Abstract
T cells require the protein tyrosine phosphatase CD45 to detect and respond to antigen because it activates the Src family kinase Lck, which phosphorylates the T cell receptor (TCR) complex. CD45 activates Lck by opposing the negative regulatory kinase Csk. Paradoxically, CD45 has also been implicated in suppressing TCR signaling by dephosphorylating the same signaling motifs within the TCR complex that Lck acts upon. We sought to reconcile these observations using chemical and genetic perturbations of the Csk/CD45 regulatory axis incorporated with computational analyses. Specifically, we titrated the activities of Csk and CD45 and assessed their influence on Lck activation, TCR-associated ζ-chain phosphorylation, and more downstream signaling events. Acute inhibition of Csk revealed that CD45 suppressed ζ-chain phosphorylation and was necessary for a regulatable pool of active Lck, thereby interconnecting the activating and suppressive roles of CD45 that tunes antigen discrimination. CD45 suppressed signaling events that were antigen-independent or induced by low-affinity antigen but not those initiated by high-affinity antigen. Overall, our findings reveal that CD45 acts as a signaling “gatekeeper”, enabling graded signaling outputs while filtering weak or spurious signaling events.
INTRODUCTION
Antigens derived from foreign pathogens or malignant cells are detected by a cognate T cell using its T cell antigen receptor (TCR). Because antigen detection is essential for a T cell response, the TCR is critical to human adaptive immunity and current efforts to harness T cells therapeutically. Antigen detection occurs when the TCR binds to agonist peptide-MHC complexes (pMHC) on the surface of an antigen presenting cell (APC). Because it lacks intrinsic kinase activity, the TCR requires the Src family kinase (SFK) Lck to detect and respond to antigen (1, 2). Lck phosphorylates immunoreceptor tyrosine-based activation motifs (ITAMs) within the TCR-associated CD3 and ζ-chains (denoted as the TCR complex). Phosphorylated ITAMs recruit the Zap70 kinase where it is then also phosphorylated by Lck to activate it and propagate signaling events that are necessary for T cell activation to occur (3–5). Because Lck is required to initiate signals through the TCR, its regulation is critical to T cell function. In T cells, Lck activity is controlled by the phosphatase CD45 whose action on Lck is opposed by the inhibitory kinase Csk.
Lck activity is regulated by modulating the conformation of its kinase domain through the phosphorylation of critical regulatory sites (6, 7). CD45 activates Lck by dephosphorylating a tyrosine in its inhibitory C-terminal tail (8–10). Dephosphorylation of the inhibitory C-terminal tail allows Lck to adopt an active open conformation which is stabilized through trans-autophosphorylation of a tyrosine in its activation loop (11). The inhibitory kinase Csk opposes CD45 and phosphorylates the C-terminal tail of Lck to stabilize the closed autoinhibited conformation (12, 13). Loss of CD45 causes hyperphosphorylation of the Lck C-terminal tail and markedly reduces the amount of active Lck. Because active Lck amounts are reduced, T cell development is impaired when TCR signaling is required, such as during positive selection (14–16). In contrast, loss of Csk activity causes increased activation of Lck and results in the aberrant survival of thymocytes lacking a functional TCR (12, 17, 18). Therefore, Csk and CD45 comprise a regulatory axis that controls active Lck amounts which is important for T cell development. In mature peripheral T cells, prior to TCR engagement, there is a basal pool of active Lck (19, 20). Consistent with active Lck amounts setting a threshold for T cell activation, T cell responses to low affinity antigen are potentiated by increasing active Lck abundance through inhibition of Csk (21). Memory T cells possess increased amounts of active Lck which corresponds with their augmented response to antigen (22). Therefore, Csk is a critical inhibitor of Lck which reduces active Lck amounts. The role of CD45, however, is less clear.
CD45 is a receptor-type protein tyrosine phosphatase (RT-PTP) that is amongst the most abundant proteins within the T cell plasma membrane – yet its role in regulating T cell function remains enigmatic (23). CD45 is required for TCR signaling because it activates Lck, which is required to phosphorylate the TCR complex. However, CD45 has also been observed to associate with the phosphorylated ζ-chain, a component of the TCR complex, and to dephosphorylate it in vitro (24, 25). Consistent with a negative regulatory role, CD45 is excluded from the site of contact when a T cell encounters a cell bearing antigen (26, 27). The segregation of CD45 within the T cell plasma membrane is thought to be important for T cell activation because when this process is disrupted T cell activation is attenuated (28, 29). Altered levels of CD45 expression can also affect T cell development within the thymus. In contrast to mice deficient for CD45, those that express low levels of CD45 possess more thymocytes undergoing positive selection which indicates increased sensitivity towards self-antigens (30). In aggregate, CD45 is required for T cell function, however, differing experimental observations suggest that it can positively or negatively affect TCR signaling.
A potential explanation for the divergent roles attributed to CD45 is that it acts on multiple substrates. Because the importance of a given substrate will likely depend upon the experimental context, assigning a specific role to CD45 has been challenging. We therefore sought to reconcile the various roles attributed to CD45 by performing a series of chemical and genetic perturbations to the Csk/CD45 regulatory axis. We reasoned that such an approach would reveal whether CD45 acts differentially on multiple substrates in T cells, including Lck and the TCR complex. Transgenic mice and cell lines modified using CRISPR/Cas9 were used to assess the consequences of CD45-deficiency and Csk inhibition on TCR signaling pathways. We report two critical and interdependent roles of CD45. CD45 is required for graded changes in active Lck amounts and suppression of ζ-chain phosphorylation in intact cells. Moreover, our findings reveal that negative regulation of the TCR by CD45 prevents antigen independent or weak signals but permits sustained signaling by high affinity antigens. Together these features are critical to graded signaling responses required for antigen discrimination.
RESULTS
Antigen discrimination is sensitive to active Lck abundance
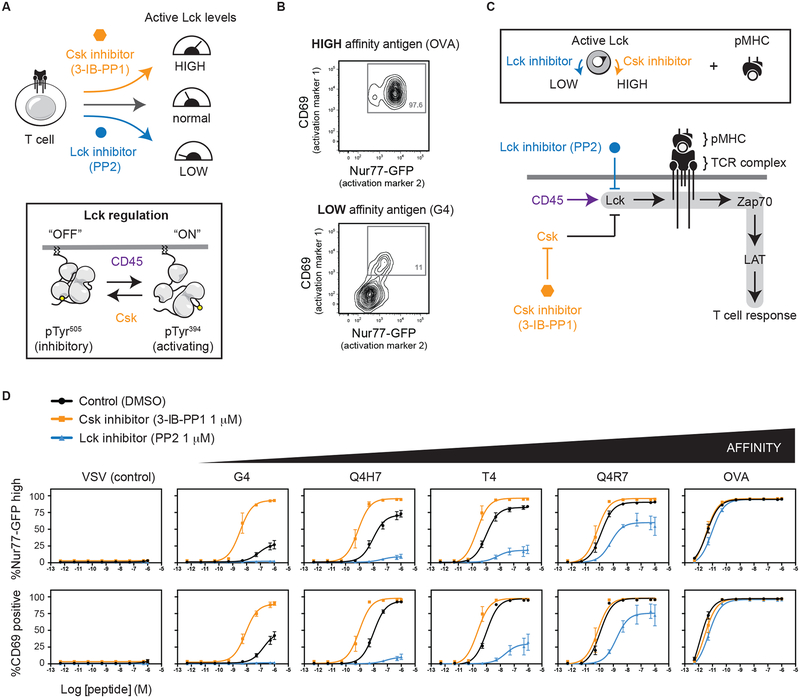
We previously reported that the T cell response to low affinity antigens is potentiated by increasing the amount of active Lck (21). Specifically, by inhibiting Csk, a negative regulator of Lck, active Lck amounts increase and TCR signaling in response to weak stimuli is augmented. Importantly, the amount of active Lck and its recruitment to TCR:pMHC is thought to set a threshold which controls the extent of signaling (31). Because antigen discrimination is a critical hallmark of the T cell response, we sought to better understand how manipulation of active Lck amounts influences T cell activation. To increase active Lck, we employed a previously described analog sensitive allele of Csk (CskAS) that can be inhibited using the small molecule 3-IB-PP1 (hereafter denoted as Csk inhibitor) (32, 33) (Fig. 1A). We also assessed the effect of decreasing active Lck amounts using a SFK inhibitor (PP2). The response of T cells to antigens of differing affinities was assessed using the OT1 TCR transgene. The OT1 TCR binds to a peptide derived from ovalbumin and variants of this peptide sequentially reduce the binding affinity. During thymic selection high affinity peptides act as agonists which cause negative selection whereas lower affinity variants act as partial or non-agonists (34, 35). Therefore, by assessing a panel of peptide antigens of differing affinities the OT1 TCR provides a readout of TCR sensitivity.
Fig. 1. The amount of active Lck alters antigen discrimination by T cells.
