Abstract.
To implement future malaria elimination strategies in French Guiana, a characterization of the infectious reservoir is recommended. A cross-sectional survey was conducted between October and December 2017 in the French Guianese municipality of St Georges de l’Oyapock, located along the Brazilian border. The prevalence of Plasmodium spp. was determined using a rapid diagnostic test (RDT) and a polymerase chain reaction (PCR). Demographic, house locations, medical history, and biological data were analyzed. Factors associated with Plasmodium spp. carriage were analyzed using logistic regression, and the carriage localization was investigated through spatial cluster analysis. Of the 1,501 samples analyzed with PCR, positive results totaled 90 and 10 for Plasmodium vivax and Plasmodium falciparum, respectively. The general PCR prevalence was 6.6% [5.3–7.9], among which 74% were asymptomatic. Only 13/1,549 were positive by RDT. In multivariate analysis, participants older than 15 years, living in a remote neighborhood, with a prior history of malaria, anemia, and thrombocytopenia were associated with an increased odds of Plasmodium spp. carriage. High-risk clusters of P. vivax carriage were detected in the most remote neighborhoods on the village outskirts and two small foci in the village center. We also detected a hot spot for both P. vivax and P. falciparum symptomatic carriers in the northwestern part of the village. The present study confirms a wide-scale presence of asymptomatic P. falciparum and P. vivax carriers in this area. Although they were more often located in remote areas, their geographic distribution was spatially heterogeneous and complex.
INTRODUCTION
In 2016, the incidence of malaria worldwide increased for the first time since 2000.1 In the Americas, this increase was largely confined to incidences in Brazil and Venezuela. French Guiana is a French overseas territory and a malaria-endemic country on the South American continent. Historically, malaria was mainly due to Plasmodium falciparum, but in the past 15 years, Plasmodium vivax infections have become predominant.2,3 This area is committed to a regional program for malaria control.4 In remote areas, diagnosis and treatment are free of charge and administered in health centers using rapid diagnostic tests (RDTs) and artemisinin combination therapies. Vector control is also conducted in all active transmission areas. In-house residual spraying is conducted in households where malaria cases are detected. In addition, permethrin insecticide–treated nets are distributed for free to pregnant women, symptomatic malaria cases, and their family members.
Although the number of malaria cases has declined over the past 10 years, the region still faces major challenges in controlling malaria transmission among socially marginalized and/or isolated populations, such as gold miners and autochthonous populations.2,3,5,6 Although French Guiana’s coastal and urban areas have very low malaria transmission, the country’s inland sites experience higher levels.3,5–7 Illegal gold miners working deep inside the rainforest are massively infected, mostly by P. falciparum.5,8 Amerindian villages along the main rivers at the borders between Suriname and Brazil represent the second largest population affected by symptomatic malaria, mostly due to P. vivax.3,5,9
Asymptomatic carriers of Plasmodium spp. provide a reservoir of infection in areas of transmission. Considering a parasitemia generally above 1 parasite/µL of blood, they may contribute to continuous transmission of the disease and are potentially responsible for the genesis of outbreaks.10,11 Therefore, to interrupt transmission, it is important to focus actions on identifying residual malaria transmission foci. Those efforts should then be intensified to eliminate any remaining foci. For P. vivax malaria infections, it has been clearly shown that both symptomatic and asymptomatic carriers are rapidly infectious.12 However, the scarcity of studies makes it difficult to identify the Amazonian region’s residual transmission areas, referred to as “malaria hotspots.” French Guiana’s population, notably gold miners, is expected to have strong malaria immunity, and many of them present asymptomatic parasitemia.5,8,13 Symptomatic malaria cases in this context occur throughout the year.7 In addition, prevalence of Plasmodium spp. is highly heterogeneous among the different mining sites.5,8 Apart from these, most of the remaining cases in French Guiana are detected at the border with Brazil (Northeastern French Guiana) and at a lower rate along the Surinamese border (Southwestern French Guiana), more specifically in Amerindian villages.6,7 Malaria transmission among these autochthonous populations is mostly seasonal, demonstrating an unstable pattern.3,7 Within these populations, it is likely that asymptomatic carriage exists.6
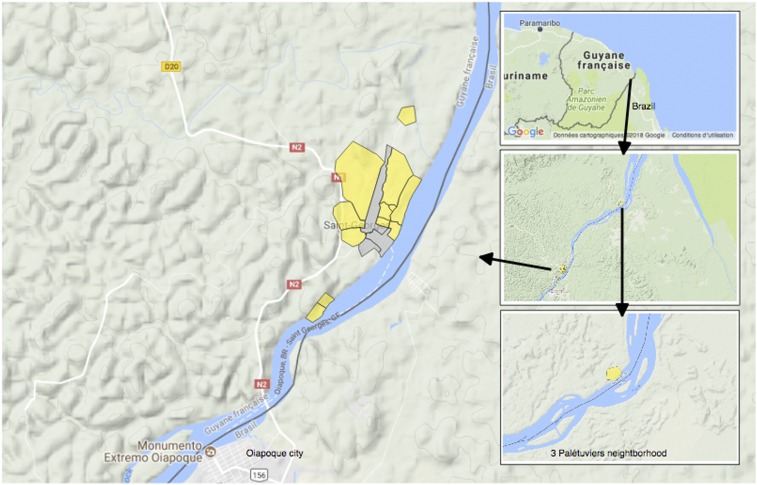
This study was conducted in the village of Saint Georges de l’Oyapock (STG), a persistent malaria-endemic area along the Brazilian border (Figure 1). Two peaks in malaria cases are generally observed in this region and are consistent with the equatorial climate. These peaks, occurring in June and November, are inter-seasonal and observed between the rainy and the dry seasons.7 In this municipality, Anopheles Darlingi was previously described as the predominant species.14 The vector density and number of breeding sites are high beginning a few weeks before the onset of symptomatic cases.14,15 However, the density of An. darlingi is heterogeneous, depending notably on the distance to the dense forest.16
Figure 1.
Study area. This figure appears in color at www.ajtmh.org.
To date, no study has investigated the distribution of Plasmodium spp. carriage in this Amazonian area, and the risk factors associated with malaria parasite carriage remain unclear. The aim of this study was to provide a better understanding of the distribution and contribution of Plasmodium spp. carriage to the overall parasite reservoir.
METHODS
Study area.
This transversal study was carried out between October and December 2017 in the municipality of STG (Figure 1), and was the first part of a before–after study called “Palustop.” It was conducted to evaluate the efficiency in treating both symptomatic and asymptomatic Plasmodium spp. carriers to decrease malaria prevalence in the study area. This period of the year was chosen to cover the epidemic peak observed annually in this municipality.
