Abstract
Background:
Matrix metalloproteinases (MMP) are being considered important mediators in cancer invasion, and plenty of research is in progress. Our objective was to evaluate the presence of MMP-2 and tissue inhibitor of metalloproteinase (TIMP-2) in oral lichen planus (OLP) and to assess its role in the pathogenesis of OLP and as an indicator of malignant transformation.
Materials and Methods:
Immunohistochemical analysis for MMP-2 and TIMP-2 was performed in thirty histopathologically confirmed, formalin-fixed, paraffin-embedded specimens of OLP (24 cases of reticular and 6 cases of erosive LP). A semi-quantitative analysis was done to assess the expression and distribution of this marker in these lesions.
Results:
In all cases of OLP, MMP-2 expression was seen mainly in areas of lymphocytic inflammatory infiltrate (100%) in the lamina propria within the overlying epithelium. TIMP-2 expression was seen more than 50% in the fibroblasts and basal and parabasal cells.
Conclusion:
The expression of MMP-2 and TIMP-2 was observed in all cases of OLP. However, a clinical 5-year follow-up of the lesion revealed no progression of the disease except for chronic exacerbation and regression of these lesions. Although our study considers MMP-2 and TIMP-2 as mediators in the pathogenesis of OLP, it still remains debatable whether they have a direct role to play in the disease process or whether they are suitable biomarkers to assess the disease progression.
Keywords: Biomarkers, excision, inflammation, lichen planus, malignancy
INTRODUCTION
Oral lichen planus (OLP) is a common chronic inflammatory disorder that affects the oral mucous membrane with an estimated prevalence of 1%–2% in the general population.[1] The disease usually manifests between the age of 50 and 70 years with a preponderance to women, with the F:M ratio being 2:15.[2,3] Lesions are most often found on the buccal mucosa, tongue and gingiva, but occasionally on the lips and palate.[4,5] These lesions do have a waxing and waning course.
Histologically, OLP is characterized by the presence of subepithelial band of lymphocytic infiltrate and epithelial basal cell destruction with formation of apoptotic bodies for which T-cell-mediated autoimmune mechanism plays an important role. It has been observed that keratinocytes undergo apoptosis and are secondary to basement membrane destruction.[6]
Immune antigen-specific and antigen nonspecific mechanisms may be involved in the pathogenesis of OLP. Nonspecific mechanisms include mast cell degranulation and matrix metalloproteinase (MMP) activation in OLP.[7] MMPs, a family of zinc- and calcium-dependent proteolytic enzymes, are involved in a wide variety of biological processes ranging from physiological (cell proliferation and differentiation) to pathological states associated with inflammation, degeneration, tumor metastasis and growth. In normal tissues, MMPs are expressed at very low levels, and their production and activation is rapidly induced when active tissue remodeling is needed. Further, their activity is regulated by a group of specific tissue inhibitors of matrix proteinases (TIMPs).[8,9]
MMP-2, the most widely distributed of all MMPs, has been identified in skin fibroblasts, keratinocytes, chondrocytes, endothelial cells, monocytes, osteoblasts and in a number of other normal and transformed cells.[10] Along with MMP-9, it is said to digest a number of extracellular matrix (ECM) molecules including type IV, V and XI collagens; laminin and aggrecan core protein and singly digests collagens I, II and III. However, because proMMP-2 is recruited to the cell surface and activated by the membrane-bound MT-MMPs, it may express reasonable collagenolytic activity on or near the cell surface.[11] There are reports on malignant transformation in OLP.[2,4] Disruption of basement membrane plays a crucial role in the migration of dysplastic cells. MMP-2 and MMP-9 play an important role in the cleavage of type IV collagen, thereby facilitating this process. This prompted us to conduct this study to assess the expression of and elucidate the role of MMP-2 and inhibitor TIMP-2 in OLP.
MATERIALS AND METHODS
This laboratory-based study involved the use of thirty buffered, formalin-fixed, paraffn-embedded tissue blocks of histologically proven cases of OLP. Diagnosis was confirmed by two oral pathologists using hematoxylin and eosin-stained sections [Figure 1]. Inclusion criteria had patients clinically and histopathologically proven disease, whereas patients with comorbidities (hypertension and diabetes) and those previously/presently being treated for OLP, lichenoid reaction, lichenoid dysplasia and lesions mimicking oral and skin LP but not confirmed by biopsy were excluded from this study. Ten apparently normal oral mucosal tissues which were age and sex matched and obtained at the time of tooth extraction (with patients’ consent) were used as controls [Table 1]. A university ethical committee approval was obtained prior to carrying out this study. Participants taking part gave written informed consent.
