Abstract
BACKGROUND
Stage III or IVA endometrial cancer carries a significant risk of systemic and locoregional recurrence.
METHODS
In this randomized phase 3 trial, we tested whether 6 months of platinum-based chemotherapy plus radiation therapy (chemoradiotherapy) is associated with longer relapse-free survival (primary end point) than six cycles of combination chemotherapy alone in patients with stage III or IVA endometrial carcinoma. Secondary end points included overall survival, acute and chronic toxic effects, and quality of life.
RESULTS
Of the 813 patients enrolled, 736 were eligible and were included in the analysis of relapse-free survival; of those patients, 707 received the randomly assigned intervention (346 received chemoradiotherapy and 361 received chemotherapy only). The median follow-up period was 47 months. At 60 months, the Kaplan–Meier estimate of the percentage of patients alive and relapse-free was 59% (95% confidence interval [CI], 53 to 65) in the chemoradiotherapy group and 58% (95% CI, 53 to 64) in the chemotherapy-only group (hazard ratio, 0.90; 90% CI, 0.74 to 1.10). Chemoradiotherapy was associated with a lower 5-year incidence of vaginal recurrence (2% vs. 7%; hazard ratio, 0.36; 95% CI, 0.16 to 0.82) and pelvic and paraaortic lymph-node recurrence (11% vs. 20%; hazard ratio, 0.43; 95% CI, 0.28 to 0.66) than chemotherapy alone, but distant recurrence was more common in association with chemoradiotherapy (27% vs. 21%; hazard ratio, 1.36; 95% CI, 1.00 to 1.86). Grade 3, 4, or 5 adverse events were reported in 202 patients (58%) in the chemoradiotherapy group and 227 patients (63%) in the chemotherapy-only group.
CONCLUSIONS
Chemotherapy plus radiation was not associated with longer relapse-free survival than chemotherapy alone in patients with stage III or IVA endometrial carcinoma. (Funded by the National Cancer Institute; ClinicalTrials.gov number, NCT00942357.)
Women with locally advanced (International Federation of Gynecology and Obstetrics [FIGO] stage III or IVA) endometrial carcinoma are a heterogeneous group of patients who are at risk for both local and systemic disease recurrence. Clinical and pathologic factors affecting the risk of recurrence include the extent of abdominal and pelvic disease, histologic subtype, nodal involvement, presence of extranodal disease, and the completeness of surgical resection.1-4 Because of the heterogeneity of this patient population, a wide range of 5-year survival estimates has been reported, and an appropriate postsurgery strategy remains unclear.5-8
Pelvic or whole abdominal radiotherapy has traditionally followed surgical resection.6,8 This approach prevented pelvic recurrence but was less effective in preventing systemic recurrence, which limited long-term survival. In a randomized trial conducted by the Gynecologic Oncology Group (GOG), GOG 122, chemotherapy was found to be superior to radiotherapy in treating locally advanced disease, and it thus became part of the standard treatment.9 However, if chemotherapy is given alone, the incidence of locoregional recurrence approaches 20%,9 heralding subsequent distant metastasis and death. Therefore, it was logical to hypothesize that an approach that combined the methods of treatment would improve outcomes by preventing local (pelvic) and distant recurrences.
This combined approach has been studied, but its efficacy relative to that of chemotherapy alone is not known. Patients with stage III endometrial carcinoma treated with chemoradiotherapy in the Radiation Therapy Oncology Group (RTOG) protocol 9708 trial had an estimated 4-year overall survival of 77% and relapse-free survival of 72%.10 In the GOG 184 trial, which compared two chemotherapy regimens after tumor volume-directed external-beam radiotherapy, the 3-year relapse-free survival estimates were 62% to 64%.11 These results supported the feasibility and efficacy of a combined treatment strategy.
The purpose of the current trial (GOG 258) was to evaluate the use of concurrent tumor volume–directed external-beam radiation therapy and chemotherapy (i.e., chemoradiotherapy) as compared with the use of chemotherapy alone.9,11-13 Here, we report on relapse-free survival, the primary end point.
METHODS
PATIENTS AND TRIAL OVERSIGHT
We enrolled women who were 18 years of age or older and who had surgical stage III or IVA endometrial carcinoma according to FIGO 2009 staging criteria of any histologic subtype or had FIGO 2009 surgical stage I or II clear-cell or serous endometrial carcinoma and peritoneal washings that were positive for cancer cells. Hysterectomy and bilateral salpingo-oophorectomy had to have been performed within 8 weeks before trial entry. No single residual tumor mass could be larger than 2 cm in greatest dimension. Pelvic and paraaortic lymph-node biopsy or dissection was optional. Normal organ function and a GOG performance status score of 2 or lower were required (scores range from 0 to 5, with higher scores reflecting greater disability). Patients with carcinosarcoma or recurrent endometrial carcinoma were excluded.
The trial was conducted in accordance with applicable regulatory requirements and the principles of the Declaration of Helsinki. Approval for the trial and for the informed-consent process from a local or central institutional review board or independent ethics committee was required for site participation. Patients provided written informed consent before enrollment.
