Abstract
Background:
Olfactory dysfunction (OD) in chronic rhinosinusitis (CRS) is common. It is likely that numerous factors such as sex, race, age, allergies, asthma, smoking, and other comorbidities play a role in CRS-related OD. In order to determine which aspects of OD are due solely to CRS and which are associated with other confounders, control populations are needed to allow appropriate risk assessments.
Methods:
Prospective, multi-institutional enrollment of patients with CRS and control subjects without CRS was performed. Demographic information, comorbidities, and olfactory testing (Sniffin’ Sticks) of threshold (T), discrimination (D) and identification (I) scores was collected.
Results:
A total of 224 patients with CRS and 164 control subjects were enrolled. Olfaction was worse in CRS patients compared to controls (mean TDI=22.4[±9.5] vs. 28.8[±7.0], respectively, p<0.001). Only 27% of CRS patients were normosmic compared to 49% of controls (p<0.001). When stratifying by nasal polyp (NP) status, CRSwNP patients had significant impairments in TDI, T, D, and I compared to controls with mean differences of 11.2, 3.3, 3.5, and 4.4 points respectively (all p<0.001). In contrast, CRSsNP patients only had impaired T when compared to controls with a mean difference of 2.2 points (p<0.001). Multivariate modeling of TDI scoring demonstrated that OD was driven by polyps, asthma, diabetes, and age. CRSsNP was not independently associated with worse TDI scores.
Conclusion:
OD in CRS patients is multi-factorial. Independent drivers appear to be polyp status, asthma, diabetes and age. OD in patients with CRSsNP is similar to controls with the exception of impaired thresholds.
Keywords: olfaction, chemosensory, sinusitis, surgery, polyp
INTRODUCTION
Chronic rhinosinusitis (CRS) is one of the most common causes of olfactory dysfunction (OD). While olfactory loss is one of the cardinal symptoms of CRS, it is relatively challenging to determine the mechanism, severity and impact of OD in patients with CRS. Olfactory loss in CRS appears heterogeneous, as OD associated with CRS with nasal polyposis (CRSwNP) differs from that associated with CRS without nasal polyposis (CRSsNP) in numerous aspects. This includes radiographic, endoscopic and local cytokine measures.1–4 Additionally, it is very difficult to predict olfactory outcomes with medical or surgical treatment of CRS and many patients suffer some degree of olfactory loss despite our best efforts5. When considering a population of patients with CRS, the question remains as to what degree of olfactory loss can be attributed to CRS and what amount may be associated with other contributing factors.
In addition to CRS, there are numerous other factors that impact olfaction including viruses, trauma, allergies, asthma, anatomic variants, toxins, smoking, neurodegenerative disorders, aging, sex, race, vascular disorders, and diabetes6. Patients presenting with CRS often have many of these other risk factors, and prior reports have implicated smoking, age and asthma7,8. It is unlikely that CRS is the only factor impacting olfaction in a given patient as many non-CRS factors can also be present. Thus CRS-related OD may very well be multifactorial, and optimal treatment outcomes and prognostication will not be obtained unless we understand all the contributing factors for a given patient. In order to determine which aspects of OD are due solely to CRS and which are associated with other confounding factors, control populations matched for these confounders are needed.
MATERIALS and METHODS
Study Population – Case Subject Selection
Case subject enrollment was conducted as part of a prospective, investigator-monitored, observational research study of human subjects funded by the National Institute on Deafness and Other Communication Disorders (Bethesda, MD.; Federal grant #R01 DC005805). Case study participants were prospectively recruited via invitation from a patient population presenting to academic, rhinology centers located in the United States at either Oregon Health and Science University (OHSU, Portland, OR), the Medical University of South Carolina (MUSC, Charleston, SC), the University of Utah (Salt Lake City, UT), the University of Colorado (Aurora, CO), and the University of Virginia (Charlottesville, VA). Symptomatic, adult study participants (≥ 18 years of age) received a confirmed diagnosis of symptomatic CRS, with or without nasal polyposis, from a fellowship trained rhinologist following criteria established by current clinical practice guidelines of the American Academy of Otolaryngology-Head and Neck Surgery9. Study participants reported experiencing at least 2 of the cardinal symptoms associated with CRS including, but not limited to: nasal congestion, mucopurulent drainage, facial pain and/or pressure, and impairment in olfactory function. Case patients provided written, informed consent after initial enrollment meetings to ensure voluntary participation involving minimal risk without deviation from the standard of care (SOC) for the treatment of CRS. Clinical measures of disease severity and olfactory dysfunction were collected and compared with data collected on control subjects without CRS.
