93 years).
Figure 1.
Study location on East Hawai’i Island and participants’ reported place of residence. This figure appears in color at www.ajtmh.org.
Self-reported diagnosis.
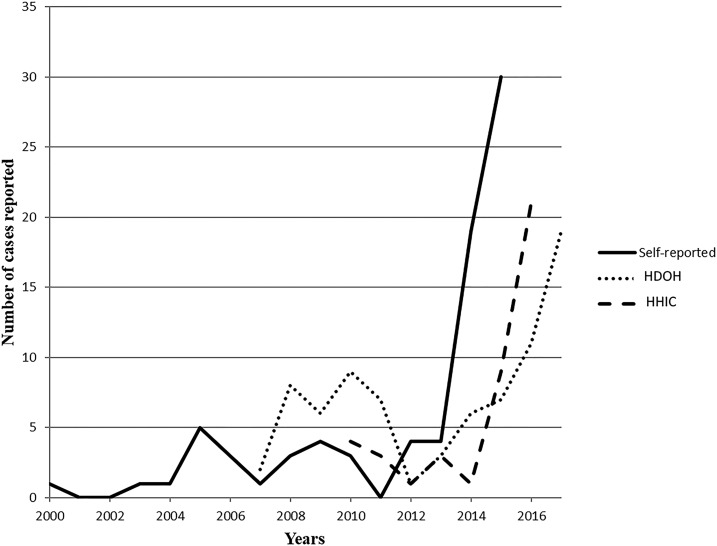
The participants were asked whether they believed they had ever had angiostrongyliasis and, if so, whether diagnosis was made by a licensed clinician. A total of 105 (24%) reported angiostrongyliasis (self-diagnosed), 315 (72%) reported no known exposure to A. cantonensis, and 15 (3.4%) reported clinical diagnosis. Of the 105 who reported having had angiostrongyliasis, 81 reported the date of onset of symptoms (Figure 2). More participants reported symptoms acquired after 2013; this is similar to incidence data reported by the HDOH and Hawaii Health Information Corporation (HHIC) (Figure 2). Self-reported demographics as well as ELISA (%EV) and dot-blot results of the 15 clinically diagnosed participants are provided in Table 1. Reported dates of clinical diagnosis ranged from 1981 to 2015.
Figure 2.
Self-reported numbers and year of infection with A. cantonensis as compared with numbers reported by the Hawai’i Department of Health and Hawai’i Health Information Corporation.
Table 1.
Self-reported demographics of clinically diagnosed participants
| Residence | Gender | Age | When infected | Where infected | Diagnosis procedure | Hospital admission | %EV | Dot blot |
|---|---|---|---|---|---|---|---|---|
| Pahoa | F | 68 | 1981 | Honokaa or Hilo | Spinal tap | HMC | 18 | P |
| Pahoa | M | 52 | 2004 | Kapoho (Puna) | Spinal tap | QMC | 58 | N |
| Pahoa | F | 63 | 2005 | Kapoho (Puna) | Antibody test | NR | 27 | N |
| Pahoa | M | 67 | 2007 | Pahoa (Puna) | Eosinophil test | NR | 60 | P |
| Hilo | M | 30 | 2008 | Kapoho (Puna) | Spinal tap | HMC | 53 | N |
| Pahoa | M | 70 | 2009 | Papaya Farms Rd (Puna) | Other | Thailand | 69 | P |
| Pahoa | F | 12 | 2009 | Puna, HI | Spinal Tap | HMC | 48 | N |
| Pahoa | M | 73 | 2009 | Pahoa (Puna) | Symptoms | NR | 16 | N |
| Keaau | F | 62 | 2010 | Hawaii | Don't Know | NR | 38 | N |
| Hilo | F | 39 | 2014 | Hilo | Spinal Tap/CT | HMC | 100 | P |
| Hilo | M | 39 | 2014 | Hilo | Spinal tap | HMC | 70 | P |
| Hilo | F | 55 | 2014 | Keaau (Puna) | Ab test | NR | 32 | N |
| Pahoa | M | 23 | 2015 | Puna | Spinal tap | HMC | 111 | P |
| Pahoa | M | 73 | 2015 | Puna | Antibody test | HMC | 87 | P |
| Pahoa | F | 59 | 2015 | Puna | Symptoms and response to treatment | NR | 69 | N |
HMC = Hilo Medical Center (Hilo); QMC = Queen’s Medical Center (Honolulu); all were diagnosed by MD or ND. NR = no response; M = male; F = female. The %EV is the ELISA value, and dot blot is listed as positive (P) or negative (N).