TRIAL DESIGN AND END POINTS
This randomized, multicenter, phase 3 trial was designed with input from the Gynecologic Oncology Group. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol, available with the full text of this article at NEJM.org. The trial treatments were paid for by medical insurance, and the trial was supported by the National Cancer Institute. All the patients were registered through the National Cancer Institute Oncology Patient Enrollment Network. Treatment was randomly assigned at the GOG Statistical and Data Center and was concealed until registration with verification of eligibility. Randomization was stratified according to age and the presence or absence of gross residual disease. The primary objective was to determine whether chemoradiotherapy would be associated with a lower incidence of recurrence or death (i.e., longer relapse-free survival) than chemotherapy alone. Secondary objectives were between-group comparisons of overall survival, the incidence and severity of acute and late toxic effects, and patient-reported quality of life.
TREATMENT AND ASSESSMENTS
The two treatment regimens were randomly assigned in a 1:1 ratio within permuted blocks. The chemoradiotherapy regimen consisted of cisplatin at a dose of 50 mg per square meter of body-surface area given intravenously on days 1 and 29 together with volume-directed external-beam radiation therapy, followed by carboplatin given at a dose to achieve an area under the concentration-time curve (AUC) of 5 to 6 plus paclitaxel at a dose of 175 mg per square meter every 21 days for four cycles, with granulocyte colony-stimulating factor (G-CSF) support. The chemotherapy-only regimen consisted of carboplatin (to achieve an AUC of 6) plus paclitaxel at a dose of 175 mg per square meter every 21 days for six cycles. In the chemoradiotherapy group, external-beam radiation therapy was delivered to the pelvis with or without paraaortic fields. The planned total dose was 4500 cGy in 25 fractions at 180 cGy per fraction. Intensity-modulated radiotherapy and vaginal brachytherapy were allowed only in the chemoradiotherapy group. Disease assessments included computed tomography (CT) of the abdomen and pelvis and chest radiography at baseline, the end of treatment, every 6 months for the first 2 years, and then annually up to 5 years. Safety assessments included recording of adverse events and concomitant medications, physical examination, and hematologic and chemical testing on the same schedule. Adverse events were graded with the use of the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Annual follow-up was planned beyond 5 years.
The Trial Outcome Index of the Functional Assessment of Cancer Therapy (FACT) for endometrial cancer (FACT-En) and the FACT/GOG-neurotoxicity (NTX) subscale were used to measure quality of life and chemotherapy-induced neurotoxic effects (see the Supplementary Appendix, available at NEJM.org). Two items from the FACT for colorectal cancer (FACT-C) combined with four items from the FACT-En Trial Outcome Index were used to assess gastrointestinal symptoms. Each item in the FACT-En Trial Outcome Index and the FACT/GOG-NTX subscale was scored with the use of a 5-point scale (0, not at all; 1, a little bit; 2, somewhat; 3, quite a bit; 4, very much). The total FACT-En Trial Outcome Index score was calculated as the sum of the subscale scores if more than 80% of the items were answered within each subscale. The total scores range from 0 to 120 for the FACT-En Trial Outcome Index and from 0 to 16 for the FACT/GOG-NTX subscale (see the Supplementary Appendix). A higher Trial Outcome Index, NTX, or gastrointestinal score suggests better quality of life or fewer symptoms. The minimally clinically important difference is 6 points for the FACT-En Trial Outcome Index and 1.2 points for the FACT/GOG-NTX subscale.14 Assessments were completed before treatment (at baseline), 1 week after completing radiation therapy or before cycle 3 of chemotherapy, and 18 and 70 weeks after the start of treatment.
QUALITY ASSURANCE
The trial was open to enrollment between June 29, 2009, and July 28, 2014, and the data cutoff for analysis was March 9, 2017. The GOG Pathology Committee verified histologic subtypes, grades, and stages for all patients. Eligibility, surgery, and radiation plans were reviewed centrally. The trial chairs monitored eligibility, chemotherapy delivery and modifications, adverse events, and radiographic assessments.
STATISTICAL ANALYSIS
The null hypothesis was that chemoradiotherapy would not achieve higher relapse-free survival percentages than chemotherapy alone. A 28.5% lower incidence of recurrence or death (relative hazard, 1.4, corresponding to relapse-free survival at 3 years of 61% in the chemotherapy-only group and 70% in the chemoradiotherapy group) was considered clinically significant. Observation of at least 252 recurrences or death events was needed to attain 85% statistical power with type I error for a one-tailed comparison at 0.05 for relapse-free and overall survival separately. Under the assumption of a decrease in the hazard of recurrence or death over time, a sample size of 804 was targeted. Independence between relapse-free or overall survival and the randomly assigned treatment was assessed with a stratified log-rank test in an intention-to-treat analysis including the eligible patients. Two interim analyses were planned when 42% (105) and 83% (210) of the expected number of recurrences or deaths had been reported. These were reported to the data and safety monitoring board in September 2013 and January 2016. No action was taken to alter the conduct of the trial on the basis of those interim analyses. A Kruskal–Wallis test corrected for ties was used to compare the maximum grade of acute and late adverse effects of therapy (see the Supplementary Appendix). Between-group differences in quality-of-life scores were assessed with a linear mixed model with adjustment for the pretreatment score, assessment time point, and age at enrollment.