Study Population – Control Subject Selection
Control study enrollment was prospectively conducted using a clinic-based population sample of healthy subjects without diagnoses of active CRS or previous endoscopic sinus surgery. Adult volunteers, 18 years or older, were recruited locally using advertisements, word-of-mouth, and self-referral techniques while data use agreements were established for data sharing purposes between OHSU and MUSC in compliance with the Health Insurance Portability and Accountability Act of 1996. The Institutional Review Board affiliated with all enrollment locations approved study protocols.
Exclusion Criteria
Patients identified as both case and control subjects were excluded for this investigation based on similar criteria including a history of comorbid conditions associated with an increased prevalence of olfactory dysfunction including: dementia, aphasia, Alzheimer’s disease, other non-specified neurocognitive disorders, Parkinson’s disease, major head trauma / traumatic brain injury, and patients on immunosuppressive medications at the time of enrollment. Study candidates were also required to demonstrate strong fluency in English as a first or second language.
Clinical Measures of Disease Severity
Case and control participants also completed a review of medical history and social history, as well as inquiries of recently completed and currently prescribed therapeutics. Comorbidities thought to potentially play a role in olfaction were assessed, including asthma, diabetes, allergies, depression, anxiety and smoking (Table 1). Additional clinical measures of disease severity were collected for diagnostic purposes and sourced as research data when provided by the SOC for treatment of CRS, including Lund-Mackay staging for computed tomography (CT) imaging (score range: 0-24)10, Lund-Kennedy staging for sinonasal endoscopy (score range: 0-20)11, and completion of the 22-item SinoNasal Outcome Test (SNOT-22) survey (score range: 0-110, ©2006, Washington University, St. Louis, MO.)12. Total scores for all three measures are summarized with higher scores reflecting worse overall disease severity.
Table 1:
Comparison of demographic measures, subject characteristics, and comorbid conditions between case subjects with CRS and control subjects without CRS.
| Characteristics at enrollment: | Case Subjects with CRS (n=224) | Control Subjects (n=164) | Test statistic | p-value | |
|---|---|---|---|---|---|
| Age in years | Mean[± SD] | 50.1 [± 16.6] | 51.3 [±17.4] | t= −0.72 | 0.471 |
| Males | N (%) | 107 (48%) | 62 (38%) | --- | --- |
| Females | 117 (52%) | 103 (62%) | χ2= 4.02 | 0.045 | |
| White/Caucasian | 201 (90%) | 118 (71%) | χ2= 21.37 | <0.001 | |
| African American | 17 (8%) | 38 (23%) | χ2= 18.66 | <0.001 | |
| Asian | 3 (1%) | 4 (2%) | χ2= 0.63 | 0.426 | |
| Hispanic/Latino ethnicity | 10 (5%) | 6 (4%) | χ2= 0.17 | 0.684 | |
| Education years completed: | ---- | ---- | ---- | ---- | |
| Less than Junior High/Middle School | 1 (<1%) | 0 (0%) | χ2< 0.01 | >0.999 | |
| Junior High / Middle School | 1 (<1%) | 0 (0%) | χ2< 0.01 | >0.999 | |
| High School | 68 (31%) | 48 (29%) | χ2= 0.07 | 0.787 | |
| Post-secondary / College / University | 94 (42%) | 74 (45%) | χ2= 0.32 | 0.570 | |
| Graduate school / Professional degree | 58 (26%) | 43 (26%) | χ2< 0.01 | 0.970 | |
| Nasal polyposis | 119 (53%) | 0 (0%) | χ2= 126.29 | <0.001 | |
| Previous sinus surgery / ESS | 102 (46%) | 0 (0%) | χ2= 101.84 | <0.001 | |
| Asthma | 99 (44%) | 14 (9%) | χ2= 58.79 | <0.001 | |
| Diabetes mellitus (Type I/II) | 22 (10%) | 15 (9%) | χ2= 0.06 | 0.808 | |
| Depression (history/self-reported) | 62 (28%) | 27 (16%) | χ2= 6.89 | 0.009 | |
| Anxiety (history/self-reported) | 50 (22%) | 20 (12%) | χ2= 6.70 | 0.010 | |
| OSA | 35 (16%) | 15 (9%) | χ2= 3.62 | 0.057 | |
| Smoking / tobacco use (current) | 9 (4%) | 19 (12%) | χ2= 8.00 | 0.005 | |
| Smoking / tobacco use (former) | 50 (22%) | 43 (26%) | χ2= 0.79 | 0.374 | |
| Alcohol use (current) | 121 (54%) | 99 (60%) | χ2= 1.27 | 0.259 | |
| Allergic rhinitis | 113 (50%) | 49 (30%) | χ2= 16.11 | <0.001 | |
| Positive allergy test (mRast/skin prick) | 118 (53%) | 38 (23%) | χ2= 34.29 | <0.001 | |
| GERD | 62 (28%) | 18 (11%) | χ2= 16.36 | <0.001 | |
| Autoimmune disease | 23 (10%) | 5 (3%) | χ2= 7.29 | 0.001 | |
| Oral corticosteroid use (past 30 days) | 50 (22%) | 1 (1%) | χ2= 39.33 | <0.001 | |
SD, standard deviation; ESS, endoscopic sinus surgery; OSA, obstructive sleep apnea; GERD, gastroesophageal Reflux disease; t, independent samples t-test statistic; χ2, chi-square test statistic; reported p-values correspond to two-sided asymptotic significance.