Self-reported symptoms.
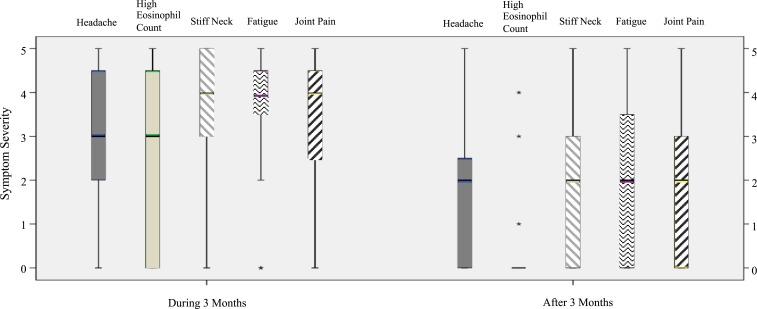
Frequency and severity of common symptoms and presentations reported by the 15 clinically diagnosed patients are summarized, and earlier stages of infection (< 3 months post-symptom onset) are compared with those reported during later infection (> 3 months post-symptom onset) (Figure 3). Severity range was ranked from 1 to 5, with 5 being the most severe. The frequency and severity of symptoms reported earlier versus later in infection appeared to decrease over time among the 15 clinically diagnosed participants; however, some participants reported severe symptoms persisting after 3 months, for example, skin sensitivity. Figure 3 displays boxplots of symptoms that demonstrated statistically significantly different levels of severity earlier versus later in infection. Specifically, nonparametric Wilcoxon signed-rank tests showed that median symptom severity was significantly greater for headaches (P = 0.0232), high eosinophil counts (P < 0.05), stiff neck (P < 0.05), fatigue (P < 0.05), and joint pain (P < 0.05) during the initial 3 months of infection than reported severity of these symptoms later than 3 months. No statistically significant differences were observed for the other symptoms.
Figure 3.
A boxplot analysis displaying mean severity of symptoms in 15 clinically diagnosed participants found to be significantly different during and after 3 months of being infected with A. cantonensis. These clinically diagnosed symptoms had higher severity during 3 months of infection than 3 months post-infection. The lines are the severity spectrum, the bars represent most of the patients’ responses, and the cross between bars represents the medians. *Extreme outliers. This figure appears in color at www.ajtmh.org.
ELISA.
Crude antigen was isolated from 10 male and 10 female adult A. cantonensis for use in ELISAs, yielding a protein concentration of 1.343 µg/µL. All serum samples were tested by ELISA for the presence of antibodies to A. cantonensis. ELISA tests were run in triplicate and data included where the SD among replicates was < 0.025. ELISA cutoff values were determined by the %EV of the samples from clinically diagnosed participants. The lowest %EV seen in samples from participants reporting recent clinical diagnoses (2014–2015) by spinal tap at Hilo Medical Center consistently showed a %EV at or greater than 70%, and this was chosen as the lowest threshold for classifying the sample “positive” by ELISA for exposure to A. cantonensis. The lowest %EV of the samples from participants reporting clinical diagnosis before 2014 by spinal tap at Hilo Medical Center (2009) was 48, with the exception of one outlier, clinically diagnosed in 1981 whose sample showed a %EV of 17. Thus, 48 %EV served as a cutoff range for testing by dot blot, and samples at or greater than this threshold were considered “likely positive.” The highest %EV within negative control samples was 16; therefore, 16 was established as the upper end of a negative result. A %EV of 16.1–48 was considered equivocal. For simplification, negative samples (%EV < 16) were numerically assigned a value of 0, equivocal (16.1–48 %EV) assigned 1, likely positive (48.1–70 %EV) assigned 2, and positive (> 70 %EV) assigned 3 in our datasets.
