Abstract
Introduction: Vitamin A deficiency (VAD) is an underreported micronutrient deficiency after bariatric surgery (BS).
Objectives: The goal of this study was to characterize VAD prevalence in patients undergoing malabsorptive and restrictive procedures up to 2 years postoperatively.
Methods: Primary sleeve gastrectomy (SG; n = 322) and gastric bypass (GB; n = 249) patients were reviewed. Levels for overall VAD (oVAD; retinol <39 mcg/dL) and moderate VAD (mVAD; retinol <30 mcg/dL) were reported preoperatively and 6, 12, and 24 months postoperatively. Differences in demographic, surgical, and postoperative data were tested between these groups.
Settings: Single-center academic institution.
Results: Serum retinol levels were documented for 56%, 74%, 61%, and 37% of patients for listed time points. Baseline retinol inversely correlated to preop body mass index (BMI) (R = −0.15, P = .007). Both oVAD and mVAD peaked 6 months postoperatively (33% vs. 15%, P < .005; 12% vs. 4%, P = .0004, respectively). oVAD remained elevated at 24 months (22% vs. 15%, P = .03). Compared to SG, oVAD was higher following GB at 6 months (39% vs. 28%, P = .001) and 12 months (26% vs. 17%, P = .04), and mVAD was greater with GB at 6 months (18% vs. 6%, P < .0005). African American patients had higher oVAD/mVAD preoperatively (26% vs. 13%, P = .02; 13% vs. 3%, P = .001, respectively) and at 6 months (19% vs. 10%, P = .04). Prior mild VAD (retinol 1.05–1.35 μM) was significantly associated with mVAD up to 12 months postoperatively.
Conclusions: Although higher following LRYGB, VAD is prevalent following both malabsorptive and restrictive procedures. Preoperative serum retinol is inversely correlated to increasing BMI, and African American race and mild VAD are associated with moderate VAD.
Keywords: bariatric surgery, vitamin A, micronutrient deficiency after bariatric surgery
Introduction
Disrupted micronutrient stores after bariatric surgery (BS) are a well-documented complication, of which vitamin B12, calcium, iron, and vitamin D deficiencies are the most common.1,2 Stores of fat-soluble vitamins (A, D, E, and K) are also at risk of decreasing after malabsorptive procedures, such as laparoscopic Roux-en-Y gastric bypass (LRNYGB), as the proximal intestine needed for digestion and absorption is bypassed.3 Given these concerns, not only are all patients placed on a multivitamin postoperatively,3 but routine evaluation of repletion of vitamin levels is recommended.4 However, since comprehensive micronutrient panels can be very costly, most providers pick specific micronutrients to follow based on their clinical relevance, thereby resulting in variability between bariatric programs vis-à-vis evaluation of particular vitamins. Arguably, most programs monitor and treat calcium and vitamin D, due to the risk of decreased bone density postprocedure,4 and B12 and iron levels to avoid the risk of anemia and peripheral neuropathy. Conversely, vitamin A, which plays a significant role in maintaining cell growth, immunity, and vision, is not routinely evaluated by many centers. Clinically, hypovitaminosis A is typically recognized for its impact on ocular health, with progression to night blindness and xerophthalmia being main concerns.5 However, retinoic acid, the active form of vitamin A, plays a significant role in stabilizing and modulating local inflammation and oxidative stress,6 which is of particular importance to patients with obesity.
The link between obesity and inflammation has garnered significant interest recently, with numerous studies elegantly demonstrating the impact of key inflammatory changes in the pathogenesis of obesity-related comorbidities.7–10 Specifically, regulatory T cells (Tregs), which are anti-inflammatory, are reduced in murine and human subjects with obesity compared to lean counterparts, and decreases in adipose tissue Treg abundance are correlated to increasing insulin resistance.7,8,11,12 Retinoic acid functions to promote and stabilize Tregs, while retinoic acid receptor antagonism significantly impedes Treg stimulation.13–16 In fact, subcutaneous injection of retinoic acid in mice has been shown to improve metabolism and glycemic control in mice placed on high fat diet.17 Taken together, these studies suggest an immunomodulatory and metabolic role for vitamin A, which may be of critical importance to patients both before and after BS.
In light of the preceding, the goal of this study was to (1) evaluate the prevalence of vitamin A deficiency (VAD) preoperatively and within 2 years after BS and (2) determine factors associated with VAD at each time point.
Methods
Data collection
All patients who underwent primary LRNYGB and laparoscopic sleeve gastrectomy (LSG) from July 2014 to June 2016 at a single academic institution were retrospectively reviewed. Data were obtained from the patient's electronic medical record. Demographic data (age, gender, race), surgical data (LRNYGB or LSG), and preoperative comorbidities (type 2 diabetes [T2D], hypertension, hyperlipidemia, obstructive sleep apnea) were obtained. Weight measures reported include preoperative excess body weight (EBW), baseline body mass index (BMI), as well as postoperative percent EBW lost (%EBWL) at 6, 12, and 24 months after BS.
Formulas for weight calculations
EBW was calculated using [preoperative weight (kg)]—[ideal body weight in kg]. The equations for ideal body weight and %EBWL at each time point were obtained via the formulas below:

Measurement of vitamin A
Serum retinol levels were obtained at the time of the initial medical evaluation and at 6, 12, and 24 months after surgery. Vitamin A levels were classified based on our institution's laboratory values, which defined normal plasma retinol levels ranging from 39 to 98 mcg/dL. Overall VAD was defined as plasma retinol <39 mcg/dL (retinol <1.35 μM); mild VAD was defined as 31–38 mcg/dL (1.05 μM < serum retinol <1.35 μM), while moderate deficiency was defined as <30 mcg/dL (serum retinol <1.05 μM). As an index for nutrition and protein stores, albumin levels were also collected at all time points and included in the analyses.
