Abstract
Background:
Episodic memory consists of different mnemonic phases, including acquisition and early and late consolidation. Each of these phases is characterised by distinct molecular processes. Although both cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are implicated in the acquisition phase, early consolidation only depends on cGMP, whereas late consolidation is mediated by cAMP. Accordingly, the cGMP-selective phosphodiesterase 5 (PDE5) inhibitor vardenafil or the cAMP-selective PDE4 inhibitor rolipram can improve memory acquisition or consolidation when applied during their respective time windows.
Aims:
Considering the important role of glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) during normal memory function, we aimed to investigate whether the differential actions of these PDE inhibitors are mediated through AMPAR dynamics.
Methods:
For biochemical analysis, mice were treated with either vardenafil or rolipram and sacrificed shortly after injection. For the behavioural studies, mice received either of the inhibitors during the different mnemonic phases, while their spatial memory was tested using the object location task, and they were sacrificed 24 hours later.
Results:
Administration of either vardenafil or rolipram causes rapid changes in AMPARs. Moreover, treatment with vardenafil during the acquisition or early consolidation of spatial memory resulted in increased surface levels of AMPARs which were still augmented 24 hours after learning. Membrane levels of AMPARs were not affected anymore 24 hours after learning when rolipram was administrated at either the acquisition or late consolidation phase.
Conclusions:
These results suggest that dissociative molecular mechanisms could mediate the pro-cognitive function of different classes of PDE inhibitors, and in the case of vardenafil, this phenomenon could be explained by changes in AMPAR dynamics.
Keywords: Phosphodiesterase inhibitors, AMPA receptors, cyclic nucleotide pathways, vardenafil, rolipram, cAMP, cGMP
Introduction
Memory is a complex cognitive process by which the brain stores and retrieves information (Kandel et al., 2014). When discussing the concept of hippocampus-dependent episodic memory, a distinction can be made between the different subtypes of memory, on the one hand, and the different memory phases (or processes), on the other. The different subtypes of memory include short-term, intermediate and long-term memory (Kesner and Hopkins, 2006). Additionally, the different memory phases can be distinguished in the acquisition, the consolidation and the retrieval phase (McGaugh, 2000). During the acquisition phase, sensory information can be processed and encoded in the brain, while retrieval is the ability to access and retrieve this information from memory storage. Consolidation represents transformation of memories or information from a labile state to a more stabilised form. Memory consolidation can be further divided in early and late consolidation (Izquierdo et al., 2002). It is suggested that conversion from short-term memory to intermediate memory and from the latter to long-term memory are mediated by early and late consolidation, respectively (Reneerkens et al., 2009). Importantly, each memory phase is governed by distinct molecular cascades (Izquierdo et al., 2006). In this respect, cyclic nucleotides, such as cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP), have a prominent role in memory formation (Bach et al., 1999; Bernabeu et al., 1996; Bourtchouladze et al., 1998).
In the study of the involvement of cyclic nucleotides in mnemonic processes, the phosphodiesterase (PDE) inhibitors are important assets. PDEs are the enzymes that hydrolyse cGMP and/or cAMP, and their inhibition can prolong the action of the nucleotides (Beavo, 1995). Therefore, the application of PDE inhibitors gained particular interest for having potential memory-enhancing effects (Heckman et al., 2015). The PDE superfamily exists out of 11 subfamilies, of which the PDE4 and PDE5 subfamilies are especially highly expressed in the rodent and human hippocampus (Lakics et al., 2010). As a result, PDE4 and PDE5 inhibitors are abundantly tested for their memory-enhancing potential (Reneerkens et al., 2009). Importantly, it was shown in rats that administration of the cGMP-specific PDE5 inhibitor vardenafil at the early consolidation time window or the cAMP-specific PDE4 inhibitor rolipram at the late consolidation time window could extend short-term memory into long-term memory (Bollen et al., 2014; Izquierdo et al., 2002; Rutten et al., 2007). The existence of these defined time windows in the action of the different cyclic nucleotides during memory consolidation was further outlined in a study in rats showing that the cognitive-enhancing effect of PDE5 inhibition was apparent when vardenafil was administered up to 45 minutes after the learning trial, whereas PDE4 inhibition via rolipram was effective when administered between three and five-and-a-half hours after the learning trial of the object recognition task (ORT; Akkerman et al., 2016). Additionally, both vardenafil and rolipram were shown to enhance memory function by improving memory acquisition when administered before the learning trial (Akkerman et al., 2016).
In the hippocampus, common downstream effectors for cGMP and cAMP are protein kinase G (PKG) and protein kinase A (PKA), respectively. In turn, both PKG and PKA share the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) as common downstream effector, which represents one of the main types of receptors in excitatory synapses (Roche et al., 1996; Serulle et al., 2007). AMPARs are mainly heterotetramers consisting of various combinations of four subunits, designated as GluA1–4 (Collingridge et al., 2009; Dingledine et al., 1999; Mayer and Armstrong, 2004). Despite the existence of several subtypes, most of the AMPARs in the hippocampus are heteromers of GluA1/GluA2 or GluA2/GluA3 (Lu et al., 2009). There is a plethora of evidence showing the importance of GluA1/GluA2 heteromers in synaptic transmission and memory formation (Kessels and Malinow, 2009; Sanderson et al., 2008). However, it has been shown only recently that GluA2/GluA3 receptors participate in homeostatic scaling in the absence of activity and are involved in hippocampal synaptic plasticity (Renner et al., 2017; Makino and Malinow, 2011). Accordingly, rapid trafficking and synaptic incorporation of AMPARs has an eminent role in these processes. Phosphorylation of AMPARs by PKG and PKA (Roche et al., 1996; Serulle et al., 2007) promotes trafficking of already existing AMPARs to extrasynaptic sites in the membrane (Oh et al., 2006) and additionally increases channel opening probability (Banke et al., 2000). Based on the model proposed by Soderling and his group, incorporation of AMPARs to extrasynaptic sites primes their delivery to synapses during induction of long-term potentiation (LTP; Oh et al., 2006) – the proposed molecular correlate of memory. Importantly, the different AMPAR subunits also participate in a biphasic process that mediates plasticity-induced trafficking of receptors to synaptic sites. Neuronal activity involves trafficking of GluA1/GluA2 heteromers to the synapses (Hayashi et al., 2000; Shi et al., 1999). Later on, these receptors are replaced by the constitutively trafficking GluA2/GluA3 heteromers, maintaining long-lasting synaptic strengthening (Shi et al., 2001). Considering that upregulation of cAMP or cGMP pathways has an essential role in promoting trafficking of AMPARs to the extrasynaptic site and subsequently enhancing synaptic plasticity, we hypothesise that the pro-cognitive action of PDE inhibitors during the specific time windows can be explained by changes in AMPAR dynamics. In order to investigate this hypothesis, we initially confirmed the previously observed pro-cognitive effect of vardenafil and rolipram when administered within specific time windows using the object location task (OLT) in mice. Thereafter, we examined whether intraperitoneal (i.p.) administration of vardenafil or rolipram could have an effect on AMPAR trafficking or synthesis in mice sacrificed shortly after drug administration or 24 hours after the OLT.