(A) Depiction of small-molecule inhibition to manipulate the amount of basally active Lck in OT1 CD8+ T cells. (B) When co-cultured with splenocytes and the high affinity OVA peptide antigen, OT1 T cells upregulate two readouts of T cell activation: Nur77-GFP reporter and CD69. A low affinity peptide variant only weakly activates Nur77-GFP and CD69. (D) OT1 T cells were co-cultured with a series of OVA peptide variants that bind with altered affinity. Active Lck amounts were manipulated by adding intermediate doses (1 μM) of PP2 or Csk inhibitor. Error bars represent means ± SEM of at least three independent experiments.
To monitor how active Lck amounts affect antigen discrimination we employed two readouts of T cell activation: Nur77-GFP and CD69 upregulation. The Nur77-GFP transcriptional reporter provides a readout of TCR integrated signaling involving several downstream pathways and is less sensitive to other mitogenic signals (36, 37). Csk analog-sensitive mice were crossed to incorporate both the OT1 TCR transgene and the Nur77-GFP reporter. CD8+ T cells from these mice upregulate Nur77-GFP and CD69 when co-cultured with splenocytes that display the high affinity OVA peptide but only weakly respond to the low affinity G4 peptide (Fig. 1B). To assess the influence of active Lck amounts on T cell activation, purified CD8 T cells were treated with an intermediate dose of either the Csk inhibitor (3-IB-PP1) or the Lck inhibitor (PP2) (Fig. 1C).
Consistent with our previous findings, inhibition of Csk potentiated responses to low affinity antigens resulting in both Nur77-GFP and CD69 upregulation. Whereas, upregulation of both Nur77-GFP and CD69 was insensitive to Csk inhibition when cells were exposed antigens of high affinity. Conversely, low affinity antigens were markedly sensitive to partial Lck inhibition by PP2 causing reduced Nur77-GFP and CD69 upregulation. The high affinity OVA antigen responded similarly regardless of whether PP2 was present (Fig. 1D). These findings demonstrate, using two readouts of T cell activation, that the capacity of the TCR to distinguish between antigens that bind with differing affinities is markedly sensitive to changes in active Lck amounts. Because Csk and CD45 cooperate to affect active Lck amounts, we employed Csk inhibition to study the role of CD45.
Csk inhibition can activate Lck when CD45 is absent
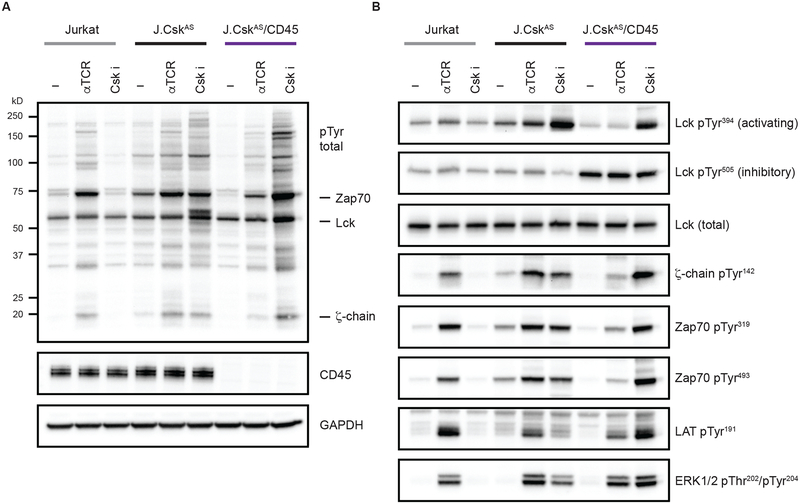
Important regulatory features of CD45 could be unmasked if Lck were activated in its absence. For example, CD45-mediated dephosphorylation of phosphorylated ITAMs within the TCR complex has been reported in vitro using purified proteins (24). However, this has been challenging to observe in T cells because the loss of CD45 impairs Lck activation. We therefore sought to uncouple Lck activation from CD45 expression using Csk inhibition. CD45 is normally required to activate Lck in T cells but inhibition of Csk can relieve negative regulation of Lck and allow for its activation when CD45 is reduced or absent. Because loss of CD45 prevents proper T cell development in mice, we employed a more genetically tractable system. Using Jurkat T cells, we were able to delete both Csk and CD45 using CRISPR/Cas9, and install a Csk analog-sensitive allele (CskAS) which can be inhibited (32, 38, 39) (fig. S1, A to E). These cells were then treated with an anti-TCR antibody to initiate signaling or with the CskAS inhibitor. To assess signaling we monitored global increases in protein tyrosine phosphorylation (Fig. 2A). Jurkat T cells respond to the anti-TCR antibody, as revealed by a marked increase in overall protein phosphorylation, but do not respond to Csk inhibition because they lack the CskAS allele. Jurkat T cells that were made deficient in Csk that were reconstituted with CskAS (J.CskAS) respond to both anti-TCR stimulation and the Csk inhibitor. Cells expressing the CskAS allele and are deficient for CD45 (J.CskAS/CD45) display attenuated signaling in response to anti-TCR stimulation consistent with impaired Lck activation. Interestingly, despite a lack of CD45, the Csk inhibitor caused robust protein tyrosine phosphorylation.
Fig. 2. Csk inhibition activates Lck in the absence of CD45.
(A) Cells were stimulated for 2 minutes by either anti-TCR crosslinking or Csk inhibition (5 μM 3-IB-PP1) and lysates analyzed by immunoblot. Total protein tyrosine phosphorylation was assessed, the mobilities of specific proteins are denoted. (B) Phosphorylation of specific regulatory sites was assessed using site-specific antibodies. Data are representative of three independent experiments.
We next monitored phosphorylation of specific signaling effectors as a readout of their activation status (Fig. 2B). When CD45 is present, TCR stimulation causes robust phosphorylation of the ζ-chain, Zap70, LAT and ERK. We also assessed Lck activation by monitoring Lck regulatory site phosphorylation and these were not substantially affected by TCR stimulation. Because CD45 activates Lck, its loss causes hyperphosphorylation of the inhibitory C-terminal tail (Tyr505) of Lck which reduces active Lck amounts and can be readout by decreased Lck autophosphorylation (Tyr394). Upon TCR stimulation, cells deficient for CD45 display impaired phosphorylation of the ζ-chain, Zap70 and LAT. Therefore, TCR stimulation causes signaling when CD45 is present and signaling is attenuated when CD45 is absent due to reduced active Lck amounts.
We contrasted signaling which occurs upon TCR stimulation with that initiated by acute Csk inhibition. When CD45 is present, Csk inhibition causes increased Lck activation and a corresponding decrease in phosphorylation of the inhibitory C-terminal tail. Because the amount of active Lck is increased, its substrates the ζ-chain and Zap70 are phosphorylated. In contrast, in resting CD45-deficient cells the inhibitory C-terminal tail of Lck is hyperphosphorylated and its activation loop is predominately unphosphorylated, indicating that it is mostly autoinhibited. Surprisingly, Csk inhibition causes a marked increase in Lck autophosphorylation, and therefore active Lck amounts, despite no appreciable dephosphorylation of the inhibitory C-terminal tail. When Lck is doubly phosphorylated on both its inhibitory C-terminal tail (Tyr505) and its activation loop (Tyr394) it is active (19, 24). In this way, inhibition of Csk could allow some activation of Lck despite the loss of CD45 if a very small pool of Lck was transiently in the open conformation due to the inefficient actions of other transmembrane or cytoplasmic PTPs. Consistent with this reasoning, we observed robust phosphorylation of the ζ-chain and Zap70. Interestingly, phosphorylation of the ζ-chain appeared increased when Lck was activated in the absence of CD45.
CD45 suppresses ζ-chain phosphorylation
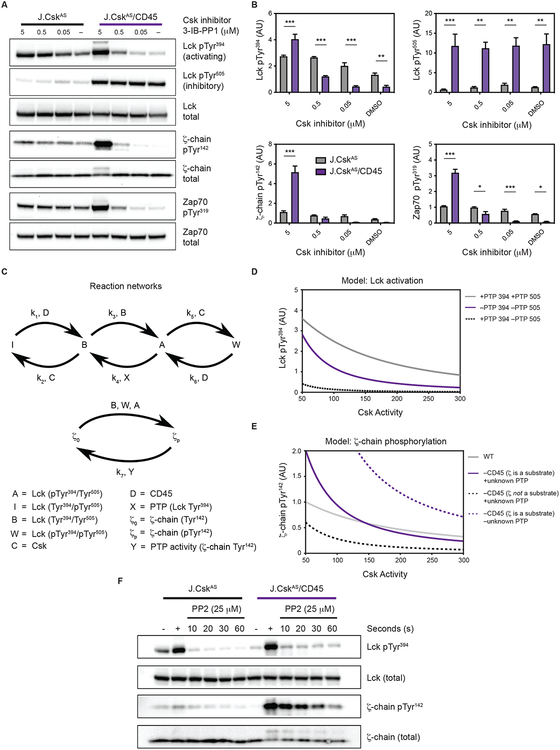
To better understand the role of CD45 in Lck activation, we evaluated the effects of the extent of Csk inhibition. We found that higher doses of Csk inhibitor was required to activate Lck in CD45-deficient cells, consistent with CD45 facilitating Lck activation (Fig. 3A and fig. S2A). Notably, in the absence of CD45, no appreciable change to phosphorylation of the inhibitory C-terminal tail (Tyr505) could be detected (Fig. 3A). However, in the absence of CD45, a small reduction in Tyr505 by other PTPs could facilitate Lck activation through trans-autophosphorylation of the activation loop (19, 24). Consistent with this reasoning, the extent of ζ-chain phosphorylation was markedly increased (4-fold) when Lck was activated in the absence of CD45 (Fig. 3, A and B). We also noted that in the absence of CD45 a more abrupt change in Lck activation occurred with higher concentrations of Csk inhibitor (fig. S2A). The extent of Lck activity was confirmed using Lck isolated from cellular extracts (fig. S2B). Because changes in Lck activity affect ζ-chain phosphorylation, and both appear to be regulated by CD45, a computational model was constructed to explore these complex behaviors.