ELISA results are shown in Table 2. Overall, 28 samples (6%) were positive, 69 (16%) were likely positive, 198 (46%) were equivocal, and 140 (32%) were negative. Combined, 97 (22%) samples tested positive or likely positive. Of the samples from the 15 individuals who had reported clinical diagnosis of angiostrongyliasis, four (27%) tested positive, six (40%) tested likely positive by ELISA, and five tested equivocal. None of the samples from clinically diagnosed participants returned results within our negative range. Of the 15 participants reporting clinical diagnosis, seven reported diagnoses by spinal tap. Six of these tested positive or likely positive and one tested equivocal. Of the 105 volunteers who reported infection with A. cantonensis by self-diagnosis, nine (9%) tested positive, 15 (14%) likely positive, 46 (44%) equivocal, and 35 (33%) tested negative. Of the 315 participants reporting no exposure to A. cantonensis, 15 (5%) tested positive, 48 (15%) likely positive, 147 (47%) equivocal, and 105 (33%) negative.
Table 2.
Results of ELISA testing on 435 serum samples and dot-blot data on 186 serum samples
| Clinically diagnosed | Self-diagnosed as positive | Self-diagnosed as negative | ||
|---|---|---|---|---|
| ELISA | n = 435 | n = 15 | n = 105 | n = 315 |
| Positive | 6% (28) | 27% (4) | 9% (9) | 5% (15) |
| Likely positive | 16% (69) | 40% (6) | 14% (15) | 15% (48) |
| Equivocal | 46% (198) | 33% (5) | 44% (46) | 47% (147) |
| Negative | 32% (140) | 0 | 33% (35) | 33% (105) |
| Dot blot | n = 186 | n = 15 | n = 45 | n = 126 |
| Positive | 30% (56) | 47% (7) | 29% (13) | 29% (36) |
| Negative | 70% (130) | 53% (8) | 71% (32) | 71% (90) |
Percent rounded to the nearest significant number. Diagnosis category is based on self-reported questionnaire responses.
Dot blot.
A subset of 186 samples was tested with the 31-kDa dot blot, including 28 samples from the ELISA-positive pool of samples, 64 samples from the likely positive group, 71 from the equivocal group, and 23 from the group testing negative. Results are presented in Table 2. Overall, 56 (30%) dot blots were positive and 130 (70%) were negative. Within the 15 samples from clinically diagnosed participants, seven (47%) were positive and eight (53%) were negative by dot blot. Of the seven participants diagnosed by spinal tap, four samples (57%) were positive and three (43%) were negative. Of the 105 participants who self-diagnosed angiostrongyliasis, 45 were tested by dot blot, with 13 (29%) testing positive and 32 (71%) negative. Of the 315 volunteers reporting no exposure or disease, 126 were tested by dot blot, with 36 (29%) testing positive and 90 (71%) negative.
Dot-blot and ELISA results are compared in Table 3. Of samples testing “positive” by ELISA, 19 (68%) tested positive and nine (32%) tested negative by dot blot. Of those that were “likely positive” by ELISA, 24 (38%) tested positive and 40 (62%) tested negative by dot blot. Of those “equivocal” by ELISA, 13 (18%) tested positive and 58 (82%) tested negative by dot blot. Of those “negative” by ELISA, zero were positive and 23 (100%) were negative by dot blot (Table 3).
Table 3.
Comparison of dot-blot results with ELISA results categorized by %EV (ELISA value)
| ELISA results | ||||
|---|---|---|---|---|
| Positive, ≥ 70 %EV | Likely positive, 48.1–70 %EV | Equivocal, 16.1–48 EV | Negative, ≤ 16 %EV | |
| Dot-blot positive | 19 (68%) | 24 (38%) | 13 (18%) | 0 (0%) |
| Dot-blot negative | 9 (32%) | 40 (62%) | 58 (82%) | 23 (100%) |
| Total tested by dot blot | 28 | 64 | 71 | 23 |
Percent rounded to the nearest significant number.