Methods
Animals
All experimental procedures were approved by the local ethics committee of Maastricht University for animal experiments and met governmental guidelines. In total, 138 male C57BL/6 mice (Charles River, Sulzfeld, Germany) aged between four and five months were tested. Specifically, 48 mice were used for the study that involved only treatment and 69 mice were used for the study that involved treatment and behavioural testing. All animals were housed individually throughout the experiment in standard green line Tecniplast individually ventilated cages on sawdust bedding. The animals were housed on a reversed 12-hour/12-hour light/dark cycle (lights on from 19:00 to 07:00 hours) and received food and water ad libitum. The mice were housed and tested in the same room. A radio, playing softly, provided background music in the room 24 hours a day, also during testing, in order to habituate the animals to background sounds.
Drug preparation
We administered two selective PDE inhibitors: PDE5 inhibitor vardenafil (kindly donated by BAYER, Wuppertal, Germany) and PDE4 inhibitor rolipram (Sigma–Aldrich, Zwijndrecht, the Netherlands). Both inhibitors were previously shown to cross the blood–brain barrier (Akkerman et al., 2016). Both PDE inhibitors were dissolved in the same vehicle (98% methyl cellulose tylose solution (0.5%) and 2% Tween80) and were administered in a volume of 4 mL/kg. The drugs were given i.p. at a dose of 0.3 mg/kg for vardenafil and 0.03 mg/kg for rolipram. Dosages, injection volumes and time of injection are based on extensive previous experience of the lab with the current drugs (Bollen et al., 2014; Izquierdo et al., 2002; Rutten et al., 2007). The solutions were prepared freshly each testing day.
OLT
The OLT is a hippocampus-dependent spatial memory task that has been derived from the ORT (Ennaceur and Delacour, 1988). The OLT was performed as previously described (Sierksma et al., 2014). In short, the apparatus consisted of a circular arena, 40 cm in diameter and 40 cm high. The back half of the wall was made of white polyvinyl chloride (PVC), and the front was made of transparent PVC. Fluorescent red tubes and a light bulb provided a constant illumination of about 20 lux on the floor of the apparatus. We used two different sets of two identical objects, which were divided in a semi-random manner between animals and over all treatment conditions in order to avoid object preferences. The objects consisted of a massive metal rectangular prism (2.5 cm×5 cm×7.5 cm) containing two holes (diameter 1.5 cm) and a massive aluminium cube with a tapering top (4.5 cm×4.5 cm×8.5 cm). The test session comprised two trials: the learning trial (T1) and the test trial (T2), each lasting four minutes. Prior to the experimental trials, the mice were put in an empty cage for four minutes to increase exploratory behaviour during testing. In both trials, mice were placed into the arena facing the transparent wall. During T1, two identical objects were placed inside the apparatus on a horizontal line in the middle of the arena (object a1 and object a2). At the end of the test, the mice were returned to their home cage for a predetermined interval of 24 hours. After this interval, the mice were put back into the arena for T2 in which one of the two objects from T1 was moved to a different position on a vertical line, to the front or back of the arena (b), while the other object was at the same position as in T1. Between the trials, the objects and arena were cleaned with 70% ethanol in order to avoid olfactory cues. The read-out parameters of the OLT are similar to the ORT (Akkerman et al., 2012) and refer to the exploration time for each object during T1 and T2. Exploration was defined in the following manner: directing the nose to the object at a distance of no more than 2 cm and/or touching the object with the nose. Sitting on the object was not considered exploratory behaviour. The exploration time (in seconds) of each object during T1 are presented as ‘a1’ and ‘a2’. The time spent exploring the familiar and the displaced object in T2 are represented as ‘a3’ and ‘b’, respectively. Using this information, the following variables were calculated: (a) the total exploration time during T1 (e1 (=a1+a2)), (b) the total exploration time during T2 (e2 (=a3+b)) and (c) the discrimination index (d2 (=b–a3/e2))]. The d2 index is a relative measure of discrimination corrected for exploratory activity and could range from −1 to 1. A significant difference from zero (i.e. chance level) indicates that the mice remembered the object locations from T1, and a difference from the vehicle condition signifies an actual memory improvement. Considering that mice require a minimum amount of exploration in order to show reliable memory performance (Akkerman et al., 2012), mice exploring for less than seven seconds during T1 or less than 10 seconds during T2 were excluded from the analysis. Prior to testing, the animals were habituated to the arena, the objects and the injections. Specifically, the habituation of the animals lasted eight days. On days 1 and 2, mice were introduced to the arena for five minutes to explore the first and second sets of objects, respectively. On day 3, we continued with the first familiarisation session in which the animals’ discrimination index was evaluated after a one-hour retention interval between T1 and T2. The rationale of this procedure was to ensure that the animals were able to discriminate between the statutory and the moved object when a short interval was introduced between trials. On the following days, we conducted the second (days 4 and 5) and the third (days 6 and 7) familiarisation session in order to establish that natural forgetting occurred when T1 and T2 were separated by a long 24-hour interval. As has been shown before, the animals could still distinguish the location of the objects during the second familiarisation session, while the discrimination was omitted during the third session (Akkerman et al., 2012). On any of the above familiarisation days, animals were placed in an empty cage for four minutes before entering the arena in order to enhance their exploration. On the last habituation day (day 8), the animals were given a saline injection i.p. at the same volume as the experimental procedures in order to get them accustomed to the injection procedure. The testing procedures were commenced after one resting day. For the behavioural test, the mice were divided into three groups: the ‘acquisition group’ in which either vardenafil or rolipram was administrated 30 minutes before T1, the ‘early consolidation group’ in which the animals received the treatment with either of the drugs 20 minutes after T1 so as not to influence acquisition/encoding (Akkerman et al., 2016) and the ‘late consolidation group’ in which drug administration was performed three hours after T1. All three groups were tested in T2 after a 24-hour inter-trial interval in order to assess the efficacy of the treatment in improving long-term spatial memory. The experimenter was always blind to the experimental conditions.