To characterize the risk factors for malaria and the prevalence of Plasmodium spp. infection in this transmission area, 13 STG neighborhoods were selected based on their history of an elevated incidence of symptomatic cases over the last 2 years. These neighborhoods also included three remote villages located in the south of the municipality, 10 minutes by canoe for Blondin, or in the north, 1 hour by canoe for Trois-Palétuviers (Figure 1).
Sample size and population.
There are approximately 4,033 inhabitants (STG health center data 2017) in STG, mainly in the municipality center. Three neighborhoods (Blondin 1, Blondin 2, and Trois-Palétuviers) are particularly isolated and only accessible by canoe. The entire population size of the 11 studied neighborhoods was ∼2,727 inhabitants, all ages and nationalities included.
Designed for the “Palustop” before–after study, the sample size was calculated at 1,200 (expected polymerase chain reaction [PCR] prevalence of 4% based on previous reported data, error margin, and an alpha risk of 0.05).6 Because of the good participation and mobility of the population in this area, we targeted a sample size of 1,500 participants for the first step of the study, expecting to finish a year later with a minimum of 1,200.6
Biological analysis.
Malaria RDTs were performed to immediately treat participants who tested positive. We applied the most commonly used RDT in French Guiana, the SD BIOLINE® Malaria Ag Pf/Pan test (pfHRP2/pLDH-based Standard Diagnostics, SD Bioline Standard Diagnostics, Gyeonggi, Republic of Korea). Malaria PCR detection was performed using a real-time method derived from Shokoples et al.17 Detection and identification of four Plasmodium species (P. falciparum, P. vivax, Plasmodium ovale, and Plasmodium malariae) were performed using a Taqman® (Vilnius, Lithuania) probe strategy, with a sensitivity of 1 parasite/µL for all the species except P. vivax at 0.5p/µL. Positive and negative controls were systematically added to the experiments. This method is certified according to the International Organization for Standardization 15189 requirements for medical examinations. Complete blood count, reticulocyte, and the glucose-6-phosphate dehydrogenase (G6PD) activity tests were also performed.
Interviews and medical care.
Participants were interviewed using a questionnaire designed to gather information on knowledge, attitudes, and practices regarding malaria. Data on demographic factors such as gender, age, and occupation were collected. Participants were also questioned about their travel habits such as frequency and location of hunting, fishing, and farming in the border area. For children, parental consent was obtained and parents were interviewed on behalf of the child. The questionnaire was administered by trained cultural mediators in the language of the participants. Their houses were georeferenced. Participants’ body temperatures were measured by an ear thermometer, and fever was defined as any temperature ≥ 38°C. All participants received a medical examination. If RDT or PCR results were positive, participants were treated according to national treatment guidelines.18
Data analysis.
Symptomatic cases were defined as RDT- or PCR-positive results associated with a history of fever in the last 48 hours or during the inclusion process. Plasmodium spp. carriage was defined as a Plasmodium PCR-positive result, regardless of body temperature.
Spatial statistics.
Bernoulli methods proposed by Kulldorff et al.19 were applied via SaTScan software (Monte-Carlo method using 999 replications, version 9.5). Clusters of high or low rates with maximum likelihood ratios were selected, and any overlapping clusters were excluded. A multi-scale analysis was performed by considering the study area as a whole (study area scale). Then, because of their relative distances, the neighborhoods of Trois-Palétuviers, Blondin (1 and 2), and the STG downtown area were considered separately (locality scale). Different analyses were also performed according to the Plasmodium species (all species, P. vivax, or P. falciparum) and the presence or absence of symptoms (symptomatic and asymptomatic cases, symptomatic cases only, and asymptomatic cases only). Map analyses were conducted in QGIS 2.12 software.
Risk factor analysis.
Associated factors of Plasmodium spp. carriage were investigated using logistic regression models. Bivariate analyses were used to select potential covariates (P < 0.1) for the multivariate model. A backward selection procedure was then applied to keep only significant associated factors (P < 0.05) in the final multivariate model. Odds ratios and associated 95% CIs were estimated. Statistical analyses were conducted with SAS version 9.4 software (SAS Institute Inc., Cary, NC).
Ethics approval and consent to participate.
The study was approved by the Comité de Protection des Personnes du Sud-Ouest et Outre-Mer 4 N° AM-36/1/CPP15-024 (French ethics committee). The database was anonymized and declared to the French Regulatory Commission (Commission Nationale Informatique et Libertés, CNIL, n°917186). Samples collected by the National Reference Center were registered by the French Ministry for Research (declaration number DC-2010-1223; collection N°2). Duly signed, informed consent was obtained from all participants involved in the study, following explanations about the study in their local languages. Treatments and medical follow-up of malaria infection were offered.
RESULTS
A total of 1,566 inhabitants were included in the study (57.4% of the study area inhabitants). Of these, 1,501 (95.8%) individuals had a blood sample taken. Venipuncture failure or fear of blood sampling was the primary reason why certain participants did not contribute to the blood samples. The median age was 22.8 years (min–max: 0.2–92.9) and the sex ratio was 0.88 (Table 1). A majority of participants declared having French nationality (56.7%), 42.7% Brazilian, and 0.6% another nationality (Table 1). The study population was multiethnic, with more than 12 different native languages reported. A third (32.8%) of the participants older than 18 years never went to school or reported having only a primary level of education. A large portion (45.2%) of the participants reported living in the same house with a large group of individuals (more than six people). A minority (18.5%) of participants did not have social coverage or reported having precarious social protection.
Table 1.