A nonparametric Wilcoxon rank-sum test demonstrated statistically significant differences in ELISA values between participants with positive versus negative dot-blot test results. Specifically, participants testing positive by the 31-kDa dot blot had higher median ELISA values than those testing negative (P < 0.0001) (Table 3). Of the 186 participants whose samples were tested by dot blot, 152 (82%) reported using rainwater catchment as a source of household water and 33 (18%) reported having a municipal water source. Because of the large difference in sample sizes, the water source data were not further analyzed.
ELISA and dot-blot results from clinically and self-diagnosed positive participants from 2001 to 2015 were summarized (data not shown). Among participants reporting clinical diagnosis, the earliest reported year of angiostrongyliasis onset our ELISA detected was 2004. By dot blot, the earliest reported year of onset detected was 2007, again with the exception of one participant reporting diagnosis in 1981 (Table 1). A two-sample t-test demonstrated that participants with recent (2014–2015) infections had a significantly higher mean %EV than those with onset of disease before 2010 (mean %EV: 78.2 versus 43, P = 0.027).
Regression analyses.
Separate multivariate logistic regression models were estimated to examine whether demographic and food preparation habit variables were associated with A. cantonensis exposure status as measured by ELISA and dot-blot tests. None of these variables was found to be significantly associated with a “positive” ELISA or dot-blot test at the P < 0.05 level.
Polymerase chain reaction.
We extracted DNA from 58 samples, including 55 positives by dot blot, and tested for the presence of A. cantonensis DNA in the blood by real-time PCR. Reliable signal was not detected in any samples except the positive controls. Negative controls remained negative throughout (data not shown).
DISCUSSION
This study represents the first attempt in the United States to estimate human exposure to A. cantonensis and to describe early symptoms (< 3 months onset) and later symptoms (> 3 months onset) of angiostrongyliasis. Initial screening of 435 serum samples from participants by Hawai’i-based crude antigen ELISA revealed a “positive” rate of 97/435 (22%). Using a previously validated 31-kDa dot-blot test developed in Thailand on samples that were previously screened by ELISA, including all positive samples and subsets of the equivocal and negative samples, we found 56/186 tested positive (30%). These results suggest the number of humans exposed to A. cantonensis may be greater than the number of cases previously reported by the HDOH.
Thailand has reported approximately 47% of all angiostrongyliasis cases worldwide.1 However, the number of cases dropped from 1,386 cases of eosinophilic meningitis in 2000 to 176 cases in 2009.18 Many of the cases in Thailand, as in China, are due to intentional ingestion of undercooked mollusks.3,18 Studies from China showed seropositivity to A. cantonensis in 20% of 459 subjects on Hainan Island,25 21% of 393 in another study,26 and 14% of 300 in Guangdong Province.27 Intentional ingestion of mollusks is rare in Hawai’i28,29; most of our subjects could not identify their infection source.
The semi-slug Parmarion martensi is a recent immigrant to the Hawaiian Islands30 and is likely a major contributor to the increase in angiostrongyliasis cases acquired on the island of Hawai’i in the past 15 years.31 Many cases have originated in the Puna district of Hawai’i Island, where P. martensi are numerous and heavily infected (> 75%) with A. cantonensis larvae.31 In a survey of wild rats in the Puna district, 54% (20/37) had adult rat lungworms visible on necropsy and 100% of rats lungs tested positive for A. cantonensis by real-time PCR.32 Rats captured in the Hilo district of Hawai’i Island were found to be 94% (512/545) positive for A. cantonensis based on findings of adult worms, encysted adult worms, L3 larvae, and/or real-time PCR analysis of brain tissue.33 The introduction of P. martensi combined with the high infection rate in rats may explain the increase in human cases of angiostrongyliasis and the high rates of human exposure in East Hawai’i Island.
Notably, East Hawai’i also has widespread use of unregulated rainwater catchment for residential as well as agricultural purposes. Our questionnaire demonstrated that some people, including five of the participants reporting clinical diagnosis in this study, believe they were infected via contaminated rainwater in catchment systems. Water transmission of A. cantonensis has been previously demonstrated in rats,34–36 and it has been suggested that infections in man can be acquired from the consumption of A. cantonensis L3 in water.37 Parmarion martensi is a robust climber and has routinely been found, drowned, inside catchment tanks. Infective larvae leave drowned hosts and can survive in water for several weeks.38 The potential exists for water transmission if effective filtration and treatment are not implemented. For example, contaminated water may be consumed directly from the tap, used for rinsing food, or during the course of performing personal hygiene functions such as showering and brushing teeth. The widespread use of rainwater catchment for household water supply may further contribute to the high exposure rate of humans to A. cantonensis on East Hawai’i Island and other regions with rainwater catchment use.