RESULTS
PATIENTS AND FOLLOW-UP
Enrollment concluded with 813 patients; 77 were deemed inelgible, most commonly because of an inappropriate cancer stage or a lack of pathological documentation. In addition, 29 eligible patients were never treated, with 24 of those having been assigned to the chemoradiotherapy group. At the time of this report, all patients were no longer receiving the trial treatment and, after treatment, 25 women had withdrawn consent for continued follow-up (12 in the chemoradiotherapy group and 13 in the chemotherapy-only group) (Fig. S1 in the Supplementary Appendix). There were 51 major protocol violations in 399 reviewed cases: 43 in the chemoradiotherapy group and 8 in the chemotherapy-only group; 15 in the chemoradiotherapy group were related to radiation delivery (see the Supplementary Appendix).
The median follow-up duration was 47 months; 295 recurrence or death events were reported in the entire study population, and 271 were reported among eligible patients. The safety analysis included all eligible and treated patients, whereas the efficacy analysis included all eligible patients, regardless of whether they received treatment.
Characteristics of the patient and the tumors are shown in Table 1. A total of 72% of patients were between 50 and 69 years of age (median age, 60 years), 90% identified as non-Hispanic, and 77% identified as white. The performance status score was 0 for 74% of enrolled patients, and the endometrioid histologic type was predominant. Stratification factors were balanced between the treatment groups, with nearly 98% of patients having no gross residual disease. Surgery to remove lymph nodes was reported in more than 94% of patients, with a median number of 13 pelvic nodes and 3 paraaortic nodes removed.
Table 1.
Characteristics of the Patients. *
| Characteristic | Chemoradiotherapy (N = 370) |
Chemotherapy Only (N = 366) |
|---|---|---|
| Mean age (range) — yr | 60.5 (31–88) | 60 (31–85) |
| Race — no. (%)† | ||
| White | 291 (78.6) | 279 (76.2) |
| Black | 37 (10.0) | 42 (11.5) |
| Asian, other, or not specified | 42 (11.4) | 45 (12.3) |
| GOG performance status score — no.(%)‡ | ||
| 0 | 278 (75.1) | 268 (73.2) |
| 1 | 88 (23.8) | 96 (26.2) |
| 2 | 4 (1.1) | 2 (0.5) |
| FIGO stage — no. (%)§ | ||
| I or II | 6 (1.6) | 10 (2.7) |
| IIIA | 70 (18.9) | 81 (22.1) |
| IIIB | 12 (3.2) | 13 (3.6) |
| IIIC1 | 189 (51.1) | 166 (45.4) |
| IIIC2 | 90 (24.3) | 93 (25.4) |
| IVA | 3 (0.8) | 3 (0.8) |
| Histology and grade — no. (%) | ||
| Endometrioid, grade 1 | 87 (23.5) | 79 (21.6) |
| Endometrioid, grade 2 | 103 (27.8) | 118 (32.2) |
| Endometrioid, grade 3 | 64 (17.3) | 61 (16.7) |
| Serous | 66 (17.8) | 65 (17.8) |
| Clear cell | 10 (2.7) | 12 (3.3) |
| Mixed epithelial or other | 40 (10.8) | 31 (8.5) |
| Gross residual disease — no. (%) | ||
| Absent | 360 (97.3) | 359 (98.1) |
| Present | 10 (2.7) | 7 (1.9) |
| Median BMI (range)¶ | 32.0 (11.2–65.3) | 32.9 (18–60.2) |
| BMI category — no. (%) | ||
| Normal or underweight | 72 (19.5) | 71 (19.4) |
| Overweight | 84 (22.7) | 81 (22.1) |
| Obesity class I, II, or III | 214 (57.8) | 214 (58.5) |
There were no significant differences in baseline characteristics between the treatment groups. Percentages may not total 100 because of rounding.
Race was reported by the patient.
A Gynecologic Oncology Group (GOG) performance status score of 2 or lower was required for enrollment (scores range from 0 to 5, with higher scores reflecting greater disability).
Stages were assigned according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 classification; stages range from I to IV, with higher stages indicating more advanced spread of cancer.
Body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
TREATMENT
Overall, 75% of patients completed all prescribed chemoradiotherapy in a median period of 21 weeks.The majority of patients in the chemoradiotherapy group received 45 cGy, intensity-modulated radiotherapy was used in 30% of cases, and 201 patients received vaginal-cuff brachytherapy. Two cycles of cisplatin were coadministered with radiation to more than 85% of patients in the chemoradiotherapy group, and 75% of the patients in that group received all four planned cycles of carboplatin and paclitaxel. In the chemotherapy-only group, 85% of women received all six cycles during a median period of 17 weeks (Table S1 in the Supplementary Appendix). Two patients in the chemotherapy-only group received additional chemotherapy and radiotherapy before progression; both patients discontinued the trial treatment early because of toxic effects. Between 8 and 10% of patients discontinued treatment due to adverse events.