Management of VAD
Currently, patients who enter the program with serum retinol levels <30 mcg/dL (<1.05 μM) are supplemented with 10,000 international units daily of retinyl esters for 2 months. However, postoperatively, patients with low serum retinol levels are advised to continue multivitamin supplementation, which if taken appropriately, delivers ∼5000 IU of retinyl ester. We currently do not screen for symptoms of VAD.
Operative technique
LSG was performed in a standard 5-port manner by dividing vessels along the greater curvature of the stomach, ∼6 cm proximal to the pylorus. The gastric sleeve was then formed over a 36F or 40F bougie using a laparoscopic stapling device (ENDO GIA™ Ultra Universal Staplers, Medtronic, MN). LRNYGB was accomplished in the following manner: (1) Creation of a jejunojejunostomy through anastomosing a 60–80 cm biliopancreatic limb ∼150 cm distal along the Roux limb, (2) creation of a 5 cm gastric pouch, and (3) formation of an anticolic, antigastric gastrojejunostomy using a 25 mm EEA circular stapler (Autosuture™ EEATM 25 mm single-use stapler with 3.5 mm or 4.8 mm staples, Medtronic, MN). Postoperatively, all patients were managed using standardized order sets specific for LSG and LRNYGB procedures.
Study endpoints
The primary endpoint of this study was to determine the prevalence of VAD preoperatively and after BS. Secondary endpoints included determining patient characteristics that may be associated VAD prevalence in bariatric patients.
Data analysis
Stata 15™ (StataCorp, College Station, TX) was utilized to carry out all statistical analyses reported in this study. Differences in linear variables between groups were determined using Student's t test or Mann–Whitney U, as appropriate, while proportions were compared using Fisher's exact or Chi squared tests. Spearman's correlations were used to test strength of the relationship between paired ordinal data for nonnormally distributed data. A P-value of <.05 was considered statistically significant.
Results
Overall cohort characteristics and availability of data
Characterization of the cohort is detailed in Table 1. A total of 571 patients consecutively admitted for primary BS from 2014 to 2016 were reviewed. The mean age was 44.8 ± 10.8 years, and the majority of patients were female (79.2%) and Caucasian (80.4%). The average body-mass index (BMI) was 49.5 ± 9.3 kg/m2, which corresponded to a baseline EBW of 79.9 ± 26.4 kg. Over half of the patients underwent LSG (56.4%), while the remaining underwent gastric bypass (43.6%). Overall mean percent excess body weight loss (%EBWL) outcomes were 44.9 ± 17.1%, 53.7 ± 18.1%, 52.3 ± 21.2%, at 6, 12, and 24 months, respectively. Due to inconsistencies in outside hospital laboratory orders, plasma retinol values were not available for the full cohort. At baseline, only 55.7% had documented plasma retinol values, while postoperatively, the availability of data were 74.2%, 60.8%, and 36.7% at 6, 12, and 24 months.
Table 1.
Cohort Characteristics and Availability of Data
| Characteristics | Cohort (n = 571) |
|---|---|
| Age (years): mean (SD) | 44.8 (10.8) |
| Sex (Female): % (n) | 79.2 (452) |
| Race | |
| African American: % (n) | 17.9 (102) |
| Hispanic: % (n) | 1.8 (10) |
| Caucasian: % (n) | 80.4 (459) |
| Baseline (Intake evaluation) BMI kg/m2: mean (SD) | 49.5 (9.3) |
| Baseline (Intake evaluation) Excess body weight (kg): mean (SD) | 79.9 (26.4) |
| Surgery type | |
| LSG: % (n) | 56.4 (322) |
| LRYGB: % (n) | 43.6 (249) |
| % Excess body weight lost | |
| 6 months: mean (SD) | 44.9 (17.1) |
| 12 months: mean (SD) | 53.7 (18.1) |
| 24 months mean (SD) | 52.3 (21.2) |
| % Total weight loss | |
| 6 months: mean (SD) | 24.6 (6.8) |
| 12 months: mean (SD) | 29.9 (9.7) |
| 24 months mean (SD) | 29.4 (12.0) |
| Plasma retinol availability | |
| Baseline: % (n) | 55.7 (318) |
| 6 months: % (n) | 74.2 (423) |
| 12 months: % (n) | 60.8 (347) |
| 24 months: % (n) | 36.7 (209) |
BMI, body mass index; LRYGB, laparoscopic Roux-en-Y gastric bypass; LSG, laparoscopic sleeve gastrectomy; SD, standard deviation.