Severity of presentation in human cases of angiostrongyliasis ranges from mild and flu-like illness to coma, long-term disability, and death.1 In the United States, symptoms such as headache, paresthesia, hyperesthesia, and numbness have been reported to last up to 10 months or longer.29 Among our participants, 24% believed that they had been infected with A. cantonensis and 72% believed that they had never been infected. Because these are self-reported data, there is some question as to the reliability of responses relating to self-diagnosis. However, self-reported data can be valuable,28,39 especially when information is otherwise unavailable. The prevalence of angiostrongyliasis in participants who self-diagnosed (Figure 2) shows a recent upward trend similar to the number of clinical cases reported by the HDOH and HHIC40; this correlation provides support for the reliability of the self-reported data.
Participants self-reporting no disease had ELISA and dot-blot results comparable to those reporting having contracted the disease. This suggests that subclinical infections may be common with mild symptoms that went undiagnosed, were absent, or were misattributed to the flu or other ailments. A low-level exposure to A. cantonensis (e.g., potentially through environmental exposure such as to contaminated water from catchment)38 over time may produce antibodies as seen in participants reporting no disease. It has been shown in rats that low exposure levels do provide immunological protection from lethal challenge with A. cantonensis.41
Polymerase chain reaction was used to attempt to detect A. cantonensis DNA in the blood of the 55 dot-blot–positive blood samples.20,21 No amplification was detected in any of the test samples, except for the positive controls. We have previously detected A. cantonensis DNA in peripheral rat blood under controlled infection at several time points using the same methods,42 and we now routinely detect A. cantonensis DNA in canine blood samples provided by local veterinarians (Jarvi et al., Unpublished data). In our human samples, the parasite had likely cleared from the peripheral blood; sample collection for most participants was at least 1 year after reported symptoms or diagnosis. It would be useful to study blood (and CSF) PCR changes over time in human angiostrongyliasis.
Of our 435 participants, 15 (3.4%) reported clinical diagnoses of angiostrongyliasis. Seven of these individuals reported diagnoses by spinal tap and provided contact information of the medical center that confirmed the diagnosis. Data from clinically diagnosed infections may provide further information on the duration of the antibody response as well as the impact of longer term symptoms. The frequency and severity of many of the symptoms in participants reporting clinical diagnoses decreased after 3 months. Some symptoms were reported as severe earlier in infection (< 3 months.) but were significantly reduced later (> 3 months). These included headache, high eosinophil count, stiff neck, fatigue, and joint pain. However, some participants reported several symptoms persisted at severe levels (e.g., skin sensitivity and memory issues). A better understanding is required of the relationships among detection, treatment, and long-term sequelae of this disease.
In comparisons of ELISA and dot-blot results from the participants reporting clinical diagnoses, higher ELISA values generally correlated with positive dot-blot results, with the exception of the person who was first infected in 1981 who had a low %EV and a positive dot blot. The %EV was significantly higher in participants more recently diagnosed, which was expected given that antibody titer generally decreases as infection senesces.43 We detected antibody by dot blot in participants reporting onset of disease and clinical diagnosis in 1981, 2007, and 2009. It is not clear whether antibodies detected were to the initial exposure in these time periods or to a more recent reexposure. All (100% or 23/23) of the samples from dot-blot negative participants were also ELISA negative, whereas 53% (49/92) of the samples from ELISA-positive participants were dot-blot negative. This suggests that, as seen with crude antigen from Thailand,17 crude antigen from Hawai’i A. cantonensis in ELISA exhibits some level of cross-reactivity. There is no true gold standard for comparison within this dataset, but if we were to consider our dot-blot results with the validated 31-kDa Thai proteins as the “gold standard,” our ELISA would show 100% sensitivity and 32% specificity. All dot-blot positives were also ELISA positive, which suggests an ELISA test using crude antigen isolated from adult A. cantonensis from Hawai’i appears to be an effective initial screening method and the 31-kDa dot blot appears to have efficacy as a diagnostic for exposure to A. cantonensis in Hawai’i.