TOXICITY
Table 2 shows acute adverse events, regardless of attribution to the trial intervention, in the 707 eligible participants who initiated treatment. Grade 3, 4, or 5 adverse events were reported in 202 patients (58%) in the chemoradiotherapy group and 227 patients (63%) in the chemotherapy-only group. A grade 4 or higher acute adverse event occurred in 48 patients (14%) in the chemoradiotherapy group and in 108 patients (30%) in the chemotherapy-only group. Two deaths were attributed to the trial treatment; both deaths occurred in the chemotherapy-only group (grade 5 sepsis and sudden death). Constitutional symptoms, fatigue, gastrointestinal events, renal or genitourinary events, and musculoskeletal events were significantly more frequent per grade in the chemoradiotherapy group. Hematologic adverse events were significantly more frequent and more severe in the chemotherapy-only group. Late toxic effects are summarized in Table S2 in the Supplementary Appendix. A grade 4 or higher late adverse event was reported in 15 patients in the chemoradiotherapy group and in 11 patients in the chemotherapy-only group. No deaths that were determined by the trial chairs to be attributable to treatment occurred during the follow-up period.
Table 2.
Acute Adverse Events.
| Adverse Event | Chemoradiotherapy (N = 346) | Chemotherapy Only (N = 361) | P Value* | ||
|---|---|---|---|---|---|
| Any Grade | Grade 3–5 | Any Grade | Grade 3–5 | ||
| percent of patients | |||||
| Constitutional symptom | 87 | 6 | 80 | 2 | 0.004 |
| Fatigue | 85 | 5 | 75 | 2 | <0.001 |
| Cardiac event | 16 | 3 | 19 | 4 | 0.71 |
| Endocrine event | 11 | 1 | 11 | 0 | 0.46 |
| Gastrointestinal event | 90 | 13 | 79 | 4 | <0.001 |
| Renal or genitourinary event | 33 | 2 | 11 | 1 | <0.001 |
| Blood or bone marrow event | 96 | 40 | 90 | 52 | 0.01 |
| Infection | 23 | 4 | 20 | 5 | 0.18 |
| Lymphatic event | 17 | <1 | 16 | <1 | 0.74 |
| Musculoskeletal event | 20 | 3 | 13 | 1 | 0.01 |
| Metabolic or laboratory event | 48 | 15 | 44 | 9 | 0.02 |
| Neurologic event | 76 | 7 | 80 | 5 | 0.99 |
| Pulmonary event | 31 | 2 | 26 | 1 | 0.83 |
| Pain | 70 | 8 | 68 | 5 | 0.04 |
P values for the between-group comparison of the maximum grade of adverse events were calculated with a Kruskal-Wallis test corrected for ties.
EFFICACY
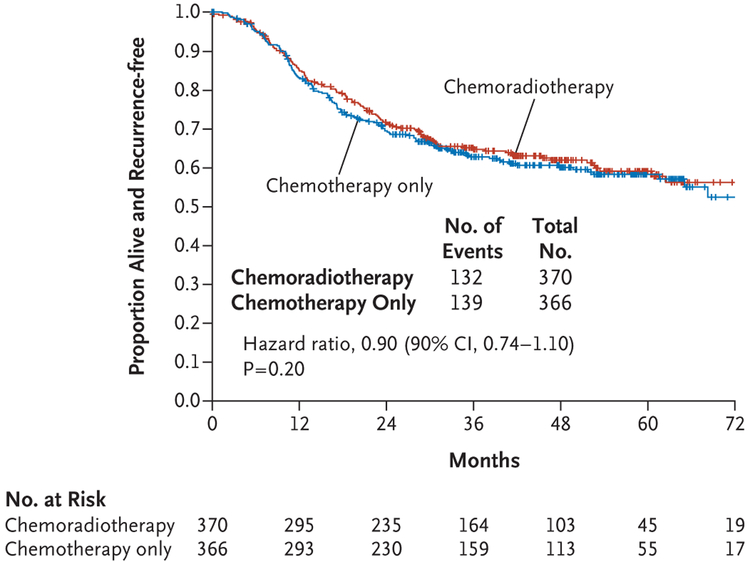
The results with regard to the primary end point did not reach significance. At 60 months, the Kaplan-Meier estimate of the percentage of patients who were alive and recurrence-free was 59% (95% confidence interval [CI], 53 to 65) in the chemoradiotherapy group and 58% (95% CI, 53 to 64) in the chemotherapy-only group (hazard ratio, 0.90; 90% Wald CI, 0.74 to 1.10). Therefore, the null hypothesis that chemoradiotherapy is not superior to chemotherapy alone could not be rejected (P=0.20 by one-tailed test) (Fig. 1). A sensitivity analysis indicated that the results observed in the eligible treated population were consistent with the results among all eligible patients.
Figure 1. Relapse-free Survival.
Tick marks indicate censored data.
