SUMMARY
Zika virus (ZIKV) has recently been associated with birth defects and pregnancy loss after maternal infection. Because dengue virus (DENV) and ZIKV co-circulate, understanding the role of antibody-dependent enhancement in the context of pregnancy is critical. Here, we showed that the presence of DENV-specific antibodies in ZIKV-infected pregnant mice significantly increased placental damage, fetal growth restriction, and fetal resorption. This was associated with enhanced viral replication in the placenta that coincided with an increased frequency of infected trophoblasts. ZIKV-infected human placental tissues also showed increased replication in the presence of DENV antibodies, which was reversed by FcγR blocking antibodies. Furthermore, ZIKV-mediated fetal pathogenesis was enhanced in mice in the presence of a DENV-reactive monoclonal antibody, but not in the presence of the LALA variant, indicating a dependence on FcγR engagement. Our data suggest a possible mechanism for the recent increase in severe pregnancy outcomes after ZIKV infection in DENV-endemic areas.
In Brief
Dengue-specific antibodies can cross-react to Zika virus. Brown et al. demonstrate that dengue-specific antibodies can enhance Zika virus pathogenesis during pregnancy by increasing damage to the placenta. These findings suggest a possible mechanism for the recent increase in severe pregnancy outcomes after Zika virus infection in areas where dengue co-circulates.
INTRODUCTION
Zika virus (ZIKV) is a mosquito-transmitted flavivirus that is closely related to dengue virus (DENV). In the 60 years after its discovery in 1947, ZIKV remained a relatively obscure pathogen, associated with only sporadic cases of human infection and mild febrile illness (Weaver et al., 2016). In the last decade, however, after its geographic spread into the Pacific Islands and then South America, ZIKV outbreaks have been larger, more frequent, and more severe (Lazear and Diamond, 2016). Most concerning are infections in pregnant women, particularly during the first and second trimesters, which have resulted in a wide range of birth defects, including microcephaly, intrauterine growth restriction, and spontaneous abortion (Brasil et al., 2016; Coyne and Lazear, 2016; Honein et al., 2017; Reynolds et al., 2017; Schaub et al., 2017). The factors involved in this increased pathogenesis of ZIKV are not fully understood but may include recently acquired viral genetic changes (Yuan et al., 2017), as well as prior exposure to closely related flaviviruses (Bardina et al., 2017). The latter is due to a phenomenon originally described between DENV serotypes, known as antibody-dependent enhancement (ADE). In humans, ADE between DENV serotypes has been shown to correlate with increased viremia and disease severity within a specific window of waning heterotypic antibody levels (Halstead, 1988; Katzelnick et al., 2017; Kliks et al., 1988; Vaughn et al., 2000); however, the extent to which antibodies against DENV and potentially other flaviviruses can cause enhanced ZIKV pathogenesis in humans has not been thoroughly evaluated.
Studies have shown that the ZIKV envelope (E) protein has high structural similarity to the E proteins of all DENV serotypes (Barba-Spaeth et al., 2016; Kostyuchenko et al., 2016; Sirohi et al., 2016). Numerous in vitro studies have demonstrated that monoclonal antibodies or sera elicited by infection with DENV can enhance ZIKV replication (Bardina et al., 2017; Dejnirattisai et al., 2016; Paul et al., 2016; Priyamvada et al., 2016; Stettler et al., 2016; Willis and Hensley, 2017). Recently, our group demonstrated that pre-existing antibodies to DENV increased ZIKV pathogenesis in adult Stat2−/− mice (Bardina et al., 2017). This raises a major question about whether DENV antibodies impart a heightened risk on the developing fetus after maternal ZIKV infection. Here, we examined this question with both an in vivo model of ZIKV ADE in pregnant Stat2−/− mice and an ex vivo model with human placental tissues. We show that the presence of DENV antibodies in mice increased damage to the placenta and worsened pregnancy outcomes in an FcγR-dependent manner. In both mouse and human placentas, the presence of DENV antibodies enhanced replication of ZIKV, which was associated with an increased frequency of infected trophoblasts. Our data suggest a possible mechanism for the enhancement of ZIKV pathogenesis in the placenta, indirectly enhancing fetal damage.
RESULTS
Dengue Virus Antibodies Increase Zika Virus Pathogenesis during Pregnancy
To address the impact of preexisting flavivirus immunity on ZIKV pathogenesis during pregnancy, we adapted a recently described animal model of ZIKV ADE using Stat2−/− mice (Bardina et al., 2017). Human immune plasma from individuals seropositive for DENV was passively transferred into pregnant Stat2−/− mice intraperitoneally at E6.5 (embryonic day 6.5). Plasma from flavivirus-naive individuals was included as a control (CTRL). Pregnant dams were then peripherally infected through intradermal injection with ZIKVPRVABC59, a strain from Puerto Rico isolated in 2015. Although uninfected mice injected with immune plasma continued to gain weight identically throughout pregnancy (Figure S1), ZIKV-infected mice started to lose weight on day 3 post infection (E9.5; Figure 1A). ZIKV-infected mice receiving CTRL plasma started to regain weight on day 5 (E11.5); however, mice receiving DENV-immune plasma did not (Figure 1A; p = 0.0012). Evaluation of 6–8 pregnant dams per condition revealed fetal resorption rates to be greatly enhanced among mice receiving DENV-immune plasma (45.2% for mice receiving DENV immune plasma versus 8.7% for CTRL; p = 0.0002; Figure 1B). Gross morphological analysis of uterine horns, fetuses, and placentas obtained at E13.5 from ZIKV-infected mice treated with DENV-immune plasma showed fetal growth restriction and altered placental development compared to those from mice receiving CTRL plasma (Figure 1C). The fetuses harvested at E13.5 from pregnant dams that received DENV-immune plasma were significantly smaller compared to those from the CTRL condition (p < 0.0001; Figure 1D), suggesting that the presence of DENV antibodies during pregnancy contributes to ZIKV-mediated fetal demise.
Figure 1. In Vivo Effects of Preexisting DENV Antibodies on ZIKV Pathogenesis during Pregnancy.
(A–H) Pregnant dams at E6.5 were infected intradermally with 5×103 PFU (plaque-forming unit) ZIKVPRVABC59 2 h after passive transfer of 20 μl CTRL or DENV immune plasma (A–D) or 2 μl purified IgG (E–H). Daily weight measurements after ZIKV infection (A and E) are shown as the percentage of maternal starting weight for uninfected mice (A, n = 8; E, n = 3) as well as ZIKV-infected mice in the presence of CTRL (A, n = 6; E, n = 4) or DENV (A, n = 8; E, n = 3) immune plasma/IgG. Uninfected mice were also injected with immune plasma or IgG and found to be identical for all parameters tested (Figure S1) and were thus combined for the corresponding analyses. Mean ± SEM. (B and F) Resorption rates at E13.5 of uninfected pregnant dams (B, n = 59; F, n = 25) or ZIKV-infected pregnant dams in the presence of CTRL (B, n = 46; F, n = 29) or DENV immune plasma/IgG (B, n = 42; F, n = 23) were measured. (C and G) Representative images of uterine horns, fetuses and placentas at E13.5 are shown. (D and H) Fetal size was assessed at E13.5 by multiplying the crown-to-rump length (CRL) by the occipital-frontal diameter (OFD). Each data point represents an individual fetus. Median ± interquartile range.
(I) Mice were monitored daily for clinical symptoms, including hesitancy to walk, unresponsiveness, and limb paralysis; vaginal bleeding was also observed in a subset of pregnant mice.
(J–L) Viral load was quantified by qPCR from maternal blood three days post infection at E9.5 (J) as well as from the maternal brains (K) and spinal cords (L) on day 7 post infection at E13.5. Median ± interquartile range.
For (A), (D), (E), and (H), significance was determined by one-way ANOVA corrected using Bonferroni’s test for multiple comparisons; Fisher’s exact test was used to calculate significance in (B) and (F). For (J)–(L), significance was determined by the Mann-Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
See also Figures S1 and S2.
Because immune plasma contains many inflammatory molecules and other undefined factors, we purified the immunoglobulin G (IgG) fraction from pooled DENV and CTRL immune plasma to employ in the in vivo experiments. To confirm the validity of this approach, we compared neutralization, binding, and enhancement activity against ZIKV in vitro before and after IgG purification. As shown in Figure S2, purified DENV IgG maintained identical neutralization potency against ZIKV when compared to the pooled DENV-immune plasma (IC50/ml = 5,012 for DENV-immune plasma versus 5,210 for purified DENV IgG), was equally capable of binding to ZIKV E protein by ELISA, and showed no alteration in the ability to enhance ZIKV infection in K562 cells, whereas CTRL immune plasma and purified IgG showed negligible activity. The neutralization capacity of both the DENV-immune plasma and purified IgG against DENV-1 and DENV-4, the two most prevalent circulating serotypes during the time the samples were collected (Tomashek et al., 2016), were found to be much higher than that of ZIKV, with no major differences observed between pre- and post-IgG purification (Figure S2). We next tested whether the in vivo enhancement activity of the DENV-immune plasma observed in Figures 1A–1D was mediated by DENV-specific antibodies. To do this, we passively transferred purified DENV IgG into pregnant Stat2−/− mice at E6.5. As shown in Figures 1E–1H, a nearly identical phenotype, with increased maternal weight loss, higher fetal resorption rates, and decreased fetal size, was observed with the purified DENV IgG compared to the unfractionated DENV-immune plasma. Maternal health monitoring throughout the course of the 7-day infection revealed only minor symptoms among the infected pregnant mice, with hesitancy to walk being the only indication of infection. This symptom was observed more frequently among mice receiving DENV antibodies compared to mice receiving CTRL antibodies (Figure 1I). Interestingly, we also observed vaginal bleeding in 22.2% of mice receiving DENV antibodies, but not in mice receiving CTRL antibodies, consistent with the increased rate of fetal resorption observed in the DENV condition. Analysis of peripheral blood obtained on day 3 post infection from ZIKV-infected dams at E9.5 showed higher viremia in mice receiving DENV antibodies (Figure 1J; p = 0.003), but no differences in tissue viral loads in the brain or spinal cord were observed in the pregnant dams (Figures 1K–1L), which notably differed from our previous report in non-pregnant Stat2−/− mice. This difference is most likely due to the increased age of the Stat2−/− mice (8–10 weeks old) used in this study compared to those used in our previous study (~5–6 weeks old) and immune alterations due to pregnancy such as hormonal changes, increased weight gain, and blood volume (Kourtis et al., 2014). Our data suggest that DENV antibodies can enhance ZIKV pathogenesis in the context of pregnancy, leading to increased fetal damage.