Clinical Measure of Olfactory Dysfunction
For the primary outcome of interest to this investigation, both case and control subjects completed a comprehensive evaluation of bilateral olfactory function at the time of enrollment using Sniffin’ Stick pens (Burghart Messtechnik, Wedel, Germany) which evaluate three separate components of olfactory function including: odorant threshold (score range: 1-16), odorant discrimination (score range: 0-16), and odorant identification (score range: 0-16)13,14. Correctly identified threshold (T), discrimination (D), and identification (I) scores, as well as a composite TDI total score, are summarized from item response scores (score range: 1-48) with higher scores reflecting superior olfactory function. Rather than adjusting normal cutoffs based upon age, we defined normal cutoffs for each component of T, D and I based upon ideal scores reported for normal subjects aged 16-35 years old. This permits us to examine the impact of age as an independent variable as previously described.15
Database Management and Statistical Methods
Investigational data was secured through the assignment of unique study identification numbers for study participants and removal of all protected health information prior to transfer into a centralized database in a closed environment at OHSU (Access; Microsoft Corporation; Redmond, WA). All descriptive and statistical comparisons were completed using SPSS software (version 24.0; IBM Corporation, Armonk, NY.). Statistical comparisons for this prevalence study were directed after confirmation of normality and linearity for all scaled measures using graphical analysis and Shapiro-Wilk testing. Comparisons between all mean values and frequency/prevalence measures were completed using either two-tailed independent samples t-testing or chi-square (χ2) testing, respectively, between cases and control subjects. Multivariate linear regression modeling was used to identify and enumerate significant associations between independent patient factors and scaled/continuous measures of olfactory dysfunction. Linear models first screened subject cofactors / candidate variables at the 0.200 α-level for preliminary model inclusion. Adjusted, final models were constructed using forward selection and backwards elimination (p<0.050) procedures with case / control subject variable as the primary exposure of interest for each outcome measure. Covariate factors meeting screening criteria were included in final models and assessed for independence and potential confounding (>10% difference in effect estimate). Multicollinearity between variables was evaluated using variance inflation factors (VIFs) while goodness-of-model fit was evaluated using coefficients of multiple determination (R2) to quantify the percentage of total explained model variance. Final linear modeling diagnostics confirmed approximate residual normality using Q-Q plots. Adjusted effect estimators (ƥ) are reported for each significant association with corresponding standard error (SE), 95% confidence intervals, t-test statistics, and type-I error probabilities (p-values).
RESULTS
Final Sample Population
Study enrollment was completed across all locations between October, 2016 and March, 2019 for a total of 388 participants meeting all inclusion and exclusion criteria, including 224 (58%) case subjects with CRS, with nasal polyposis (CRSwNP) and without nasal polyposis (CRSsNP), and 164 (42%) control subjects without a diagnosis of CRS. Associations of both prevalence and mean values of reported demographic measures, patient characteristics, and medical comorbidity between case and control subjects were identified and described in Table 1. Significant independent associations (p<0.050) between case and control subjects in the prevalence of gender, race, nasal polyposis, previous endoscopic sinus surgery, numerous comorbid conditions, and recent oral corticosteroid use (within past 30 days) were identified and considered potential confounders of olfactory dysfunction scores. Comparisons in clinical measure of disease severity between CRSwNP and CRSsNP identified significantly worse mean Lund-Mackay CT scores in CRSwNP compared to CRSsNP (16.3 [±4.7] vs. 11.0 [±4.7]; p<0.001) as well as Lund-Kennedy endoscopy scores (9.2 [±3.5] vs. 4.9 [±2.8]; p<0.001), respectively. No significant differences (p=0.088) in average SNOT-22 total scores were found between CRSwNP (47.4 [±22.8]) and CRSsNP (42.5 [±19.4]).
Unadjusted Comparisons of Sniffin’ Sticks Scores
Bivariate associations in average Sniffin’ Stick scores between case and control subjects were evaluated without adjustment (Table 2). Case subjects with CRS were found to have significantly worse Sniffin’ Stick total scores, as well as worse mean threshold, discrimination, and identification scores. Following olfactory diagnostic category assignment by overall TDI, the CRS (case) group was found to have lower prevalence of normosmia and higher prevalence of functional anosmia when compared to controls (p<0.001).