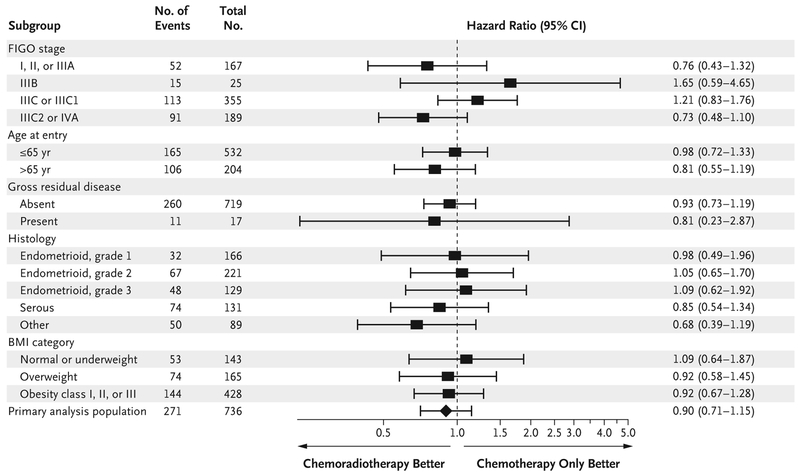
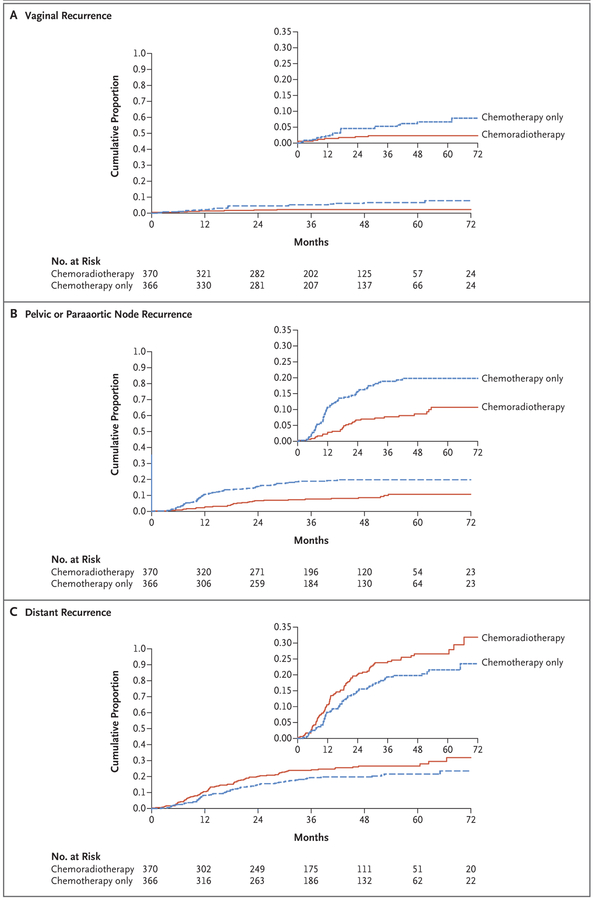
A total of 165 deaths have been reported to date — 86 in the chemoradiotherapy group and 79 in the chemotherapy-only group. Of those deaths, 73% and 81%, respectively, were due to endometrial cancer progression. The data on overall survival are not sufficiently mature to allow comparison between the groups. Exploratory subgroup analyses of relapse-free survival did not identify a subgroup of patients who may have benefited more from chemoradiotherapy than from chemotherapy alone, when age, histologic subtype, surgical stage, body-mass index, and the presence or absence of gross residual disease were taken into consideration (Fig. 2). The cumulative incidence of vaginal disease recurrence at 60 months of follow-up was 2% in the chemoradiotherapy group and 7% in the chemotherapy-only group (hazard ratio, 0.36; 95% CI, 0.16 to 0.82) (Fig. 3A). The cumulative incidence of pelvic or paraaortic node recurrence at 60 months was 11% in the chemoradiotherapy group and 20% in the chemotherapy-only group (hazard ratio, 0.43; 95% CI, 0.28 to 0.66) (Fig. 3B). The cumulative incidence of distant recurrence at 60 months was 27% in the chemoradiotherapy group and 21% in the chemotherapy-only group (hazard ratio, 1.36; 95% CI, 1.00 to 1.86) (Fig. 3C). Coincident local and distant recurrences at first presentation were found in 2.2% of patients in the chemoradiotherapy group and in 4.9% of patients in the chemotherapy-only group; the sites of initial recurrence are shown in Table S3 in the Supplementary Appendix.
Figure 2. Subgroup Analysis According to Recognized Prognostic Factors.
Stages were assigned according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 classification; stages range from I to IV, with higher stages indicating more advanced spread of cancer. BMI denotes body-mass index.
Figure 3 (facing page). Cumulative Risk of Recurrence.
The insets show the same data on an enlarged y axis.
QUALITY OF LIFE
Adherence to the quality-of-life assessments was 95% at baseline, 90% at 6 weeks, 87% at 18 weeks, and 78% at 70 weeks. The patients who could be evaluated were those with a valid baseline assessment and at least one follow-up assessment (332 in the chemoradiotherapy group and 349 in the chemotherapy-only group). After adjustment for age and baseline scores, the least-squares mean Trial Outcome Index score at 18 weeks in the chemoradiotherapy group was 5.2 points lower (97.5% CI, 2.7 to 7.8) than that in the chemotherapy-only group. The difference in this score remained significant at 70 weeks (3.4 points lower in the chemoradiotherapy group; 97.5% CI, 0.7 to 6.2) but did not exceed the 6-point difference that had been preset as clinically meaningful.14 Patients in both groups reported symptoms of neurotoxicity in association with treatment, but the least-squares mean FACT/GOG-NTX subscale score at 6 weeks among patients receiving chemotherapy only was 2.0 points lower (97.5% CI, 1.4 to 2.6) than that in the chemoradiotherapy group (i.e., reflecting worse symptoms in the chemotherapy-only group), whereas patients receiving chemoradiotherapy reported gastrointestinal symptoms at both 6 weeks and 18 weeks that were significantly worse than those in the chemotherapy-only group.