Main characteristics of study participants, STG, 2017
| Patients characteristics (n = 1,566) | Number or median | % or min–max |
|---|---|---|
| Age (years) | 22.8 | (0.2–92.9) |
| Age categories (years) | ||
| 0–14 | 710 | 45.3 |
| 15–24 | 232 | 14.8 |
| ≥ 25 | 624 | 39.8 |
| Male | 735 | 47 |
| Level of education if age > 18 years (n = 786) | ||
| No formal education | 122 | 15.5 |
| Primary | 136 | 17.3 |
| College | 318 | 40.4 |
| High school | 169 | 21.5 |
| University | 41 | 5.2 |
| Occupation | ||
| Farmer | 111 | 7.1 |
| Hunter | 38 | 2.4 |
| Work at home | 333 | 21.3 |
| Student | 572 | 36.5 |
| Gold miner | 1 | 0.06 |
| Fisherman | 28 | 1.8 |
| Canoe driver | 4 | 0.3 |
| Pensioner | 37 | 2.4 |
| Employee in the center of STG | 88 | 2.6 |
| Employee in the center of Oiapoque city | 5 | 0.3 |
| Others | 349 | 22.3 |
| Number of people in a household (n = 1,562) | ||
| 1–3 | 244 | 15.6 |
| 4–6 | 612 | 39.2 |
| 6–10 | 529 | 33.9 |
| Greater than 10 | 177 | 11.3 |
| Nationality | ||
| French | 888 | 56.7 |
| Brazilian | 668 | 42.7 |
| Surinamese | 3 | 0.3 |
| Haitian | 2 | 0.1 |
| Guyanese | 1 | 0.06 |
| Other | 4 | 0.3 |
| Native language | ||
| Brazilian | 513 | 32.8 |
| French | 98 | 6.3 |
| Creoles from French Guiana | 371 | 23.7 |
| Creoles from Haiti | 4 | 0.3 |
| Palikur | 372 | 23.7 |
| Karipuna | 70 | 4.5 |
| Kalina | 2 | 0.1 |
| Wayampi | 15 | 0.9 |
| Teko | 39 | 2.5 |
| Others | 82 | 5.2 |
| Social coverage | ||
| French State Medical Assistance* | 144 | 7.3 |
| French Universal Health Coverage | 1,089 | 69.5 |
| Brazilian social coverage | 57 | 3.6 |
| French social security | 79 | 5 |
| No social coverage | 175 | 11.2 |
| Unknown | 52 | 3.3 |
| Sleeping under bednets | ||
| No | 408 | 26 |
| Yes | 1,558 | 74 |
| Medical history of confirmed malaria† | ||
| No | 846 | 54 |
| Yes | 720 | 46 |
| If age older than 18 years | ||
| No | 241 | 31.8 |
| Yes | 517 | 68.2 |
| Number of episodes of malaria (N = 720) | 3.0 | (2.8–3.6) |
| Year of last episode | ||
| 2017 | 132 | 18.3 |
| 2014–2016 | 123 | 17 |
| 2000–2014 | 358 | 49.7 |
| ≤ 2000 | 107 | 14.9 |
| Malaria species of the last episode | ||
| Plasmodium falciparum | 94 | 13 |
| Plasmodium vivax | 394 | 54.7 |
| Unknown | 226 | 31.4 |
| Other | 6 | 0.8 |
STG = Saint Georges de l’Oyapock.
* State Medical Assistance: Social coverage for an immigrant without a residency permit or a document proving that the immigrant has begun the application process for legal residency.
† Malaria confirmed by a test in a health center.
Common biological characteristics.
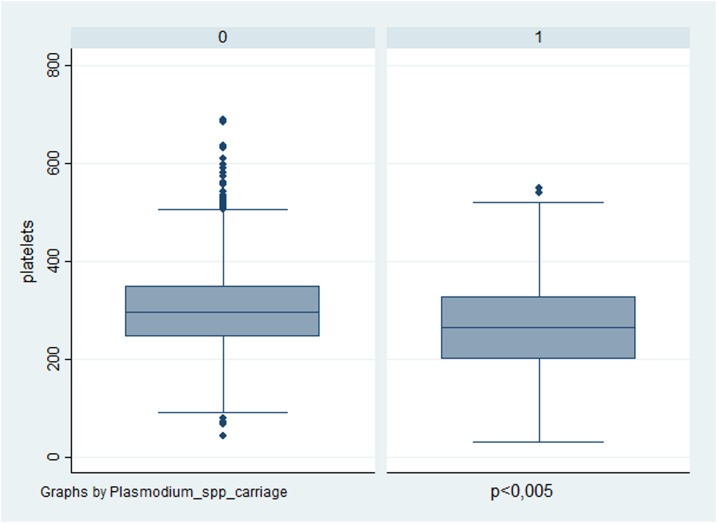
Severe anemia (< 8g/dL) was found in only two participants (0.14%). Anemia (< 10g/dL) and thrombocytopenia (< 150 109/L) were associated with Plasmodium spp. carriage (Table 2 and Figure 2). Hemolysis was uncommon; only 34 participants (2.3%) had more than 2.5% of reticulocytes. Participants (n = 1,470) were screened for G6PD deficiency (N > 10 U/g Hg). The median value of G6PD was 11.9 [11.7–12.2] in males and 12.2 [12.0–12.5] in females. According to the WHO classification for G6PD activity ranges, the majority of them (91.2%, n = 1,345/1,474) had a normal G6PD activity (> 80%), 67 females (4.5%, n = 67/1,474) had an intermediate activity (30–80%), and only three females (0.2%, n = 3/1,474) and one male (0.06%, n = 1/1,474) had an activity less than 30% and 10%, respectively.20
Table 2.
Factors associated with Plasmodium spp. carriage based on PCR results in univariate analysis, STG, 2017
| Plasmodium spp. PCR-negative (n = =1,401) | Plasmodium spp. PCR-positive (n = =100) | P-value | |
|---|---|---|---|
| Age (years)† | 23.5 (22.7–24.73) | 26.63 (23.6–30.19) | 0.04 |
| ≤ 14 | 639 (95.7%) | 29 (4.3%) | < 0.005 |
| 15–24 | 197 (89.1%) | 24 (10.9%) | |
| 25–49 | 410 (92.1%) | 35 (7.9%) | |
| ≥ 50 | 155 (92.8%) | 12 (7.2%) | |
| Gender | 0.624 | ||
| Female | 750 (93.6%) | 51 (6.4%) | |
| Male | 651 (93.0%) | 49 (7.0%) | |
| Nationality | 0.943 | ||
| French | 784 (93.2%) | 60 (6.8%) | |
| Brazilian | 607 (93.8%) | 40 (6.2%) | |
| Guyanese | 1 (100.0%) | 0 (0.0%) | |
| Haitian | 2 (100.0%) | 0 (0.0%) | |
| Surinamese | 3 (100.0%) | 0 (0.0%) | |
| Others | 4 (100.0%) | 0 (0.0%) | |
| Mother tongue† | < 0.005 | ||
| French | 90 (97.8%) | 2 (2.2%) | |
| Creole from French Guiana | 315 (88.0%) | 43 (12.0%) | |