Fig. 3. CD45 suppresses ζ-chain phosphorylation.
(A and B) Cells were treated with decreasing amounts of Csk inhibitor and protein phosphorylation assessed after 2 minutes by immunoblot, represented in (A) and quantified in (B). Protein phosphorylation was normalized to total protein. Data were pooled from three independent experiments (N=3). Error bars represent the means ± SEM and * P < 0.05, ** P < 0.01, *** P < 0.001 (two-way ANOVA Bonferroni multiple comparisons test). (C) Reaction network which describes Lck in its active (“A”), basal (“B”), inactive (“I”) and doubly phosphorylated state (“W”); “D” is CD45, and “C” is Csk; “X” and “Y” are phosphatases whose identities were explored. (D) Computational model to assess PTP activities affecting Lck regulatory sites. (E) Computational model to evaluate effects of CD45 on ζ-chain phosphorylation. (F) Cells were treated with either DMSO control (–) or Csk inhibitor (5 μM) for 0.5 min. To assess the extent of phosphorylation cells were treated with Csk inhibitor alone (+). Following Lck activation with Csk inhibitor, PP2 (25 μM) was added to block Lck activity, and dephosphorylation was assessed over time by immunoblot. Data are representative of two independent experiments.
Our model considers phosphorylation of regulatory sites within Lck because these sites control its activity. To deactivate Lck, the C-terminal tail of Lck (Tyr505) is phosphorylated by Csk, and CD45 reverses this modification. Dephosphorylation of Lck Tyr505 by CD45 results in a basally active unphosphorylated state. When trans-autophosphorylation of the basal state occurs (pTyr394) it becomes fully active. Our model considers the doubly phosphorylated form of Lck (pTyr394/pTyr505) because it is reported to possess similar activity to basally active Lck (19, 24). Thus, in the computational model there are four possible states for Lck: Activated, A (pTyr394, Tyr505); Basal state, B (Tyr394/Tyr505); Doubly phosphorylated, W (pTyr394/pTyr505); and Inactive, I (Tyr394, pTyr505).
A reaction network was constructed to compute changes in Lck and ζ-chain phosphorylation as Csk activity is reduced (Fig. 3C). The kinetic processes within the reaction network were described mathematically using mass action kinetics and the resulting differential equations were solved (text S1). We considered different identities for the phosphatase (denoted X) that predominately acts on the activation loop of Lck. Using our model, we evaluated whether our experimental findings were best recapitulated with X being CD45 or another phosphatase. We found our model best recapitulated the experimental changes in Lck autophosphorylation if the identity of X is CD45, consistent with previous findings (Fig. 3D) (24, 40).
We next computationally evaluated the consequences of Lck activation on ζ-chain phosphorylation. Two states for ζ-chain Tyr142 were designated: ζ0 (unphosphorylated) and ζp (phosphorylated). Within our model, the ζ chain is dephosphorylated by phosphatase(s) that we label, Y. Our calculations explored three scenarios to evaluate whether our experimental data are consistent with Y being: (i) CD45, (ii) another unknown phosphatase, or (iii) CD45 and another phosphatase which both act on ζ (Fig. 3E). Our model predicts that if CD45 is the only phosphatase that acts on the ζ chain, then active ζ chain should be higher in CD45-deficient cells at all levels of Csk activity due to the loss negative regulation. Alternatively, if ζ is not a substrate for CD45, and Y is an unknown phosphatase, then ζ chain phosphorylation is predicted to be lower in CD45-deficient cells at all levels of Csk activity because CD45-mediated activation of Lck is lost while the capacity to dephosphorylate ζ is unaffected. Only in the case where CD45 and another phosphatase both act on ζ do the computational results mirror our experimental findings. Within this scenario, in CD45-deficient cells, a transition occurs where ζ phosphorylation is lower at high levels of Csk activity, and as Csk activity is reduced ζ phosphorylation becomes higher. Such a transition occurs because at high levels of Csk activity, only a small amount of Lck is active and therefore ζ chain phosphorylation is low and another phosphatase is present to act on ζ. When Csk activity is reduced, the amount of active Lck is increased and ζ-chain phosphorylation is also increased because CD45 is absent. Therefore, our experimental findings and computational analysis reveal that both CD45 and an unknown phosphatase regulate ζ-chain phosphorylation.
To further evaluate suppression of ζ-chain phosphorylation by CD45 experimentally, we inhibited Csk to phosphorylate the ζ-chain and then inhibited Lck. We monitored Lck and ζ-chain dephosphorylation and found that activation loop (Tyr394) phosphorylation was rapidly diminished following Lck inhibition. Despite the loss of Lck autophosphorylation, ζ-chain phosphorylation was dephosphorylated very slowly in the absence of CD45, implicating the importance of CD45 in ζ-chain dephosphorylation but also implicating another PTP (Fig. 3F).
Titration of CD45 expression unmasks opposing regulatory roles
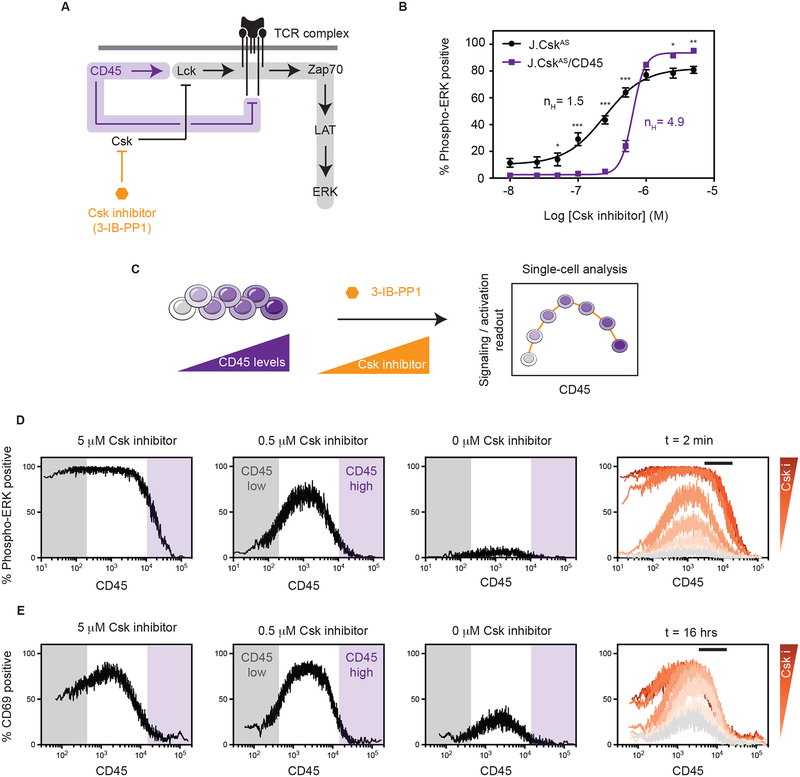
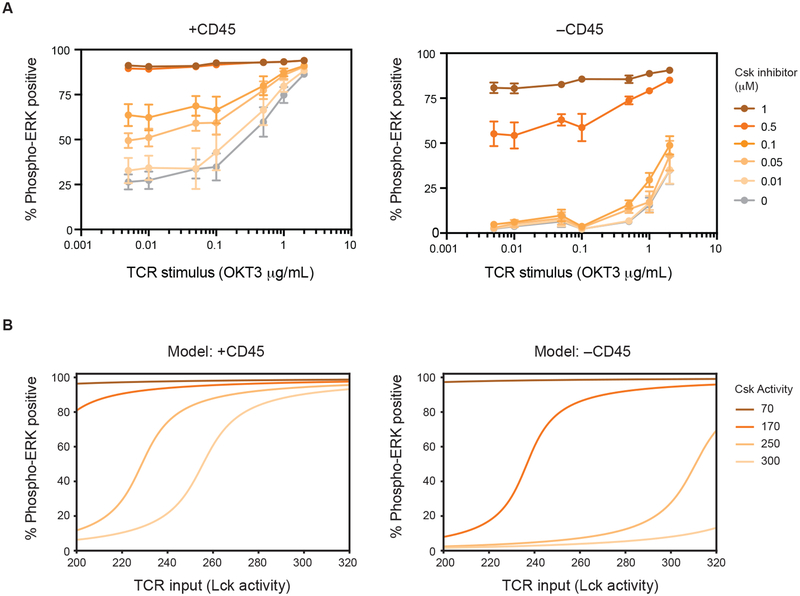
Having found that CD45 can affect dephosphorylation of both Lck and ζ we explored a more downstream readout of TCR signaling, Erk phosphorylation (Fig. 4A). We reasoned that Erk would provide an integrated readout of the influence of CD45 on TCR signaling. We therefore assessed the proportion of phospho-ERK positive cells in response to Csk inhibition. We found that in the presence or absence of CD45, at high levels of Csk inhibition, cells were phospho-ERK positive. However, as Csk inhibition was reduced the proportion of cells that became phospho-ERK positive drastically declined in the absence of CD45. It is apparent that more graded activation of signaling occurs in the presence of CD45. In contrast, in the absence of CD45, switch-like or cooperative signaling occurs (Hill coefficient nH=4.9) (Fig. 4B).