DISCUSSION
The role of radiotherapy in local control of endometrial carcinoma has been firmly established15-18; however, external-beam radiotherapy does not significantly improve overall survival in patients with early-stage, lower-risk disease. This was shown in both the Post Operative Radiation Therapy in Endometrial Carcinoma (PORTEC) 1 trial and the GOG 99 trial,17,19 and its use in this context remains tailored to women who are considered to be at high risk for relapse.20 Because the risk of local relapse is higher among women with stage III uterine cancer than among those with early-stage disease,21 whole abdominal or pelvic radiotherapy has traditionally been incorporated in the standard postoperative approach for those with stage III disease.6, 22 However, given the competing risk of distant metastasis, which has led to the implementation of chemotherapy as the standard of care,9 the role of radiotherapy remains uncertain. This trial prospectively evaluated the effect of combined chemotherapy plus radiotherapy in patients with stage III or IVA endometrial carcinoma as compared with the effect of chemotherapy alone. The results show that the combined regimen was not superior to chemotherapy alone in prolonging relapse-free survival, although locoregional relapses were less frequent than with chemotherapy alone.
An important strength of this trial is the rigorous definition of the patient population, which includes only patients with adnexal, lymph-node, and pelvic, nonperitoneal metastasis. With the revision of the FIGO staging system in 2009,23 the trial was amended to exclude patients with peritoneal washings that were positive for cancer cells but with no evidence of extrauterine endometrioid tumor, because such patients have better outcomes than other patients with stage III disease.24,25 One exception was the group of patients with positive peritoneal washings and carcinoma of the clear-cell or serous histologic type, who are recognized as having a high risk of recurrence26-30; these patients were included in this trial and represented less than 3% of the trial population. Nearly 75% of the patient population had endometrial carcinoma with lymph-node involvement. Although surgical staging was not mandated, the majority of patients underwent full staging procedures.
Chemotherapy became the mainstay of treatment for high-risk endometrial carcinoma after it became clear that distant metastasis is a key determinant of survival in patients with locally advanced endometrial carcinoma. In the GOG 122 trial, overall survival at 60 months was 10 percentage points higher among patients who received doxorubicin and platinum than among those treated with whole abdominal radiotherapy.9 The role of chemotherapy has been studied by other groups.5, 31-33 Maggi et al. reported no significant difference in overall survival between patients who received cyclophosphamide, doxorubicin, and cisplatin (CAP) and those who received externalbeam radiotherapy.31 In the Japanese Gynecologic Oncology Group trial, which involved patients with high-risk endometrial carcinoma, the percentages of patients surviving without progression (83% vs. 66%) and surviving overall (89% vs. 73%) were significantly higher among those who were randomly assigned to receive CAP than among those who received pelvic external-beam radiotherapy.32 In a pooled analysis of trials conducted by the Nordic Society of Gynecological Oncology (NSGO)–European Organization for Research and Treatment of Cancer (EORTC) and the Mario Negri Gynecologic Oncology (MaNGO) group, sequential chemotherapy and pelvic external-beam radiotherapy approached statistical superiority to pelvic radiotherapy alone (hazard ratio, 0.69; P = 0.07).33 Adjuvant chemoradiotherapy was compared with external-beam radiotherapy in patients with early-stage high-risk and stage III endometrial carcinoma in the recently reported PORTEC 3 trial. Overall survival was not affected by the addition of chemotherapy, but the combined regimen improved relapse-free survival relative to radiotherapy (75% vs. 68%), with most of the benefit found in patients with stage III disease.34
Several regimens have been tested in patients with locally advanced or metastatic endometrial carcinoma, including those regimens named above, paclitaxel–doxorubicin–cisplatin, and carboplatin–paclitaxel.11,13,31,33,35 Because carboplatin–paclitaxel induced similar outcomes with less toxicity,12 this regimen was adopted in clinical practice and in this trial. More than 85% of patients in this trial who were in the chemotherapy-only group received the planned six cycles, whereas only 63% completed all cycles of treatment in GOG 122, as a result of treatment-related toxic effects. Full delivery of chemotherapy was diminished by the addition of radiotherapy, with only 75% of women completing the four planned courses in the chemoradiotherapy group. These results are similar to previous observations in GOG 184, in which only 80% of patients completed the intended chemotherapy after radiotherapy,11 and may have contributed to the higher-than-anticipated frequency of distant metastases.