Prevalence of overall and moderate VAD preoperatively and after BS
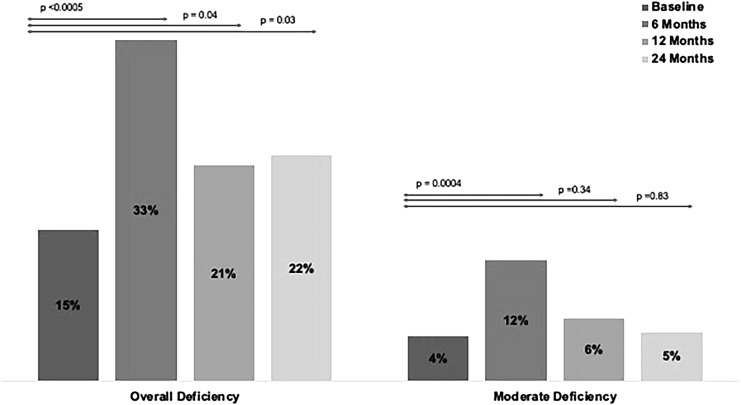
The prevalence of overall and moderate VAD preoperatively and for each time point after surgery are summarized in Figure 1. Overall VAD (plasma retinol <39 mcg/dL) was present in 15.0% of patients preoperatively and increased significantly 6 months postoperatively (33.3% vs. 14.8%, P < .0005). At 12 and 24 months prevalence of VAD remained elevated, although to a lesser extent than at 6 months, but remained significantly higher than that observed preoperatively (21.0% vs. 14.8%, P = .04, and 22.0% vs. 14.8%, P = .03; for 12 and 24 months, respectively). The prevalence of moderate VAD (plasma retinol ≤30 mcg/dL) was 4.4% preoperatively and increased significantly to 11.8% at 6 months postoperatively (11.8% vs. 4.4%, P = .0004). By 12 and 24 months postsurgery, the degree of deficiency was comparable to preoperative levels.
FIG. 1.
Overall and Moderate Vitamin A Deficiency by Time Point for the Overall Cohort. Overall Vitamin A Deficiency: plasma retinol <39 mcg/dL. Moderate deficiency: plasma retinol ≤30 mcg/dL.
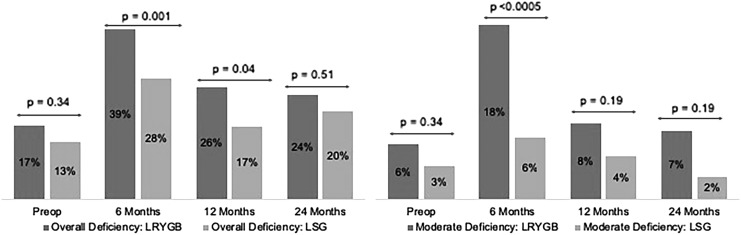
Stratification of VAD by surgery type is detailed in Figure 2. Preoperatively, there was no significant difference in rates of overall and moderate VAD for patients who elected to undergo either gastric bypass or sleeve gastrectomy. However, post-operatively the prevalence of overall VAD was significantly higher in gastric bypass patients at 6 and 12 months compared to sleeve gastrectomy patients (39.3% vs. 27.9%, P = .001 and 25.8% vs. 16.7%, P = .04; for 6 and 12 months, respectively) but was comparable at 2 years (24.0% vs. 20.2%, P = .51). While prevalence of moderate VAD was significantly higher in gastric bypass compared to sleeve gastrectomy patients at 6 months (17.9% vs. 6.3%, P < .0005), there was no difference at later time points.
FIG. 2.
Pre- and Postsurgical Vitamin A Deficiency (Left: Overall and Right: Moderate Deficiency) by Postoperative Time Point and Surgery Type. LRYGB, laparoscopic Roux-en-Y gastric bypass; LSG, laparoscopic sleeve gastrectomy.
Characteristics associated with preoperative overall and moderate VAD
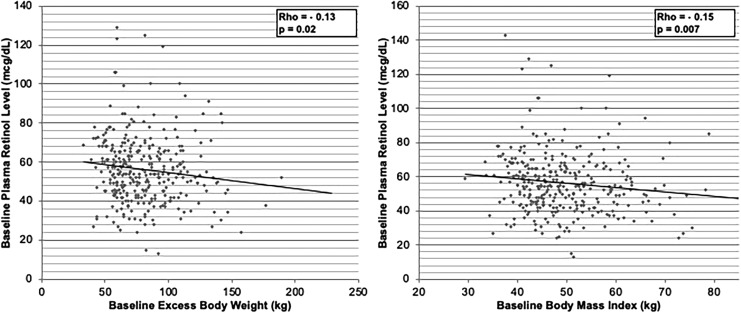
Preoperatively, compared to those who were not deficient, a higher proportion of African American patients were either overall or moderately vitamin A deficient (29.8% vs. 14.8%, P = .04; 50.0% vs. 15.4%, P = .009; respectively). In addition, both overall and moderate VAD trended toward being associated with higher baseline BMI (51.5 ± 10.3 kg/m2 vs. 49.0 ± 8.6 kg/m2, P = .07; 53.5 ± 12.0 kg/m2 vs. 49.2 ± 8.7 kg/m2, P = .08; respectively). However, only overall VAD trended toward an association with a higher baseline EBW (87.0 ± 30.8 kg vs. 79.3 ± 24.8 kg, P = .06). These results are summarized in Table 2. Examination of the strength of the association between preoperative plasma retinol levels and baseline weight demonstrated a similar inverse correlation of lower plasma retinol levels and increasing baseline EBW (Rho = −0.13, P = .02) and BMI (Rho = −0.15, P = .0007). These results are depicted in Figure 3.
Table 2.