| Brazilian | 465 (94.3%) | 28 (5.7%) | |
| Palikur Indians | 344 (95.3%) | 17 (4.7%) | |
| Karipuna Indians | 63 (94.0%) | 4 (6.0%) | |
| Teko or Wayãpi Indians | 51 (98.0%) | 1 (2.0%) | |
| Saramaka (Maroons) | 0 (0.0%) | 1 (100.0%) | |
| Others | 73 (94.8%) | 4 (5.2%) | |
| Amerindian or creole ethnicity* | 0.030 | ||
| No | 208 (96.7) | 7 (3.3%) | |
| Yes | 1,193 (92.8%) | 93 (7.2%) | |
| School level | 0.179 | ||
| Any level | 249 (94.3%) | 15 (5.7%) | |
| Nursery school | 106 (95.5%) | 5 (4.5%) | |
| Elementary school | 368 (93.9%) | 24 (6.2%) | |
| High school | 178 (89.0%) | 22 (11.0%) | |
| University | 38 (92.7%) | 3 (7.3%) | |
| Occupation† | 0.023 | ||
| Farmers | 99 (90.0%) | 11 (10.0%) | |
| Hunters | 31 (83.8%) | 6 (16.2%) | |
| Canoe driver or fisherman | 27 (84.4%) | 5 (15.6%) | |
| Occupation in downtown area | 85 (93.4%) | 6 (6.6%) | |
| Student | 526 (95.1%) | 27 (4.9%) | |
| Work at home (housewife notably) | 309 (93.9%) | 20 (6.1%) | |
| Others | 324 (92.8%) | 25 (7.2%) | |
| Residence area† | < 0.005 | ||
| Blondin 1 and 2 neighborhoods | 42 (73.4%) | 13 (23.6%) | |
| Trois-Palétuviers neighborhood | 133 (73.9%) | 47 (26.1%) | |
| Other neighborhood (village center) | 1,266 (96.9%) | 40 (3.1%) | |
| Time spent in STG (years) | 0.799 | ||
| < 2 | 164 (93.2%) | 12 (6.8%) | |
| 2–4 | 187 (94.4%) | 11 (5.6%) | |
| ≥ 4 | 1,050 (98.0%) | 77 (2.0%) | |
| Mean of people in a household (n = 1,497) | 6.4 (6.2–6.5) | 7.6 (6.8–8.3) | < 0.005 |
| Bednets use† | < 0.005 | ||
| No | 374 (96.4%) | 14 (3.6%) | |
| Yes | 1,027 (92.3%) | 86 (7.7%) | |
| Bednets with holes (n = 1,073) | < 0.005 | ||
| Yes | 666 (90.6%) | 69 (9.4%) | |
| No | 322 (95.3%) | 16 (4.7%) | |
| Medical history of malaria† | < 0.005 | ||
| Yes | 769 (97.5%) | 20 (2.5%) | |
| No | 632 (88.8%) | 80 (11.2%) | |
| Complete antimalarial treatment during the last event (n = 712) | 0.48 | ||
| Yes | 42 (87.7%) | 7 (14.3%) | |
| No | 590 (89.0%) | 73 (11.0%) | |
| Use traditional plants† | < 0.005 | ||
| Never | 913 (94.7%) | 51 (5.3%) | |
| Sometimes | 387 (90.6%) | 40 (9.4%) | |
| Often | 110 (92.4%) | 9 (7.6%) | |
| Hunting practice† | < 0.005 | ||
| Yes | 221 (89.1%) | 27 (10.9%) | |
| No | 1,180 (94.2%) | 73 (5.8%) | |
| Fishing practice† | < 0.005 | ||
| Yes | 385 (88.9%) | 48 (11.1%) | |
| No | 1,016 (87.0%) | 52 (3.0%) | |
| Farming activity in the rain forest† | < 0.005 | ||
| Yes | 639 (90.1%) | 70 (9.9%) | |
| No | 762 (96.2%) | 30 (3.8%) | |
| Gold mine activity or spent time in a gold mine† | 0.021 | ||
| No | 1,356 (93.6%) | 92 (6.4%) | |
| Yes | 45 (84.9%) | 8 (15.1%) | |
| History of fever in the last 48 hours† | < 0.005 | ||
| Yes | 115 (81.6%) | 26 (18.4%) | |
| No | 1,286 (88.1%) | 74 (11.9%) | |
| Temperature† | 0.007 | ||
| < 38 | 1,379 (93.6%) | 94 (6.4%) | |
| ≥ 38 | 21 (77.8%) | 6 (22.2%) | |
| Physical examination† | 0.003 | ||
| Normal | 1,073 (93.9%) | 62 (6.1%) | |
| Not normal | 327 (89.6%) | 38 (10.4%) | |
| Missing data | 1 (100.0%) | 0 (0.0%) | |
| Rapid diagnostic test† | < 0.005 | ||
| Negative | 1,390 (93.9%) | 91 (6.1%) | |
| Positive | 4 (30.8%) | 9 (69.2%) | |
| Missing data or cannot be interpreted | 7 (100.0%) | 0 (0.0%) | |
| Hemoglobin count (g/dL)† | < 0.005 | ||
| < 10 | 16 (72.7%) | 6 (27.3%) | |
| ≥ 10 | 1,358 (93.5%) | 94 (6.5%) | |
| Missing data | 27 (100.0%) | 0 (0.0%) | |
| Platelets count† | < 0.005 | ||
| < 150 109/L | 24 (63.2%) | 14 (36.8%) | |
| ≥ 150 109/L | 1,350 (94.0%) | 86 (6.0%) | |
| Missing data | 27 (100.0%) | 0 (0.0%) | |
| Eosinophil count (G/L) | 0.439 | ||
| ≤ 0.5 | 668 (93.4%) | 47 (6.6%) | |
| > 0.5 | 675 (94.4%) | 40 (5.6%) | |
| Missing data | 58 (56.3%) | 13 (43.7%) | |
| Glucose-6-phosphate dehydrogenase activity | 0.793 | ||
| < 80% | 120 (93.8%) | 8 (6.2%) | |
| ≥ 80% | 1,249 (93.1%) | 92 (6.9%) | |
| Missing data | 32 (100.0%) | 0 (0.0%) |
PCR = polymerase chain reaction; STG = Saint Georges de l’Oyapock.
* Wayana, Palikur, Kalina, Wayãpi, Teko, and Karipuna Amerindian communities were grouped on one side, Haitian and French Guianese Creole on the other side.
† Variable used in multivariable analysis.
Figure 2.
Level of platelets in participants with or without Plasmodium spp. carriage. This figure appears in color at www.ajtmh.org.
Plasmodium spp. carriers.