Biotinylation assay and sample preparation
After completing the behavioural testing and following a sufficient washout period of four days, the animals received a single injection of either vardenafil or rolipram at the abovementioned time points in order to mimic the conditions of the behavioural test. This time, the animals were sacrificed after 24 hours for biochemical analysis without undergoing T2. In order to examine the effect of vardenafil or rolipram in AMPARs dynamics per se, a different cohort of mice was treated with either vardenafil or rolipram, and the animals were sacrificed at different time points (i.e. 15, 40 and 60 minutes after treatment). Animals were sacrificed by means of cervical dislocation, and the brains were excised and both hippocampi were isolated. Coronal hippocampal slices 400 µm thick were obtained using a McIlwain tissue chopper. The slices were transferred in ice-cold ACSF (124 mM NaCl, 4.4 mM KCl, 1 mM Na2HPO4, 25 mM NaHCO3, 2 mM CaCl, 2 mM MgSO4 and 10 mM glucose) and incubated with 1 mM sulfo-NHS-SS-biotin (#21328; Thermo Fisher Scientific, Bleiswijk, The Netherlands) for 60 minutes on ice. Following biotin incubation, slices were washed with cold 100 mM glycine to remove the excess biotin and were then flash-frozen in liquid nitrogen. Frozen hippocampal slices were mechanically dissociated in lysis buffer (1 mM EDTA, 1 mM EGTA, 1% glycerol, 0.1% triton and 1% IGEPAL CA-630 in phosphate-buffered saline (PBS)) containing protease and phosphatase inhibitors. Protein concentration was determined with Lowry protein assay (Bio-Rad Laboratories, Veenendaal, The Netherlands). For the membrane fractions, protein lysates (60 µg) were incubated overnight with streptavidin-coated Dynabeads (#65601; Thermo Fisher Scientific) at 4°C under constant rotation. Dynabeads containing surface biotinylated proteins were separated from cytosolic proteins by magnetic precipitation. Biotinylated proteins were eluted from streptavidin beads with 1× sodium dodecyl sulphate (SDS) loading buffer (1 M Tris HCL, 75% glycerol, 6% SDS, 15%-β-mercaptoethanol and 0.025% brome phenol blue in milliQ) at 95°C for five minutes.
Western blotting
Surface protein fractions (60 µg) and their corresponding total protein samples (8 µg) were resolved in 10% SDS polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked (50% Odyssey blocking buffer in PBS; Li-Cor, Lincoln, NE) for one hour at room temperature, followed by overnight incubation with the primary antibodies at 4°C. The primary antibodies consisted of mouse anti-glutamate receptor 1 N-terminus (1:1000, #MAB2263; Merck Millipore, Burlington, MA), rabbit anti-GluA2 (1:1.000, #MAB5306S; Cell Signaling Technology, Danvers, MA) and mouse anti-GAPDH (1:1,000,000, #10R-G109A; Fitzgerald Industries, Acton, MA) as loading control. Membranes were subsequently incubated with secondary antibodies for one hour at room temperature: goat anti-rabbit IRDye 800 (1:10,000; Li-Cor) and donkey anti-mouse IRDye 680 (1:10,000; Li-Cor). Membranes were visualised using the Odyssey Infrared Imaging System (Li-Cor), and protein bands were quantified using ImageJ (National Institutes of Health, Bethesda, MD). Raw intensity measures were normalised to GAPDH to control for loading differences.
Statistics
For the behavioural test, one-sample t-tests were performed to compare the d2 index of vardenafil or rolipram to zero (i.e. chance level). For both the behavioural experiments and Western blots, statistical differences were evaluated with one-way analysis of variance (ANOVA) followed by post hoc Dunnett’s t-tests (GraphPad Prism; GraphPad Software, La Jolla, CA). Outliers were excluded based on a Dixon Q-test for outliers.
Results
Treatment with vardenafil or rolipram improves long-term spatial memory in mice when administered within specific time frames
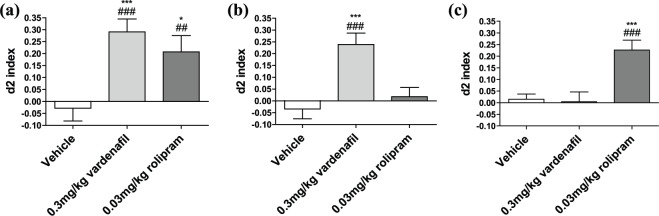
Animals treated with vehicle 30 minutes before T1, to target the acquisition process, were not able to remember the location of the new object when tested after 24 hours, as their respective d2 value did not significantly different from zero (i.e. chance level; Figure 1(a)). When vardenafil or rolipram were given 30 minutes before T1, both treatments were effective in improving the animals’ spatial memory when tested after 24 hours (Figure 1(a)). The d2 values of the animals treated with vardenafil or rolipram differed significantly from the chance level as measured with one-sample t-tests (vardenafil: p<0.0001; rolipram: p=0.005), indicating improved spatial memory. Additionally, a one-way ANOVA comparing the d2 value of every group showed a significant difference between group performance (F(2, 51)=7.838; p=0.001). The post hoc Dunnett’s t-tests, comparing every condition to vehicle treatment, indicated that treatment with vardenafil (p=0.001) or rolipram (p=0.013) significantly enhanced the OLT performance of the mice.
Figure 1.
The effect of vardenafil and rolipram treatment given intraperitoneally in the object location task (OLT) at different memory stages.
(a) OLT performance after treatment with vardenafil or rolipram 30 minutes before T1 (acquisition phase) and with a 24-hour retention interval showed that both treated groups are able to discriminate between the old and the new location of the object compared to zero (chance level). Additionally, treated animals exhibit improved d2 index in comparison to that of the vehicle. N=18 for all three groups. (b) OLT performance when the treatment was given 20 minutes after T1 (early consolidation phase), and the animals tested after a 24-hour inter-trial interval showed that only treatment with vardenafil was able to improve the animals’ performance in comparison to the chance level, as well as the d2 index in comparison to that of the vehicle. N=18 for all the groups. (c) OLT performance when treatment was administered three hours after T1 (late consolidation phase) showed that only treatment with rolipram improved the animals’ performance in comparison to the chance level. Additionally, the d2 index differs significantly from that of the vehicle-treated group. Vehicle group: N=17; vardenafil group: N=19; rolipram group: N=20. Data are shown as the mean±standard error of the mean (SEM). A significant difference from zero is depicted with hashes (one sample t-tests, ##p<0.01; ###p<0.001). A significant difference from the vehicle condition is depicted with asterisks (one-way analysis of variance (ANOVA) followed by post hoc Dunnett’s test, *p<0.05; ***p<0.001).
Administration of vardenafil or rolipram 20 minutes after T1, at the early consolidation phase, resulted in improved spatial memory only for the vardenafil-treated animals (p<0.001; Figure 1(b)). The d2 value of the animals that received either vehicle or rolipram did not differ from the chance level. A one-way ANOVA revealed a significant difference between the experimental groups (F(2, 51)=11.239; p<0.0001). Furthermore, a post hoc Dunnett’s t-test confirmed that mice treated with vardenafil performed significantly better than vehicle animals (p<0.0001), while the performance of rolipram-treated animals was not different from vehicle. Treatment with vardenafil or rolipram three hours after T1, at the late consolidation phase, showed that only rolipram (p<0.0001) could enhance spatial memory at this time point, while there was no statistically significant difference between the d2 value for vardenafil-treated animals when compared to the chance level (Figure 1(c)). A one-way ANOVA comparing the performance between the different treatment groups showed a significant treatment effect (F(2, 53)=11.285; p<0.0001). A post hoc Dunnett’s t-test additionally confirmed that that rolipram-treated animals performed significantly better in comparison to the vehicle condition (p=0.001), whereas no difference was observed for the vardenafil condition.