Table 2:
Unadjusted comparisons of Sniffin’ Stick TDI scores between all case subjects with CRS (n=224) and control subjects without CRS (n=164).
| Case Subjects with CRS Mean [±SD] | Control Subjects Mean [±SD] | Difference Mean [±SE] | Difference 95% CI | Test statistic | p-value | |
|---|---|---|---|---|---|---|
| TDI Total Score | 22.4 [± 9.5] | 28.8 [±7.0] | −6.4 [±0.9] | (−8.0, −4.7) | t= 7.61 | <0.001 |
| Threshold score | 3.8 [± 3.1] | 6.1 [±2.7] | −2.3 [±0.3] | (−2.9, −1.7) | t= 7.82 | <0.001 |
| Discrimination score | 9.2 [± 3.5] | 10.9 [±2.7] | −1.7 [±0.3] | (−2.3, −1.1) | t= 5.37 | <0.001 |
| Identification score | 9.4 [±4.2] | 11.8 [±2.8] | −2.4 [±0.4] | (−3.1, −1.7) | t= 6.71 | <0.001 |
| TDI total score classification: | N (%) | |||||
| Normosmia | 60 (27%) | 81 (49%) | ---- | ---- | χ2= 20.91 | <0.001 |
| Hyposmia / microsmia | 90 (40%) | 73 (45%) | ---- | ---- | χ2= 0.73 | 0.393 |
| Functional anosmia | 74 (33%) | 10 (6%) | ---- | ---- | χ2= 40.51 | <0.001 |
CRS, chronic rhinosinusitis; SE, standard error of the mean; CI, confidence interval of the mean difference, SD, standard deviation; t, independent samples t-test statistic; χ2, chi-square test statistic; reported p-values correspond to two-sided significance. T, olfactory threshold; D, olfactory discrimination; I, olfactory identification.
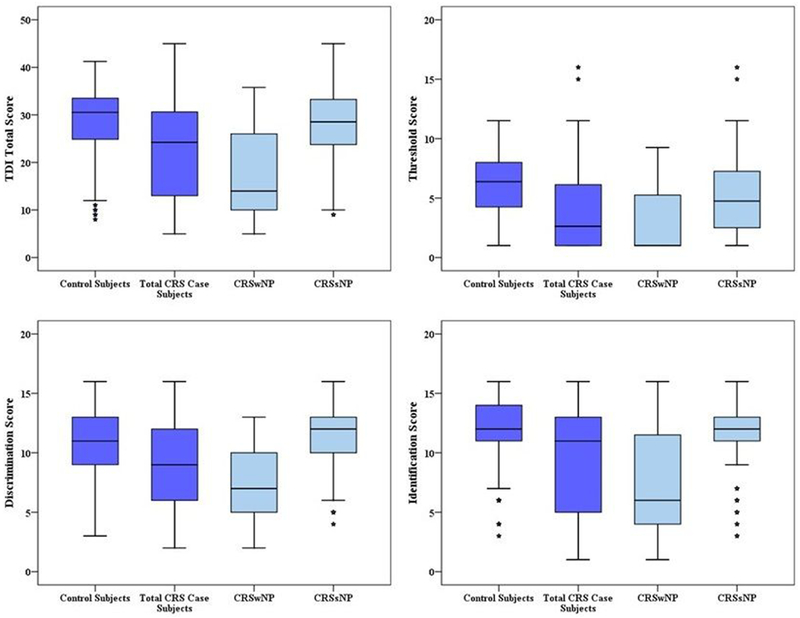
In order to examine differences in CRS patients based upon polyp status, we then compared mean olfactory scores, as well as diagnostic TDI categories between control subjects and CRS patients subdivided by polyp status. CRSsNP patients had mean threshold scores approximately 1.2 points worse than controls, however all other olfactory components and overall TDI classification were similar to controls. In contrast, CRSwNP patients had olfactory loss that affected every single component of olfaction and resulted in significantly worse TDI classification with the majority of CRSwNP being classified as anosmic (Figure 1; Table 3). Further examination of component classification demonstrates again the similarities between control subjects and patients with CRSsNP. Approximately ¾ of both groups had normal discrimination and identification. The only difference between these groups was threshold classification, as 46% of controls had impaired threshold compared to 64% of CRSsNP patients (Table 3). CRSwNP patients once again demonstrated a stark contrast when compared to controls, as over 70% of CRSwNP patients had abnormalities in each individual component of olfaction.
Figure 1:
Boxplot comparisons of TDI olfactory scores between control subjects and case subjects with both CRSwNP and CRSsNP. TDI, Threshold, Discrimination, and Identification. CRS, chronic rhinosinusitis; CRSwNP, chronic rhinosinusitis with nasal polyposis; CRSsNP, chronic rhinosinusitis without nasal polyposis. Subjects with CRSwNP had significantly lower TDI total scores and T,D, and I component scores compared to controls (all p<0.001) on average. Subjects with CRSsNP were found to only have significantly lower T component scores compared to controls subjects (4.9 [±3.2] vs. 6.1 [±2.7]; p=0.003).