Dengue Virus Antibodies Increase ZIKV Replication and Inflammation in the Placenta
To further characterize how DENV-specific antibodies alter pregnancy outcomes, we next measured viral replication in both the fetus and the placenta. As shown in Figure 2A, mice receiving DENV-immune plasma showed significantly higher ZIKV replication in the placenta (~6-fold increase) compared to CTRL-treated animals (p < 0.0001), whereas the fetal head showed no differences between the groups (Figure 2B; p = 0.8423). The increased viral load observed within placentas obtained from DENV-antibody-treated mice coincided with a stronger proinflammatory response in those placentas (Figure 2C). Histopathological analysis of placentas obtained at E13.5 from ZIKV-infected pregnant dams treated with DENV antibodies revealed more severe damage caused by infection compared to placentas of CTRL-treated animals, with a smaller, less-defined labyrinth zone (Figure 2D, black outline) and a more pronounced loss of structural integrity visible through immunofluorescence staining for vimentin, a marker for endothelium in fetal capillaries (Figure 2E). Collectively, these results suggest that rather than directly enhancing ZIKV replication within the fetal brain, DENV antibodies exacerbate damage to the fetus by enhancing ZIKV replication and inflammation in the placenta.
Figure 2. DENV Antibodies Increase ZIKV Replication and Inflammation in the Placenta In Vivo.
(A and B) Viral loads were measured in placentas (A) and fetal heads (B) collected on day 7 post infection (E13.5) by qPCR. Significance was determined using the Mann-Whitney test. Median ± interquartile range.
(C) Heat map visualization of inflammatory cytokines and chemokines measured from placental homogenates on day 7 post infection from ZIKV-infected mice that received CTRL (n = 38) or DENV immune plasma (n = 28). Data are represented as log-transformed fold induction over uninfected placentas (n = 18). Cluster analysis was based on heirarchical clustering, using the hclut function in R.
(D and E) H&E staining (D) and immunofluorescence staining of vimentin (E) were conducted on placentas harvested on day 7 post infection. For (D), the black line denotes the labyrinth zone and the gray line denotes the boundary between the decidua and the giant trophoblast layer. For (E), blue = DAPI, green = vimentin. Scale bars indicate 500 mm. ****p < 0.0001
Dengue Virus Antibodies Enhance ZIKV Pathogenesis at Advanced Gestational Age
In mice infected with ZIKV on E6.5, peak viremia occurs 3 days post infection at E9.5, which coincides with the initial formation of the placenta (~E9–E10). This raises the question of whether cross-reactive DENV-specific antibodies could also impact ZIKV infection later in gestation, after full placentation has occurred. This is particularly interesting because recent studies have shown that ZIKV-induced fetal damage in both mice and non-human primates is highly dependent on gestational age, with susceptibility decreasing as gestation progresses (Dudley et al., 2018; Jagger et al., 2017). To this end, we infected Stat2−/− pregnant dams at E10.5 in the presence of DENV or CTRL immune plasma. Consistent with previously published results (Jagger et al., 2017), mice infected at this advanced gestational age showed more resistance to ZIKV, with weight gain, resorption rates, and fetal sizes among the ZIKV-infected group receiving CTRL antibodies nearly identical to that of uninfected mice (Figures 3A–3D). Fetal resorption rates in dams infected in the presence of DENV antibodies also remained unaltered. However, the presence of DENV-specific antibodies notably increased maternal weight loss (p = 0.04) and resulted in smaller fetuses at E16.5 (p = 0.001; Figures 3A–3D). Maternal health monitoring revealed no symptom development or vaginal bleeding in any experimental group (data not shown), consistent with the unaltered resorption rates. Likewise, no significant differences in maternal viral loads were observed (Figures 3E–3G). We observed approximately a 5-fold increase in viral load in the placentas of mice receiving DENV antibodies compared to mice receiving CTRL antibodies (p = 0.0001; Figure 3H), but no difference in viral load in the fetal head was observed between the DENV and CTRL groups (p = 0.3134; Figure 3I), similar to our results from mice infected at E6.5. This increased viral load in the placentas was associated with increased placental damage, marked by a reduced labyrinth zone (Figure 3J, black outline) and greater breakdown of the endothelial network (Figure 3K). Together, our data suggest that although mice are more resistant to fetal damage with increased gestational age, the presence of DENV antibodies still exacerbates ZIKV-mediated damage to the fetus that correlates with increased viral replication and damage to the placenta.
Figure 3. Impact of DENV Antibodies on ZIKV Pathogenesis at Advanced Gestational Age.
Pregnant dams were infected intradermally with 5×103 PFU ZIKVPRVABC59 at E10.5 2 h after passive transfer of 20 μl CTRL or DENV immune plasma.
(A) Daily weight measurements after ZIKV infection are shown as the percentage of maternal starting weight for uninfected as well as ZIKV-infected pregnant dams in the presence of CTRL or DENV immune plasma (n = 3 per group). Mean ± SEM.
(B) Resorption rates were measured at E16.5 of uninfected pregnant dams (n = 19) or ZIKV-infected pregnant dams in the presence of CTRL (n = 21) or DENV (n = 22) immune plasma. Median ± interquartile range.
(C) Representative images of uterine horns, fetuses, and placentas at E16.5 are shown.
(D) Fetal size assessed at E16.5 by the CRL multiplied by the OFD was recorded; each dot represents one fetus (median ± interquartile range).
(E–I) Maternal blood (E) was collected 3 days post infection at E13.5, and maternal spinal cords (F), maternal brains (G), placentas (H), and fetal heads (I) were collected at day 6 post infection (E16.5) for viral RNA quantification by qPCR. Median ± interquartile range.
(J and K) H&E staining (J) and immunofluorescence staining of vimentin (K) were conducted on placentas harvested on day 6 post infection. For (J), the black line denotes the labyrinth zone and the gray line denotes the boundary between the decidua and the giant trophoblast layer. For (K), blue = DAPI, green = vimentin. Scale bars indicate 500 μm.
For (A) and (D), significance was determined by one-way ANOVA corrected using Bonferroni’s test for multiple comparisons; Fisher’s exact test was used to calculate significance in (B). For (E)–(I), significance was determined by the Mann-Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001.
Cellular Tropism of ZIKV Is Expanded in the Placenta in the Presence of DENV Antibodies
Evidence of placental pathology has been shown in mice, nonhuman primates, and humans after ZIKV infection, suggesting that placental damage may play a key role in ZIKV-mediated damage to the developing fetus (Dudley et al., 2018; Hirsch et al., 2018; Jagger et al., 2017; Martines et al., 2016; Martinot et al., 2018; Miner et al., 2016; Mlakar et al., 2016; Nguyen et al., 2017; Noronha et al., 2016; Seferovic et al., 2018; Yockey et al., 2018). Several previous studies, in both mice and humans, have identified macrophages (Hofbauer cells) and trophoblasts as potential in vivo targets for ZIKV within the placenta (Aagaard et al., 2017; El Costa et al., 2016; Miner et al., 2016; Quicke et al., 2016; Sheridan et al., 2017; Zimmerman et al., 2018). Given the increased viral load within the placenta in the presence of DENV-specific antibodies (Figure 2A), we sought to determine whether antibody-mediated entry of ZIKV altered the nature of the infected cell population within the placenta. To evaluate ZIKV infection among macrophages and trophoblasts, we conducted in situ hybridization for ZIKV RNA in conjunction with immunofluorescence staining for the macrophage marker MAC-2 or the trophoblast marker cytokeratin 19 (CK19) to identify infected cells. In placentas from mice receiving CTRL antibodies, cells positive for ZIKV RNA were found largely at the placenta-decidua interface, whereas in the presence of DENV antibodies, ZIKV-infected cells could be found infiltrated throughout the placenta (Figures 4A and S3A). Although we observed a greater number of ZIKV-infected macrophages in the placentas of mice receiving DENV antibodies, the overall proportion of ZIKV-infected macrophages was not significantly altered (5.0% for DENV condition versus 2.6% for CTRL condition; p = 0.8976; Figure 4B). When evaluating ZIKV infection among trophoblasts, we observed essentially no co-staining in the placentas from mice that received CTRL antibodies. However, in the placentas from mice receiving DENV antibodies, we observed a striking increase in the proportion of ZIKV-infected trophoblasts (10.8% for DENV condition versus 0.13% for CTRL condition; p = 0.0028; Figures 4C, 4D, and S3B). These data show that the presence of DENV antibodies greatly increases the infection of trophoblasts, which may contribute to increased viral replication and structural damage to the placenta.