Administration of vardenafil or rolipram results in a time-dependent differential effect on GluA1-AMPAR dynamics
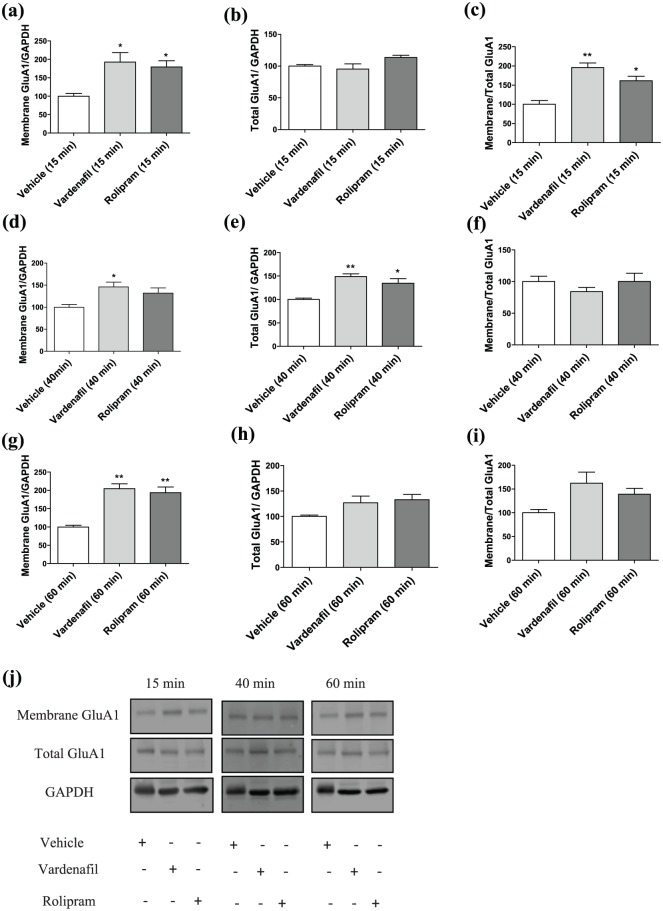
Before investigating the underlying mechanism of the temporally distinct action of vardenafil and rolipram, we first examined the effect of the compounds on GluA1-AMPAR dynamics over time. When the animals were sacrificed 15 minutes after drug administration, we observed a significant treatment effect for the surface expression (F(2, 13)=4.918; p=0.026; Figure 2(a)) and trafficking of GluA1-AMPARs (F(2, 10)=12.445; p=0.002; Figure 2(c)). Specifically, treatment with both vardenafil (p=0.019) and rolipram (p=0.041) significantly increased the amount of GluA1-AMPARs in the membrane. In addition, treatment with both drugs upregulated trafficking of GluA1-AMPARs (vardenafil: p=0.001; rolipram: p=0.011). Unlike these measurements, administration of either drugs did not affect the total levels of GluA1-AMPARs (F(2, 11)=2.265; p=0.150; Figure 2(b)).
Figure 2.
Effects of vardenafil or rolipram treatment on GluA1-AMPAR dynamics at different time points.
(a)–(c) Administration of either vardenafil or rolipram resulted in an increased GluA1-AMPAR membrane/total ratio in the hippocampus of mice sacrificed 15 minutes after treatment, while total levels of GluA1-AMPARs remain unaffected. Additionally, increased surface expression of GluA1-AMPARs was observed for the vardenafil-treated animals in comparison to the vehicle. (a) N=4, 6, 6 for vehicle, vardenafil and rolipram, respectively; (b) N=3, 6, 5 for vehicle, vardenafil and rolipram, respectively; (c) N=3, 4, 6 for vehicle vardenafil and rolipram, respectively. (d)–(f) When the brains were harvested 40 minutes after drug administration, both treatments resulted in increased total levels of GluA1-AMPARs and a concomitant upregulation of the surface fraction of the receptors only for the vardenafil-treated animals. At this time point, there was no difference in the membrane/total ratio of GluA1-AMPARs between the groups. (d) and (e) N=4, 6, 6 for vehicle, vardenafil and rolipram, respectively; (f) N=4, 5, 6 for vehicle, vardenafil and rolipram, respectively. (g)–(i) Waiting 60 minutes before harvesting the brains resulted in upregulation of membrane levels of GluA1-AMPARs for both treatments, while the total levels and the membrane/total ratio did not differ from the vehicle. (g) and (h) N=3, 6, 6 for vehicle, vardenafil and rolipram, respectively; (i) N=4, 6, 6 for vehicle, vardenafil and rolipram, respectively. (j) Representative blots for each time point. Data are shown as the mean±SEM. A significant difference from the vehicle condition is depicted with asterisks (one-way ANOVA followed by post hoc Dunnett’s test, *p<0.05; **p<0.01).
Harvesting the brains 40 minutes after treatment administration resulted in significant treatment effects for the surface (F(2, 13)=4.011; p=0.044) and total levels (F(2, 12)=8.965; p=0.004) of GluA1-AMPARs (Figure 2(d) and (e)), whereas there was no significant treatment effect for the ratio membrane/total GluA1 (F(2, 12)=0.774; p=0.483; i.e. trafficking; Figure 2(f)). Although both treatments upregulated the total levels of GluA1-AMPARs (vardenafil: p=0.002; rolipram: p=0.018), only treatment with vardenafil resulted in increased surface levels of GluA1-AMPARs (vardenafil: p=0.026; rolipram: p=0.124). Finally, sacrificing the mice 60 minutes after treatment revealed a significant effect only for the membrane levels of GluA1-AMPARs (F(2, 12)=11.293; p=0.002; Figure 2(g)). Specifically, administration of both vardenafil (p=0.001) and rolipram (p=0.003) promoted the surface expression of GluA1-AMPARs. On the contrary, there was no significant treatment effect for the total levels (F(2, 12)=1.6; p=0.242) or trafficking of GluA1-AMPARs (F(2, 13)=2.821; p=0.096; Figure 2(h) and (i)).