Fig. 4. Titration of CD45 expression unmasks opposing regulatory roles.
(A) Revised model where CD45 acts to both activate Lck kinase but also dephosphorylate its substrate, the TCR complex. (B) The proportion of phospho-ERK positive cells was quantified as an integrated readout of TCR signaling by flow cytometry using a range of Csk inhibition. Error bars represent the means ± SEM (N=3) and * P < 0.05, *** P < 0.001 (two-way ANOVA Bonferroni multiple comparisons test). (C) CD45-deficient cells (J.CskAS/CD45) were transfected with CD45 to generate a broad range of CD45 expression levels. Cells were then treated with Csk inhibitor and assessed by flow cytometry. (D) Analysis of cells with high and low CD45 levels treated with 5 μM of Csk inhibitor (3-IB-PP1) for 2 minutes using phospho-ERK as a readout. The percentage of phospho-ERK positive cells was plotted as a moving average versus CD45 levels over a range of inhibitor concentrations. (E) To assess sustained signaling, cells were treated with Csk inhibitor for 16 hours. The percentage of activated (CD69 positive) cells was plotted as a moving average versus CD45 levels. For (D and E), the far-right panel depicts an overlay of results obtained with different Csk inhibitor concentrations. The black bar denotes approximate WT level of CD45. Histograms can be found in supplementary materials. All data are representative of three independent experiments.
Because reducing CD45 levels influenced both Lck activation and signaling, we sought to unmask regulatory trends by surveying a broad range of CD45 levels. We therefore titrated CD45 expression and assessed sensitivity to Csk inhibition. We used single cell analysis to monitor the amount of CD45 on a cell and its response to Csk inhibition. Specifically, CD45-deficient cells (J.CskAS/CD45) were reconstituted with CD45 to generate a diverse expression profile. The proportion of phospho-ERK positive cells provided a readout of TCR signaling which was plotted as a moving average versus CD45 levels (Fig. 4C). When CD45 was greatly diminished or absent, we again observed all-or-none activation, where high levels of Csk inhibition caused signaling and low levels did not (Fig. 4D & fig. S3, A and B). Notably, however, as CD45 levels increased to an intermediate amount hypersensitivity to Csk inhibition occurred. Within this range of CD45, even small amounts of Csk inhibition cause signaling. Finally, when CD45 is very abundant, signaling was suppressed even at the highest level of Csk inhibition.
Erk phosphorylation occurs rapidly so we also evaluated a readout of sustained signaling, CD69 upregulation. We performed similar single cell analysis using CD45-deficient cells reconstituted with variable levels of CD45. Cells were then cultured in the presence of Csk inhibitor for 16 hours and upregulation of CD69 assessed. The obtained activation profile was qualitatively similar to that observed using phospho-ERK as a readout (Fig. 4E & fig. S3C). Specifically, upon Csk inhibition, cells displayed a more switch-like response in the absence of CD45, intermediate CD45 levels caused hypersensitivity, and high amounts of CD45 suppressed signaling. Overall, our findings indicate changes in CD45 activity can cause divergent responses to Csk inhibition that range from hypersensitivity to suppression of activation.
Mice that express a CD45 variant (LL) that results in reduced levels of CD45 (~ 10–14% of WT) were anticipated to be hyper-responsive to Csk inhibition, much as immature thymocytes from the LL mice have been shown to be hyperresponsive to TCR stimulation. We therefore crossed CD45 low (LL) mice to incorporate the CskAS allele (30). Consistent with increased reactivity, we found that CD8+ T cells isolated from mice with low CD45 possessed reduced TCR levels and an increased proportion of memory T cells which are hallmarks of chronic stimulation (fig. S4, A to C). CD8+ T cells were isolated and treated with Csk inhibitor at different concentrations. Csk inhibition caused an increase in ζ-chain phosphorylation in mice with WT or reduced CD45 levels (fig. S4D). However, the extent of ζ-chain phosphorylation in CD45 low mice did not reach that of WT, perhaps due to chronic TCR stimulation and down-regulation of the TCR in vivo. Despite a lower extent of ζ-chain phosphorylation, CD45 low mice display increased Zap70 activation and LAT phosphorylation, as well as increased global protein tyrosine phosphorylation. Of note, and not seen in CD45 null Jurkat cells, the phosphorylation of Lck Tyr505 decreased at high levels of Csk inhibition, perhaps reflective of the action of the low level of CD45 in these cells (Fig. 3A and fig. S4D).
CD45 enables graded signaling outputs
Because changes in CD45 expression affect sensitivity to Csk inhibition (Fig. 4D & fig. S2A), CD45 appears necessary to maintain a basally active pool of Lck while suppressing antigen-independent signals. To further evaluate this model, the TCR was stimulated while Csk was inhibited. We reasoned that loss of CD45 would narrow the range of TCR stimuli which cells could respond to. We treated cells with differing concentrations of stimulatory anti-CD3 antibody and Csk inhibitor and assessed ERK phosphorylation (Fig. 5A). In the presence of CD45, Csk inhibition causes an incremental increase in the proportion of phospho-ERK positive cells. Despite this basal increase, except at the highest levels of Csk inhibition, TCR stimulation further increases the proportion of phospho-ERK positive cells. In contrast, CD45-deficient cells were markedly less responsive to TCR stimulation. At higher levels of Csk inhibition, cells were predominately activated, and therefore could not respond to further stimuli, and when Csk inhibition is decreased CD45-deficient cells respond only weakly to TCR stimulation.
Fig. 5. CD45 is necessary for regulatable activation of Lck and TCR responsiveness.
(A) The amount of active Lck was perturbed through Csk inhibition of cells that were CD45 positive (left panel) and CD45 negative (right panel) in combination with TCR stimulation using anti-CD3 cross-linking. Signaling was assessed by monitoring the proportion of phospho-ERK positive cells. Error bars represent the means ± SEM of four independent experiments (N=4). (B) A computational model was generated to evaluate the effects of CD45 on the SOS/RAS/ERK pathway.
To better understand ERK activation in the absence of CD45 we constructed a computational model. We previously reported that Ras/SOS/ERK pathway behavior in T cells can be recapitulated computationally (41). Importantly, positive feedback mediated by SOS-catalyzed Ras activation creates a digital response (i.e. within a given cell ERK is either activated or not). Using this model, we sought to explore how changes in Lck activity and ζ-chain phosphorylation that occur in the presence or absence of CD45 affect ERK activation (Fig. 5B). We therefore incorporated the Csk/CD45 regulatory axis into a simplified model of ERK activation. Within our model, we considered the amount of active Lck as a proxy for TCR input or stimulation level. We found that our experimental observations could be broadly recapitulated by considering a linear relationship between the amount of phosphorylated ζ-chain and active SOS. The increased sensitivity of CD45-sufficient cells can be attributed to the role of CD45 in activating Lck. When cells are CD45-deficient, low basally active Lck amounts propagate through the system. These findings highlight the importance of the positive regulatory role of CD45 upon Lck activation at high levels of Csk activity, and of its negative regulatory roles, such as ζ-chain dephosphorylation, at low levels Csk activity. These roles underlie the experimental changes in Erk activation which occur as CD45 levels are altered (Fig. 4D). Using the model described above, our calculations recapitulate the non-monotonic dependence of ERK activation on the level of CD45 (text S1).