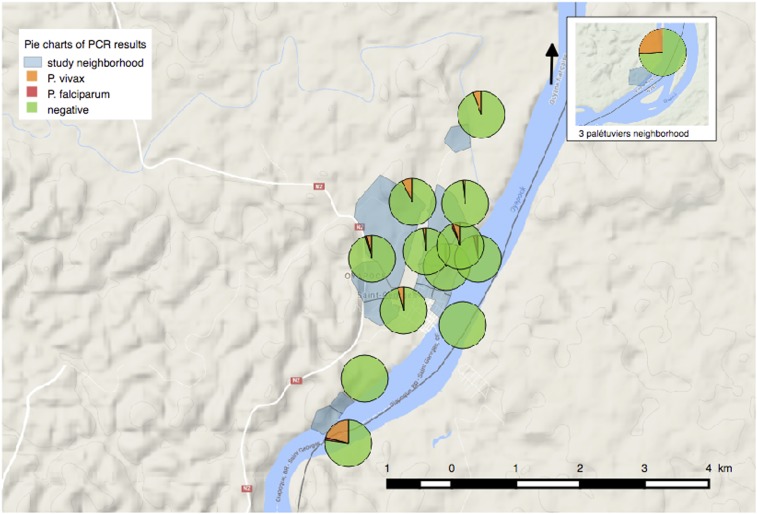
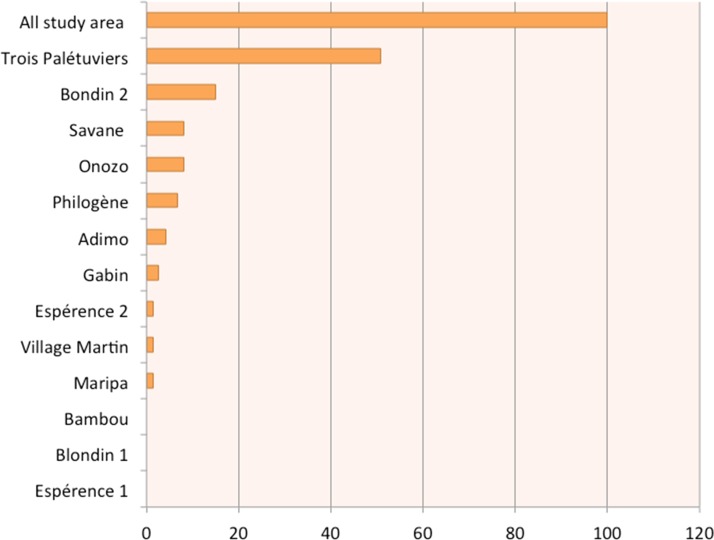
The prevalence of Plasmodium spp. carriers was 6.6% [5.3–7.9], with the 100 positive cases being predominantly P. vivax (n = 90). In comparison, malaria prevalence using RDTs was 0.84% ([0.38–1.3], n = 13/1,540). The median age of the PCR-positive population was 23.5 years (min–max: 0.8–74.3), significantly higher than that of the PCR-negative population (P = 0.04, Table 2). Asymptomatic carriers represented 73.0% (n = 73/100) and 38.5% (n = 5/13) of the PCR- and RDT-positive population, respectively. Asymptomatic carriage also varied according to the Plasmodium species: 100% (n = 10/10) for P. falciparum and 70.0% (n = 63/90) for P. vivax (P < 0.005). A strong heterogeneity of Plasmodium spp. carriage between neighborhoods ranged from 0.0% to 29.5% (Table 3, Figures 3 and 4).
Table 3.
Plasmodium spp. prevalence per neighborhood, STG, 2017
| STG neighborhoods | Results of polymerase chain reaction | Studied participants (n) | Exhaustivity* | |||
|---|---|---|---|---|---|---|
| Plasmodium spp. prevalence % (IC 95%) | Negative | Plasmodium vivax | Plasmodium falciparum | |||
| Total | 6.4 (5.3–7.9) | 1,401 | 90 | 10 | 1,566 | 57.4% |
| Blondin 2 | 29.5 (15.9–43.2) | 31 | 12 | 1 | 44 | 91.7% |
| Trois-Palétuviers | 26.1 (19.6–32.5) | 133 | 46 | 1 | 183 | 98.9% |
| Adimo | 7.5 (2.4–12.6) | 98 | 8 | 0 | 111 | 41.7% |
| Philogène | 6.5 (0.9–12.0) | 72 | 4 | 1 | 77 | 90.6% |
| Village Martin | 5.7 (2.0–13.5) | 33 | 2 | 0 | 35 | 42.3% |
| Gabin | 4.7 (0.6–8.7) | 102 | 4 | 1 | 113 | 78.2% |
| Maripa | 3.9 (1.4–9.3) | 49 | 2 | 0 | 54 | 38.6% |
| Onozo | 3.0 (0.8–5.3) | 221 | 2 | 5 | 252 | 53.5% |
| Esperance 2 | 2.4 (0.3–5.0) | 124 | 3 | 0 | 137 | 44.1% |
| Savane | 1.7 (0.4–2.9) | 409 | 6 | 1 | 425 | 65.4% |
| Esperance 1 | 1.3 (1.3–3.9) | 75 | 1 | 0 | 79 | 67.5% |
| Bambou | 0.0 | 43 | 0 | 0 | 45 | 24.2% |
| Blondin 1 | 0.0 | 11 | 0 | 0 | 11 | 68.7% |
IC = interval confident; n = number; STG = Saint Georges de l’Oyapock.
* Total numbers of inhabitants per neighborhood (n = 2,727) were derived from the STG health center data to calculate the exhaustivity level, 2017.
Figure 3.
Plasmodium spp. polymerase chain reaction (PCR) results by neighborhood and number of PCR-positive results per study participant. This figure appears in color at www.ajtmh.org.
Figure 4.
Percentage of asymptomatic Plasmodium spp. carriage by neighborhood. This figure appears in color at www.ajtmh.org.
In bivariate analysis, asymptomatic carriage was more common in participants older than 15 years as well as in the Amerindian and Creole communities (Table 2). Autochthonous practices such as hunting, fishing, farming, and using traditional plants were also associated with asymptomatic malaria carriage (Table 2). In bivariate analysis, sleeping under bednets and the presence of holes in bednets among those who reported using them were related to a higher Plasmodium spp. prevalence. Previous history of malaria was associated with Plasmodium spp. carriage.
Spatial distribution of Plasmodium spp. carriers.
Spatial data from thirteen individuals, all associated with negative PCR results, were removed from the analysis because of either missing (n = 5) or erroneous (n = 8) global positioning system localizations.