Effect of vardenafil or rolipram administration on GluA1- and GluA2-AMPAR dynamics at the acquisition phase
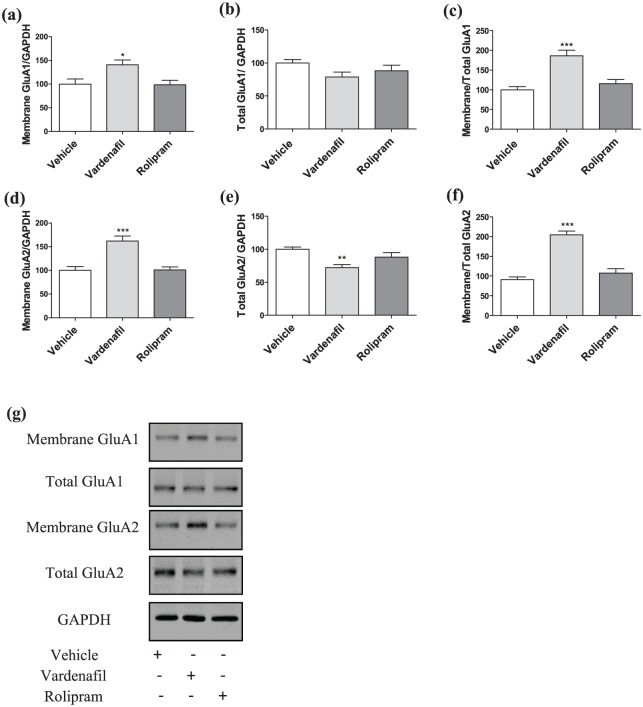
To determine the molecular basis of the pro-cognitive effect of vardenafil or rolipram at the acquisition phase of spatial memory, animals were treated with one of the drugs 30 minutes before T1, and their brains were collected after 24 hours. A one-way ANOVA showed a significant treatment effect for the surface expression (F(2, 18)=5.5; p=0.014; Figure 3(a)) and trafficking (F(2, 18)=16.72; p<0.0001) of GluA1-AMPARs (Figure 3(c)), while no significant difference was detected for the total levels (F(2, 18)=2.232; p=0.136; Figure 3(b)). Accordingly, post hoc Dunnett’s t-tests indicated that only vardenafil, but not rolipram, led to increased surface expression (vardenafil: p=0.021; rolipram: p=0.991) and trafficking of GluA1-AMPARs (vardenafil: p<0.0001; rolipram: p=0.526). Regarding GluA2-AMPARs, a significant treatment effect was observed for the surface levels (F(2, 18)=10.014; p<0.0001; Figure 3(d)) and the ratio membrane/total GluA2 (F(2, 18)=41.447; p=0.000; Figure 3(f)). In both cases, the post hoc Dunnett’s t-test revealed a significant upregulation in the membrane levels (p<0.0001) and trafficking of GluA2-AMPARs (p<0.0001) for the vardenafil-treated animals. However, no difference was observed for the rolipram-treated animals in comparison to the vehicle conditions (membrane GluA2/GAPDH: p=0.994; membrane/total GluA2: p=0.371). Additionally, a significant treatment effect was detected for the total levels of GluA2-AMPARs (F(2, 18)=7.316; p=0.005; Figure 3(e)). This effect was attributed to decreased total levels of GluA2-AMPARs in the vardenafil condition (p=0.002), while the rolipram condition did not differ from vehicle (p=0.2015). Finally, no treatment effect was detected for the ratio membrane GluA2/GluA1 (F(2, 18)=1.525; p=0.244; data not shown) and total GluA2/GluA1 (F(2, 18)=10.453; p=0.643; data not shown).
Figure 3.
Effects of vardenafil or rolipram treatment on GluA1 and GluA2 AMPAR subunits 24 hours after T1, while the treatment was given 30 minutes before T1.
(a)–(c) Administration of vardenafil before T1 resulted in increased surface expression of GluA1-AMPARs that was accompanied by upregulated membrane/total ratio, while the total levels of GluA1-AMPARs did not differ from the vehicle-treated animals. Administration of rolipram did not affect any of these values. (d)–(f) Treatment with vardenafil at the same time point as before increased the surface/GAPDH and membrane/total ratio of GluA2-AMPARs, while the total levels were decreased. Treatment with rolipram had no effect to these values. N=7 for all the conditions. (g) Representative blots for the acquisition treatments. Data are shown as the mean±SEM. A significant difference from the vehicle condition is depicted with asterisks (one-way ANOVA followed by post hoc Dunnett’s test, *p<0.05; **p<0.01; ***p<0.001).
Effect of vardenafil or rolipram administration on GluA1- and GluA2-AMPAR dynamics at the early consolidation phase
In order to examine the cognitive-enhancing effects of vardenafil or rolipram administration at the early consolidation phase, mice received one of the drugs 20 minutes after T1, and their brains were excised 24 hours after T1.
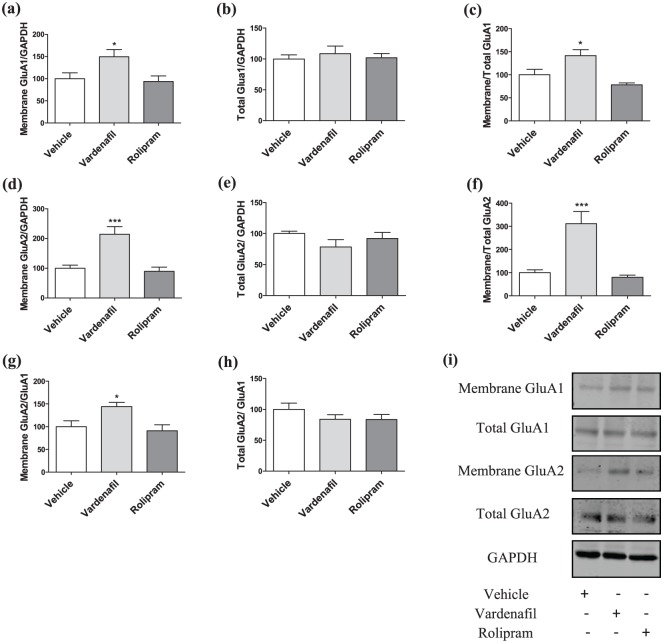
At this time point, we detected a significant treatment effect for the membrane levels (Figure 4(a) and (d)) and trafficking (Figure 4(c) and (f)) for both GluA1- and GluA2-AMPARs (one-way ANOVA; membrane GluA1/GAPDH: F(2, 21)=4.697, p=0.021; membrane/total GluA1: F(2, 20)=8.885, p=0.002; membrane GluA2/GAPDH: F(2, 21)=14.622, p<0.0001; membrane/total GluA2: F(2, 20)=15.187, p<0.0001). There was no significant treatment effect for the total levels for both GluA1-and GluA2-AMPARs (F(2, 21)=0.255, p=0.777; F(2, 21)=1.450, p=0.257; Figure 4(b) and (e)). Subsequently, post hoc Dunnett’s t-tests showed a significant increase in the membrane levels (vardenafil: p=0.04; rolipram: p=0.930) and trafficking (vardenafil: p=0.021; rolipram: p=0.289) for the vardenafil but not for the rolipram condition. Similarly, a significant difference was detected between vehicle- and vardenafil-treated animals with regard to the surface expression (vardenafil: p<0.0001; rolipram: p=0.889) and trafficking (vardenafil: p<0.0001; rolipram: p=0.878) of GluA2-AMPARs, while treatment with rolipram did not affect these values.