Table 3:
Comparisons in the frequency of normal and abnormal olfactory scores between case subjects with CRS and control subjects without CRS.
| TDI total score classification: | Control Subjects (n=164) | Case Subjects with CRSsNP (n=105) | χ2 | p-value vs control | Case Subjects with CRSwNP (n=119) | χ2 | p-value vs control |
|---|---|---|---|---|---|---|---|
| Normosmia | 81 (49%) | 43 (41%) | 1.83 | 0.176 | 17 (14%) | <0.001 | |
| Hyposmia | 73 (45%) | 54 (51%) | 1.23 | 0.268 | 36 (30%) | 0.015 | |
| Anosmia | 10 (6%) | 8 (8%) | 0.24 | 0.626 | 66 (56%) | <0.001 | |
| TDI components | |||||||
| Normal T | 89 (54%) | 38 (36%) | 8.39 | 0.004 | 23 (19%) | 35.21 | <0.001 |
| Abnormal T | 75 (46%) | 67 (64%) | 96 (81%) | ||||
| Normal D | 121 (74%) | 79 (75%) | 0.07 | 0.789 | 31 (26%) | 63.19 | <0.001 |
| Abnormal D | 43 (26%) | 26 (25%) | 88 (74%) | ||||
| Normal I | 127 (77%) | 79 (75%) | 0.17 | 0.678 | 35 (29%) | 64.99 | <0.001 |
| Abnormal I | 37 (23%) | 26 (25%) | 84 (71%) |
CRSsNP, chronic rhinosinusitis without nasal polyposis; CRSwNP, chronic rhinosinusitis with nasal polyposis; SE, standard error of the mean; CI, confidence interval of the mean difference, SD, standard deviation; t, independent samples t-test statistic; χ2, chi-square test statistic; reported p-values correspond to two-sided significance. T, olfactory threshold; D, olfactory discrimination; I, olfactory identification.
Multivariate Modeling of Sniffin’ Stick Scores
Final multivariate linear regression modeling provided further elucidation of significant associations between patient cofactors and TDI olfactory outcome scores (Table 4). CRS of any type was associated with significantly lower threshold scores on average. However, CRS was not found to be independently associated with TDI total scores, discrimination, or identification component scores. The presence of nasal polyps, asthma and age were each independent drivers of TDI and all components of olfaction. Diabetes mellitus was also significantly associated with worse TDI total scores as well as lower identification component scores on average. Race, ethnicity and allergy status had statistically significant but variable impacts. No evidence of multicollinearity was found in any final regression model (all VIF<2.0) and standardized residual diagnostic plotting suggested adequate linearity and no violations of modeling assumption. Coefficient of multiple determination (R2) indicated the total variance explained by each final model to be between 29-40%.
Table 4:
Final adjusted multivariate modeling results for Sniffin’ Stick TDI scoring
| TDI total score (outcome): | Adjusted ƥ | SE | 95% CI | t-test statistic | p-value | R2 | VIF |
|---|---|---|---|---|---|---|---|
| ---- | ---- | ---- | ---- | ---- | ---- | ---- | |
| Constant term | 35.08 | 1.25 | 32.62, 37.54 | 28.06 | <0.001 | ---- | ---- |
| Any CRS (case subjects) | −0.15 | 0.92 | −1.97, 1.66 | −0.17 | 0.868 | ---- | 1.58 |
| Age (years) | −0.11 | 0.02 | −0.15, −0.07 | −5.01 | <0.001 | ---- | 1.06 |
| Nasal polyposis | −9.07 | 0.98 | −10.99, −7.15 | −9.28 | <0.001 | ---- | 1.55 |
| Asthma | −4.36 | 0.88 | −6.10, −2.63 | −4.96 | <0.001 | ---- | 1.22 |
| Diabetes mellitus (Type I/II) | −2.74 | 1.27 | −5.23, −0.25 | −2.16 | 0.031 | 0.396 | 1.06 |
| Threshold score (outcome): | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Constant term | 7.86 | 0.47 | 6.93, 8.79 | 16.64 | <0.001 | ---- | ---- |
| Any CRS (case subjects) | −1.05 | 0.34 | −1.73, −0.37 | −3.04 | 0.003 | ---- | 1.59 |
| Age (years) | −0.04 | 0.01 | −0.05, −0.02 | −4.60 | <0.001 | ---- | 1.03 |
| Hispanic/Latino ethnicity | 1.50 | 0.69 | 0.15, 2.84 | 2.18 | 0.030 | ---- | 1.03 |
| Nasal polyposis | −1.89 | 0.37 | −2.61, −1.18 | −5.19 | <0.001 | ---- | 1.56 |
| Asthma | −1.44 | 0.34 | −2.11, −0.77 | −4.24 | <0.001 | ---- | 1.31 |
| Positive allergy test (mRast/skin prick) | 0.75 | 0.30 | 0.16, 1.35 | 2.48 | 0.013 | 0.289 | 1.21 |
| Discrimination score (outcome): | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Constant term | 12.51 | 0.52 | 11.49, 13.52 | 24.30 | <0.001 | ---- | ---- |
| Any CRS (case subjects) | 0.37 | 0.36 | −0.34, 1.07 | 1.03 | 0.306 | ---- | 1.68 |