Characteristics Associated with Preoperative Overall and Moderate (Top, <39 mcg/dL) and Moderate (Bottom, <30 mcg/dL) Vitamin A Deficiency
| Preop overall VAD (n = 48) | Control (n = 271) | P | |
|---|---|---|---|
| Age (years): mean (SD) | 42.4 (10.5) | 44.8 (10.9) | .15 |
| Sex (Female): % (n) | 85.1 (40) | 75.7 (205) | .16 |
| Race | |||
| African American: % (n) | 29.8 (14) | 14.8 (40) | .04 |
| Hispanic: % (n) | 0 | 3.0 (8) | |
| Caucasian: % (n) | 70.2 (33) | 82.2 (223) | |
| Baseline body mass index (kg/m2) | 51.5 (10.3) | 49.0 (8.6) | .07 |
| Baseline excess body weight (kg): mean (SD) | 87.0 (30.8) | 79.3 (24.8) | .06 |
| Preop moderate VAD (n = 14) | Control (n = 304) | P | |
|---|---|---|---|
| Age (years): mean (SD) | 44.3 (9.5) | 44.5 (10.9) | .95 |
| Sex (Female): % (n) | 92.9 (13) | 76.3 (232) | .20 |
| Race | |||
| African American: % (n) | 50.0 (7) | 15.4 (47) | .009 |
| Hispanic: % (n) | 0 | 2.6 (8) | |
| Caucasian: % (n) | 50.0 (7) | 82.0 (249) | |
| Baseline body mass index (kg/m2) | 53.5 (12.0) | 49.2 (8.7) | .08 |
| Baseline excess body weight (kg): mean (SD) | 88.3 (3.8) | 80.1 (25.5) | .24 |
SD, standard deviation; VAD, vitamin A deficiency.
FIG. 3.
Preoperative Vitamin A Levels are Inversely Correlated to Increasing Baseline Excess Body Weight (Left) and Baseline Body-Mass-Index (Right). Spearman's correlations were utilized in these analyses.
Characteristics associated with overall VAD postoperatively
Demographically, younger patient age was significantly associated with overall VAD 6 months after surgery (42.2 ± 10.8 years vs. 46.2 ± 10.9 years, P = .02) but not at later time points. In addition, female patients were significantly more likely to demonstrate deficiency overall at 12 months (90.4% vs. 77.4%, P = .01). Clinically, overall VAD preoperatively was significantly associated with overall VAD at every postoperative time point (30.4% vs. 8.6%, P < .0005; 36.4% vs. 12.5%, P < .0005; 35.7% vs. 7.1%, P < .005; for 6, 12, and 24 months, respectively). In addition, overall VAD at 12 months was associated with overall VAD at 6 months (74.2% vs. 23%, P < .0005), and overall VAD at 24 months was associated with overall VAD at both 6 (60.5% vs. 25%, P < .0005) and 12 months (51.2% vs. 14.1%, P < .0005). While there was no correlation with EBW and VAD at any postoperative time point, %EBWL was positively associated with increasing VAD at 12 and 24 months postoperatively for patients who underwent LRYGB (62.7% ± 17.5% vs. 56.9% ± 16.3%, P = .05, and 67.7% ± 17.4% vs. 58.3% ± 18.1%, P = .03; for 12 and 24 months, respectively). These results are summarized in Table 3.
Table 3.
Characteristics Associated with Overall Vitamin A Deficiency (<39 mcg/dL)
| Postoperative time point |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 Months |
12 Months |
24 Months |
|||||||
| VAD (n = 141) | Control (n = 282) | P | VAD (n = 73) | Control (n = 274) | P | VAD (n = 46) | Control (n = 163) | P | |
| Age (years): mean (SD) | 43.0 (10.5) | 46.3 (10.9) | .003 | 43.8 (10.6) | 45.6 (11.1) | .20 | 43.5 (10.0) | 45.1 (11.2) | .38 |
| Sex (Female): % (n) | 83.0 (117) | 75.9 (214) | .10 | 90.4 (66) | 77.4 (212) | .01 | 82.6 (38) | 80.4 (131) | .73 |
| Racea | |||||||||
| Caucasian: % (n) | 78.8 (108) | 84.2 (234) | .18 | 76.7 (56) | 84.3 (231) | .09 | 78.3 (36) | 79.2 (126) | .89 |
| African American: % (n) | 21.2 (29) | 15.8 (44) | 21.9 (16) | 13.9 (38) | 21.7 (10) | 20.8 (33) | |||
| Vitamin A deficient | |||||||||
| Preoperative: % (n) | 30.4 (24) | 8.6 (14) | <.0005 | 36.4 (16) | 12.5 (19) | <.0005 | 35.7 (10) | 7.1 (6) | <.0005 |
| 6M: % (n) | — | — | — | 74.2 (49) | 23.0 (53) | <.0005 | 60.5 (23) | 25.0 (34) | <.0005 |
| 12M: % (n) | — | — | — | — | — | — | 51.2 (21) | 14.1 (19) | <.0005 |
| %EBWL LRYGB | |||||||||
| 6M: mean (SD) | 49.1 (18.8) | 45.7 (11.1) | .14 | — | — | — | — | — | — |
| 12M: mean (SD) | — | — | — | 62.7 (17.5) | 56.9 (16.3) | 0.05 | — | — | — |
| 24M: mean (SD) | — | — | — | — | — | — | 67.7 (17.4) | 58.3 (18.1) | .03 |
| %EBWL LSG | |||||||||
| 6M: mean (SD) | 44.6 (11.9) | 43.3 (21.8) | .66 | — | — | — | — | — | — |
| 12M: mean (SD) | — | — | — | 55.0 (14.0) | 50.1 (17.6) | .16 | — | — | — |
| 24M: mean (SD) | — | — | — | — | — | — | 48.1 (20.0) | 48.9 (21.6) | .88 |
| Excess body weight (kg) | |||||||||
| 6M: p50 (IQR) | 45.1 (29.6–60.4) | 42.0 (31.7–57.0) | 0.86 | — | — | — | — | — | — |
| 12M: p50 (IQR) | — | — | — | 35.6 (21.5–49.1) | 35.2 (23.8–52.2) | .38 | — | — | — |
| 24 Months: p50 (IQR) | — | — | — | — | — | — | 28.4 (21.5–50.3) | 33.6 (21.7–50.8) | .43 |
Hispanic removed due to small n.