Figure 4.
Effects of vardenafil or rolipram treatment on GluA1 and GluA2 AMPAR subunits 24 hours after T1, while the treatment was given 20 minutes after T1.
(a)–(c) Administration of vardenafil 20 minutes after T1 resulted in increased membrane/GAPDH and membrane/total ratio of GluA1-AMPARs, while the total levels of GluA1-AMPARs remained unaffected. Administration of rolipram did not affect any of these values. (a) and (b) N=8 for all the groups; (c) N=8, 8, 7 for vehicle, vardenafil and rolipram, respectively. (d)–(f) Similar to GluA1-AMPARs, treatment with vardenafil at the same time point upregulated both the membrane/GAPDH and membrane/total ratio of GluA2-AMPARs without changing the total levels. Treatment with rolipram did not affect these values. (d) and (e) N=8 for all the groups; (f) N=8, 8, 7 for vehicle, vardenafil and rolipram, respectively. (g) and (h) Vardenafil-treated animals exhibited increase in membrane GluA2/GluA1 ratio, whereas no changes are observed in the total GluA2/GluA1 ratio. (g) N=8 for all the groups; (h) N=8, 7, 8 for vehicle, vardenafil and rolipram, respectively. (i) Representative blots for the early consolidation treatments. Data are shown as the mean±SEM. A significant difference from the vehicle condition is depicted with asterisks (one-way ANOVA followed by post hoc Dunnett’s test, *p<0.05; ***p<0.001).
Additionally, we detected a significant treatment effect for the ratio membrane GluA2/GluA1 (F(2, 21)=5.774; p=0.010; Figure 4(g)). A post hoc Dunnett’s t-test revealed a significant difference between the vehicle and vardenafil conditions (p=0.028), whereas no difference was observed for the rolipram condition (p=0.818). There was no significant treatment effect for the ratio total GluA2/GluA1 (F(2, 20)=1.190; p=0.325; Figure 4(h)).
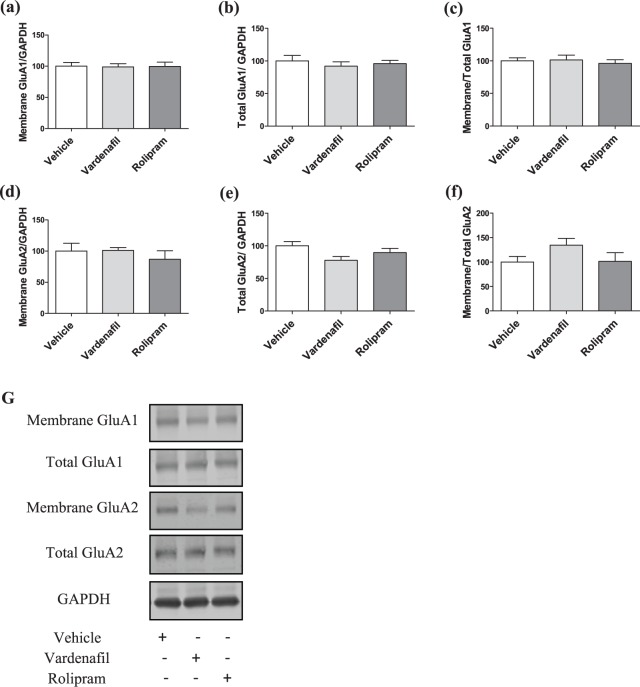
Effect of vardenafil or rolipram administration on GluA1- and GluA2-AMPAR dynamics at the late consolidation phase
The cognitive-enhancing effect of vardenafil or rolipram at the late consolidation phase was examined in mice that received the treatment three hours after T1 and were sacrificed 24 hours after the test. A one-way ANOVA showed no difference for the surface (F(2, 20)=0.012; p=0.988), total levels (F(2, 21)=0.349; p=0.349) and trafficking (F(2, 21)=0.21; p=0.812) of GluA1-AMPARs (Figure 5(a)–(c)). Similarly, there was no significant treatment effect for the surface (F(2, 21)=0.526; p=0.597), total levels (F(2, 21)=3.144; p=0.064) and trafficking of GluA2-AMPARs (F(2, 21)=1.799; p=0.19; Figure 5(d)–(f)). Finally, no significant treatment effect was observed for the ratios membrane GluA2/GluA1 (F(2, 21)=0.893; p=0.567; data not shown) and total GluA2/GluA1 (F(2, 21)=0.584; p=0.424; data not shown).
Figure 5.
Effects of vardenafil or rolipram treatment on GluA1 and GluA2 AMPAR subunits 24 hours after T1, while the treatment was given three hours after T1.
(a)–(c) Administration of vardenafil or rolipram three hours after T1 did not affect the surface levels, total levels and the membrane/total ratio of GluA1-AMPARs. (a) N=7, 8, 8 for vehicle, vardenafil and rolipram, respectively. (b) and (c) N=8 for all the groups. (d)–(f) Treatment with vardenafil or rolipram at the same time point did not change GluA2-AMPARs dynamics. N=8 for all the groups. (g) Representative blots for the late consolidation treatments. Data are shown as the mean±SEM.
Discussion
The present study replicated previous findings showing that administration of PDE4 or PDE5 inhibitors within specific time windows of spatial memory can enhance memory performance in rodents (Akkerman et al., 2016; Bollen et al., 2014; Rutten et al., 2007). More specifically, administration of the cGMP-specific PDE5 inhibitor vardenafil or the cAMP-specific PDE4 inhibitor rolipram enhanced long-term object location memory when the drugs were given during the acquisition phase (i.e. 30 minutes before T1). Despite the similar effect at the acquisition phase, a temporal distinction in the action of the above inhibitors was observed when they were given at the consolidation phase. Administration of vardenafil at the early (20 minutes after T1), but not late (three hours after T1), consolidation phase and rolipram at the late, but not early, consolidation phase counteracted natural forgetting in the OLT.