| Age (years) | −0.04 | 0.01 | −0.06, −0.03 | −5.04 | <0.001 | ---- | 1.02 |
| White/Caucasian | 0.92 | 0.37 | 0.19, 1.65 | 2.47 | 0.014 | ---- | 1.11 |
| Nasal polyposis | −3.28 | 0.37 | −4.00, −2.55 | −8.82 | <0.001 | ---- | 1.58 |
| Asthma | −1.50 | 0.33 | −2.16, −0.85 | −4.51 | <0.001 | 0.343 | 1.24 |
| Identification score (outcome): | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Constant term | 13.99 | 0.55 | 12.90, 15.07 | 25.37 | <0.001 | ---- | ---- |
| Any CRS (case subjects) | 0.19 | 0.41 | −0.61, 0.99 | 0.47 | 0.640 | ---- | 1.58 |
| Age (years) | −0.04 | 0.01 | −0.06, −0.02 | −3.77 | <0.001 | ---- | 1.06 |
| Nasal polyposis | −3.75 | 0.43 | −4.59, −2.90 | −8.70 | <0.001 | ---- | 1.55 |
| Asthma | −1.79 | 0.39 | −2.56, −1.03 | −4.62 | <0.001 | ---- | 1.22 |
| Diabetes mellitus (Type I/II) | −1.77 | 0.56 | −2.87, −0.67 | −3.16 | 0.002 | 0.348 | 1.06 |
T, olfactory threshold; D, olfactory discrimination; I, olfactory identification; ƥ, effect estimate; SE, standard error; CI, confidence interval; CRS, chronic rhinosinusitis; R2, coefficient of multiple determination (% model variance explained by included cofactors); VIF, variance inflation factor.
DISCUSSION
Our results confirm that a large proportion of patients with CRS have some degree of OD. They also confirm that OD in most CRS patients is multi-factorial and not due solely to the presence or absence of CRS. Prior reports of OD in CRS patients seeking care at tertiary centers range from 67 to 78% 15,16. While these studies were useful in identifying the prevalence of OD and contributing factors, they lacked a control group without CRS and only examined olfactory identification. Drivers of impaired olfactory identification were polyps, asthma, AERD, non-deviated septums, age and smoking7,8. Our study used more comprehensive olfactory assessments (Sniffin’ Sticks) and a control population to evaluate confounders. Again, nasal polyps were a major driver of OD in CRS. When examining CRSwNP patients we found that polyp status impacts all components of olfaction (T, D and I), and carries the largest effect of the documented clinical factors. The implications and mechanisms of this are unclear. OD has previously been thought of as conductive and/or sensorineural. Theoretically, one would think that OD secondary to mechanical obstruction of airflow may still allow patients to retain relatively normal discrimination and identification, as suprathreshold stimuli are delivered. This may vary depending upon the size of polyps and the severity of obstruction, but at present, it appears that broad dysfunction is present in CRSwNP. It is also possible that specific endotypes within the CRSwNP phenotype have varying impacts upon olfaction. Prior studies have shown that specific olfactory cytokines are associated with OD2 and this remains an area for further study.
Perhaps most interestingly, CRSsNP has some OD, particularly with mean threshold scores, but actually appears to have overall olfactory function similar to control subjects. Thus, OD in the CRSsNP appears to be multi-factorial with significant contribution from non-CRS factors. Only loss of threshold seems to be related specifically to CRSsNP, as discrimination and identification scores are similar to controls. It is possible that CRSsNP patients have such mild CRS that olfaction was not impacted. When examining metrics of CRS severity, not surprisingly, CRSwNP had more severe endoscopy and CT scores. However, our CRSsNP cohort still had fairly significant inflammation as evidenced by CT, endoscopy and SNOT22 measures. Again, this raises a number of questions as threshold is classically attributed to peripheral olfactory epithelial function. Mechanistically, it is possible that some intact neuroepithelium remains responsive to suprathreshold stimuli measured by discrimination and identification assessments. Impaired threshold may be due to modified airflow, altered mucus, decreased quantity of functional olfactory neurons or other factors. Future studies using matched control populations will need to explore these mechanisms specifically.