CD45 is required for antigen affinity discrimination
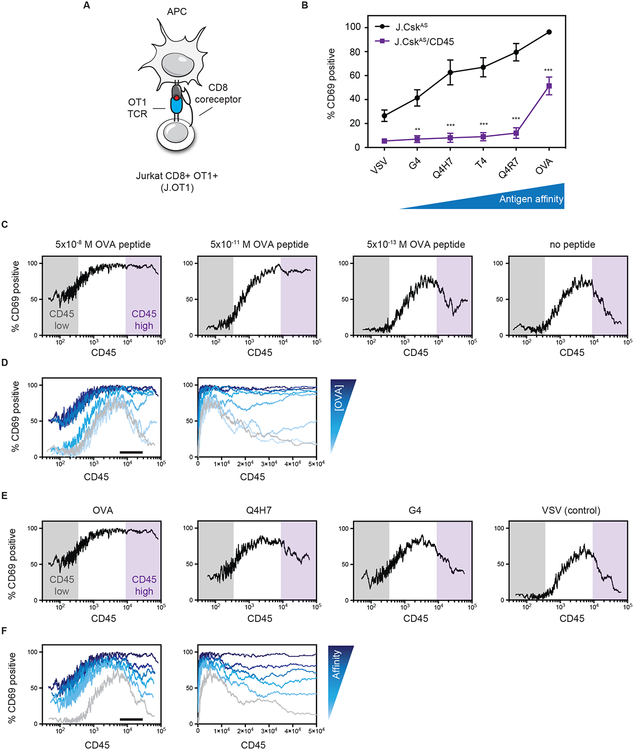
High levels of CD45 suppress antigen-independent signals (Fig. 4). We therefore sought to evaluate whether antigen-dependent signals were affected similarly. Antigen-dependent signaling was tuned using peptide antigens that bind the TCR with differing affinities (Fig. 1). We employed Jurkat T cells that were engineered to express the OT1 TCR transgene and CD8 coreceptor. Jurkat OT1+ CD8+ (J.OT1) cells were used to generate a CD45-deficient variant (J.OT1/CD45) (Fig. 6A & fig. S5, A to C) (42). Similar to primary T cells, J.OT1 cells display a graded response to antigen-presenting cells displaying altered peptide ligands (Fig. 6B). The high affinity OVA epitope elicited robust activation as read out by CD69 upregulation. In contrast, the low affinity antigen resulted in only weak activation. When compared to CD45-deficient cells however only the highest affinity OVA antigen was able to elicit partial activation. Even when no peptide is present an increased proportion of activated cells is observed when CD45 is reduced which is consistent with CD45 suppressing weak signals that occur in the absence of cognate antigen in cells where Lck is active. Consistent with the ability of high affinity antigens to elicit activation, we observed surfaces coated with immobilized high affinity OVA pMHC or anti-TCR facilitated robust cell spreading when a synapse is formed with the surface (fig. S6, A and B). In contrast, surfaces displaying lower affinity peptide displayed reduced spreading. Interestingly, the capacity of CD45-deficient cells to form a synapse was reduced, particularly in response to lower affinity antigen (fig. S6, A and B).
Fig. 6. CD45 is required for affinity discrimination of antigen.
(A) Jurkat T cells were engineered to express the OT1 TCR transgene and the CD8 coreceptor (J.OT1). CD45 was deleted from J.OT1 cells using CRISPR/Cas9 (J.OT1/CD45). (B) Cells were co-cultured with T2-Kb antigen presenting cells pulsed with the indicated peptide antigens (0.05 nM) and upregulation of CD69 was assessed as a readout of T cell activation. Error bars represent the means ± SEM (N=3) and ** P < 0.01, *** P < 0.001 (two-way ANOVA Bonferroni multiple comparisons test). (C to F) CD45-deficient cells (J.OT1/CD45) were transiently transfected with CD45 to generate a broad range of CD45 levels. Cells were then co-cultured with T2-Kb cells and peptide antigen for 16 hours. The percentage of CD69 positive cells was plotted as a moving average versus CD45 expression levels. Differing concentrations of OVA peptide (C) and overlay of OVA concentrations on a log scale (left) or linear (right) axis (D). Black bar denotes approximate WT level of CD45. (E) Altered peptide ligands at a fixed concentration (50 nM) and (F) overlay of altered peptide ligands on a log scale (left) or linear (right) axis. Black bar denotes approximate WT level of CD45. Histograms can be found in supplemental information. All data are representative of three independent experiments.
We next reconstituted CD45-deficient cells to generate a range of CD45 levels. Activation of single cells was monitored as a function of CD45 level in response to differing doses of the OVA peptide (Fig. 6, C and D; & fig. S7A). At low levels of CD45 cells were less responsive to antigen, particularly as the antigen concentration was reduced. At intermediate levels of CD45 expression, cells were increasingly sensitive with substantial activation even when antigen was greatly reduced or absent. In contrast to Csk inhibition, we observed that the high affinity OVA peptide could cause robust activation even at high levels of CD45. To expand this finding, we assessed variants of the OVA peptide that bind with reduced affinity (Fig. 6, E and F; & fig. S7, B and C). Remarkably, at high levels of CD45, the proportion of cells that became activated declined as antigen affinity was decreased.
DISCUSSION
T cells can distinguish between antigens of differing affinities (35, 43, 44). Recent efforts have revealed that not only the affinity of the TCR:pMHC interaction is critical but also its strength under exerted force (45–49). How TCR signaling events enforce antigen discrimination remains unclear. The kinetic proofreading model proposes that once initiated signaling must accumulate to a point of commitment prior to dissociation of the TCR from pMHC (50, 51). An expanded kinetic proofreading model has found recruitment of active Lck and coreceptor to the TCR:pMHC to be critical for this process (31, 49). Studies have implicated active Lck abundance in setting a threshold for T cell activation (21, 22, 52). We demonstrate using two readouts of T cell activation that increasing active Lck amounts potentiate responses to low affinity antigens, and conversely, the response to these weak antigens is attenuated when active Lck is reduced. In contrast to low affinity antigens, high affinity antigens are less sensitive to changes in the abundance of active Lck. Although we focus predominately on Lck, T cells also possess another less abundant SFK, Fyn (23, 53). Fyn is dispensable for T cell development but is reported to contribute to antigen recognition in the periphery (52, 54). Overall, our findings are consistent with Csk repressing active SFK amounts to set a threshold for T cell activation.
Because Csk-mediated inhibition of Lck is opposed by CD45, we investigated the contributions of both regulators simultaneously using intact cells. We found that inhibition of Csk could activate Lck in the absence of CD45 despite no appreciable dephosphorylation of the inhibitory C-terminal tail (Tyr505). Because a greater extent of Csk inhibition is required when CD45 is absent, we speculate that another phosphatase may weakly act on this site, and when Csk activity is abolished, Lck trans-autophosphorylation can occur independently of CD45. Phosphorylation of Lck (Tyr394) has been demonstrated to activate the kinase even when its inhibitory C-terminal tail remains phosphorylated (19, 24). Activation of Lck in this way allowed us to assess the role of CD45. Notably, our findings indicate that CD45 acts on multiple substrates – facilitating Lck activation while also suppressing phosphorylation of its substrate the ζ-chain. Computational modeling of our Csk inhibition results confirm that suppression of ζ-chain phosphorylation is recapitulated only if ζ is a CD45 substrate. Importantly, our computational analysis also implicates an additional phosphatase which can dephosphorylate the ζ-chain because deletion of CD45 would otherwise be predicted to cause constitutive ζ-chain phosphorylation. The contribution of an additional phosphatase to suppression of TCR signaling is consistent with roles attributed to phosphatases, including SHP1, PTPN22 and others, thought be important for regulating T cell reactivity in the periphery (5, 55, 56). Here and in previous reports, when CD45 was reduced but not absent, cells became hyper-responsive to stimuli (30). Consistent with increased reactivity, T cells that we isolated from mice with low levels of CD45 displayed hallmarks of chronic stimulation: reduced TCR and a higher proportion of memory T cells. Together these observations suggest that numerous adaptations can occur in the periphery to suppress TCR signaling when CD45 is reduced, which could affect ζ-chain phosphorylation.