Figure 4. DENV Antibodies Increase the Susceptibility of Trophoblasts to ZIKV Infection In Vivo.
(A–D) Placenta sections from ZIKV-infected mice receiving either CTRL or DENV immune plasma or placentas from uninfected pregnant dams were co-stained for ZIKV RNA by in situ hybridization (red) with either macrophage marker (MAC-2; A) or trophoblast marker CK19 (C) in green; nuclei were stained with DAPI (blue). Scale bars indicate 500 μm for the top panels and 100 mm for the remaining panels. Cells double positive for ZIKV RNA and either MAC-2 (B) or CK19 (D) were quantified. Nine placentas were imaged in the DENV condition and five placentas were imaged in the CTRL condition. The Mann-Whitney test was used to determine significance. **p < 0.01 See also Figure S3.
DENV Antibodies Increase ZIKV Replication in Human Placentas Ex Vivo
To determine whether our observations in the murine placenta may be relevant for human ZIKV infections, we adopted an ex vivo model of ZIKV infection using human placental tissues as previously described (Corry et al., 2017; Platt et al., 2018; Weisblum et al., 2017). We compared ZIKV replication in the presence of either DENV- or CTRL immune plasma using first and second trimester placentas from healthy human donors. Fetal-derived chorionic villi were isolated, cut, and placed in tissue culture overnight (Figure S4A). Tissues were then infected with ZIKV that had been pre-incubated with CTRL or DENV immune plasma. Culture supernatants were sampled throughout the 6-day culture, during which time production of human placental lactogen and chorionic gonadotropin remained robust, demonstrating that placental tissue remained functional (Figures S4B and S4C). At the earliest time point (day 2 post infection), all 11 donor placentas showed an increase in viral replication in the presence of DENV-immune plasma that ranged between 3- to 1,000-fold above ZIKV titers in the presence of CTRL immune plasma (p = 0.001; Figure 5A). This increase continued throughout the course of the infection for all donor placentas on day 4 (p = 0.001; Figure 5B) and was maintained in 10 of the 11 donor placentas through day 6 (p = 0.018; Figure 5C). We repeated these experiments using purified IgG, which showed a similar increase in viral replication in the presence of DENV IgG in all donors tested (Figures 5D–5F). To determine the dependence of this activity on Fcγ receptor (FcγR) engagement as well as on neonatal Fc receptor (FcRn) engagement, we pre-incubated the placental tissues with blocking antibodies against FcγRI (anti-CD64), FcγRII (anti-CD32), FcγRIII (anti-CD16), or anti-FcRn prior to infection. All blocking antibodies were able to decrease ZIKV replication in human placentas (Figures 5G–5J). Our data show that DENV antibodies can enhance ZIKV replication in human placentas in a manner that is Fc-receptor dependent.
Figure 5. DENV Antibodies Enhance ZIKV Infection in Human Placentas Ex Vivo.
(A–F) First and second trimester human placentas were obtained after elective terminations. Tissues were infected with 1×104 PFU ZIKVPRVABC59 after a pre-incubation of the virus with either CTRL or DENV immune plasma (A–C) or purified IgG (D–F) at a dilution of 1:250. Immune plasma or IgG were also added to the media at the same concentration every other day during the media change. Each condition was tested using triplicate wells (27 blocks of tissue per condition in total) along with an uninfected donor-matched condition. The total amount of virus produced was measured by plaque assay on days 2 (A and D), 4 (B and E), and 6 (C and F) post infection.
(G–J) Placental tissues were incubated with blocking antibodies against FcRn (G), CD64 (FcγRI; H), CD32 (FcγRII; I), or CD16 (FcγRIII; J) at a concentration of 1 μg/mL for 24 h prior to infection with ZIKV that was pre-incubated with DENV-immune plasma; blocking antibodies were maintained in culture media and the total virus production over all 6 days is shown. Dotted line indicates the limit of detection. Significance was determined using the Wilcoxon matched pairs signed-rank test. *p < 0.05, ***p < 0.001. See also Figure S4.
Dengue Virus-Specific Antibodies Promote ZIKV Infection of Trophoblasts in Human Placentas
To further tease apart the mechanism of ZIKV ADE in human placentas, we evaluated the ZIKV-infected cells using both in situ hybridization for ZIKV RNA and ZIKV NS3 antibody staining combined with immunofluorescence staining for macrophages and trophoblasts. Evaluation of co-stained cells revealed that the overall proportion of macrophages infected with ZIKV remained unchanged in the presence or absence of DENV antibodies (3.64% in the CTRL condition versus 3.65% in the DENV condition; p = 0.9375; Figures 6A, 6B, and S5A), consistent with our observations in mice. Furthermore, co-staining for ZIKV and CK19 revealed an approximately 10-fold increase in the proportion of placental trophoblasts infected with ZIKV, with 1.2% of trophoblasts co-staining with ZIKV in the absence of enhancing antibodies (CTRL-treated) and 12.4% in the presence of DENV antibodies (p = 0.0156; Figures 6C, 6D, and S5B), again consistent with our observations in placentas from ZIKV-infected Stat2−/− mice. To distinguish between syncytiotrophoblasts and cytotrophoblasts, we co-stained placental tissue for ZIKV RNA and syncytin, a marker for syncytiotrophoblasts in the placenta. We observed a trend of increased ZIKV-infected syncytiotrophoblasts in the presence of DENV antibodies in all three donors tested, but this increase was not as striking as the overall increase in CK19-stained trophoblasts, with 1.9% of syncytiotrophoblasts infected in the presence of CTRL antibodies and 6.5% infected in the presence of DENV-specific antibodies (p = 0.25; Figures S6A and S6B), suggesting that the majority of infected trophoblasts in the presence of DENV antibodies are likely cytotrophoblasts. These data suggest that DENV antibodies may facilitate infection of human trophoblasts, consistent with our data in mice.
Figure 6. DENV Antibodies Increase the Susceptibility of Human Placental Trophoblasts to ZIKV Infection Ex Vivo.
(A–D) Placental tissue infected with ZIKV in the presence of DENV or CTRL immune plasma or donor-matched uninfected controls was collected on day 2 post infection and paraffin embedded. Tissue sections were co-stained for ZIKV RNA using in situ hybridization (red) and either MAC-2 for macrophages (A and B) or CK19 for trophoblasts (C and D) shown in green. DAPI was used to identify nuclei (blue). Scale bars indicate 100 μm. Cells double positive for ZIKV and either MAC-2 (B) or CK19 (D) were quantified across the three donors that were co-stained for ZIKV RNA using in situ hybridization (blue circles) combined with four donors that were co-stained for ZIKV NS3 using immunofluorescence (red squares). The Wilcoxon matched pairs signed-rank test was used to determine significance. *p < 0.05 See also Figures S5 and S6.
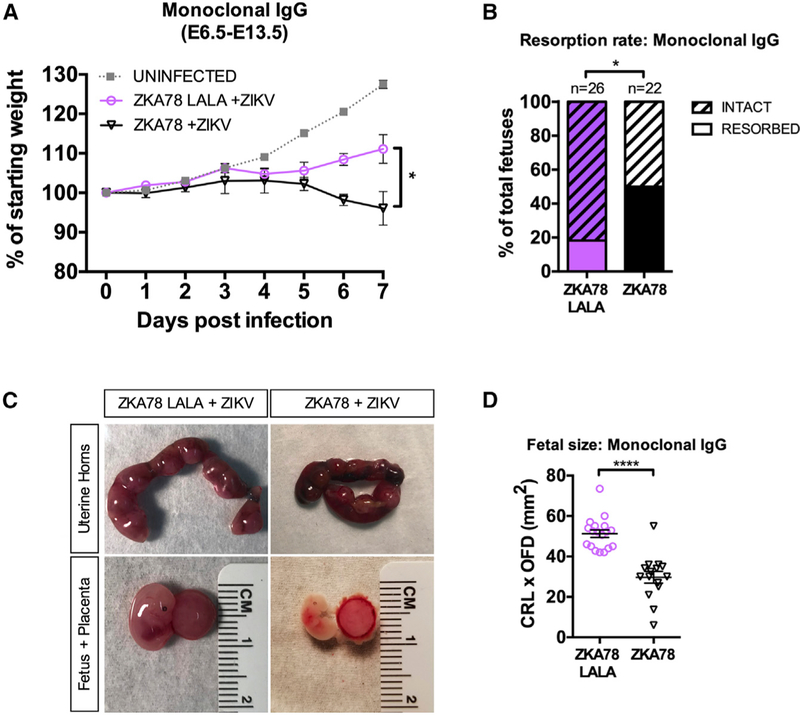
Enhancement of ZIKV Pathogenesis by Dengue Virus-Reactive Antibodies Is FcγR Mediated In Vivo
Our in vivo studies clearly demonstrate that DENV-specific IgG mediates enhancement of ZIKV pathogenicity, and data using FcγR blocking antibodies in human placental explants further point to a role for FcγRs in mediating this effect. To address this, we obtained a previously described human monoclonal antibody clone, ZKA78, which is cross-reactive to and can enhance both DENV and ZIKV in vitro (Stettler et al., 2016), as well as its LALA (leucine [L] to alanine [A] substitution at position 234 and 235) counterpart, which is incapable of engaging FcγRs. We injected 1 μg/mouse (0.05 mg/kg) of ZKA78 or ZKA78 LALA into pregnant Stat2−/− mice at E6.5 2 h prior to infection with ZIKV, as we have previously done in Figure 1. As shown in Figure 7A, mice that received the ZKA78 antibody prior to ZIKV infection showed increased maternal weight loss post infection compared to mice receiving the ZKA78 LALA antibody (p = 0.027). This was also accompanied by a significant increase in fetal resorption rates, with pregnant dams that received ZKA78 having a resorption rate of 50% compared to 18.2% observed in mice that received the ZKA78 LALA (p = 0.034; Figure 7B). Fetuses harvested at E13.5 from ZIKV-infected pregnant dams receiving ZKA78 were significantly smaller than those harvested from mice that had received ZKA78 LALA (p < 0.0001; Figures 7C and 7D). Overall, these data provide compelling evidence that cross-reactive antibodies are capable of enhancing ZIKV-mediated fetal damage in an FcγR-dependent fashion.