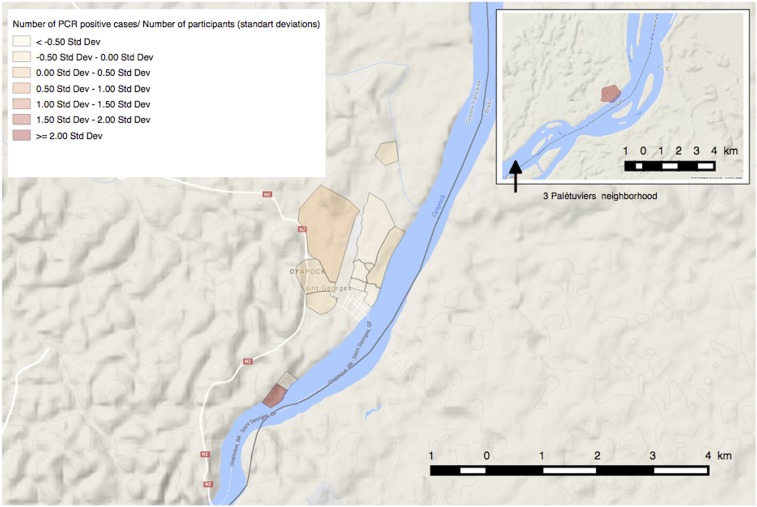
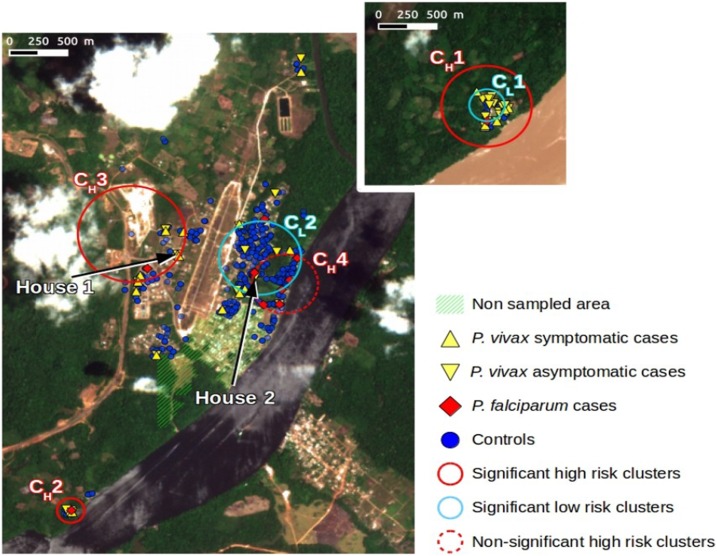
At the whole study area scale, the Trois-Palétuviers neighborhood alone formed a high-risk cluster for asymptomatic P. vivax cases (P < 0.005, see cluster CH1 in Figure 5). The relative risks were 6.97, 8.21, and 9.29 for the positive Plasmodium spp. cases, P. vivax cases only (asymptomatic and symptomatic), and Plasmodium spp. asymptomatic cases, respectively.
Figure 5.
Results summary of spatial clustering using multiple outcomes. This figure appears in color at www.ajtmh.org.
The study of the Trois-Palétuviers neighborhood alone (locality scale) indicated that the “center” of the neighborhood formed a low-risk cluster (see Figure 5, cluster CL1) (RR=0, P-value = 0.038) for symptomatic cases (considering Plasmodium spp.).
At the whole study area scale, the southern part of the Blondin neighborhood (Blondin 2) formed a high-risk cluster for P. vivax asymptomatic cases also (see cluster CH2 in Figure 5). The relative risks were 6.63 (P < 0.005), 6.67 (P < 0.005), and 10.71 (P-value = 0.0002) for the positive Plasmodium spp. cases, P. vivax cases only (asymptomatic and symptomatic), and Plasmodium spp. asymptomatic cases, respectively. No other significant results were derived from the local scale analysis for this neighborhood.
Two high-risk clusters in the Saint Georges downtown area corresponded, in fact, to specific homes (denoted as House 1 and House 2 in Figure 5). One house was located in the Adimo neighborhood, which counted four P. vivax carriers (two asymptomatics and two symptomatics) of the five household members. The second significant high-risk cluster, located in the Philogène quarter, included two neighboring houses, which had four asymptomatic P. vivax carriers among the eight household members. Except for these two very small hot spots, the surrounding neighborhoods presented significantly low relative risks. One of the most significant (P < 0.005) low-risk clusters (RR = 0.14), denoted as CL2, is represented in Figure 5.
By considering symptomatic cases for Plasmodium spp. at the local scale, a significant high-risk cluster was identified in the northwestern part of the village’s urban area (the western part of the Adimo quarter and the northern part of the Gabin quarter; see cluster CH3 in Figure 5). The associated relative risk was 10.82 (P = 0.046). All the symptomatic cases in this area were P. vivax cases. What is more, among the total number of cases in this cluster (12), only one was associated with P. falciparum. Thus, these results reveal that this cluster predominantly comprised symptomatic P. vivax cases.
Plasmodium falciparum cases were pooled in the eastern part of the Saint Georges locality (CH4 in Figure 5). However, this cluster was not statistically significant (P = 0.417).
Analysis of factors associated with Plasmodium spp. carriage.
Independent associated factors of Plasmodium spp. carriage are shown in Table 4. The main factors were identified as “living in an outlying neighborhood” (Blondin or Trois-Palétuviers), “being older than 14 years,” “having reported a previous history of malaria,” and “having reported a history of fever in the last 48 hours.” Anemia and thrombocytopenia were also found to be associated with Plasmodium spp. carriage in the final multivariate model.
Table 4.
Risk factors for Plasmodium spp. carriage in multivariable analysis, Saint Georges de l’Oyapock, 2017
| Variable | Odds ratio | 95% Wald confidence limits | |
|---|---|---|---|
| Neighborhood | Trois-Palétuviers vs. downtown | 13.40 | 7.86–22.88 |
| Blondin 2 vs. downtown | 9.85 | 4.51–21.88 | |
| Age (years) | [15–24] vs. ≤ 14 | 2.49 | 1.64–4.89 |
| ≥ 25 vs. ≤ 14 | 1.86 | 1.05–3.30 | |
| Medical history of malaria | Yes vs. no | 2.66 | 1.05–4.72 |
| History of fever in the last 48 hours | Yes vs. no | 3.66 | 1.99–6.74 |
| Anemia (g/dL) | < 10 vs. ≥ 10 | 10.38 | 3.11–34.57 |
| Thrombocytopenia | < 150 109/L vs. ≥ 150 109/L | 6.87 | 2.87–16.45 |
DISCUSSION
Active case detection of malaria in this border area revealed a heterogeneous Plasmodium spp. prevalence, ranging from 0.0% to 29.5% depending mainly on the neighborhood. The general prevalence was 6.6% IC95% (5.3–7.9). The majority carried P. vivax (90.0%) and was asymptomatic (74.0%).
Specificity of Plasmodium spp. carriage in this population.
Numerous behavioral factors can increase the risk of infection. The present study showed that people aged from 14 to 25 years formed the most significant at-risk group. People older than the age of 14 may have more at-risk behaviors such as night hunting or fishing. Other contributing factors include activities in high transmission sites such as gold mines or even staying outside with friends during sunset, a known Anopheles-vector biting time (4). The present study surprisingly revealed that the use of bednets is associated with Plasmodium spp. carriage in bivariate analysis. Also, when bednets were used, the presence of holes was associated with Plasmodium spp. carriage. In the same way, participants who reported the use of plants for medicinal purposes had a higher Plasmodium spp. carriage prevalence. This may be due to a confounding bias: Amerindian and Creole communities were the hardest hit by malaria and had the greatest numbers of users of traditional medicine and bednets.