Figure 1.
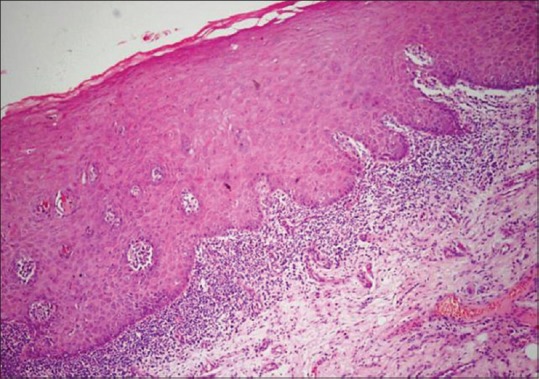
Photomicrograph showing hyperplastic parakeratinized stratified squamous epithelium with band like lymphocytic infiltration (H&E, ×200)
Table 1.
Clinical data of the patients included in the study
| n | Age | Sex | Habits | Site | Type | HP |
|---|---|---|---|---|---|---|
| 1 | 40 | Male | Occasional tobacco smoking | Buccal mucosa | Reticular | LP |
| 2 | 38 | Male | No habits | Buccal mucosa | Reticular | LP |
| 3 | 45 | Female | No habits | Buccal mucosa | Reticular | LP |
| 4 | 47 | Female | No habits | Buccal mucosa | Erosive | LP |
| 5 | 42 | Male | No habits | Buccal mucosa | Reticular | LP |
| 6 | 44 | Male | No habits | Buccal mucosa | Reticular | LP |
| 7 | 36 | Male | No habits | Buccal mucosa | Reticular | LP |
| 8 | 43 | Male | No habits | Buccal mucosa | Reticular | LP |
| 9 | 45 | Male | No habits | Buccal mucosa | Reticular | LP |
| 10 | 42 | Female | No habits | Buccal mucosa | Reticular | LP |
| 11 | 45 | Male | Occasional tobacco smoking | Buccal mucosa | Erosive | LP |
| 12 | 43 | Female | No habits | Buccal mucosa | Reticular | LP |
| 13 | 46 | Male | Occasional tobacco smoking | Buccal mucosa | Reticular | LP |
| 14 | 38 | Male | Occasional tobacco smoking | Buccal mucosa | Reticular | LP |
| 15 | 43 | Female | No habits | Lateral border of tongue | Erosive | LP |
| 16 | 42 | Male | No habits | Buccal mucosa | Reticular | LP |
| 17 | 43 | Male | No habits | Buccal mucosa | Reticular | LP |
| 18 | 44 | Female | No habits | Buccal mucosa | Reticular | LP |
| 19 | 42 | Male | No habits | Buccal mucosa | Erosive | LP |
| 20 | 46 | Male | No habits | Buccal mucosa | Reticular | LP |
| 21 | 45 | Male | No habits | Buccal mucosa | Reticular | LP |
| 22 | 42 | Male | No habits | Buccal mucosa | Reticular | LP |
| 23 | 41 | Male | No habits | Buccal mucosa | Reticular | LP |
| 24 | 41 | Female | No habits | Buccal mucosa | Erosive | LP |
| 25 | 43 | Male | No habits | Buccal mucosa | Reticular | LP |
| 26 | 46 | Male | No habits | Buccal mucosa | Reticular | LP |
| 27 | 45 | Male | No habits | Buccal mucosa | Reticular | LP |
| 28 | 45 | Male | No habits | Buccal mucosa | Erosive | LP |
| 29 | 42 | Male | No habits | Buccal mucosa | Reticular | LP |
| 30 | 47 | Male | No habits | Buccal mucosa | Reticular | LP |
LP: Lichen planus, HP: Helicobacter pylori
Immunohistochemistry
Immunohistochemical analysis for MMP-2 and TIMP-2 was performed using streptavidin-biotin-peroxidase technique. Paraffn-embedded OLP tissues were cut into 4-μm thick sections and were taken onto 2% 3-aminopropyltriethoxysilane solution (Sigma Aldrich Chemical Co., St Louis, MO, USA) adhesive-coated micro slides. Antigen retrieval was carried out using commercial microwave antigen retrieval system where the sections were placed in a container containing 10 mM Tris ethylenediaminetetraacetic acid buffer (pH 9) at 96°C for three cycles of 6 min each (SAMSUNG, India). After rinsing in Tris-buffered saline (TBS), these sections were treated with peroxidase block consisting of 3% H2O2 in water for 15 min to block the endogenous peroxidase activity. This was followed by a power block for 20 min to block any nonspecific antigenic sites. The sections were subsequently incubated at room temperature for 3 h with primary anti-sera mouse monoclonal anti-MMP-2 antibody diluted at 1:20 (NCL-MMP2-507, Clone: 17B11, Leica Biosystems New Castle Ltd., New Castle, UK) and