Figure 7. Enhanced ZIKV-Mediated Damage in the Presence of DENV Antibodies Is FcγR Dependent.
(A–D) Pregnant dams at E6.5 were injected intraperitoneally with 1 μg/mouse (0.05 mg/kg) of ZKA78 or ZKA78 LALA human monoclonal antibody prior to infection with 5×103 PFU ZIKVPRVABC59. (A) Daily weight measurements after ZIKV infection are shown as the percentage of maternal starting weight for ZIKV-infected mice in the presence of ZKA78 (n = 3) or ZKA78 LALA (n = 3) IgG. Uninfected mice from Figure 1A are shown for the purposes of comparison and were not included for statistical analysis. Mean ± SEM. (B) Resorption rates at E13.5 of ZIKV-infected pregnant dams in the presence of ZKA78 (n = 22) or ZKA78 LALA (n = 26) were measured. (C) Representative images of uterine horns, fetuses, and placentas at E13.5 are shown. (D) Fetal size was assessed at E13.5 by multiplying the CRL by the OFD. Each data point represents an individual fetus. Median ± interquartile range. Significance was determined by two-way ANOVA corrected using Bonferroni’s test for multiple comparisons (A), Fisher’s exact test (B), or Mann-Whitney test (D). *p < 0.05, ****p < 0.0001 See also Figure S6.
DISCUSSION
This study reports evidence linking prior DENV infection with the severe disease phenotype caused by ZIKV during pregnancy. In both the mouse and human infection models, the hallmark of enhanced ZIKV infection caused by DENV antibodies was the increase in infected trophoblasts. Trophoblasts are critical for normal placental function and fetal development—dysfunction of these cells has been shown to lead to intrauterine growth restriction, impaired neurological development, and miscarriage (Gil-Sánchez et al., 2011; Tabata et al., 2015; Woods et al., 2017; Yougbaré et al., 2017), much like the pathology observed in ZIKV infection in humans during pregnancy. In fact, trophoblasts become less susceptible to ZIKV infection during mid to late gestation (Bayer et al., 2016; Corry et al., 2017; Sheridan et al., 2017; Tabata et al., 2016) because they have been shown to constitutively express IFNλ, which is consistent with the increased resistance to ZIKV infection observed in mice infected later in gestation. This is also supported by human epidemiologic data showing fewer birth defects in women infected with ZIKV later in gestation (Brasil et al., 2016; Hoen et al., 2018; Pacheco et al., 2016; Reynolds et al., 2017). Increased susceptibility of and/or access to early-gestation placental trophoblasts in the presence of DENV-specific antibodies may explain the high rates of ZIKV-induced birth defects and spontaneous abortions that have occurred in DENV-endemic regions (Brasil et al., 2016). Infected trophoblasts may exacerbate damage to the developing fetus indirectly by restricting transfer of oxygen and nutrients to the fetus. Our data also show that DENV antibodies increase the breakdown of placental architecture during ZIKV infection, which may also directly impact fetal development. Although we did not observe a significant difference in the proportion of infected macrophages relative to all infected cells between mice that received CTRL antibodies and mice that received DENV antibodies, we did see an increase in the total number of infected macrophages in the DENV antibody condition, which may contribute to the overall heightened viral load in the placenta. This is consistent with a recently published study showing that DENV antibodies can increase infection of full-term placental macrophages (Zimmerman et al., 2018). However, our data suggest that the majority of the increase in viral load is due to increased infection of trophoblasts.
Our data suggest that the worsened fetal outcome during ZIKV infection in the presence of DENV antibodies is FcγR mediated. It is well established that FcRn expression increases as gestational age increases and that FcRn is responsible for the trans-placental transfer of IgG (Lozano et al., 2018; van den Berg et al., 2011). However, ablation of the ability for antibody to bind to FcγRs decreased ZIKV replication in human tissue and pathogenesis in mice. This suggests that despite the low expression levels of FcRn during early gestation, the binding of DENV antibodies to FcγRs is still sufficient to exacerbate pathogenesis during pregnancy. We can envision several possible mechanisms for ADE of ZIKV in the placenta: (1) syncytiotrophoblasts are first infected in an FcγR-dependent fashion, allowing the virus to disseminate into the placenta; (2) peripheral enhancement of ZIKV infection combined with inflammation may promote tissue damage and weaken the placental barrier; and (3) low levels of FcRn expression during early gestation are sufficient for the delivery of enhancing concentrations of DENV antibodies into the placenta (Simister, 1998). In order to determine the precise role of individual Fc receptors during ADE of ZIKV in the context of pregnancy, tissue-specific knockout mice will likely be important tools to investigate this further.
It is important to note that our study only evaluates the role of antibodies in mediating ADE of ZIKV. Recent studies have also shown that anti-DENV CD8+ T cells can offer protection against subsequent ZIKV infections in mice (Wen et al., 2017). Although natural immunity to DENV involves both antibody and T cell responses, in the context of pregnancy, a passive transfer model like the one used here, which solely evaluates the role of antibodies, may be more relevant because antibodies, but not T cells, can be transferred across the placenta. The interplay between the humoral and cellular immune responses against DENV, the timing of the ZIKV infection after an initial DENV infection, and the immune state at the time of infection (particularly during pregnancy) are all likely to impact the extent of ADE of ZIKV that may occur in humans.
Currently, it is not clear whether prior exposure to DENV, either through infection or vaccination, impacts clinical syndromes during ZIKV infection in humans, nor is there a clear time frame between sequential infections identified to observe such an effect. These questions are very challenging to address because of the high level of cross-reactivity among flaviviruses, which makes it difficult to serologically differentiate ZIKV and DENV infection. One human study with a relatively small patient population (Terzian et al., 2017) and two macaque studies (McCracken et al., 2017; Pantoja et al., 2017) evaluating how DENV immunity impacts ZIKV pathogenesis showed no increase in viremia. However, identification of enhancement of ZIKV infection caused by preexisting DENV immunity is complex and may be highly dependent on numerous factors, such as the timing of sequential infections, circulating antibody levels, cell-mediated responses, or disease parameter definitions. Furthermore, ADE only occurs at subneutralizing concentrations of cross-reactive antibodies (Katzelnick et al., 2017; Kliks et al., 1988); high levels of DENV-specific antibodies are protective against ZIKV at high concentrations (Bardina et al., 2017; Fernandez et al., 2017; Robbiani et al., 2017). Epidemiologic studies that evaluate the extent to which congenital ZIKV disease is impacted by prior infection with DENV should consider circulating DENV antibody levels at the time of infection because DENV immunity can be both protective and enhancing. This is exemplified by a recent study evaluating the incidence of ADE after sequential DENV infections, which showed that waning DENV antibodies can enhance subsequent infection with a heterotypic DENV serotype within a distinct window of antibody levels (Katzelnick et al., 2017). Thus, human epidemiologic studies that do not characterize DENV antibody titers at the time of ZIKV infection will likely see no differences (Halai et al., 2017).
It is possible that ADE of ZIKV may only be apparent in humans and non-human primates under specific immunologic circumstances, such as pregnancy. This is highlighted by the fact that the recent ZIKV outbreaks only gained prominence as the virus’s association with microcephaly and other birth defects became apparent. Numerous non-human primate studies have now shown that ZIKV can cause placental pathology, with detectable virus in the placental chorionic villi (Adams Waldorf et al., 2016; Dudley et al., 2018; Hirsch et al., 2018; Martinot et al., 2018; Nguyen et al., 2017; Seferovic et al., 2018). Further, primary placental macrophages have been shown to be significantly more susceptible to ZIKV in the presence of DENV antibodies (Zimmerman et al., 2018). Thus, studies evaluating ADE of ZIKV in the context of pregnancy should be conducted in primates and examined epidemiologically in humans.