Hawai’i Island has extremely high infection rates in rats33 and semi-slugs,31 and this study reveals human exposure levels higher than those reported in endemic areas (China).25–27 Effective preventive measures are needed, especially in areas of high transmission. Effective, less invasive diagnostics are also needed in these areas to facilitate treatments. Currently, HDOH requires PCR-based detection of parasite DNA in the CSF to definitively diagnose angiostrongyliasis which is expensive, invasive, and can be inconclusive. Antibody-based testing requires only a blood sample and provides a reasonable or supplemental alternative to a lumbar puncture test, although one has to wait some time until antibodies are produced in sufficient levels for detection. Detection of parasite DNA in the blood provides an early diagnostic test and has been demonstrated in rats,42 dogs, and horses (Jarvi et al., Unpublished data). Availability of a reliable blood-based diagnostic test for exposure to A. cantonensis in humans, pets, and livestock would benefit the public’s health wherever the disease exists or emerges.
Supplemental file
Acknowledgments:
We would like to thank Puna Community Medical Center and staff for assistance in participant completion of the forms and questionnaires necessary for conducting this study, and Clinical Laboratories of Hawai’i for their assistance with blood sample collection. We would also like to thank Peter Hoffman, PhD, for his helpful comments in reviewing this manuscript and the volunteers who assisted with conducting this study including Patrick Edwards, Smiley Burrows, Doran Vaughan, Patricia Macomber, Judy Hartshom, Jeremai Cann, and Shawzy Cann. We would also like to thank all the volunteer participants who donated blood samples and took the time to complete the questionnaire.
Note: Supplemental file appears at www.ajtmh.org.
REFERENCES
- 1.Wang QP, Lai DH, Zhu XU, Chen X-G, Lun Z-R, 2008. Human angiostrongyliasis. Lancet Infect Dis 8: 621–630. [DOI] [PubMed] [Google Scholar]
- 2.Chen HT, 1935. Un nouveau nematode pulmonaire, pulmonema cantonensis, NGN sp. Des rats de Canton. Ann Parasit 13: 312. [Google Scholar]
- 3.Barratt J, Chan D, Sandaradura I, Malik R, Spielman D, Lee R, Marriott D, Harkness J, Ellis J, Stark D, 2016. Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 143: 1087–1118. [DOI] [PubMed] [Google Scholar]
- 4.Stockdale-Walden HD, Slapcinsky JD, Roff S, Mendieta Calle J, Goodwin ZD, Stern J, Corlett R, Conway J, McIntosh A, 2017. Geographic distribution of Angiostrongylus cantonensis in wild rats (Rattus rattus) and terrestrial snails in Florida, USA. PLoS One 12: e0177910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackerras MJ, Sandars DF, 1955. The life history of the rat lung-worm, Angiostrongylus cantonensis (Chen) (Nematoda: Metastrongylidae). Aust J Zool 3: 1–21. [Google Scholar]
- 6.Wang QP, Wu ZD, Wei RL, Owen ZR, Lun ZR, 2012. Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis 31: 389–395. [DOI] [PubMed] [Google Scholar]
- 7.Prociv P, 1999. Parasitic meningitis: crossing paths with the rat lungworm (Angiostrongylus cantonensis). Med J Aust 170: 517–518. [DOI] [PubMed] [Google Scholar]
- 8.Yii CY, 1976. Clinical observations of eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis on Taiwan. Am J Trop Med Hyg 25: 233–249. [DOI] [PubMed] [Google Scholar]
- 9.Cooke-Yarborough CM, Kornberg AJ, Hogg GG, Spratt DM, Forsyth JRL, 1999. A fatal case of angiostrongyliasis in an 11-month–old infant. Med J Aust 170: 541–543. [DOI] [PubMed] [Google Scholar]
- 10.Lindo JF, Escoffery CT, Reid B, Codrington G, Cunningham-Myrie C, Eberhard ML, 2004. Fatal autochthonous eosinophilic meningitis in a Jamaican child caused by Angiostrongylus cantonensis. Am J Trop Med Hyg 70: 425–428. [PubMed] [Google Scholar]