It is suggested that the time-dependent effect in the action of PDE inhibitors is related to the differential involvement of their corresponding cyclic nucleotide signalling cascade in memory consolidation. Intrahippocampal infusion of cAMP or cGMP analogues in different behavioural paradigms resulted in similar temporal dissociation in the action of cyclic nucleotides (Bernabeu et al., 1996; Bernabeu et al., 1997; Prickaerts et al., 2002). Additionally, it was shown that the cognitive-enhancing properties of PDE4 or PDE5 inhibitor administration at the specific mnemonic phases during consolidation could be abolished by intrahippocampal inhibition of PKG or PKA, the main downstream effectors of cyclic nucleotides (Bollen et al., 2014). In line with these previous observations, a temporally differential effect was observed in the effectiveness of vardenafil and rolipram at the consolidation phase, whereas both drugs are equally effective on memory acquisition when given before the learning trial. Although the underlying mechanism for this phenomenon is yet unknown, it is possible that the effect at acquisition is predominantly related to changes in cyclic nucleotide signalling in the presynaptic cell, while the effect during the consolidation phase is mainly induced postsynaptically. In this respect, it was found that presynaptic activation of cGMP or cAMP promotes the synthesis and/or release of neurotransmitters including glutamate (Arancio et al., 1995; Imanishi et al., 1997;), increasing the release probability of the synapses. On the other hand, administration of either vardenafil or rolipram at the consolidation phase probably enhances ongoing postsynaptic events, including synthesis of new proteins via activation of cAMP response element-binding protein (CREB). Of note, the relationship between presynaptic and postsynaptic mechanisms during acquisition and early consolidation could be sequential rather than discrete, since AMPAR trafficking is involved in both phases. This is similar to the relationship between late and early consolidation based on a study showing that activation of the cAMP/PKA pathway at the late consolidation phase of object recognition memory requires intact cGMP/PKG signalling at the early consolidation phase for the formation of long-term memories (Bollen et al., 2014).
Due to the apparent relationship between GluA1-AMPARs and cyclic nucleotide signalling, we sought to determine whether upregulation of cAMP/PKA or cGMP/PKG signalling via rolipram of vardenafil, respectively, could affect GluA1-AMPAR dynamics. Interestingly, administration of either vardenafil or rolipram had a distinguished effect on AMPARs over time. More specifically, 15 minutes after treatment with either of the drugs, there was an increase in surface expression of GluA1-AMPARs that was also depicted in increased trafficking; 40 minutes after treatment, the total levels of GluA1-AMPARs were upregulated, resulting in increased surface expression for the vardenafil-treated mice; and 60 min after treatment, there was an increase in the surface expression of GluA1-AMPARs for both treatments. The above findings suggest that there is a ‘wave’ in the trafficking synthesis of GluA1-AMPARs. The initial upregulation in trafficking and surface expression of GluA1-AMPARs could be the result of increased mobilisation of receptors that reside in synaptic endosomes (Park et al., 2004). Later on, the observed increased synthesis of GluA1-AMPARs for both vardenafil- and rolipram-treated animals could be mediated via CREB-dependent transcription or via translation of local pools of GluA1 mRNAs. Several studies have shown that CREB is the convergent point between cAMP/PKA and cGMP/PKG signalling, and an increase in CREB phosphorylation is a critical step in the commencement of protein transcription (Lu and Hawkins, 2002; Navakkode et al., 2004). Additionally, there are several lines of research indicating that mRNA of AMPARs subunits can be found in hippocampal dendrites (Grooms et al., 2006; Miyashiro et al., 1994; Steward and Schuman, 2001), where it is locally translated and subsequently incorporated into or close to the synapse (Ju et al., 2004; Sutton et al., 2006). This observation was confirmed further by a study showing that local translation of GluA1- and GluA2-containing AMPARs could be triggered be pharmacological manipulations that could elicit LTP, like activation of metabotropic glutamate receptors (mGluRs) or application of high levels of potassium (Ju et al., 2004). Finally, 60 minutes after upregulation of cAMP/PKA or cGMP/PKG cascades, a significant increase in surface expression of GluA1-AMPARs was observed. Considering the previously observed increase in the total levels of GluA1-AMPARs at 40 minutes, this indicates that the synthesis of new receptors started mitigating, and more receptors, most likely newly synthetised, are inserted into the membrane. In addition to the time-dependent effect of PDE4 and PDE5 inhibition on GluA1-AMPAR dynamics, our study demonstrates that increased surface expression and trafficking of GluA1- and GluA2-AMPARs could explain the pro-cognitive effect of vardenafil when administrated either at the acquisition or at the early consolidation phase, but not at the late consolidation phase, of spatial memory. The ratio of surface GluA2/GluA1 receptors did not differ when vardenafil treatment was given during the acquisition phase. However, there was a significant increase in GluA2-AMPARs in comparison to GluA1-AMPARs when vardenafil was administered at the early consolidation phase. These findings indicate that different types of receptors are upregulated when the cGMP/PKG pathway is stimulated at different phases of the mnemonic process. Considering that GluA1/GluA2 and GluA2/GluA3 heteromers are the prevailing types of AMPARs in pyramidal hippocampal neurons (Lu et al., 2009), we could hypothesise that at the acquisition phase, there is mainly an increase in surface expression of GluA1/GluA2 heterotetramers, while at the early consolidation phase, there is possibly an additional increase in GluA2/GluA3 heterotetramers. Nevertheless, we cannot be conclusive about whether the receptors are incorporated at the synapse or they still reside at extrasynaptic sites. Unlike vardenafil, administration of rolipram during the two mnemonic phases (i.e. acquisition and late consolidation), in which it exerts pro-cognitive function, did not affect total levels or trafficking of AMPARs. The distinguished function between vardenafil and rolipram should be related to their downstream effectors, which, in turn, impact on AMPAR trafficking. Although both inhibitors increased surface expression of GluA1-AMPARs 60 minutes after their administration, this effect was still apparent after 24 hours only for vardenafil. This finding was particularly surprising for rolipram, since several studies showed that activation of cAMP/PKA pathway promotes trafficking of AMPARs (Shi et al., 1999; Banke et al., 2000; Lee et al., 2003; Makino et al., 2011; Esteban et al., 2003). Importantly, the majority of these studies have been conducted in in vitro or ex vivo systems, and trafficking of AMPARs has been monitored for only a few hours after plasticity-inducing pharmacological treatments (Esteban et al., 2003; Shi et al., 1999). In our study the effect of treatment in AMPAR dynamics was examined 24 hours after the mnemonic test. Thereafter, despite the initial effect of rolipram on AMPARs trafficking, another mechanism seems to be responsible for its long-term cognitive-enhancing properties. Upregulation of the cAMP pathway via the PDE4 inhibitor rolipram could also result in activation of the exchange protein activated by cAMP (Epac; Grandoch et al., 2010). Epacs have a multifactorial role in plasticity, enhancing release of neurotransmitter and facilitating both LTP and long-term depression (Gekel and Neher, 2008; Gelinas et al., 2008; Ster et al., 2009). Interestingly, in cell cultures of rat cortical neurons, activation of Epac2 induced spine shrinkage by promoting endocytosis of GluA2/GluA3 AMPARs (Woolfrey et al., 2009). Considering this finding, it is possible that activation of the cAMP pathway via rolipram leads to activation of several intracellular pathways that promote the initial trafficking of AMPARs to the synapse and, later on, their endocytosis into the cell. In turn, other receptors than AMPARs could be inserted into the synapse, maintaining the increased synaptic size.