Understanding the interplay between sequential DENV-ZIKV infections in humans will have profound implications for the development of flavivirus vaccines and antibody therapeutics and could help identify at-risk populations for future outbreaks. Our data highlight the potential for antibody responses generated through DENV infection or vaccination to increase the risk of ZIKV-related diseases. Although it may take years or decades to conclusively evaluate whether ADE of ZIKV occurs in human populations, the Dengvaxia vaccine trial tells a cautionary tale for flavivirus vaccine development (Halstead, 2017). As such, all flavivirus vaccines should consider the potential risk for ADE. Additionally, our study opens up novel opportunities for specific interventions to protect pregnant women after exposure to ZIKV, particularly in DENV endemic regions.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jean Lim (jean.lim@mssm.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal Models
Mouse studies were carried out in an animal biosafety level 2 plus facility under a protocol approved by the Icahn School of Medicine at Mount Sinai Animal Care and Use Committee. Breeding pairs were formed using Stat2−/− mice (provided by Christian Schindler; 8–10 week old nulliparous females and >8 week old non-virgin males). Females were then separated after plugs were detected (defined as E0.5) and infected at E6.5 or E10.5 as indicated.
Human Models
IgG-positive plasma from DENV-infected (n =141) blood donors was identified through screening of blood donations in the United States Territories as follows: 270,049 blood donations isolated between March 2010–August 2013 were initially screened using a DENV NS1 antigen ELISA or individual donation transcription-mediated amplification (TMA) testing (Gen-Probe, San Diego, CA). All positive hits by ELISA were further validated by TMA (both nucleic acid testing and PCR). IgM and IgG antibody testing (performed by the CDC-Dengue Branch in San Juan PR), along with DENV serotype specific assay was also performed. 141 samples tested positive for DENV-specific IgG and were thus used. Random blood donor plasma samples (n =54) that were negative for all infectious pathogens were used as negative controls. All plasma samples were individually tested for reactivity to ZIKV E protein by ELISA with recombinant ZIKV envelope protein (Protein Sciences Corp., Meriden, CT) as previously described (Bardina et al., 2017). Immune plasma used for experiments consisted of pooled plasma samples from individuals with the highest reactivity to ZIKV E protein from 15 DENV-seropositive donors. All control plasma failed to react to ZIKV E protein, and 15 donors were pooled and used for the CTRL immune plasma condition.
For placental studies, first and second trimester human placental tissue, ranging from 8 to 22 weeks of gestation (n = 17), was obtained from donors undergoing elective termination under an IRB-approved protocol (HS: 12–00145). Tissue samples from 7 week terminations were excluded from the study as no ZIKV replication was observed. Placentas were processed and cultured as expounded upon in the Method Detail section.
Cell Lines
Vero (ATCC® CCL81™) cells were used at low passage for all virus quantification by plaque assay. These cells, originally derived from kidney epithelium of a female green monkey, were obtained from the ATCC for the purposes of this study. K562 (ATCC® CCL-243™) cells, a human female lymphoblast line derived from a patient with Chronic Myelogenous Leukemia (CML) were used at low passage for all in vitro ADE experiments. K562 cells were grown at 37°C, and 5% CO2 in RPMI supplemented with 10% FBS, 10U/ml penicillin and 10μg/ml streptomycin and 2mM L-Glutamine.
METHOD DETAILS
ZIKV Infection of Stat2–/– Mice
Pregnant Stat2−/− mice were injected intraperitoneally with 20 μl of CTRL or DENV immune plasma two hours prior to intradermal infection with 5×103 PFU ZIKV (strain PRVABC59, GenBank: KU501215.1) at E6.5 or E10.5. ZIKV was obtained originally from the ATCC, passaged once in Vero cells, and sequence verified. Mice were monitored daily for weight loss and symptom development, following the clinical score system used in our previous study (Bardina et al., 2017). Fetal and maternal tissues were harvested at either E13.5 or E16.5 for analysis. Plasma samples from DENV-seropositive individuals that showed high reactivity for ZIKV E protein were pooled from 15 donors, heat inactivated, and 0.22 μm filtered. CTRL plasma samples where no reactivity to ZIKV E was detected were also pooled from 15 donors. For infections using purified IgG, pregnant Stat2−/− mice were injected intraperitoneally with 2 ml of CTRL or DENV IgG antibodies reconstituted into PBS to the original plasma volume used for purification. Dose down experiments were conducted for in vivo experiments, and 2 μl of purified IgG was identified as the optimal amount. For infections using ZKA78 or ZKA78 LALA, 1μg/mouse (0.05mg/kg) of antibody in a volume of 200μl in PBS was injected intraperitoneally, and mice were subsequently infected as above.
ZIKV Quantification
Total RNA was isolated from tissue homogenates using Direct-zol RNA MiniPrep Plus (Zymo Research) or plasma using QIAamp viral RNA kit (Qiagen) according to the manufacturer’s protocol. The following ZIKV-specific primers (5′-TTGGTCATGATACTGCTGATTGC-3′ and 5′-CCYTCCACRAAGTCYCTATTGC-3′) and probe (5′−6FAM-CGGCATACAGYATCAGGTGCATWGGAG-MGBN FQ-3′) were used for qRT-PCR (Schwarz et al., 2016) using the Lightcycler 480 RNA Master Hydrolysis Probes kit (Roche). For plasma, 5μl of vRNA was used for ZIKV RNA quantification; for maternal organs 100ng total RNA was used as input for ZIKV RNA quantification. For fetal heads and placentas, 1μg RNA was first reverse transcribed using High Capacity cDNA Reverse Transcription Kits (Applied Biosciences), followed by PCR using SYBR green (Roche) using the ZIKV-specific primers described above. RNA quantification was determined by fitting to an in vitro transcribed RNA standard. PCR amplification was conducted in 384-well format using the Roche LightCycler 480 System. ZIKV plaque forming units (PFU) were quantified on Vero cell monolayers whereby 250 μl of tissue culture supernatant was adsorbed for 1 hour at 37°C in 12 well plates, and cells were overlaid with 1 ml DMEM (Invitrogen) supplemented with 0.8% methyl cellulose, 2% FBS, and 50 μg/ml gentamicin sulfate. Cells were incubated for 5 days at 37°C, fixed with 4% PFA, and stained with crystal violet for plaque visualization.
ZIKV RNA Detection by In Situ Hybridization and Immunofluorescence Co-staining
Tissues were fixed in 10% neutral buffered formalin for 24 hours and placed back into PBS until paraffin embedding. In situ hybridization using RNAscope® was performed on 5 μm paraffin-embedded sections. Deparaffinization was performed by baking slides at 55°C for twenty minutes. Following this step, slides were washed twice with xylene for five minutes each, twice in 100% ethanol for two minutes each, and lastly slides were dried for five minutes at 60°C. Slides were then incubated with hydrogen peroxide for 10 minutes at RT, and were subsequently washed in diH2O. Target retrieval was performed by incubating slides in Target Retrieval solution at 100°C for 15 minutes. Lastly, slides were washed with water and transferred into 100% ethanol for three minutes, then were allowed to dry. Sections were treated with RNAscope® Protease Plus and incubated at 40°C for 30 minutes. Slides were then washed with diH2O. Fluorescence in situ hybridization was subsequently performed according to the manufacturer’s protocol (ACD# 323110) with RNAscope® Probe V-ZIKVsph2015 (ACD #467871; binds sense RNA) as previously described (Cao et al., 2017). Following in situ hybridization, slides were washed twice for five minutes in Tris-buffered Solution containing 0.01% Tween-20 (TBST) and subsequently blocked in a solution of 2.5% goat serum and 2.5% BSA in TBST for 30 minutes at RT. For co-stains, sections were incubated for 1 hour at 4°C with monoclonal rat anti-MAC2 antibody (1:500, clone M3/38, Cedarlane) or rabbit monoclonal anti-cytokeratin 19 (1:100, clone EP1580Y, Abcam), or overnight at 4°C with rabbit polyclonal anti-syncytin (1:50, ab71115, Abcam) and then mounted with Vectashield hard-set mounting medium with DAPI (Vector Laboratories). Slides were analyzed using an AxioImager Z2 microscope (Zeiss) and Zen 2012 software (Zeiss). Cell quantifications were conducted using CellProfiler Analyst software. For mouse placentas, cells were quantified across the entire placental slice, with 4,000 cells counted on average per slice. The percentage of ZIKV+ macrophages or trophoblasts was calculated for each placenta, and the percentages were averaged. For the CTRL condition, 5 individual placentas were quantified; for DENV condition, 9 individual placentas were quantified. For the quantification of human placentas, three placental donors were evaluated by RNA ISH, and four placental donors were evaluate using NS3 staining (described below). Because nearly identical quantifications were found using both methods, these quantifications were combined as human placental tissues were limited. On average, 3,000 cells were counted per donor per condition, with image analysis spanning the entire tissue slice. Significance was calculated using the Mann-Whitney test for mouse placentas and Wilcoxon matched pairs signed-rank test for human placentas.
H&E and Vimentin Staining
Tissues were fixed in 10% neutral buffered formalin before embedding into paraffin and cutting 5μm sections. Deparaffinization and antigen retrieval was performed as previously described (Bardina et al., 2017). Slides were stained with hematoxylin (Gill’s formula, Vector Laboratories H3401) and eosin Y (Sigma Aldrich E4009) according to manufacturer’s instructions. For vimentin staining, slides were blocked in TBST containing 2.5% normal goat serum for 40 minutes at RT, washed and incubated with rabbit monoclonal anti-vimentin antibody (1:1000, clone EPR3776, Abcam) for 1 hour at 4°C, then washed and incubated with goat anti-rabbit Alexa Fluor 488 before mounting with Vectashield as described above and imaging with an AxioImager Z2 microscope (Zeiss) and Zen 2012 software.