p50, median; IQR, interquartile range; LRYGB, laparoscopic Roux-en-Y gastric bypass; LSG, laparoscopic sleeve gastrectomy; SD, standard deviation; VAD, vitamin A deficiency.
Characteristics associated with moderate VAD postoperatively
Six months postoperatively, patients with moderate VAD were significantly more likely to be younger (42.2 ± 10.8 years vs. 46.2 ± 10.9 years, P = .02), female (92% vs. 76.4%, P = .01) and African American (28% vs. 16.2%, P = .04). Clinically, moderate VAD at each time point was significantly associated with moderate VAD preoperatively up to 12 months (15.4% vs. 4.2%, P = .04, and 27.3% vs. 3.8%, P = .01; for 6 and 12 months, respectively). In addition, as seen with overall VAD (Table 3), moderate VAD (Table 4) at 12 months was associated with moderate VAD at 6 months (68.4% vs. 9.4%, P < .0005), and moderate VAD at 24 months was associated with moderate VAD at both 6 (71.4% vs. 11.4%, P = .001) and 12 months (44.4% vs. 4.2%, P = .001). Again, while there was no association between moderate VAD and EBW at each postoperative time point, %EBWL at 24 months post-LRYGB was positively associated with moderate VAD (74.6% vs. 59.5%, P = .03). These results are summarized in Table 4.
Table 4.
Characteristics Associated with Moderate Vitamin A Deficiency (<30 mcg/dL) Compared to Normal Levels (≥39 mcg/dL)
| Postoperative time point |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 Months |
12 Months |
24 Months |
|||||||
| VAD (n = 50) | Control (n = 296) | P | VAD (n = 21) | Control (n = 285) | P | VAD (n = 10) | Control (n = 170) | P | |
| Age (years): mean (SD) | 42.2 (10.8) | 46.2 (10.9) | .02 | 41.3 (10.8) | 45.9 (11.2) | .07 | 45.6 (9.9) | 45.1 (11.1) | .90 |
| Sex (Female): % (n) | 92.0 (46) | 76.4 (226) | .01 | 85.7 (18) | 77.5 (221) | .58 | 80.0 (8) | 80.9 (161) | 1.0 |
| Racea | |||||||||
| Caucasian: % (n) | 72.0 (36) | 83.8 (306) | .04 | 76.2 (16) | 84.7 (271) | .30 | 70.0 (7) | 79.5 (155) | .44 |
| African American: % (n) | 28.0 (14) | 16.2 (59) | 23.8 (5) | 15.3 (49) | 30.0 (3) | 20.5 (40) | |||
| Vitamin A Deficient | |||||||||
| Preoperative: % (n) | 15.4 (4) | 4.2 (9) | .04 | 27.3 (3) | 3.8 (7) | .01 | 20.0 (1) | 2.8 (3) | .17 |
| 6 Months: % (n) | — | — | — | 68.4 (13) | 9.4 (26) | <.0005 | 71.4 (5) | 11.4 (19) | .001 |
| 12 Months: % (n) | — | — | — | — | — | — | 44.4 (4) | 4.2 (7) | .001 |
| %EBWL for LRYGB | |||||||||
| 6M: mean (SD) | 48.6 (14.0) | 47.0 (15.0) | .52 | — | — | — | — | — | — |
| 12M: mean (SD) | — | — | — | 57.9 (16.5) | 64.5 (19.3) | .19 | — | — | — |
| 24M: mean (SD) | — | — | — | — | — | — | 74.6 (18.0) | 59.5 (18.0) | .03 |
| %EBWL for LSG | |||||||||
| 6M: mean (SD) | 44.8 (14.4) | 43.6 (20.0) | .83 | — | — | — | — | — | — |
| 12M: mean (SD) | — | — | — | 50.4 (10.2) | 51.0 (17.4) | .93 | — | — | — |
| 24M: mean (SD) | — | — | — | — | — | — | 44.3 (0.2) | 48.9 (21.5) | .72 |
| Excess body weight (kg) | |||||||||
| 6M: p50 (IQR) | 47.0 (30.1–60.8) | 41.7 (31.2–56.2) | .62 | — | — | — | — | — | — |
| 12M: p50 (IQR) | — | — | — | 37.1 (28.5–58.3) | 35.8 (23.8–52.2) | .36 | — | — | — |
| 24M: p50 (IQR) | — | — | — | — | — | — | 30.9 (8.2–51.5) | 33.7 (21.7–50.8) | .70 |
Hispanic removed due to small n.
p50, median; IQR, interquartile range; LSG, laparoscopic sleeve gastrectomy; LRYGB, laparoscopic Roux-en-Y gastric bypass; SD, standard deviation; VAD, vitamin A deficiency.