In conclusion, our study showed that upregulation of the cGMP/PKG or cAMP/PKA signalling cascades via the PDE5-specific inhibitor vardenafil and the PDE4-specific inhibitor rolipram causes immediate and versatile changes in GluA1-AMPAR dynamics. Additionally, administration of vardenafil at the acquisition and early consolidation phase of object location memory led to formation of long-term memory that could be explained by increased surface levels and trafficking of GluA1- and GluA2-AMPARs. Nevertheless, the long-lasting pro-cognitive effect of rolipram, when administered at either the acquisition or late consolidation phase, is not related to changes in AMPARs. Collectively, these results suggest that there is a differential underlying mechanism mediating the cognitive enhancing properties induced by upregulation of cyclic nucleotide signalling. Future studies are required to determine the molecular components of these pathways.
Footnotes
Authors’ note: Pim RA Heckman is now affiliated with Department of Neurobiology, Groningen Institute for Evolutionary Life Sciences (GELIFES), University of Groningen, Groningen, The Netherlands.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: PRAH and BTJvH were financially supported by the Human Enhancement and Learning (HEaL) initiative of Maastricht University.
ORCID iD: Pim RA Heckman  https://orcid.org/0000-0002-7319-8690
https://orcid.org/0000-0002-7319-8690
References
- Akkerman S, Blokland A, Prickaerts J. (2016) Possible overlapping time frames of acquisition and consolidation phases in object memory processes: a pharmacological approach. Learn Memory 23: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman S, Blokland A, Reneerkens O, et al. (2012) Object recognition testing: methodological considerations on exploration and discrimination measures. Behav Brain Res 232: 335–347. [DOI] [PubMed] [Google Scholar]
- Arancio O, Kandel E, Hawkins R. (1995) Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature 376: 74. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, et al. (1999) Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A 96: 5280–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke T, Bowie D, Lee H-K, et al. (2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20: 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA. (1995) Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 75: 725–748. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, et al. (1997) Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci U S A 94: 7041–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu R, Schmitz P, Faillace MP, et al. (1996) Hippocampal cGMP and cAMP are differentially involved in memory processing of inhibitory avoidance learning. Neuroreport 7: 585–588. [DOI] [PubMed] [Google Scholar]
- Bollen E, Puzzo D, Rutten K, et al. (2014) Improved long-term memory via enhancing cGMP-PKG signaling requires cAMP-PKA signaling. Neuropsychopharmacology 39: 2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, et al. (1998) Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Memory 5: 365–374. [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, et al. (2009) A nomenclature for ligand-gated ion channels. Neuropharmacology 56: 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, et al. (1999) The glutamate receptor ion channels. Pharmacol Rev 51: 7–62. [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. (1988) A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31: 47–59. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi S-H, Wilson C, et al. (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136. [DOI] [PubMed] [Google Scholar]
- Gekel I, Neher E. (2008) Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci 28: 7991–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Peters MM, et al. (2008) Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus. Learn Memory 15: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandoch M, Roscioni SS, Schmidt M. (2010) The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol 159: 265–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooms SY, Noh K-M, Regis R, et al. (2006) Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci 26: 8339–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi S-H, Esteban JA, et al. (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267. [DOI] [PubMed] [Google Scholar]
- Heckman P, Wouters C, Prickaerts J. (2015) Phosphodiesterase inhibitors as a target for cognition enhancement in aging and Alzheimer’s disease: a translational overview. Curr Pharm Des 21: 317–331. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Sawa A, Ichimaru Y, et al. (1997) Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur J Pharmacol 321: 273–278. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, et al. (2006) Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci 29: 496–505. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Barros DM, Vianna MR, et al. (2002) Molecular pharmacological dissection of short-and long-term memory. Cell Mol Neurobiol 22: 269–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, et al. (2004) Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci 7: 244. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR. (2014) The molecular and systems biology of memory. Cell 157: 163–186. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO. (2006) Mnemonic functions of the hippocampus: a comparison between animals and humans. Biol Psychol 73: 3–18. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61: 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakics V, Karran EH, Boess FG. (2010) Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 59: 367–374. [DOI] [PubMed] [Google Scholar]
- Lee H-K, Takamiya K, Han J-S, et al. (2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631–643. [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, et al. (2009) Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62: 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-F, Hawkins RD. (2002) Ryanodine receptors contribute to cGMP-induced late-phase LTP and CREB phosphorylation in the hippocampus. J Neurophysiol 88: 1270–1278. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. (2011) Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron 72: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Johnson RC, Yu Y, et al. (2011) Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proc Natl Acad Sci U S A 201105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. (2004) Structure and function of glutamate receptor ion channels. Annu Rev Physiol 66: 161–181. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. (2000) Memory – a century of consolidation. Science 287: 248–251. [DOI] [PubMed] [Google Scholar]
- Miyashiro K, Dichter M, Eberwine J. (1994) On the nature and differential distribution of mRNAs in hippocampal neurites: implications for neuronal functioning. Proc Natl Acad Sci U S A 91: 10800–10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU. (2004) The type IV-specific phosphodiesterase inhibitor rolipram and its effect on hippocampal long-term potentiation and synaptic tagging. J Neurosci 24: 7740–7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, et al. (2006) Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem 281: 752–758. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, et al. (2004) Recycling endosomes supply AMPA receptors for LTP. Science 305: 1972–1975. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, de Vente J, Honig W, et al. (2002) cGMP, but not cAMP, in rat hippocampus is involved in early stages of object memory consolidation. Eur J Pharmacol 436: 83–87. [DOI] [PubMed] [Google Scholar]
- Reneerkens OA, Rutten K, Steinbusch HW, et al. (2009) Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology 202: 419–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner MC, Albers EH, Gutierrez-Castellanos N, et al. (2017) Synaptic plasticity through activation of GluA3-containing AMPA-receptors. elife 6: e25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, et al. (1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16: 1179–1188. [DOI] [PubMed] [Google Scholar]
- Rutten K, Prickaerts J, Hendrix M, et al. (2007) Time-dependent involvement of cAMP and cGMP in consolidation of object memory: studies using selective phosphodiesterase type 2, 4 and 5 inhibitors. Eur J Pharmacol 558: 107–112. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Good MA, Seeburg PH, et al. (2008) The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory. Prog Brain Res 169: 159–178. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, et al. (2007) A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron 56: 670–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S-H, Hayashi Y, Esteban JA, et al. (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331–343. [DOI] [PubMed] [Google Scholar]
- Shi S-H, Hayashi Y, Petralia RS, et al. (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284: 1811–1816. [DOI] [PubMed] [Google Scholar]
- Sierksma A, Van Den Hove D, Pfau F, et al. (2014) Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D. Neuropharmacology 77: 120–130. [DOI] [PubMed] [Google Scholar]
- Ster J, De Bock F, Bertaso F, et al. (2009) Epac mediates PACAP-dependent long-term depression in the hippocampus. J Physiol 587: 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Schuman EM. (2001) Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci 24: 299–325. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, et al. (2006) Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125: 785–799. [DOI] [PubMed] [Google Scholar]
- Woolfrey KM, Srivastava DP, Photowala H, et al. (2009) Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci 12: 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]