These findings may also have direct clinical implications. Providers treating patients with CRSsNP may be inclined to attribute any OD to the inflammatory/infectious pathology noted in CRS. Patients may similarly blame their olfactory loss on CRS. Traditionally, therapeutic efforts have focused largely upon medical and/or surgical treatments to decrease inflammation/infection or improve anatomy, yet many patients with CRSsNP related OD fail to improve despite our best efforts at managing CRS. The most dramatic improvements in objective olfaction after sinus surgery are found in anosmic CRSwNP patients17. Hyposmic CRSwNP and CRSsNP patients with any degree of OD improve somewhat, but generally remain in the hyposmic range postoperatively. In light of our study, it seems reasonable to attribute some degree of OD in these patients to non-CRS factors, such as comorbid diabetes, asthma or the aging process, which is directly relevant for patient counseling4,18,19. Thus, our expectations for treatment outcomes and our overall therapeutic strategy for treating OD in CRSsNP may require reassessment, as it may not be realistic for all patients to return to normosmia unless other confounders and comorbid conditions are also addressed.
Multivariate modeling demonstrated a few surprising results. While it was expected that polyps would be a major driver in CRS-associated OD, the independent, broad impacts of asthma, diabetes and aging are interesting. It is possible comorbid asthma has systemic inflammatory effects that impact olfaction in ways independent of polyp status. Others have reported an association between asthma and sensorineural hearing loss 20 and visual loss21. Similarly, DM has been associated with OD22,15 as well as other forms of sensory loss, including auditory dysfunction23 and visual loss 24. A variety of mechanisms have been suggested, including microvascular and macrovascular changes, and diabetic neuropathy25,26. Age is known to impact OD and we found that these associations generally were consistent for all components of TDI27. It is possible that these factors impact olfactory neuroepithelium, olfactory neurons or central olfactory processing sites. Precise mechanisms for these associations are unknown, but the mean age of our CRS cohort was 50, an age above which olfactory loss becomes more prevalent across the population. Our modeling explained 29-40% of data variation in olfaction scores. While that is relatively high for this type of modelling, it does not explain all of the variation. Clearly, there are unmeasured confounding factors, such as genetics, cognition, olfactory exposures, and others, that may impact olfaction across a population and these will be important to identify and model with future investigations. Interestingly, allergy had small positive effect on threshold. Litvack et al. previously examined a CRS only cohort using smell identification and failed to find any impact due to allergy, but did find positive impact of septal deviation and turbinate hypertrophy8. It is unclear if these factors somehow impact olfactory airflow or if medications for turbinate hypertrophy improve olfaction via other mechanisms. We also examined the potential impact of prior sinus surgery upon olfaction. When placed into multivariate modeling, it was not significantly associated with any aspect of olfaction. This does not mean that resection of olfactory bearing tissue, such as complete middle turbinate resections, or postsurgical alteration of airflow to the olfactory cleft does not impact olfaction for an individual patient. However, it was not a significant factor across our large cohort.
Prior studies using olfactory function tests have described normative values that vary by age and gender 14,28 and generally report worse olfaction in males and older subjects. However, this type of classification inherently assumes that such olfactory loss is “normal”. It is also in contrast to testing for other sensory functions, such as vision or hearing, where ideal values are established and normal vision or normal hearing do not vary with age or gender. While use of age-adjusted normative values would have altered the reported prevalence of TDI classifications, we treated olfactory scores as continuous variables for multivariate modeling (Table 4) and comparison of absolute values (Table 2) and our findings ultimately would not have changed.
Conclusions
Olfactory loss in CRS is largely driven by polyp status, but also by asthma, DM and age. Patients with CRSsNP have olfactory function that is similar to matched control subjects with the exception of decreased olfactory threshold. Successful counseling and treatment of OD in patients with CRSsNP should focus upon other contributing comorbidities in addition to management of CRS.
Acknowledgments
Funding source: National Institute on Deafness and Other Communication Disorders, National Institutes of Health (R01 DC005805 [PI: T.L.S./Z.M.S.] to J.A.A., R.J.S., J.C.M., T.L.S., V.R.R. and Z.M.S.). This funding organization did not contribute to the design or conduct of this study, preparation, review, approval, or decision to submit this manuscript for publication
Footnotes
Presented at American Rhinologic Society Meeting, New Orleans, LA, September 14, 2019
The abstract for this manuscript was submitted for oral / podium presentation to the American Rhinologic Society during the American Academy of Otolaryngology-Head and Neck Surgery Annual Meeting & OTO Experience in New Orleans, LA., September 13-14, 2019.
Financial disclosures and conflict of interest: ZMS: Olympus, OptiNose, Regeneron, Healthy Humming, and Novartis, consultant (not affiliated with this study); JAA.: Medtronic, OptiNose, Spirox, and GlycoMira Therapeutics, consultant (not affiliated with this study); RJS.: OptiNose, Olympus, Stryker, Regeneron, and Healthy Humming, consultant (not affiliated with this study); VRR: OptiNose and Medtronic, Inc, consultant (not affiliated with this study); DMB: none.