The results of our trial could lead to speculation that external-beam radiotherapy should be delivered after completion of chemotherapy. Single-institution retrospective studies using a “sandwich” radiotherapy-chemotherapy approach have suggested a reasonable side-effect profile and estimated 5-year overall and distant metastasis-free survival of 77% and 85%, respectively.36,37 Because these results have not been validated prospectively, they should not be adopted without further study. Likewise, substituting vaginal brachytherapy for external-beam radiotherapy may be tempting, but because the risk of vaginal recurrence is low, intracavitary radiotherapy should be reserved for women who are at high risk for vaginal relapse. Finally, the short-term and long-term effects of treatment on quality of life should be considered. Although acute toxic effects were more common in the chemoradiotherapy group than in the chemotherapy-only group in our trial, most were low-grade and reversible on treatment discontinuation. Chronic toxic effects included diarrhea, lymphedema, and musculoskeletal events and were more common with chemoradiotherapy, which affected patient-reported outcomes. Late second cancers are also a risk.
In summary, in this randomized trial, the combined regimen of chemotherapy plus radiation did not provide a benefit over chemotherapy alone with respect to relapse-free survival in patients with stage III or IVA endometrial carcinoma. Our data are compatible with the hypothesis from previous studies that completion of chemotherapy is important for the prevention of distant relapse.
Supplementary Material
Acknowledgments
Supported by grants from the National Cancer Institute (NCI) to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), NRG Oncology (1 U10 CA180822), NRG Operations (U10 CA180868), and the NCI Community Oncology Research Program (NCORP) (UG1 CA189867).
Footnotes
Contributor Information
Daniela Matei, Northwestern University, Chicago
Virginia Filiaci, NRG Oncology Statistical and Data Center, Roswell Park Comprehensive Cancer Center, Buffalo, NY
Marcus E. Randall, University of Kentucky, Lexington
David Mutch, Washington University School of Medicine, Siteman Cancer Center, St. Louis
Margaret M. Steinhoff, Women and Infants Hospital in Rhode Island–The Warren Alpert Medical School of Brown University, Providence
Paul A. DiSilvestro, Women and Infants Hospital in Rhode Island–The Warren Alpert Medical School of Brown University, Providence
Katherine M. Moxley, Stephenson Cancer Center Gynecologic Cancers Clinic, University of Oklahoma Health Sciences Center, Oklahoma City
Yong M. Kim, Asan Medical Center, University of Ulsan, Songpa-gu, Seoul, South Korea
Matthew A. Powell, Washington University School of Medicine, Siteman Cancer Center, St. Louis
David M. O’Malley, Ohio State University, Columbus
Nick M. Spirtos, Women’s Cancer Center of Nevada, Las Vegas
William Small, Jr., Loyola University, Chicago
Krishnansu S. Tewari, University of California Irvine Medical Center, Irvine
William E. Richards, Lewis Cancer and Research Pavilion at St. Joseph’s–Candler, Savannah, GA
John Nakayama, Case Western Reserve University Hospital, Cleveland
Ursula A. Matulonis, Dana–Farber Cancer Institute, Boston
Helen Q. Huang, NRG Oncology Statistical and Data Center, Roswell Park Comprehensive Cancer Center, Buffalo, NY
David S. Miller, the University of Texas Southwestern Medical Center, Dallas
REFERENCES
- 1.Mariani A, Webb MJ, Keeney GL, Aletti G, Podratz KC. Assessment of prognostic factors in stage IIIA endometrial cancer. Gynecol Oncol 2002;86:38–44. [DOI] [PubMed] [Google Scholar]
- 2.Mariani A, Webb MJ, Keeney GL, Haddock MG, Aletti G, Podratz KC. Stage IIIC endometrioid corpus cancer includes distinct subgroups. Gynecol Oncol 2002;87: 112–7. [DOI] [PubMed] [Google Scholar]
- 3.McMeekin DS, Lashbrook D, Gold M, Johnson G, Walker JL, Mannel R. Analysis of FIGO stage IIIc endometrial cancer patients. Gynecol Oncol 2001;81:273–8. [DOI] [PubMed] [Google Scholar]
- 4.McMeekin DS, Lashbrook D, Gold M, et al. Nodal distribution and its significance in FIGO stage IIIc endometrial cancer. Gynecol Oncol 2001;82:375–9. [DOI] [PubMed] [Google Scholar]
- 5.Bruzzone M, Miglietta L, Franzone P, Gadducci A, Boccardo F. Combined treatment with chemotherapy and radiotherapy in high-risk FIGO stage III-IV endometrial cancer patients. Gynecol Oncol 2004; 93:345–52. [DOI] [PubMed] [Google Scholar]
- 6.Mundt AJ, Murphy KT, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Surgery and postoperative radiation therapy in FIGO Stage IIIC endometrial carcinoma. Int J Radiat Oncol Biol Phys 2001;50:1154–60. [DOI] [PubMed] [Google Scholar]
- 7.Nelson G, Randall M, Sutton G, Moore D, Hurteau J, Look K. FIGO stage IIIC endometrial carcinoma with metastases confined to pelvic lymph nodes: analysis of treatment outcomes, prognostic variables, and failure patterns following adjuvant radiation therapy. Gynecol Oncol 1999;75: 211–4. [DOI] [PubMed] [Google Scholar]