Heterogeneous Plasmodium spp. prevalence in the same village.
As the current global context moves toward malaria elimination, shifts toward heterogeneous transmission of the disease are generally observed.21,22 The situation in this part of French Guiana reflects a similar trend. Blondin 2 and Trois-Palétuviers, the most remote neighborhoods, appeared to be the largest hot spots. Indeed, these results indicated that living near the forest and vector-breeding sites is associated with elevated Plasmodium spp. carriage, as previously identified along the Oyapock River.14,23 Concordantly, in the Trois-Palétuviers neighborhood, symptomatic cases were reported more significantly in the outlying areas, near high vector density sites. The role of vector density and human behaviors in sustaining symptomatic cases in this micro-area deserves to be explored. It is worth noting that complex malaria transmission foci were also found in the STG village center within Amerindian families. In this context, travel or certain practices (frequent fishing and hunting expeditions in this community) would explain those cases. Similarly, most P. falciparum carriage appeared in Onozo, a fishing neighborhood that may have hosted gold miners. Determining additional risk factors may help guide effective control programs among these border populations. Such risk factors include travel to specific regions or villages linked to imported malaria cases and potentially resulting in the start of transmission chains.24 In addition, the present study describes a fine-scale spatial distribution of Plasmodium spp. carriage, but further studies are needed to determine whether or not these hot spots are stable and potentially responsible for the seasonal transmission observed each year.
A majority of asymptomatic carriage with frequent biological abnormalities.
Plasmodium spp. carriage, notably asymptomatic malaria, has significant health consequences. For example, anemia and thrombopenia are observed in both symptomatic and asymptomatic infections. Asymptomatic carriage can result in a low-grade hemolysis.25 In areas where asymptomatic carriage is highly prevalent, malaria-attributable anemia can be challenging. Helminth infections are common and can exacerbate anemia while simultaneously providing a degree of protection from symptomatic malaria.26 However, in our study, hypereosinophilia, which is a marker of helminth infection, was not associated with a lower rate of Plasmodium spp. carriage. Thrombocytopenia in asymptomatic carriage was previously described in Africa.27 The mechanism behind thrombocytopenia may be attributed to peripheral destruction and consumption of platelets.28 Thus, some authors propose replacing the term “asymptomatic malaria” with “chronic malaria infection” and suggest treating all cases of Plasmodium spp. carriage because of their health and social consequences.29
Implications for malaria transmission.
A challenge is to identify asymptomatic individuals with a level of gametocytemia sufficient to transmit infection to mosquitoes. Asymptomatic carriers could transmit malaria even at low submicroscopic parasitemias. The limits for transmission are difficult to determine because of a combination of variables, including gametocyte maturity, Plasmodium species, and degree of immunity. In our study, the PCR deployed to detect parasite carriers was not an ultrasensitive method. With its detection limit around parasite/µL of blood, we could assume that patients detected positive have a high probability to transmit the disease. Moreover, P. vivax parasites generally generate gametocytes earlier than P. falciparum.
Implications for public health.
Whatever the country, Plasmodium spp. carriage is challenging. Control programs based on passive case detection do not detect asymptomatic infections, and, therefore, these carriers remain untreated. Because of the absence of symptoms, most infected people do not go to health facilities for testing or are under the detection limit of RDTs. Therefore, one option is to identify and treat asymptomatic patients. In our study, two remote neighborhoods represented 66.0% of the asymptomatic cases of this municipality. Such a high concentration of cases can positively influence efforts to implement control measures. Despite their distance from the STG village center, health authorities should consider these transmission hot spots if they expect better malaria control in this region. Remote-area residents were previously described as hard-to-reach populations often missed by control measures.30 Nonetheless, Trois-Palétuviers and Blondin 2 achieved the highest participation rates (96.3% and 80.0%, respectively). These rates suggest that these communities have a high level of concerns regarding this disease and expect action from health authorities. This highlights the importance of providing easier access to diagnosis and care in these areas. Improvements to consider include training community residents in malaria testing, as is already carried out in Brazil, and facilitating travel between Trois-Palétuviers and health centers. Finally, there are additional elimination strategies worth examining. For example, intermittent preventive treatment should be considered, in particular for certain high-risk parasite carriage populations such as Trois-Palétuviers and Blondin 2 inhabitants older than 14 years. Other strategies to consider could also include seasonal malaria chemoprevention and mass drug administration.31–33 However, these approaches require rigorous risk-benefit analysis before any implementation. In fact, the associated risks include an increased drug pressure that may impose selection in favor of resistance and eventual side effects in asymptomatic patients. Benefits could lead to a decreased morbidity for participants and to malaria elimination in the community. However, at this time, in France and Brazil, WHO recommendations are followed and diagnostic testing for malaria is a prerequisite to receiving treatment in symptomatic cases.4 Detection of asymptomatic cases is not part of the control programs. To be addressed, policymakers must commit to elimination. In addition to these asymptomatic carriage hot spots, small foci around households are observed in other neighborhoods. Generally, in low-endemic settings, reactive case detection is a viable option for febrile and RDT-positive malaria patients.34 In these neighborhoods, reactive case detection by PCR should be accompanied by cultural mediator-assisted discussions with the entire household. The objective is to explain the purpose of this screening and to facilitate adherence to treatment and health recommendations.
CONCLUSION
This study allowed us to identify the main risk factors for Plasmodium spp. carriage in this area, where data were scare. These identified risk factors can help policymakers develop targeted intervention strategies along this border area in relation to the heterogeneous distribution. These findings may support a scientific-based choice for the future malaria program in France and Brazil, such as outreach initiatives in isolated neighborhoods that offer new points of community information, better access to malaria diagnosis, and treatment adapted to the level of transmission.
Acknowledgments:
We are grateful to the study participants in STG, French Guiana; the NGO, DAAC, provided culturally adapted mediators, offering crucial support throughout this study; and Franck De Laval, Gaelle Walter, Bastien Bidaud, Loïc Epelboin, Alessia Melzani, and Mylene Cebe also contributed to data collection.