- 11.Prociv P, Turner M, 2017. Neuroangiostrongyliasis: the “subarachnoid phase” and its implications for anthelmintic therapy. Am J Trop Med Hyg 98: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qvarnstrom Y, et al. 2016. Real-time polymerase chain reaction detection of Angiostrongylus cantonensis DNA in cerebrospinal fluid from patients with eosinophilic meningitis. Am J Trop Med Hyg 94: 176–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eamsobhana P, Tungtrongchitr A, Wanachiwanawin D, Yong HS, Mak JW, 1998. Characterization of a 31-kDa specific antigen from Parastrongylus cantonensis (Nematoda: Metastrongylidae). Int Med Res J 2: 9–12. [Google Scholar]
- 14.Eamsobhana P, Yoolek A, Suvouttho S, Suvuttho S, 2001. Purification of a specific immunodiagnostic Parastrongylus cantonensis antigen by electroelution from SDS-polyacrylamide gels. Southeast Asian J Trop Med Public Health Jun 32: 308–313. [PubMed] [Google Scholar]
- 15.Eamsobhana P, Yoolek A, Kreethapon N, 2003. Blinded multi-laboratory evaluation of an in-house dot-blot ELISA kit for diagnosing human parastrongyliasis. Southeast Asian J Trop Med Public Health 34: 1–6. [PubMed] [Google Scholar]
- 16.Eamsobhana P, Tungtrongchitr A, Wanachiwanawin D, Yong HS, 2018. Immunochromatographic test for rapid serological diagnosis of human angiostrongyliasis. Int J Inf Dis 73: 69–71. [DOI] [PubMed] [Google Scholar]
- 17.Eamsobhana P, Yoolek A, Punthuprapasa P, 2003. Dot-blot ELISA for the immunological detection of specific antibody to Parastrongylus cantonensis. Trop Biomed 20: 1–6. [Google Scholar]
- 18.Eamsobhana P, 2013. Angiostrongyliaia in Thailand: epidemiology and laboratory investigations. Hawaii J Med Public Health 72: 28–32. [PMC free article] [PubMed] [Google Scholar]
- 19.Eamsobhana P, Watthanakulpanich D, Punthuprapasa P, Yoolek A, Suvuttho S, 1999. Detection of antibodies to Parastrongylus cantonensis in human sera by gelatin particle indirect agglutination test. Jpn J Trop Med Hyg 27: 1–5. [Google Scholar]
- 20.Jarvi SI, Farias MEM, Howe K, Jacquier S, Hollingsworth R, Pitt W, 2012. Quantitative PCR estimates Angiostrongylus cantonensis infection levels in semi-slugs (Parmarion martensi). Mol Biochem Parasitol 185: 174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qvarnstrom Y, Aramburu da Silva AC, Teem JL, Hollingsworth R, Bishop H, Graeff-Teixeira, da Silva AJ, 2010. Improved molecular detection of Angiostrongylus cantonensis in mollusks and other environmental samples with a species-specific internal transcribed spacer 1-based TaqMan assay. Appl Environ Microbiol 76: 5287–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed GF, Lynn F, Meade BD, 2002. Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol 9: 1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team , 2014. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 24.IBMCorp Released , 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. [Google Scholar]
- 25.Hu X, Du J, Tong C, Wang S, Liu J, Li Y, He C, 2011. Epidemic status of Angiostrongylus cantonensis in hainan island, China. Asian Pac J Trop Med 4: 275–277. [DOI] [PubMed] [Google Scholar]
- 26.Li Y C, Hu XM, Tong CJ, Liu J, Li MT, Wang SQ, 2011. Investigation on serology, risk factor and awareness of Angiostrongylus cantonensis in Hainan province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 29: 74–75. [PubMed] [Google Scholar]
- 27.Zhang Y, Huang D, Tan QM, Chen DX, Zhan XM, 2008. Epidemiological investigation of Angiostrongylus cantonensis in Jiangmen of Guangdong province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 26: 370–373. [PubMed] [Google Scholar]