References
- 1.Soler ZM, Hyer JM, Karnezis TT, Schlosser RJ. The Olfactory Cleft Endoscopy Scale correlates with olfactory metrics in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(3):293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlosser RJ, Mulligan JK, Hyer JM, et al. Mucous cytokine levels in chronic rhinosinusitis-associated olfactory loss. JAMA Otolaryngol - Head Neck Surg. 2016;142(8):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soler ZM, Pallanch JF, Sansoni ER, et al. Volumetric computed tomography analysis of the olfactory cleft in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5(9):846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattos JL, Schlosser RJ, Storck KA, Soler ZM. Understanding the relationship between olfactory-specific quality of life, objective olfactory loss, and patient factors in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7(7):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soler ZM, Smith TL, Alt JA, et al. Olfactory-specific quality of life outcomes after endoscopic sinus surgery. Int Forum Allergy Rhinol. 2016;6(4):407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinology Suppl. 2017;54(26):1–30. [DOI] [PubMed] [Google Scholar]
- 7.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23(2):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litvack JR, Fong K, Mace J, James KE, Smith TL. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope. 2008;118(12):2225–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical Practice Guideline (Update): Adult Sinusitis. Otolaryngol Neck Surg. 2015;152(2_suppl):S1–S39. [DOI] [PubMed] [Google Scholar]
- 10.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology 1993;31(4):183–4. [PubMed] [Google Scholar]
- 11.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg 1997;117(3Pt2):S35–40. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric Validity of the 22-Item Sinonasal Outcome Test. Vol 34 Blackwell Publishing Ltd; 2009. [DOI] [PubMed] [Google Scholar]
- 13.Kobal G, Hummel T, Sekinger B, et al. Sniffin’ sticks": screening of olfactory performance. Rhinology 1996;34(4):222–226. [PubMed] [Google Scholar]
- 14.Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3,000 subjects. Eur Arch Oto-Rhino-Laryngology 2007;264(3):237–243. [DOI] [PubMed] [Google Scholar]
- 15.Soler ZM, Kohli P, Storck KA, Schlosser RJ. Olfactory Impairment in Chronic Rhinosinusitis Using Threshold, Discrimination, and Identification Scores. Chem Senses. 2016;41(9):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohli P, Naik AN, Harruff EE, et al. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope 2017;127(2):309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litvack JR, Mace J, Smith TL. Does olfactory function improve after endoscopic sinus surgery? Otolaryngol - Head Neck Surg. 2009;140(3):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattos JL, Schlosser RJ, Mace JC, Smith TL, Soler ZM. Establishing the minimal clinically important difference for the Questionnaire of Olfactory Disorders. Int Forum Allergy Rhinol. 2018;8(9):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattos JL, Rudmik L, Schlosser RJ, et al. Symptom importance, patient expectations, and satisfaction in chronic rhinosinusitis. Int Forum Allergy Rhinol 2019;9(6):593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilic T, Karatas E, Toplu Y, et al. Evaluation of auditory functions in patients with asthma. Eur Rev Med Pharmacol Sci 2014;18(18):2615–20. [PubMed] [Google Scholar]
- 21.André R, Cottin V, Saraux JL, et al. Central nervous system involvement in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Report of 26 patients and review of the literature. Autoimmun Rev 2017;16(9):963–9. [DOI] [PubMed] [Google Scholar]
- 22.Gouveri E, Katotomichelakis M, Gouveris H, et al. Olfactory dysfunction in type 2 diabetes mellitus: An additional manifestation of microvascular disease? Angiology 2014;65(10):869–76. [DOI] [PubMed] [Google Scholar]
- 23.Teng ZP, Tian R, Xing FL, et al. An association of type 1 diabetes mellitus with auditory dysfunction: A systematic review and meta-analysis. Laryngoscope 2017;127(7):1689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan A, Petropoulos IN, Ponirakis G, Malik RA. Visual complications in diabetes mellitus: beyond retinopathy. Diabet Med 2017;34(4):478–84. [DOI] [PubMed] [Google Scholar]
- 25.Zaghloul H, Pallayova M, Al-Nuaimi O, et al. Association between diabetes mellitus and olfactory dysfunction: current perspectives and future directions. Diabet Med 2018;35(1):41–52. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi M, Zochodne DW. Diabetic neuropathy and the sensory neuron: New aspects of pathogenesis and their treatment implications. J Diabetes Investig 2018;9(6):1239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto JM, Wroblewski KE, Kern DW, et al. The Rate of Age-Related Olfactory Decline Among the General Population of Older U.S. Adults. Journals Gerontol - Ser A Biol Sci Med Sci. 2015;70(11):1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doty RL, Kamath V. The influences of age on olfaction: A review. Front Psychol 2014;5:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]