Because CD45 acts on ζ, Lck, and potentially additional substrates, we altered Csk and CD45 activity and assessed an integrated readout of TCR signaling (Erk phosphorylation). Critical features of CD45-mediated regulation emerged: (i) a switch-like response in its absence; (ii) hypersensitivity to changes in Csk activity at reduced CD45 levels; and, (iii) suppression at high levels of CD45. These findings reveal a striking interdependence between CD45-mediated activation of Lck and suppression of TCR signaling. Our findings highlight how quantitative manipulation of Csk and CD45 activities can achieve distinct signaling behaviors. We also found that CD45 was required for TCR agonist-induced signaling. Our findings were modelled computationally and reveal that CD45 is required to suppress ζ-chain phosphorylation when Csk activity is low. Specifically, when CD45 is absent, and Csk activity is reduced to activate Lck, the loss of ζ-chain suppression causes cells to signal in the absence of TCR stimuli rendering them unresponsive to further TCR stimuli. Therefore, CD45 is required for inducible signaling because it provides for a regulatable pool of basally active Lck while suppressing phosphorylation of its substrate, the ζ-chain, until TCR stimuli are encountered. In vitro analyses of CD45 have generally emphasized its negative regulatory role (ζ-chain dephosphorylation). However, our findings reveal the importance of CD45 in maintaining a regulatable pool of active Lck. Loss of Lck regulation, for example, in CD45-deficient or CD45 low mice and cells, affects the capacity of T cells to properly discern antigen strength. In this way, the capacity of a T cell to initiate signals that correspond to the appropriate cellular response is disrupted. Moreover, feedback mechanisms which tune TCR signaling have been implicated in regulating active Lck amounts. For example, Csk resides within the cytoplasm unless it is recruited to the plasma membrane where it can inhibit Lck. Recruitment of Csk is mediated by phosphorylated adaptor proteins, such as PAG, (57, 58). Additionally, a Zap70-dependent negative regulatory loop is thought to phosphorylate a conserved site (Tyr192) within the SH2 domain of Lck (59, 60). Modification of Tyr192 disrupts the ability of CD45 to interact with Lck and dephosphorylate its inhibitory C-terminal tail (59). Such negative feedback mechanisms highlight the capacity of a T cell to tune its basal pool of active Lck. It is anticipated that tuning Lck activity is important for T cells to generate graded signaling outputs that ultimately influence cellular programs (5, 61–63).
CD45 has been previously reported to suppress T cell activation because it is excluded from the TCR as a synapse is formed between a T cell and an APC bearing cognate antigen. Within the synapse many proteins are redistributed within the plasma membrane of the T cell. Over time, the TCR becomes concentrated at the center of the synapse whereas CD45 is excluded (27). Because CD45 is excluded it has been proposed that the segregation of CD45 itself may be sufficient to initiate TCR signaling (26). However, studies using tethered antibodies that bind to the TCR with high affinity indicate that exclusion of CD45 is not a strict requirement (64). Additional studies have reported that the T cell:APC synapse also functions as a site of sustained signaling and receptor downregulation upon strong stimulation (65, 66). We did not investigate whether the exclusion of CD45 initiates TCR signaling, however, we found that high affinity antigens can overcome suppression that occurs at high levels of CD45 expression. In contrast, low affinity antigens and antigen-independent signaling (Csk inhibition) are suppressed by CD45. Our findings appear consistent with the T cell:APC synapse facilitating sustained signaling. Specifically, we anticipate that sustained signaling over time through a synapse could overcome CD45-mediated suppression through exclusion of CD45 and/or the local concentration of signaling components. Importantly, this would not occur during antigen-independent signaling (Csk inhibition).
Overall our findings reveal that CD45 acts as a signaling gatekeeper in T cells because it enables a regulatable pool of basally active Lck while suppressing weak or spurious TCR signaling. We anticipate that CD45 could fulfill a similar role in other immune cells possessing ITAM receptors, and by extension, other receptor-mediated signaling pathways possessing RT-PTPs.
MATERIALS AND METHODS
Mice.
All mice were bred and maintained on the C57BL/6 genetic background. For experiments mice were used at between 6–12 weeks of age. All animals were housed in a specific-pathogen-free facility at UCSF and were treated according to protocols that were approved by UCSF animal care ethics and veterinary committees and are in accordance with NIH guidelines. The OT1 TCR transgene was crossed to CskAS transgenic and Nur77-GFP reporter mice (33, 67). The lightning allele (LL or CD45 low) was similarly crossed to mice harboring the CskAS transgene (30). To assess cell populations, spleen and lymph nodes were isolated from mice and organs disassociated in complete medium (RPMI supplemented with 10% FBS, 2 mM glutamine, non-essential amino acids (Gibco), penicillin and streptomycin (Gibco), 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, and 10 mM HEPEs). Red blood cells were removed using ACK lysis. Cells were washed and resuspended in FACs buffer (PBS supplemented with 2% FBS and 2 mM EDTA) and stained using fluorescently labelled antibodies. Cells were analyzed using a BD Fortessa and quantification carried out using FlowJo software.
Antibodies.
A table of antibodies used in this study can be found in table S1.
Cell lines.
Jurkat T cell lines were maintained by the Weiss laboratory and are routinely analyzed for the expression of CD3 or TCRβ and other surface markers by flow cytometry. Cells were maintained in a tissue culture incubator at 37 °C with 5% CO2 in culture medium (RPMI supplemented with 10% fetal bovine serum and 2 mM glutamine). Jurkat variants deficient for specific proteins of interest were generated using CRISPR/Cas9 targeted gene deletion. J.CskAS (B2C9–26) and J.CskAS/CD45 (C14) were maintained in medium supplemented with blasticidin (10 μg/mL).
CRISPR/Cas9.
CD45-deficient cell lines were generated using the pX330 vector (38) and gRNAs targeting CD45 (59). The pX330 vector was introduced using electroporation and cultured for ~4 days. Cells were stained for surface CD45 and cells with low CD45 expression sorted into 96-well plates using a BD FACs ARIA. Cells were expanded and analyzed for CD45 expression using flow cytometry and immunoblot to confirm CD45-deficient clones. Mutations to PTPRC, which encodes CD45, were confirmed by sequencing. The target site was amplified by PCR using genomic DNA isolated from cell lines and cloned into a Topo vector. Cell lines that were reconstituted with CskAS were confirmed by immunoblot using anti-Csk and anti-Myc tag antibodies.
Electroporation.
DNA constructs were introduced into Jurkat T cell lines using a BioRad Gene Pulser Xcell. Cells were washed and resuspended with RPMI medium. 400 μL of cells (15×106 total cells) were added to a 0.4 cm cuvette. Typically, 10 μg of DNA was added and cells electroporated (260 V, 1250 μF). Immediately after electroporation cells were recovered into pre-warmed culture medium and incubated at 37 °C for 48 hours. For transient expression of CD45, cells were electroporated with a pEF CD45RO construct. For stable reconstitution, cells were electroporated with pEF CskAS and recovered for 48 hours. Stable clones were isolated using limiting dilution. Cells were diluted and cultured in selective medium containing blasticidin (10 μg/mL) in 96-well plates. After ~3 weeks clones were assessed for Csk expression using immunoblot and expanded further.
Immunoblotting.
Jurkat T cells were rinsed with RPMI and resuspended at 5 × 106 cells/mL and rested for 15 minutes at 37 °C. Cells were treated with anti-TCR antibody (C305, 0.85 μg/mL), or CskAS inhibitor 3-IB-PP1 (generously provided by the Shokat laboratory), PP2 (Tocris), or DMSO. Cells were then lysed through addition of lysis buffer containing a final concentration of: 1% NP-40, NaVO4 (2 mM), NaF (10 mM), EDTA (5 mM), PMSF (2 mM), Aprotinin (10 μg/mL), Pepstatin (1 μg/mL), Leupeptin (1 μg/mL), and PP2 (25 μM). Lysates were placed on ice and debris pelleted at 13,000 x g. Primary T cells were resuspended at 20–40 × 106 cells/mL and lysed using 6x SDS-PAGE sample buffer. DNA was pelleted in an ultracentrifuge at 70,000 rpm. Supernatants were run on 4–12% NuPage or 10% Bis-Tris gels and transferred to PVDF membranes. Membranes were incubated with blocking buffer (2% BSA TBS-T) and then probed with primary antibodies overnight at 4 °C. The following day blots were rinsed and incubated with HRP-conjugated secondary antibodies (diluted 1:5000). All antibodies used for immunoblotting were diluted 1:2000 in blocking buffer unless otherwise stated. Blots were detected using chemiluminescent substrate and a Chemi-Doc (BioRad) or iBright (Invitrogen) imaging system. Phosphorylation was assessed at 2 minutes following acute treatment with inhibitor or anti-TCR stimulation unless otherwise stated. Quantification was performed using Image Lab or iBright analysis software.
OT1 co-culture.
Cells were isolated from the spleen and lymph node. CD8+ OT1 T were purified by negative selection using biotinylated antibodies and magnetic beads as previously described (68). Splenocytes were used as antigen-presenting cells and were isolated from T cell-deficient mice (Cα −/− or Zap70 −/−). Prior to culture red blood cells removed using ACK lysis. Splenocytes were incubated with peptide antigens for 1 hr at 37 °C. Small molecule inhibitors (3-IB-PP1 or PP2) or DMSO control were then added followed immediately by OT1 T cells at a ratio of 5:1 (APC: T cell). Cells were cultured overnight at 37 °C for a total of 16 hrs and placed on ice prior to staining for CD69 and other surface markers (CD19/B220, CD8α, TCRβ or Vα2) using an antibody dilution of 1:200 in FACs buffer and Fc blocking antibody (2.4G2) at 1:1000. Upregulation of CD69 or Nur77-GFP was assessed by flow cytometry using a BD Fortessa and quantified using FlowJo software. The OVA peptide (SIINFEKL), its variants (Q4R7, T4, Q4H7, G4), and the VSV peptide were synthesized by Genescript.