Association between prior overall and mild VAD on incidence of moderate VAD
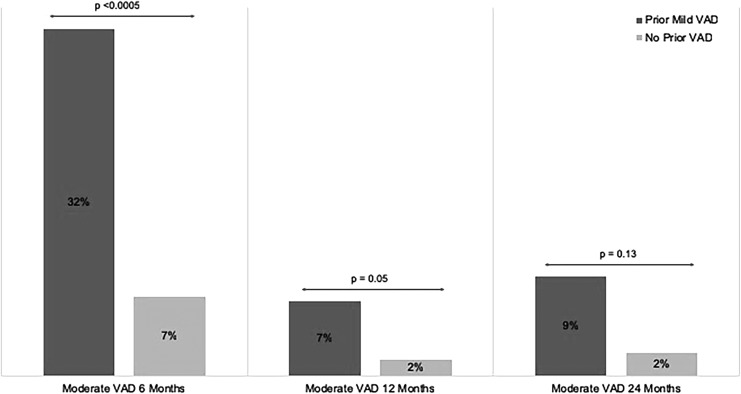
As detailed above, overall VAD preoperatively was significantly associated with overall postoperative VAD at 6 12, and 24 months (Table 3). Likewise, preoperative moderate VAD status was significantly associated with moderate VAD up to one year after BS (Table 4) but not at two years. To determine whether there was an association between mild VAD (plasma retinol 31–38 mcg/dL) and the development of moderate VAD, patients were stratified as either having mild VAD or no VAD preoperatively, and at 6 and 12 months postoperatively. These groups were then examined at the subsequent time point to determine the prevalence in development of moderate VAD. (Fig. 4). Compared to those with normal preoperative vitamin A levels, patients with mild VAD preoperatively demonstrated an over four-fold increase in the incidence of moderate VAD 6 months after BS (32% vs. 7%, P < .005). Patients with mild VAD at 6 months postoperatively were 3.5× more likely to have moderate VAD at 12 months (7% vs. 2%, P = .05). Similarly, mild VAD at 12 months was associated with an over four-fold increase in the incidence of moderate VAD at 2 years postoperatively, although this did not reach statistical significance (9% vs. 2%, P = .13).
FIG. 4.
Association Between Prior Mild Deficiency (plasma retinol 30–39 mcg/dL) and Future Development of Moderate Vitamin Deficiency (plasma retinol <30 mcg/dL) at Postoperative Time Points (6, 12, and 24 months).
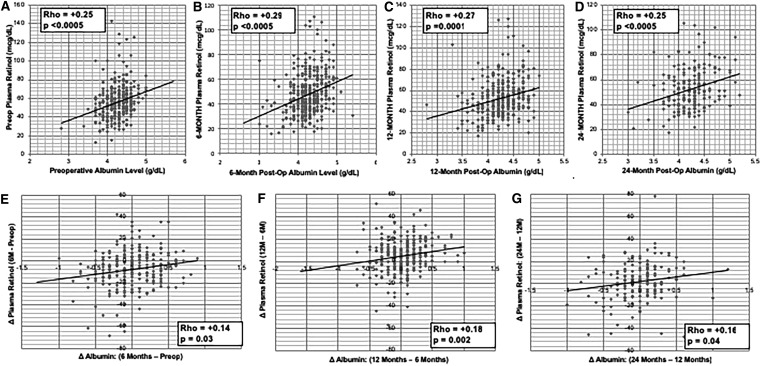
Plasma retinol levels are positively correlated with albumin levels preoperatively and after BS
Albumin was used as a surrogate for overall nutritional status, and its correlation with plasma retinol levels was examined. Plasma retinol values (Fig. 5A–G) were significantly and positively correlated preoperatively (R = 0.25, P < .0005), and after BS at 6 months (R = 0.29, P < .0005), 12 months (R = 0.27, P = .0001), and 24 months (R = 0.25, P < .0005). In addition, postoperative changes in plasma retinol values were positively correlated with changes in albumin between time points, including (1) 6 months–preop, (2) 12 months–6 months, and (3) 24 months–12 months (Fig. 5E–G).
FIG. 5.
Postoperative Plasma Retinol and Albumin Levels. (A–D) Correlations between Vitamin A Levels and Albumin Levels Preoperatively, and at 6, 12, and 24 months Postbariatric Surgery. (E–G) Correlations between Changes in Plasma Retinol and Changes in Albumin between Postsurgical Time Points. Spearman's Correlations were utilized for these analyses. Units of albumin concentration. Plasma retinol units (mcg/dL). Plasma Albumin units (g/dL). postop, postoperative; preop, preoperative.
Discussion
While micronutrient deficiencies are a known complication after BS, there is no established consensus regarding which nutrients should be followed pre- or postoperatively. In fact, routine comprehensive micronutrient panels are controversial, as they are costly, and the clinical relevance of these deficiencies and their treatment algorithms are not well-established. Some nutrients, such as vitamin D, calcium, iron, and B12, are uniformly followed after surgery, while others, such as vitamin A, are subject to provider preference. Given the clinically relevant roles of vitamin A and the paucity of data on VAD prevalence after gastric bypass and sleeve gastrectomy, the primary objective of this study was to determine the prevalence of overall and moderate VAD pre- and postoperatively in a large cohort of BS patients with two-year follow-up. Secondary goals included identifying characteristics associated with these deficiency categories, and evaluating how mild VAD (plasma retinol 30–39 mcg/dL), which is not routinely managed at our institution, was associated with development of moderate VAD.