- 8.Schorge JO, Molpus KL, Goodman A, Nikrui N, Fuller AF Jr. The effect of post-surgical therapy on stage III endometrial carcinoma. Gynecol Oncol 1996;63:34–9. [DOI] [PubMed] [Google Scholar]
- 9.Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 2006; 24:36–44. [DOI] [PubMed] [Google Scholar]
- 10.Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol 2006;103:155–9. [DOI] [PubMed] [Google Scholar]
- 11.Homesley HD, Filiaci V, Gibbons SK, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Gynecol Oncol 2009;112:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller D, Filiaci V, Fleming G, et al. Randomized phase III noninferiority trial of first-line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study.Gynecol Oncol 2012;125:771. abstract. [Google Scholar]
- 13.Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 2004;22:2159–66. [DOI] [PubMed] [Google Scholar]
- 14.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof 2005;28:172–91. [DOI] [PubMed] [Google Scholar]
- 15.Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol 1980;56:419–27. [PubMed] [Google Scholar]
- 16.Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet 2009;373: 137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Lancet 2000;355: 1404–11. [DOI] [PubMed] [Google Scholar]
- 18.Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet 2010;375: 816–23. [DOI] [PubMed] [Google Scholar]
- 19.Keys HMRJ, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004; 92:744–51. [DOI] [PubMed] [Google Scholar]
- 20.Meyer LA, Bohlke K, Powell MA, et al. Postoperative radiation therapy for endometrial cancer: American Society of Clinical Oncology clinical practice guideline endorsement of the American Society for Radiation Oncology evidence-based guideline. J Clin Oncol 2015;33:2908–13. [DOI] [PubMed] [Google Scholar]
- 21.Mundt AJ, McBride R, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Significant pelvic recurrence in high-risk pathologic stage I-IV endometrial carcinoma patients after adjuvant chemotherapy alone: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys 2001; 50:1145–53. [DOI] [PubMed] [Google Scholar]
- 22.Stewart KD, Martinez AA, Weiner S, et al. Ten-year outcome including patterns of failure and toxicity for adjuvant whole abdominopelvic irradiation in high-risk and poor histologic feature patients with endometrial carcinoma. Int J Radiat Oncol Biol Phys 2002;54:527–35. [DOI] [PubMed] [Google Scholar]
- 23.Lewin SN. Revised FIGO staging system for endometrial cancer. Clin Obstet Gynecol 2011;54:215–8. [DOI] [PubMed] [Google Scholar]
- 24.Havrilesky LJ, Cragun JM, Calingaert B, et al. The prognostic significance of positive peritoneal cytology and adnexal/serosal metastasis in stage IIIA endometrial cancer. Gynecol Oncol 2007;104:401–5. [DOI] [PubMed] [Google Scholar]
- 25.Takeshima N, Nishida H, Tabata T, Hirai Y, Hasumi K. Positive peritoneal cytology in endometrial cancer: enhancement of other prognostic indicators. Gynecol Oncol 2001;82:470–3. [DOI] [PubMed] [Google Scholar]
- 26.Sood BM, Jones J, Gupta S, et al. Patterns of failure after the multimodality treatment of uterine papillary serous carcinoma. Int J Radiat Oncol Biol Phys 2003; 57:208–16. [DOI] [PubMed] [Google Scholar]
- 27.Alektiar KM, Makker V, Abu-Rustum NR, et al. Concurrent carboplatin/paclitaxel and intravaginal radiation in surgical stage I-II serous endometrial cancer. Gynecol Oncol 2009;112:142–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alektiar KM, McKee A, Lin O, et al. Is there a difference in outcome between stage I-II endometrial cancer of papillary serous/clear cell and endometrioid FIGO grade 3 cancer? Int J Radiat Oncol Biol Phys 2002;54:79–85. [DOI] [PubMed] [Google Scholar]
- 29.Foerster R, Kluck R, Rief H, Rieken S, Debus J, Lindel K. Survival of women with clear cell and papillary serous endometrial cancer after adjuvant radiotherapy. Radiat Oncol 2014;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiess AP, Damast S, Makker V, et al. Five-year outcomes of adjuvant carboplatin/paclitaxel chemotherapy and intravaginal radiation for stage I-II papillary serous endometrial cancer. Gynecol Oncol 2012; 127:321–5. [DOI] [PubMed] [Google Scholar]
- 31.Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer 2006;95: 266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol 2008;108:226–33. [DOI] [PubMed] [Google Scholar]
- 33.Hogberg T, Signorelli M, de Oliveira CF, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer — results from two randomised studies. Eur J Cancer 2010;46:2422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 2004;22:3902–8. [DOI] [PubMed] [Google Scholar]
- 36.Geller MA, Ivy JJ, Ghebre R, et al. A phase II trial of carboplatin and docetaxel followed by radiotherapy given in a “Sandwich” method for stage III, IV, and recurrent endometrial cancer. Gynecol Oncol 2011;121:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu SM, Chang-Halpenny C, Hwang-Graziano J. Sequential versus “sandwich” sequencing of adjuvant chemoradiation for the treatment of stage III uterine endometrioid adenocarcinoma. Gynecol Oncol 2015;137:28–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.