Cytokine and Chemokine Protein Quantification
Placental tissue homogenates were evaluated for 21 cytokines/chemokines by multiplex ELISA for the following analytes: CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, CCL7/MCP-3, CCL20/MIP-3α, CXCLl1/GROα, CXCL2/GROβ, CXCL9/MIG, CXCL10/IP-10, GM-CSF, IFNγ, IL1β, IL-4, IL-6, IL-10, IL-15, IL-17A, IL-22, IL-2, and TNFα as previously described (Aguado et al., 2015). Samples were analyzed on a Luminex MAGPIX platform. For each sample, >50 beads were collected per analyte. The median fluorescence intensity of these beads was recorded and used for analysis with the Milliplex Analyst software using a 5P regression algorithm. Heat maps for fold induction were generated in R by logarithmic transformation of the mean cytokine level in the ZIKV-infected conditions divided by the mean cytokine level of uninfected controls, normalized per gram of tissue. Hierarchical clustering was performed using the hclust function in R, which stratifies Euclidian distance.
Ex Vivo Infection of Human Placental Tissue
First and second trimester human placental tissue was obtained within two hours of surgery from donors undergoing elective termination under an IRB-approved protocol (HS: 12–00145). Chorionic villi adjacent to the fetal chorionic plate were placed into pre-warmed DMEM containing 25% F-12 media, 10% FBS, 5 mM HEPES, 2 mM Glutamine, 100 IU/ml Penicillin, 100 μg/ml Streptomycin, 2.5 μg/ml Fungizone, and 300 ng/ml Timentin as previously described (Weisblum et al., 2017). After removal of the amnion and decidua, the chorionic villi were cut into 0.2 cm3 blocks; nine blocks were plated per well of a six-well plate onto collagen gelfoams (Cardinal Health) in 3 ml media, and 3 wells were used per condition (27 tissue blocks). Following an overnight incubation at 37°C, tissue blocks were individually infected with 1×104 PFU ZIKVPRVABC59 per tissue block in a volume of 5 ml that was pre-incubated with CTRL or DENV immune plasma or IgG (diluted 1:250) for 1 hour. Supernatants were collected and media changed every other day; immune plasma or IgG was replenished in the culture media at a dilution of 1:250 upon media change. For inhibition experiments, anti-FcRn (Genetex, clone 1G3), anti-CD16 (eBioscience, clone eBioCB16), anti-CD32 (eBioscience, clone 6C4), or anti-CD64 (eBioscience, clone 10.1) antibodies were added into the culture media at a concentration of 1μg/mL for 24 hours prior to infection. FcR inhibitors were maintained in culture media throughout the experiment.
Purification of IgG from Human Plasma
Heat-inactivated pooled human plasma samples were diluted approximately 1:10 in PBS and filtered through a 0.22μ filter unit. The diluted plasma was then run through a sepharose-G (GE Healthcare) column three times and the flow through was collected. The column was washed with three column volumes PBS (approximately 150 ml) and IgG was eluted with 45 ml of 0.1M glycine-HCl buffer (pH 2.7) into 5 ml of 2M Tris-HCl buffer (pH 10). The resulting 50 ml of IgG solution were then concentrated using Amicon Ultra centrifugation units (30 kDa cutoff, Millipore) and washed three times with 15 ml of PBS. Finally, the purified IgG was taken up in a volume of PBS corresponding to the original plasma volume used for purification.
ZIKV E Protein ELISAs
ZIKV E protein ELISA was performed as described previously (Bardina et al, 2017). Briefly, recombinant ZIKV E protein (Protein Sciences Corp., Meriden, CT) was coated on Immulon plates overnight at 4°C at a concentration of 2 μg/ml in coating buffer(0.1 M Na2CO3-NaHCO3, pH 9.4). Plates were then blocked for 1 hour at RT in 3% dry milk powder in PBS containing 0.1% Tween 20 (PBS-T). Meanwhile, heat-inactivated plasma or purified IgG was diluted 1:3 starting with a 1:100 dilution in blocking buffer in a separate plate. Positive controls (4G2, murine hybridoma-derived anti-flavivirus E) and negative controls (murine isotype control antibodies) were used on each plate to evaluate standardization. All control monoclonal antibodies were diluted 1:3 with a 10 μg/mL initial concentration in blocking buffer. Blocking buffer was then removed and purified IgG or immune plasma added. After a two-hour incubation phase, plates were washed four times with PBS-T and a secondary stain containing horseradish peroxidase conjugated anti-human IgG antibody (Sigma A0293, 1:3,000) was diluted in blocking buffer and added. After a one-hour incubation in secondary antibody, plates were washed four times with PBS-T and developed with SigmaFast o-phenylenediamine dihydrochloride (OPD) (Sigma, 100 μl per well). After 10 minutes of development, the reaction was stopped with 3 M HCl. The optical density (OD) at 490 nm was read on a plate reader and background levels were analyzed in Microsoft Excel, and net optical density over background graphed in GraphPad PRISM with four-parameter logarithmic regressions.
In Vitro Antibody-Dependent Enhancement of ZIKV Infection
Antibody-dependent enhancement of ZIKV infection was measured using a flowcytometry-based assay. Briefly, different dilutions of plasma or purified IgG were mixed with ZIKVPRVABC59 (MOI of 1) for 1 hour at 37 °C and then added to K562 cells (5 × 104) in 96 well U bottom plates in RPMI 1640 media supplemented with 10% FBS, 2mM L-glutamine, and 10U/ml penicillin and 10μg/ml streptomycin. After 2 days, cells were fixed with 4% PFA, permeabilized with PBS containing 0.2% BSA and 0.05% saponin, and stained with 4G2 antibody (1 μg/ml) for 1 hour at room temperature. After washing, cells were incubated with goat anti-mouse IgG conjugated to phycoerythrin (1μg/ml, Invitrogen) for 1 hr at room temperature. The number of infected cells was determined by flow cytometry using a FACS Caliber and analyzed using FlowJo2 software version 10.1.r7.
HCGβ and hPL ELISA
Supernatants from uninfected placental tissues were collected on day 2, day 4, and day 6 post culture. HCGβ (human beta chorionic gonadotropin) and human placental lactogen (hPL) levels were determined using R&D Systems DuoSet® ELISA Development System and Alpco® HPL ELISA kits, respectively. Procedures were followed per manufacturer’s instructions. The kit-provided standards and samples were measured with each assay in duplicate. For both kits, supernatants from human tonsil explants cultured ex vivo were used as a negative control.
Neutralization Assays
Neutralization titers were determined by serially-diluting heat-inactivated filter sterilized pooled immune plasma or purified IgG and mixing with 10 TCID50 of ZIKV strain PRVABC59, DENV-1 strain BC89/94, or DENV-4 strain PR 06-65-740 in DMEM with 2% FBS. After incubation for 1 hour at 37°C, the virus-antibody mixture was added to a monolayer of Vero cells in 96 well format. Following a two (ZIKV) or five (DENV) day incubation at 37°C, cells were detached from the plate using trypsin/EDTA, fixed with 4% PFA, permeabilized with PBS containing 0.2% BSA and 0.05% saponin, and stained with 4G2 antibody (1 μg/ml) for 1 hour at room temperature. After washing, cells were incubated with goat anti-mouse IgG conjugated to phycoerythrin (1μg/ml, Invitrogen) for 1 hr at room temperature. The number of infected cells was determined by flow cytometry using a FACS Caliber and analyzed using FlowJo2 software version 10.1.r7. The 50% inhibitory doses (IC50) of purified IgG and immune plasma were calculated by using the four-parameter logarithmic regression in GraphPad Prism. IC50s for undefined regressions were estimated as below a limit of detection via extrapolation from the highest concentration values. 50% tissue culture infectious doses (TCID50) were determined likewise, with ten-fold serial dilution of virus in the absence of antibody/plasma.
Immunofluorescence Assay
Tissues were fixed in 10% neutral buffered formalin, and immunofluorescence was performed on 5 mm paraffin-embedded sections. Deparaffinization and antigen retrieval was performed as previously described (Bardina et al., 2017). All washes were done in TBST and all antibody solutions were diluted in TBST containing 2.5% BSA (for MAC2) or 2.5% normal goat serum (all other stains). For ZIKV stains, slides were incubated with biotinylated rabbit polyclonal anti-ZIKV NS3 antibody (1:5,000) overnight at 4°C. Slides were washed and incubated with streptavidin conjugated to R-phycoerythrin (1:200, Invitrogen) for 40 min at room temperature, then mounted with Vectashield hard-set mounting medium with DAPI (Vector Laboratories). For co-stains, sections were blocked for 1 hour at room temperature, and incubated for 1 hour at RT with monoclonal rat anti-MAC2 antibody (1:500, clone M3/38, Cedarlane) or rabbit monoclonal anti-cytokeratin 19 (1:100, clone EP1580Y, Abcam). Slides were then incubated for 40 minutes at room temperature with donkey anti-rat or goat anti-rabbit Alexa Fluor 488 (1:200, Jackson Immuno Research Laboratories). Slides were analyzed using an AxioImager Z2 microscope (Zeiss) and Zen 2012 software (Zeiss). ZIKV-infected trophoblasts, and macrophages were quantified using CellProfiler Analyst software. On average, 4,000 cells were counted per mouse placental sample, and 3,000 cells were counted per human placental sample. Images analyzed spanned the entire tissue slice.