- 28.Hochberg NS, Blackburn BG, Park SY, Sejvar JJ, Effler PV, Herwaldt BL, 2011. Eosinophilic meningitis attributable to Angiostrongylus cantonensis infection in Hawaii: clinical characteristics and potential exposures. Am J Trop Med Hyg 85: 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slom TJ, et al. 2002. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travellers returning from the Caribbean. N Engl J Med 346: 668–675. [DOI] [PubMed] [Google Scholar]
- 30.Cowie RH, 1997. Catalog and bibliography of the nonindigenous nonmarine snails and slugs of the Hawai’ian Islands. Bishop Mus Occas Pap 50: 1–66. [Google Scholar]
- 31.Hollingsworth RG, Kaneta RK, Sullivan JJ, Bishop HS, Qvarnstrom Y, da Silva AJ, Robinson DG, 2007. Distribution of Parmarion cf. martensi, a new semi-slug pest on Hawai’i Island, and its potential as a vector for human angiostrongyliasis. Pac Sci 61: 457–468. [Google Scholar]
- 32.Qvarnstrom Y, Bishop BS, da Silva AJ, 2013. Detection of rat lungworm in intermediate, definitive, and paratenic hosts obtained from environmental sources. Hawaii J Med Public Health 72: 63–69. [PMC free article] [PubMed] [Google Scholar]
- 33.Jarvi SI, Quarta S, Jacquier S, Howe K, Bicakci D, DaSalla C, Lovesy N, Snook K, McHugh R, Niebuhr C, 2017. High prevalence of Angiostrongylus cantonensis (rat lungworm) on eastern Hawaii Island: a closer look at life cycle traits and patterns of infection in wild rats (Rattus spp). PLoS One 12: e0189458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng TC, Alicata JE, 1964. Possible role of water in the transmission of Angiostrongylus cantonensis (Nematoda: Metastrongylidae). J Parasitol 50 (Suppl 2): 39–40. [Google Scholar]
- 35.Richards CS, Merritt JW, 1967. Studies on Angiostrongylus cantonensis in molluscan intermediate hosts. J Parasitol 53: 382–388. [PubMed] [Google Scholar]
- 36.Crook JR, Fulton SE, Supanwong K, 1971. The infectivity of third stage Angiostrongylus cantonensis larvae shed from drowned Achatina fulica snails and the effect of chemical agents on infectivity. Trans R Soc Trop Med Hyg 65: 602–605. [DOI] [PubMed] [Google Scholar]
- 37.Wallace GD, Rosen L, 1969. Studies on eosinophilic meningitis V. Molluscan hosts of Angiostrongylus cantonensis on pacific islands. AM J Trop Med Hyg 18: 206–216. [PubMed] [Google Scholar]
- 38.Howe K, Kaluna L, Lozano A, Torres Fischer B, Tagami Y, McHugh R, Jarvi S, 2019. Water transmission potential of Angiostrongylus cantonensis: larval viability and effectiveness of rainwater catchment sediment filters. PLoS One 14: e0209813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan D, 2009. So why ask me? Are self-reported data really that bad? Lance CE, Vandenberg JR, eds. Statistical and Methodological Myths and Urban Legends: Doctrine, Verity and Fable in the Organizational and Social Sciences. New York, NY: Routledge, 309–335. [Google Scholar]
- 40.Howe K, Jarvi SI, 2017. Angiostrongyliasis (rat lungworm disease): viewpoints from Hawai’i Island. ACS Chem Neurosci 8: 1820–1822. [DOI] [PubMed] [Google Scholar]
- 41.Heyneman D, Liat LB, 1965. Prolonged survival in rats immunized by a small number of low level doses of Angiostrongylus cantonensis and challenged with a lethal level of infective larvae. Med J Malaya 20: 162–163. [Google Scholar]
- 42.Jarvi SI, et al. 2015. Detection of Angiostrongylus cantonensis in the blood and peripheral tissues of wild Hawaiian rats (Rattus rattus) by a quantitative PCR (qPCR) assay. PLoS One 10: e0123064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch JS, Dobson C, Campbell GR, 1980. Immunodiagnosis and seroepidemiology of Angiostrongylus cantonensis zoonoses in man. Trans R Soc Trop Med Hyg 74: 614–623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.