REFERENCES
- 1.World Health Organization , 2018. World Malaria Report 2018. Available at: https://www.who.int/malaria/publications/world-malaria-report-2018/en/. Accessed August 22, 2019. [Google Scholar]
- 2.World Health Organization , 2017. WHO Malaria Report, 2017. Available at: https://www.who.int/malaria/publications/world-malaria-report-2017/en. Accessed August 22, 2019. [Google Scholar]
- 3.Musset L, Pelleau S, Girod R, Ardillon V, Carvalho L, Dusfour I, Gomes MS, Djossou F, Legrand E, 2014. Malaria on the Guiana Shield: a review of the situation in French Guiana. Mem Inst Oswaldo Cruz 109: 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathalie M, 2015. Plan de Lutte Contre Le Paludisme En Guyane. Cayenne, French Guiana: ARS Guyane. [Google Scholar]
- 5.Douine M, et al. 2016. Prevalence of Plasmodium spp. in illegal gold miners in French Guiana in 2015: a hidden but critical malaria reservoir. Malar J 15: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosnier E, et al. 2017. Asymptomatic Plasmodium falciparum and vivax infection in the neighborhood of Blondin, Saint-Georges-de-l’Oyapock district, French Guiana. Bull Soc Pathol Exot 110: 265–269. [DOI] [PubMed] [Google Scholar]
- 7.Ardillon V, 2018. Surveillance du paludisme. Bulletin Périodique: novembre 2017 à janvier 2019. Le point épidémiologique N°01/2018. Cayenne, French Guiana: Ardillon V. [Google Scholar]
- 8.Pommier de Santi V, et al. 2016. Malaria hyperendemicity and risk for artemisinin resistance among illegal gold miners, French Guiana. Emerg Infect Dis 22: 903–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanf M, Stéphani A, Basurko C, Nacher M, Carme B, 2009. Determination of the Plasmodium vivax relapse pattern in Camopi, French Guiana. Malar J 8: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coura JR, Suárez-Mutis M, Ladeia-Andrade S, 2006. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection–a review. Mem Inst Oswaldo Cruz 101: 229–237. [DOI] [PubMed] [Google Scholar]
- 11.Bousema T, Okell L, Felger I, Drakeley C, 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 12.Kiattibutr K, et al. 2017. Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a southeast Asian vector, Anopheles dirus. Int J Parasitol 47: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pommier de Santi V, et al. 2016. Malaria in French Guiana linked to illegal gold mining. Emerg Infect Dis 22: 344–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adde A, Dusfour I, Vezenegho SB, Carinci R, Issaly J, Gaborit P, Nguyen C, Ardillon V, Girod R, Briolant S, 2017. Spatial and seasonal dynamics of Anopheles mosquitoes in Saint-Georges de l’Oyapock, French Guiana: influence of environmental factors. J Med Entomol 54: 597–605. [DOI] [PubMed] [Google Scholar]
- 15.Moua Y, Roux E, Girod R, Dusfour I, de Thoisy B, Seyler F, Briolant S, 2017. Distribution of the habitat suitability of the main malaria vector in French Guiana using maximum entropy modeling. J Med Entomol 54: 606–621. [DOI] [PubMed] [Google Scholar]
- 16.Adde A, Roux E, Mangeas M, Dessay N, Nacher M, Dusfour I, Girod R, Briolant S, 2016. Dynamical mapping of Anopheles darlingi densities in a residual malaria transmission area of French Guiana by using remote sensing and meteorological data. PLoS One 11: e0164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK, 2009. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol 47: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groupe recommadations de la Société de Pathologie Infectieuse de Langue Française , 2017. Prise en Charge et Prévention du Paludisme d’Importation Mise à Jour 2017 des RCP 2007. Available at: http://www.infectiologie.com/fr/recommandations.html. Accessed August 22, 2019. [Google Scholar]
- 19.Kulldorff M, Nagarwalla N, 1995. Spatial disease clusters: detection and inference. Stat Med 14: 799–810. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization , 2017. Dépistage Du Déficit En G6PD Pour Une Utilisation sans Risque de La Primaquine Dans Le Traitement Radical Du Paludisme à P. Vivax Ou P. Ovale: Note d’orientation. Available at: https://www.who.int/malaria/publications/atoz/g6pd-testing-pq-radical-cure-vivax/fr/. Accessed August 22, 2019. [Google Scholar]
- 21.Harris I, et al. 2010. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J 9: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kangoye DT, et al. 2016. Malaria hotspots defined by clinical malaria, asymptomatic carriage, PCR and vector numbers in a low transmission area on the Kenyan Coast. Malar J 15: 1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefani A, Roux E, Fotsing JM, Carme B, 2011. Studying relationships between environment and malaria incidence in Camopi (French Guiana) through the objective selection of buffer-based landscape characterisations. Int J Health Geogr 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiner RC, Le Menach A, Kunene S, Ntshalintshali N, Hsiang MS, Perkins TA, Greenhouse B, Tatem AJ, Cohen JM, Smith DL, 2015. Mapping residual transmission for malaria elimination. Elife 4: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pava Z, et al. 2016. Submicroscopic and asymptomatic Plasmodium parasitaemia associated with significant risk of anaemia in Papua, Indonesia. PLoS One 11: e0165340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefani A, Cheuret M, N’Guyen D, Simon S, Brousse P, Carme B, Nacher M, 2017. Interactions between gastrointestinal nematodes and malaria in a cohort of children in an Amazonian village. J Trop Pediatr 63: 144–147. [DOI] [PubMed] [Google Scholar]
- 27.Jeremiah ZA, Uko EK, 2007. Depression of platelet counts in apparently healthy children with asymptomatic malaria infection in a Nigerian metropolitan city. Platelets 18: 469–471. [DOI] [PubMed] [Google Scholar]
- 28.Erhart LM, Yingyuen K, Chuanak N, Buathong N, Laoboonchai A, Miller RS, Meshnick SR, Gasser RA, Wongsrichanalai C, 2004. Hematologic and clinical indices of malaria in a semi-immune population of western Thailand. Am J Trop Med Hyg 70: 8–14. [PubMed] [Google Scholar]
- 29.Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, O’Meara W, Price RN, Riley EM, 2016. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS Med 13: e1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gryseels C, et al. 2015. Re-imagining malaria: heterogeneity of human and mosquito behaviour in relation to residual malaria transmission in Cambodia. Malar J 14: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newby G, et al. 2015. Review of mass drug administration for malaria and its operational challenges. Am J Trop Med Hyg 93: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization , 2015. Mass Drug Administration, Mass Screening and Treatment and Focal Screening and Treatment for Malaria. Available at: https://www.who.int/malaria/mpac/mpac-sept2015-erg-mda-report.pdf. Accessed August 22, 2019. [Google Scholar]
- 33.World Health Organization , 2008. Global Malaria Control and Elimination: Report of a Technical Review. Available at: https://www.who.int/malaria/publications/atoz/9789241596756/en/. Accessed August 22, 2019. [Google Scholar]
- 34.Rossi G, et al. 2018. Closing in on the reservoir: proactive case detection in high-risk groups as a strategy to detect Plasmodium falciparum asymptomatic carriers in Cambodia. Clin Infect Dis 66: 1610–1617. [DOI] [PubMed] [Google Scholar]