J.OT1 co-culture.
J.OT1 activation assays were performed similarly to primary CD8+ OT1 T cell co-cultures. 5×104 J.OT1 cells were combined with T2-Kb cells (APCs) that had been incubated with peptide antigen for 1 hour at a ratio of 3:1 (APC:J.OT1). Cells were cultured for 16 hours and then placed on ice and stained for CD69 (1:200 in FACs buffer). CD69 upregulation was assessed by flow cytometry using a BD Fortessa and quantified using FlowJo software.
Phospho-ERK staining.
Cells were stimulated in RPMI and fixed by adding 4% formaldehyde PBS (1:1) and incubating for 12 mins at room temperature. Cells were pelleted and rinsed with FACs buffer (PBS supplemented with 2% FBS and 2 mM EDTA). Cells were then placed on ice and ice cold 90% methanol added to permeabilize the cells for 45 mins. Cells were then rinsed 3x with FACs buffer and resuspended in staining solution (anti-phospho-ERK 1:100 in FACs buffer). Cells were stained for either 1 hour at room temperature or overnight at 4 °C. Cells were rinsed 3x and stained with anti-Rabbit PE antibody and anti-CD45 AF647 antibody (1:100 in FACs buffer) for 45 mins at room temperature. Cells were rinsed 2x and analyzed by flow cytometry using a BD Fortessa and quantification performed using FlowJo software. For Csk inhibitor dose-response curves, data were fit using agonist versus response with variable slope in Graphpad Prism.
Single cell analysis.
FACs data was exported from FlowJo in CSV format and analyzed with R Studio using the Zoo package. The proportion of phospho-ERK or CD69 positive cells were calculated as a moving average. Graphs were generated using Graphpad Prism software.
In vitro kinase assay.
Cells were washed and resuspended in RPMI. Cells were incubated at 37 °C for 10–15 mins before treatment with inhibitors (25 μM or 5 μM 3-IP-PP1) for 1 min. Cells were lysed by adding concentrated lysis buffer to a final concentration of : 1% NP-40, NaVO4 (2 mM), NaF (10 mM), EDTA (5 mM), and HALT protease inhibitor cocktail (Invitrogen). Samples were vortexed briefly, and placed on ice for 10 minutes and then centrifuged to pellet debris. Anti-Lck beads (25 uL per IP) were added and mixed for 1.5 hours at 4 °C. Beads were rinsed with ice cold lysis buffer twice followed by kinase buffer (50 mM HEPEs pH 7.0, 2 mM DTT, 5 mM MgCl2, 0.2 mM NaVO4, 0.5 mM β-glycerophosphate). Beads were resuspended in 60 uL of kinase buffer containing substrate (1 ug GST CD3 ζ, Sino Biological) and ATP added to 0.2 mM. Samples were incubated with agitation at 25 °C for 5 minutes and placed on ice. Supernatant containing substrate was collected. Phosphorylation status of substrate (GST CD3 ζ) and immunoprecipitated Lck were assessed by immunoblot.
Anti-Lck resin.
Protein G Dynabeads (1 mL, Invitrogen) were washed and incubated with 200 ug of anti-Lck (1F6) in binding buffer (sodium borate pH 9) for 1 hour at room temperature. Beads were rinsed and resuspended in 10 mL binding buffer. DMP (50 mg) was added and mixed for 30 minutes. Beads were rinsed and resuspended in 0.1 M ethanolamine pH 8 and incubated for 30 minutes. Unconjugated antibody was released by washing with 0.1 M glycine pH 3. Beads were washed twice with TBS pH 7.4. Beads were resuspended in 1 mL of TBS and stored at 4 °C.
Microscopy.
Glass surfaces were coated with either stimulatory antibody (C305) or streptavidin (SA). Surfaces were then washed and biotinylated monomeric pMHC complexes were incubated with streptavidin surfaces to immobilize them. Surfaces were then washed and used for antigen presentation. To assess synapse formation, J.OT1 cells were dropped onto cover glasses coated with various stimulatory reagents for 30 minutes at 37 °C. Cells were subsequently fixed with 4% paraformaldehyde. Following fixation, the cell membrane was stained using either AF-594 anti-human-CD45 (Biolegend) or AF-594 wheat germ agglutinin (ThermoFisher). Fixed immunological synapses formed on glasses were imaged with the Nikon Ti Microscope with TIRF at the UCSF Nikon Imaging Center. The areas of synapses were processed and quantified using ImageJ software. The pMHC complexes were generously provided by the Palmer lab and the NIH tetramer core.
Supplementary Material
Text S1. Details of the computational model.
Figure S1. Csk analog-sensitive Jurkat T cell characterization.
Figure S2. Effects of Csk inhibition on CD45-deficient Jurkat T cells.
Figure S3. FACs analysis of Csk inhibition.
Figure S4. CD45-low (LL) mice possess increased memory CD8+ T cells and lower TCR levels.
Figure S5. OT1 Jurkat T cells.
Figure S6. Contact area is influenced by antigen affinity and CD45.
Figure S7. FACs analysis of J.OT1/CD45 cells.
Table S1. Antibodies.
Acknowledgements:
We thank the Shokat laboratory at UCSF for providing the 3-IB-PP1 compound, the Palmer laboratory at the University of Basel and the NIH tetramer core for providing pMHC. We thank Byron Au-Yeung and Emily Jutkiewicz for providing feedback on the manuscript. Microscopy was conducted at the UCSF Nikon Imaging Center and cell sorting carried out using the flow cytometry core at UCSF. We thank Al Roque for assisting with animal husbandry.
Funding: A.H.C. was supported by a Robertson Foundation / Cancer Research Institute fellowship. G.G. was supported by the doctoral training program GRK1660 from the German Research Foundation (DFG). This work was supported in part by the Howard Hughes Medical Institute, the National Institutes of Health (NIH), NIAID PO1 AI091580 (A.W. and A.C.), and the Czech Science Foundation, 16-09208Y (O.S.).
Footnotes
Publisher's Disclaimer: This manuscript has been accepted for publication in Science Signaling. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencesignaling.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, Davidson D, Mak TW, Profound block in thymocyte development in mice lacking p56lck. Nature 357, 161–164 (1992). [DOI] [PubMed] [Google Scholar]
- 2.Straus DB, Weiss A, Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 70, 585–593 (1992). [DOI] [PubMed] [Google Scholar]
- 3.Courtney AH, Lo WL, Weiss A, TCR Signaling: Mechanisms of Initiation and Propagation. Trends Biochem Sci 43, 108–123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith-Garvin JE, Koretzky GA, Jordan MS, T cell activation. Annu Rev Immunol 27, 591–619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaud G, Lesourne R, Love PE, Regulatory mechanisms in T cell receptor signalling. Nat Rev Immunol 18, 485–497 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Sicheri F, Moarefi I, Kuriyan J, Crystal structure of the Src family tyrosine kinase Hck. Nature 385, 602–609 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Harrison SC, Eck MJ, Three-dimensional structure of the tyrosine kinase c-Src. Nature 385, 595–602 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Mustelin T, Coggeshall KM, Altman A, Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A 86, 6302–6306 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostergaard HL, Shackelford DA, Hurley TR, Johnson P, Hyman R, Sefton BM, Trowbridge IS, Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A 86, 8959–8963 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sieh M, Bolen JB, Weiss A, CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J 12, 315–321 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi H, Hendrickson WA, Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature 384, 484–489 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Bergman M, Mustelin T, Oetken C, Partanen J, Flint NA, Amrein KE, Autero M, Burn P, Alitalo K, The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J 11, 2919–2924 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levinson NM, Seeliger MA, Cole PA, Kuriyan J, Structural basis for the recognition of c-Src by its inactivator Csk. Cell 134, 124–134 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishihara K, Penninger J, Wallace VA, Kündig TM, Kawai K, Wakeham A, Timms E, Pfeffer K, Ohashi PS, Thomas ML, Furlonger C, Paige CJ, Mak TW, Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell 74, 143–156 (1993). [DOI] [PubMed] [Google Scholar]
- 15.Pingel JT, Thomas ML, Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell 58, 1055–1065 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koretzky GA, Picus J, Thomas ML, Weiss A, Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature 346, 66–68 (1990). [DOI] [PubMed] [Google Scholar]
- 17.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S, Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell 73, 1125–1135 (1993). [DOI] [PubMed] [Google Scholar]
- 18.Schmedt C, Saijo K, Niidome T, Kuehn R, Aizawa S, Tarakhovsky A, Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature 394, 901–904 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Höfer T, Viola A, Acuto O, Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32, 766–777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stirnweiss A, Hartig R, Gieseler S, Lindquist JA, Reichardt P, Philipsen L, Simeoni L, Poltorak M, Merten C, Zuschratter W, Prokazov Y, Paster W, Stockinger H, Harder T, Gunzer M, Schraven B, T cell activation results in conformational changes in the Src family kinase Lck to induce its activation. Sci Signal 6, ra13(2013). [DOI] [PubMed] [Google Scholar]