QUANTIFICATION AND STATISTICAL ANALYSIS
For all statistical significance indications in this manuscript, ****p < 0.0001, ***p < 0.001, **p < 0.01, and *p < 0.05. All sample sizes, replicate numbers, statistical tests, and significance can be found in the figures and figure legends of this manuscript.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Biotinylated rabbit polyclonal anti-ZIKV NS3 antibody | Tortorella lab at Mount Sinai | N/A |
| Streptavidin R-phycoerythrin conjugate (SA-PE) | Invitrogen | S866 |
| Monoclonal rat anti-MAC2 antibody | Cedarlane | clone M3/38; RRID:AB_10060357 |
| Rabbit monoclonal anti-cytokeratin19 | Abcam | clone EP1580Y; RRID:AB_2281020 |
| Donkey/Goat anti-Rat/Rabbit Alexa Fluor 488 | Jackson Immuno Research Laboratories | 712545150; RRID:AB_2340683 |
| Rabbit monoclonal anti-vimentin antibody | Abcam | Clone EPR3776 |
| Rabbit polyclonal anti-HERV (syncytin) antibody | Abcam | Ab71115; RRID:AB_1269066 |
| Polyclonal anti-human IgG | eBioscience | Ref. no. 12-4998; RRID:AB_465926 |
| Anti-CD16 antibody | eBioscience | Clone eBioCB16; RRID:AB_10804882 |
| Anti-CD32 antibody | eBioscience | Clone 6C4 |
| Anti-CD64 antibody | eBioscience | Clone 10.1 |
| Anti-FcRn antibody | eBioscience | Clone 1G3 |
| ZKA78 antibody | Absolute Antibody | Ab00780-10.0 |
| ZKA78 LALA antibody | Absolute Antibody | Ab00780-10.16 |
| Bacterial and Virus Strains | ||
| ZIKV strain PRVABC59 | ATCC | VR-1843 |
| DENV-1 strain BC89/94 | BEI Resources | NR-3787 |
| DENV-4 strain PR 06-65-740 | BEI Resources | NR-49757 |
| Biological Samples | ||
| Human Plasma samples | American Red Cross | N/A |
| Human Placenta Tissue | Mount Sinai Biorepository | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Recombinant ZIKV envelope protein | Protein Sciences Corp. | 4002 |
| VectaShield hard-set mounting medium with DAPI | Vector Laboratories | Cat# H1500 |
| Hematoxylin (Gill’s formula) | Vector Laboratories | Cat# H-3401 |
| Eosin Y | Sigma | E4009-5G |
| DPX Mountant | Sigma | 06522 |
| Critical Commercial Assays | ||
| High Capacity cDNA Reverse Transcription Kits | Applied Biosystems | Cat# 4368814 |
| Direct-zol RNA MiniPrep Plus | Zymo Research | Cat# R2072 |
| QIAamp viral RNA kit | Qiagen | Cat# 52904 |
| SYBR green | Roche | Cat# Pack Size 04707516001 |
| LightCycler 480 RNA Master Hydrolysis Probes | Roche | Cat# 4991885001 |
| Miltenyi Biotec Biotinylation kit | Miltenyi Biotec | Order no. 130-093-385 |
| hCGβ DuoSet ELISA kit | R&D | Cat# DY9034-05 |
| Human Placental Lactogen ELISA kit | Alpco | Cat# 20-HPLHU-E01 |
| RNAscope Multiplex Fluorescent Reagent Kit v2 | ACD | Cat# 323100 |
| RNAscope Probe- V-ZIKVsph2015 | ACD | Cat# 467871 |
| Experimental Models: Cell Lines | ||
| Vero Cells | ATCC | ATCC CCL81 |
| K562 Cells | ATCC | ATCC CCL-243 |
| Experimental Models: Organisms/Strains | ||
| Stat2−/− mice, strain B6.129-Stat2 tm1Shnd/J (Bred from C57Bl/6J background) | Kindly provided by Christian Schindler | N/A |
| Oligonucleotides | ||
| 5′-TTGGTCATGATACTGCTGATTGC-3′ | Thermo Fisher | N/A |
| 5′-CCYTCCACRAAGTCYCTATTGC-3′ | Thermo Fisher | N/A |
| 5′−6FAM-CGGCATACAGYATCAGGTGCATWGGAG-MGBNFQ-3′ | Thermo Fisher | N/A |
| Software and Algorithms | ||
| GraphPad Prism | N/A | |
| Zen 2012 software | N/A | |
| CellProfiler Analyst 2.2.0 software | N/A | |
| Milliplex software | N/A | |
| Other | ||
| 10% neutral buffered formalin | VWR | 89370-094 |
| RPMI 1640 | Gibco | 11875-093 |
| DMEM | Gibco | 10313-021 |
| F-12 media | Gibco | 11765-047 |
| FBS | VWR | 97068-085 |
| HEPES | Gibco | 15630-080 |
| L-Glutamine | VWR | 02-0131-0100 |
| Penicillin-Streptomycin | Corning | Ref# 30-002-CI |
| Fungizone | HyClone | SV30078.01 |
| Timentin | Bioworld | NC9588884 |
| TBST (Tris Buffered Saline with 0.01%Tween 20) | Chem Cruz | SC-281695 |
Highlights.
Dengue-specific antibodies can enhance Zika virus pathogenesis during pregnancy
Enhancement of Zika pathogenesis was observed in the placenta, not in the fetus
Placental damage was associated with an increase in infected trophoblasts
This process was found to be FcγR mediated
ACKNOWLEDGMENTS
We thank Drs. Matthew J. Evan and Marion Sourisseau for providing the RNA standards for ZIKV quantification, Dr. Christian Schindler for providing the original Stat2−/− mice, and Dr. Domenico Tortorella for providing the NS3 antibody. We also thank David Sachs for assistance with R and Justin Taft for assistance with CellProfiler software. We are grateful to Drs. Dusan Bogunovic and Christopher J. Obara for invaluable evaluation of our manuscript. We thank Dr. Michael Donovan, Olha Fedoryshyn, Anastasiya Dzhun, and Josefa Lopez from the Biorepository and Pathology CoRE at the Mount Sinai Health System. Microscopy and image analysis were performed at the Microscopy CoRE at the Icahn School of Medicine at Mount Sinai. This work was supported by NIAID grants R21AI130299, R21AI129486, R21AI139593, and R21AI127955; NIAID training grant T32AI007647; and a supplement to NIAID grant U19AI118610. The following reagents were obtained through BEI Resources, NIAID, and NIH, as part of the WRCEVA program: Dengue Virus Type 4, PR 06-65-740, NR-49757; Dengue Virus Type 1, BC89/94, NR-3787. Zika virus strain PRVABC59 was obtained through ATCC (VR-1843).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and can be found with this article online at https://doi.org/10.1016/j.immuni.2019.01.005.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Aagaard KM, Lahon A, Suter MA, Arya RP, Seferovic MD, Vogt MB, Hu M, Stossi F, Mancini MA, Harris RA, et al. (2017). Primary Human Placental Trophoblasts are Permissive for Zika Virus (ZIKV) Replication. Sci. Rep 7, 41389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, et al. (2016). Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat. Med 22, 1256–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado LC, Schmid S, Sachs D, Shim JV, Lim JK, and tenOever BR (2015). microRNA Function Is Limited to Cytokine Control in the Acute Response to Virus Infection. Cell Host Microbe 18, 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. (2016). Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536, 48–53. [DOI] [PubMed] [Google Scholar]
- Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, et al. (2017). Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr., Cherry S, Sadovsky Y, and Coyne CB (2016). Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe 19, 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, et al. (2016). Zika Virus Infection in Pregnant Women in Rio de Janeiro.N. Engl. J. Med 375, 2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Parnell LA, Diamond MS, and Mysorekar IU (2017). Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J. Exp. Med 214, 2303–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry J, Arora N, Good CA, Sadovsky Y, and Coyne CB (2017). Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal-fetal interface. Proc. Natl. Acad. Sci. USA 114, 9433–9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, and Lazear HM (2016). Zika virus - reigniting the TORCH. Nat. Rev. Microbiol 14, 707–715. [DOI] [PubMed] [Google Scholar]
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, et al. (2016). Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol 17, 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DM, Van Rompay KK, Coffey LL, Ardeshir A, Keesler RI, Bliss-Moreau E, Grigsby PL, Steinbach RJ, Hirsch AJ, MacAllister RP, et al. (2018). Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat. Med 24, 1104–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, Veas F, Al-Daccak R, Izopet J, and Jabrane-Ferrat N (2016). ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci. Rep 6, 35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, Dejnirattisai W, Cao B, Scheaffer SM, Supasa P, Wongwiwat W, Esakky P, Drury A, Mongkolsapaya J, Moley KH, et al. (2017). Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat. Immunol 18, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Sánchez A, Demmelmair H, Parrilla JJ, Koletzko B, and Larqué E (2011). Mechanisms involved in the selective transfer of long chain polyunsaturated Fatty acids to the fetus. Front. Genet 2, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai UA, Nielsen-Saines K, Moreira ML, de Sequeira PC, Junior JPP, de Araujo Zin A, Cherry J, Gabaglia CR, Gaw SL, Adachi K, et al. (2017). Maternal Zika Virus Disease Severity, Virus Load, Prior Dengue Antibodies, and Their Relationship to Birth Outcomes. Clin. Infect. Dis 65, 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB (1988). Pathogenesis of dengue: challenges to molecular biology. Science 239, 476–481. [DOI] [PubMed] [Google Scholar]
- Halstead SB (2017). Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 35, 6355–6358. [DOI] [PubMed] [Google Scholar]
- Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, Wang X, Lo JO, Liu Z, Kroenke CD, Smith JL, et al. (2018). Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun 9, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen B, Schaub B, Funk AL, Ardillon V, Boullard M, Cabié A, Callier C, Carles G, Cassadou S, Césaire R, et al. (2018). Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N. Engl. J. Med 378, 985–994. [DOI] [PubMed] [Google Scholar]
- Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A, et al. ; US Zika Pregnancy Registry Collaboration (2017). Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA 317, 59–68. [DOI] [PubMed] [Google Scholar]