To the best of our knowledge, this is the first study, with a large cohort of patients, examining the association of both malabsorptive and restrictive bariatric procedures on vitamin A levels with two-year follow-up. Although we were surprised by the high prevalence of overall VAD preoperatively, prior studies have reported similarly high rates, ranging from 12.5% to 14.0%, and also support our finding that low vitamin A levels correlate with higher baseline weight.6,18 Reasons for this phenomenon are unclear but may include altered vitamin A physiology in obesity, both in terms of hepatic and adipocyte storage, or inadequate intake of beta-carotene or other carotenoids, the primary sources of dietary vitamin A.19
While studies on VAD after BS are sparse, our post-operative data are consistent with one other study reporting rates of deficiency as high as 34% 6 months after gastric bypass.20 Postoperatively, compared to overall VAD, rates of moderate VAD were much lower, but did follow a similar distribution, peaking at 6 months with a near tripling in the prevalence compared to the preoperative time point (11.8% vs. 4.4%). This suggests that a significant portion of patients are falling into the mild to moderate deficiency zone. Importantly, we showed that prior mild VAD was significantly associated with an over three- to four-fold increase in the incidence of moderate VAD at later time points (6 and 12 months postoperatively), which highlights a gap in our current supplementation guidelines.
Compared to sleeve gastrectomy patients, the gastric bypass cohort was significantly more likely to experience overall VAD at 6 and 12 months postoperatively, but was equally as likely to be deficient at 2 years (24.0% vs. 22.0%). Likewise, moderate VAD was associated with gastric bypass at 6 months but not at one or two years postoperatively, although the significance of these results is unclear given the small sample size. Despite higher rates in gastric bypass patients, overall VAD was prevalent in sleeve patients, accounting for 17%–28% of the cohort at any given postoperative time point. Similarly, Caron et al. reported peaks in VAD for LSG patients as early as 3 months after surgery.21 These results suggest that fat-soluble vitamin deficiencies are not exclusively found after malabsorptive bariatric procedures, such as the Roux-en-Y gastric bypass, although they may be less prevalent and severe after restrictive procedures, such as the sleeve gastrectomy. We additionally uncovered that African American patients demonstrated higher rates of overall and moderate VAD preoperatively and higher prevalence of moderate VAD 6 months after surgery. Compared to their Caucasian counterparts, African American patients were three times as likely to be moderately VAD at 6 months after LSG (12.5% vs. 4.8%, P = .055) and twice as likely after LRYGB (32.0% vs. 16.1%, P = .053). Given the higher risk of postoperative VAD, African American patients undergoing BS may require more aggressive screening and supplementation in the pre- and postoperative periods.
We hypothesized that malnutrition may play a role in VAD seen after BS. To this point, we found that changes in vitamin A levels were positively correlated with changes in albumin, at each postoperative time point, such that lower albumin levels correlated to lower plasma retinol levels. Zalesin et al. found a similar positive relationship when examining plasma retinol levels and prealbumin at 6 weeks and 12 months after gastric bypass.20 In fact, decreased intake of other essential macro- and micronutrients, including proteins, lipids, and zinc, during catabolic periods can contribute to VAD by disrupting absorption and transport of vitamin A, which may play a role in the development of VAD after LSG and LRYGB.6 As a caveat to this finding, protein metabolism is intimately related with plasma retinol levels, as plasma retinol is bound to retinol-binding protein 4 (RBP4).19,22 During times of protein malnutrition, reductions in RBP4 can lead to artificially low plasma retinol values that normalize when adjusted to serum RBP4.20,22 Although some have suggested monitoring serum RBP4 along with plasma retinol for this reason, RBP4 levels may fluctuate significantly with changes in weight loss and inflammatory status, which may not necessarily reflect hepatic retinol stores.23,24
To the best of our knowledge, ours is the first study to examine VAD in a large cohort of bariatric patients preoperatively and postoperatively after both LSG and LRYGB, and herein lies its strength. However, this study is limited by several factors. First, it is a retrospective analysis and describes a single-institution experience. While vitamin A levels were routinely ordered as part of our institution's pre- and postoperative assessment, many patients obtained their laboratories from outside hospital orders, which often did not include plasma retinol. This significantly impacted the availability of our data and reduced our sample sizes. Future studies are needed to validate these results.
Despite these concerns, this study has provided us with clinically relevant findings that have changed our current postbariatric management. Specifically, the variability in obtaining serum vitamin A laboratories was underappreciated before this study and now is carefully included in all pre- and postoperative laboratory requests. Second, the impact of prior mild VAD on the development VAD has put into question our current protocol of only supplementing patients with serum retinol levels less than 30 mcg/dL. A current study evaluating supplementation in patients with mild VAD is currently underway. Third, we demonstrated that postoperative VAD is not unique to gastric bypass patients, which suggests that this deficiency should be monitored after restrictive procedures as well. Finally, we recognize that a major gap in this study is the lack of clinical correlate to VAD status. This was difficult to accomplish given that screening for hypovitaminosis A was not conducted and therefore hard to obtain retrospectively. This does not suggest that patients were asymptomatic, and in fact, a small study evaluating gastric bypass patients one year after surgery reported that low vitamin A levels correlated to ocular complaints.25 As such, these findings emphasize the need for more rigorous pre- and postoperative assessment and management of hypovitaminosis A.