- 21.Manz BN, Tan YX, Courtney AH, Rutaganira F, Palmer E, Shokat KM, Weiss A, Small molecule inhibition of Csk alters affinity recognition by T cells. Elife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moogk D, Zhong S, Yu Z, Liadi I, Rittase W, Fang V, Dougherty J, Perez-Garcia A, Osman I, Zhu C, Varadarajan N, Restifo NP, Frey AB, Krogsgaard M, Constitutive Lck Activity Drives Sensitivity Differences between CD8+ Memory T Cell Subsets. J Immunol 197, 644–654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hukelmann JL, Anderson KE, Sinclair LV, Grzes KM, Murillo AB, Hawkins PT, Stephens LR, Lamond AI, Cantrell DA, The cytotoxic T cell proteome and its shaping by the kinase mTOR. Nat Immunol 17, 104–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui E, Vale RD, In vitro membrane reconstitution of the T-cell receptor proximal signaling network. Nat Struct Mol Biol 21, 133–142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furukawa T, Itoh M, Krueger NX, Streuli M, Saito H, Specific interaction of the CD45 protein-tyrosine phosphatase with tyrosine-phosphorylated CD3 zeta chain. Proc Natl Acad Sci U S A 91, 10928–10932 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anton P Merwe van der, Davis SJ, Shaw AS, Dustin ML, Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin Immunol 12, 5–21 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML, The immunological synapse. Annu Rev Immunol 19, 375–396 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Chang VT, Fernandes RA, Ganzinger KA, Lee SF, Siebold C, McColl J, Jönsson P, Palayre M, Harlos K, Coles CH, Jones EY, Lui Y, Huang E, Gilbert RJC, Klenerman D, Aricescu AR, Davis SJ, Initiation of T cell signaling by CD45 segregation at ‘close contacts’. Nat Immunol 17, 574–582 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhuri K, Wiseman D, Brown MH, Gould K, van der Merwe PA, T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature 436, 578–582 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Zikherman J, Jenne C, Watson S, Doan K, Raschke W, Goodnow CC, Weiss A, CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity 32, 342–354 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepanek O, Prabhakar AS, Osswald C, King CG, Bulek A, Naeher D, Beaufils-Hugot M, Abanto ML, Galati V, Hausmann B, Lang R, Cole DK, Huseby ES, Sewell AK, Chakraborty AK, Palmer E, Coreceptor scanning by the T cell receptor provides a mechanism for T cell tolerance. Cell 159, 333–345 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenborn JR, Tan YX, Zhang C, Shokat KM, Weiss A, Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling. Sci Signal 4, ra59(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A, Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol 15, 186–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR, T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994). [DOI] [PubMed] [Google Scholar]
- 35.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Holländer GA, Gascoigne NR, Palmer E, Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444, 724–729 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA, T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 208, 1279–1289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noviski M, Mueller JL, Satterthwaite A, Garrett-Sinha LA, Brombacher F, Zikherman J, IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. Elife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F, Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E, A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNeill L, Salmond RJ, Cooper JC, Carret CK, Cassady-Cain RL, Roche-Molina M, Tandon P, Holmes N, Alexander DR, The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity 27, 425–437 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Das J, Ho M, Zikherman J, Govern C, Yang M, Weiss A, Chakraborty AK, Roose JP, Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell 136, 337–351 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo WL, Shah NH, Ahsan N, Horkova V, Stepanek O, Salomon AR, Kuriyan J, Weiss A, Lck promotes Zap70-dependent LAT phosphorylation by bridging Zap70 to LAT. Nat Immunol, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zehn D, Lee SY, Bevan MJ, Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, Evavold BD, Zhu C, The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464, 932–936 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sibener LV, Fernandes RA, Kolawole EM, Carbone CB, Liu F, McAffee D, Birnbaum ME, Yang X, Su LF, Yu W, Dong S, Gee MH, Jude KM, Davis MM, Groves JT, Goddard WA 3rd, Heath JR, Evavold BD, Vale RD, Garcia KC, Isolation of a Structural Mechanism for Uncoupling T Cell Receptor Signaling from Peptide-MHC Binding. Cell 174, 672–687 e627 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim ST, Takeuchi K, Sun ZY, Touma M, Castro CE, Fahmy A, Lang MJ, Wagner G, Reinherz EL, The alphabeta T cell receptor is an anisotropic mechanosensor. J Biol Chem 284, 31028–31037 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng Y, Reinherz EL, Lang MJ, alphabeta T Cell Receptor Mechanosensing Forces out Serial Engagement. Trends Immunol 39, 596–609 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu B, Chen W, Evavold BD, Zhu C, Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell 157, 357–368 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong J, Ge C, Jothikumar P, Yuan Z, Liu B, Bai K, Li K, Rittase W, Shinzawa M, Zhang Y, Palin A, Love P, Yu X, Salaita K, Evavold BD, Singer A, Zhu C, A TCR mechanotransduction signaling loop induces negative selection in the thymus. Nat Immunol 19, 1379–1390 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKeithan TW, Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci U S A 92, 5042–5046 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lever M, Maini PK, van der Merwe PA, Dushek O, Phenotypic models of T cell activation. Nat Rev Immunol 14, 619–629 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Lovatt M, Filby A, Parravicini V, Werlen G, Palmer E, Zamoyska R, Lck regulates the threshold of activation in primary T cells, while both Lck and Fyn contribute to the magnitude of the extracellular signal-related kinase response. Mol Cell Biol 26, 8655–8665 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ley SC, Marsh M, Bebbington CR, Proudfoot K, Jordan P, Distinct intracellular localization of Lck and Fyn protein tyrosine kinases in human T lymphocytes. J Cell Biol 125, 639–649 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B, The influence of the src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation. Immunol Rev 191, 107–118 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Bounab Y, Getahun A, Cambier JC, Daeron M, Phosphatase regulation of immunoreceptor signaling in T cells, B cells and mast cells. Curr Opin Immunol 25, 313–320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salmond RJ, Brownlie RJ, Morrison VL, Zamoyska R, The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat Immunol 15, 875–883 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simeoni L, Kliche S, Lindquist J, Schraven B, Adaptors and linkers in T and B cells. Curr Opin Immunol 16, 304–313 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Chow LM, Fournel M, Davidson D, Veillette A, Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature 365, 156–160 (1993). [DOI] [PubMed] [Google Scholar]
- 59.Courtney AH, Amacher JF, Kadlecek TA, Mollenauer MN, Au-Yeung BB, Kuriyan J, Weiss A, A Phosphosite within the SH2 Domain of Lck Regulates Its Activation by CD45. Mol Cell 67, 498–511 e496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sjolin-Goodfellow H, Frushicheva MP, Ji Q, Cheng DA, Kadlecek TA, Cantor AJ, Kuriyan J, Chakraborty AK, Salomon A, Weiss A, The catalytic activity of the kinase ZAP-70 mediates basal signaling and negative feedback of the T cell receptor pathway. Sci Signal 8, ra49(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conley JM, Gallagher MP, Berg LJ, T Cells and Gene Regulation: The Switching On and Turning Up of Genes after T Cell Receptor Stimulation in CD8 T Cells. Front Immunol 7, 76(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nayar R, Schutten E, Bautista B, Daniels K, Prince AL, Enos M, Brehm MA, Swain SL, Welsh RM, Berg LJ, Graded levels of IRF4 regulate CD8+ T cell differentiation and expansion, but not attrition, in response to acute virus infection. J Immunol 192, 5881–5893 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S, Pellegrini M, Belz GT, Smyth GK, Febbraio MA, Nutt SL, Kallies A, The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol 14, 1155–1165 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Al-Aghbar MA, Chu YS, Chen BM, Roffler SR, High-Affinity Ligands Can Trigger T Cell Receptor Signaling Without CD45 Segregation. Front Immunol 9, 713(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS, The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity 29, 414–422 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T, Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol 6, 1253–1262 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Zikherman J, Parameswaran R, Weiss A, Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature 489, 160–164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Au-Yeung BB, Smith GA, Mueller JL, Heyn CS, Jaszczak RG, Weiss A, Zikherman J IL-2 Modulates the TCR Signaling Threshold for CD8 but Not CD4 T Cell Proliferation on a Single-Cell Level. J Immunol 198, 2445–2456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1. Details of the computational model.
Figure S1. Csk analog-sensitive Jurkat T cell characterization.
Figure S2. Effects of Csk inhibition on CD45-deficient Jurkat T cells.
Figure S3. FACs analysis of Csk inhibition.
Figure S4. CD45-low (LL) mice possess increased memory CD8+ T cells and lower TCR levels.
Figure S5. OT1 Jurkat T cells.
Figure S6. Contact area is influenced by antigen affinity and CD45.
Figure S7. FACs analysis of J.OT1/CD45 cells.
Table S1. Antibodies.