- Jagger BW, Miner JJ, Cao B, Arora N, Smith AM, Kovacs A, Mysorekar IU, Coyne CB, and Diamond MS (2017). Gestational Stage and IFN-λ Signaling Regulate ZIKV Infection In Utero. Cell Host Microbe 22, 366–376.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, and Harris E (2017). Antibody-dependent enhancement of severe dengue disease in humans. Science 358, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliks SC, Nimmanitya S, Nisalak A, and Burke DS (1988). Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg 38, 411–419. [DOI] [PubMed] [Google Scholar]
- Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, Shi J, and Lok SM (2016). Structure of the thermally stable Zika virus. Nature 533, 425–428. [DOI] [PubMed] [Google Scholar]
- Kourtis AP, Read JS, and Jamieson DJ (2014). Pregnancy and infection.N. Engl. J. Med 371, 1077. [DOI] [PubMed] [Google Scholar]
- Lazear HM, and Diamond MS (2016). Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J. Virol 90, 4864–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano NA, Lozano A, Marini V, Saranz RJ, Blumberg RS, Baker K, Agresta MF, and Ponzio MF (2018). Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and preterm newborns. Am. J. Reprod. Immunol 80, e12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HP, Iglezias SD, Kanamura CT, Keating MK, Hale G, Silva-Flannery L, Muehlenbachs A, et al. (2016). Pathology of congenital Zika syndrome in Brazil: a case series. Lancet 388, 898–904. [DOI] [PubMed] [Google Scholar]
- Martinot AJ, Abbink P, Afacan O, Prohl AK, Bronson R, Hecht JL, Borducchi EN, Larocca RA, Peterson RL, Rinaldi W, et al. (2018). Fetal Neuropathology in Zika Virus-Infected Pregnant Female Rhesus Monkeys. Cell 173, 1111–1122.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken MK, Gromowski GD, Friberg HL, Lin X, Abbink P, De La Barrera R, Eckles KH, Garver LS, Boyd M, Jetton D, et al. (2017). Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog. 13, e1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. (2016). Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 165, 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodušek V, et al. (2016). Zika Virus Associated with Microcephaly. N. Engl. J. Med 374, 951–958. [DOI] [PubMed] [Google Scholar]
- Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, et al. (2017). Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 13, e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha Ld., Zanluca C, Azevedo ML, Luz KG, and Santos CN (2016). Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem. Inst. Oswaldo Cruz 111, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco O, Beltrán M, Nelson CA, Valencia D, Tolosa N, Farr SL, Padilla AV, Tong VT, Cuevas EL, Espinosa-Bode A, et al. (2016). Zika Virus Disease in Colombia - Preliminary Report. N. Engl. J. Med Published online June 15, 2016. 10.1056/NEJMoa1604037. [DOI] [PubMed] [Google Scholar]
- Pantoja P, Pérez-Guzmán EX, Rodríguez IV, White LJ, González O, Serrano C, Giavedoni L, Hodara V, Cruz L, Arana T, et al. (2017). Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat. Commun 8, 15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LM, Carlin ER, Jenkins MM, Tan AL, Barcellona CM, Nicholson CO, Michael SF, and Isern S (2016). Dengue virus antibodies enhance Zika virus infection. Clin. Transl. Immunology 5, e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DJ, Smith AM, Arora N, Diamond MS, Coyne CB, and Miner JJ (2018). Zika virus-related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci. Transl. Med 10, eaao7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, et al. (2016). Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 113, 7852–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wramert J, Rimawi BH, Pulendran B, et al. (2016). Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 20, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, Ellington SR, Evert N, Reagan-Steiner S, Oduyebo T, et al. ; U.S. Zika Pregnancy Registry Collaboration (2017). Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure - U.S. Zika Pregnancy Registry, 2016. MMWR Morb. Mortal. Wkly. Rep 66, 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Bozzacco L, Keeffe JR, Khouri R, Olsen PC, Gazumyan A, Schaefer-Babajew D, Avila-Rios S, Nogueira L, Patel R, et al. (2017). Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico. Cell 169, 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub B, Vouga M, Najioullah F, Gueneret M, Monthieux A, Harte C, Muller F, Jolivet E, Adenet C, Dreux S, et al. (2017). Analysis of blood from Zika virus-infected fetuses: a prospective case series. Lancet Infect. Dis 17, 520–527. [DOI] [PubMed] [Google Scholar]
- Schwarz MC, Sourisseau M, Espino MM, Gray ES, Chambers MT, Tortorella D, and Evans MJ (2016). Rescue of the 1947 Zika Virus Prototype Strain with a Cytomegalovirus Promoter-Driven cDNA Clone. MSphere 1, e00246–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seferovic M, Sánchez-San Martín C, Tardif SD, Rutherford J, Castro ECC, Li T, Hodara VL, Parodi LM, Giavedoni L, Layne-Colon D, et al. (2018). Experimental Zika Virus Infection in the Pregnant Common Marmoset Induces Spontaneous Fetal Loss and Neurodevelopmental Abnormalities. Sci. Rep 8, 6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, Schust DJ, Franz AW, Sadovsky Y, Ezashi T, and Roberts RM (2017). Vulnerability of primitive human placental trophoblast to Zika virus. Proc. Natl. Acad. Sci. USA 114, E1587–E1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister NE (1998). Human placental Fc receptors and the trapping of immune complexes. Vaccine 16, 1451–1455. [DOI] [PubMed] [Google Scholar]
- Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, and Kuhn RJ (2016). The 3.8 Å resolution cryo-EM structure of Zika virus. Science 352, 467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. (2016). Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353, 823–826. [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Zydek M, Fang-Hoover J, Larocque N, Tsuge M, Gormley M, Kauvar LM, and Pereira L (2015). Human cytomegalovirus infection interferes with the maintenance and differentiation of trophoblast progenitor cells of the human placenta. J. Virol 89, 5134–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, and Pereira L (2016). Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 20, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzian ACB, Schanoski AS, Mota MTO, da Silva RA, Estofolete CF, Colombo TE, Rahal P, Hanley KA, Vasilakis N, Kalil J, and Nogueira ML (2017). Viral Load and Cytokine Response Profile Does Not Support Antibody-Dependent Enhancement in Dengue-Primed Zika Virus-Infected Patients. Clin. Infect. Dis 65, 1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomashek KM, Rivera A, Torres-Velasquez B, Hunsperger EA, Munoz-Jordan JL, Sharp TM, Rivera I, Sanabria D, Blau DM, Galloway R, et al. (2016). Enhanced Surveillance for Fatal Dengue-Like Acute Febrile Illness in Puerto Rico, 2010–2012. PLoS Negl. Trop. Dis 10, e0005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg JP, Westerbeek EA, van der Klis FR, Berbers GA, and van Elburg RM (2011). Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum. Dev 87, 67–72. [DOI] [PubMed] [Google Scholar]
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, and Nisalak A (2000). Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis 181, 2–9. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, and Vasilakis N (2016). Zika virus: History, emergence, biology, and prospects for control. Antiviral Res 130, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y, Oiknine-Djian E, Vorontsov OM, Haimov-Kochman R, Zakay-Rones Z, Meir K, Shveiky D, Elgavish S, Nevo Y, Roseman M, et al. (2017). Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface. J. Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Elong Ngono A, Regla-Nava JA, Kim K, Gorman MJ, Diamond MS, and Shresta S (2017). Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun 8, 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis E, and Hensley SE (2017). Characterization of Zika virus binding and enhancement potential of a large panel of flavivirus murine monoclonal antibodies. Virology 508, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L, Perez-Garcia V, Kieckbusch J, Wang X, DeMayo F, Colucci F, and Hemberger M (2017). Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat. Commun 8, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Jurado KA, Arora N, Millet, Rakib T, Milano KM, Hastings AK, Fikrig E, Kong Y, Horvath TL, et al. (2018). Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol 3, eaao1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yougbaré I, Tai WS, Zdravic D, Oswald BE, Lang S, Zhu G, Leong-Poi H, Qu D, Yu L, Dunk C, et al. (2017). Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat. Commun 8, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, Ji X, Xu YP, Li G, Li C, et al. (2017). A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 358, 933–936. [DOI] [PubMed] [Google Scholar]
- Zimmerman MG, Quicke KM, O’Neal JT, Arora N, Machiah D, Priyamvada L, Kauffman RC, Register E, Adekunle O, Swieboda D, et al. (2018). Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 24, 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.