Conclusion
Preoperative vitamin A levels correlate to increasing EBW and BMI, suggesting a positive metabolic association. After BS, overall VAD is highly prevalent after both LSG and LRYGB and remains elevated up to 2 years postoperatively. Moderate VAD, while less prevalent, rises significantly 6 months after surgery and is significantly more likely after LRYGB. Prior mild VAD is significantly associated with the incidence of moderate VAD at later time point, suggesting a role for more aggressive supplementation. Albumin levels are highly correlated to plasma vitamin A levels at all postsurgical time points, suggesting a role for malnutrition in the development of VAD. Given the wide-ranging functions of vitamin A, further studies are needed to elucidate the impact of this deficiency on metabolic and physiologic outcomes after BS.
Author Contributions
A.D. Jalilvand, W. Hsueh, and S.F. Noria equally contributed to the conception and design of the research; A. Blaszczak and A.D. Jalilvand contributed to the acquisition and analysis of the data; A.D. Jalilvand, S.F. Noria, B.J. Needleman, and W. Hsueh contributed to the interpretation of the data; and A.D. Jalilvand drafted the article. All authors critically revised the article, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final article.
Disclosure Statement
No competing financial interests exist.
Funding Information
This study is supported by T32 NIH NIH:1T32AI106704-01A1.
References
- 1. Malinowski SS. Nutritional and Metabolic Complications of Bariatric Surgery. Am J Med Sci 2006;331:219–225 [DOI] [PubMed] [Google Scholar]
- 2. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010;26:1031–1037 [DOI] [PubMed] [Google Scholar]
- 3. Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am 2009;56:1105–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis 2017;13:727–741 [DOI] [PubMed] [Google Scholar]
- 5. Lee WB, Hamilton SM, Harris JP, Schwab IR. Ocular complications of hypovitaminosis A after bariatric surgery. Ophthalmology 2005;112:1031–1034 [DOI] [PubMed] [Google Scholar]
- 6. Pereira S, Saboya C, Chaves G, Ramalho A. Class III Obesity and its Relationship with the Nutritional Status of Vitamin A in Pre- and Postoperative Gastric Bypass. Obes Surg 2009;19:738–744 [DOI] [PubMed] [Google Scholar]
- 7. Deng T, Lyon CJ, Minze LJ, et al. . Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab 2013;17:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng T, Liu J, Deng Y, et al. . Adipocyte adaptive immunity mediates diet-induced adipose inflammation and insulin resistance by decreasing adipose Treg cells. Nat Commun 2017;8:15725 [Google Scholar]
- 9. Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 2017;13:633–643 [DOI] [PubMed] [Google Scholar]
- 10. Engin A. The pathogenesis of obesity-associated adipose tissue inflammation. Adv Exp Med Biol 2017;960:221–245 [DOI] [PubMed] [Google Scholar]
- 11. Zeng Q, Sun X, Xiao L, Xie Z, Bettini M, Deng T. A unique population: Adipose-resident regulatory T cells. Front Immunol 2018;9:2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feuerer M, Herrero L, Cipolletta D, et al. . Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu L, Lan Q, Li Z, et al. . Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci U S A 2014;111:E3432–E3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma J, Liu Y, Li Y, et al. . Differential role of all-trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells. J Leukoc Biol 2014;95:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao S, Jin H, Korn T, et al. . Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol Baltim Md 1950 2008;181:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of Vitamin A in the immune system. J Clin Med 2018;7:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mercader J, Ribot J, Murano I, et al. . Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology 2006;147:5325–5332 [DOI] [PubMed] [Google Scholar]
- 18. de Souza Valente da Silva L, da Veiga GV, Ramalho RA. Association of serum concentrations of retinol and carotenoids with overweight in children and adolescents. Nutrition 2007;23:392–397 [DOI] [PubMed] [Google Scholar]
- 19. Hennekens CH, Mayrent SL WW. Vitamin A, carotenoids, and retinoids. Cancer 1986;58:1837–1841 [DOI] [PubMed] [Google Scholar]
- 20. Zalesin KC, Miller WM, Franklin B, et al. . Vitamin A deficiency after gastric bypass surgery: An underreported postoperative complication. J Obes 2011;2011:760695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caron M, Hould FS, Lescelleur O, et al. . Long-term nutritional impact of sleeve gastrectomy. Surg Obes Relat Dis 2017;13:1664–1673 [DOI] [PubMed] [Google Scholar]
- 22. Cuesta M, Pelaz L, Pérez C, et al. . Fat-soluble vitamin deficiencies after bariatric surgery could be misleading if they are not appropriately adjusted. Nutr Hosp 2014;30:118–123 [DOI] [PubMed] [Google Scholar]
- 23. Vink RG, Roumans NJ, Mariman EC, van Baak MA. Dietary weight loss-induced changes in RBP4, FFA, and ACE predict weight regain in people with overweight and obesity. Physiol Rep 2017;5:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Güdücü N, Görmüş U, Telatar B, Dünde I. Retinol-binding protein 4, as a negative acute-phase reactant in polycystic ovary syndrome. Minerva Endocrinol 2014;39:299–304 [PubMed] [Google Scholar]
- 25. Eckert MJ, Perry JT, Sohn VY, et al. . Incidence of low vitamin A levels and ocular symptoms after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2010;6:653–657 [DOI] [PubMed] [Google Scholar]