anti-TIMP-2 antibodies diluted at 1:20 (NCL-TIMP2-487, Clone: 46E5, Leica Biosystems, New Castle Ltd., UK), in a moist chamber washed with TBS followed by postprimary block, after which they were incubated with a secondary antibody for 30 min in a moist chamber applying NovoLink™ polymer reagent. Visualization was performed using freshly prepared 3,3-diaminobenzidine tetrachloride (Dako Cytomation, Glostruop, Denmark) chromogen for 10 min. The slides were counterstained with Harris hematoxylin, subsequent to which sections were dehydrated, cleared and mounted with dibutylpthalate xylene.
For each batch of staining, positive and negative controls were run simultaneously with the study specimens. Five cases of inflammatory bowel were taken as positive control, while internal positive control being endothelial cells, whereas the primary antibodies were replaced by nonimmune mouse serum at the same dilutions for the negative controls. Normal mucosa was taken as normal control.
Immunohistochemical analysis
The expression of MMP-2 and TIMP-2 was assessed separately in the epithelium and lamina propria. In the epithelium, the basal and suprabasal layers were found immune positive for these markers. A total of 100 cells each of basal and para-basal layers were examined in each of the randomly selected five different fields at ×40 magnification to study the expression pattern. In the lamina propria, inflammatory cells, fibroblasts and endothelial cells were positive. To eliminate any interobserver bias, the expression of MMP-2 and TIMP-2 was studied independently. The percentage of staining was analyzed semiquantitatively according to the method of Fregnani et al.[11]
Statistical analysis
Statistical analysis was performed using SPSS version 15 (Package for Social Sciences, Chicago, Illinois, USA) software for Windows. To remove any possible bias and to minimize the interobserver variability, the scores of both observers were subjected to Kendall's tau-b test. The P value thus obtained was found to be nonsignificant, and thus the expression of MMP-2 and TIMP-2 graded by one observer was considered for further statistical analysis. Chi-square test was used to compare epithelial and mesenchymal staining for MMP-2 and TIMP-2. P = 0.05 was considered statistically significant.
RESULTS
The expression of MMP-2 and TIMP-2 was observed in the epithelial and mesenchymal components of OLP [Figures 2 and 3]. In all cases of OLP, MMP-2 expression was seen mainly in the lymphocytic band in the lamina propria with few lymphocytes which infiltrate the overlying epithelium [Figure 2]. A stained percentage of >50% of cells positive for MMP-2 predominantly were the inflammatory cells in all the thirty cases (100%), while fibroblasts, endothelial, basal and parabasal stained less than 50% in descending order. There was statistical significance in the comparison of MMP-2 and TIMP-2 in basal, parabasal, inflammatory, fibroblast and endothelial cells of LP versus normal (P < 0.05) [Table 2]. In normal mucosa, the MMP-2 expression was seen in the endothelial cells (seven out of the ten cases) [Figure 4]. The expression of MMP-2 was also observed in the cytoplasm of the columnar cells, secretory cells and inflammatory cells of the stroma of inflammatory bowel [Figure 5]. The expression of TIMP-2 (>50% cells) was observed predominantly in basal and parabasal cells followed by fibroblasts [Table 2].
Figure 2.
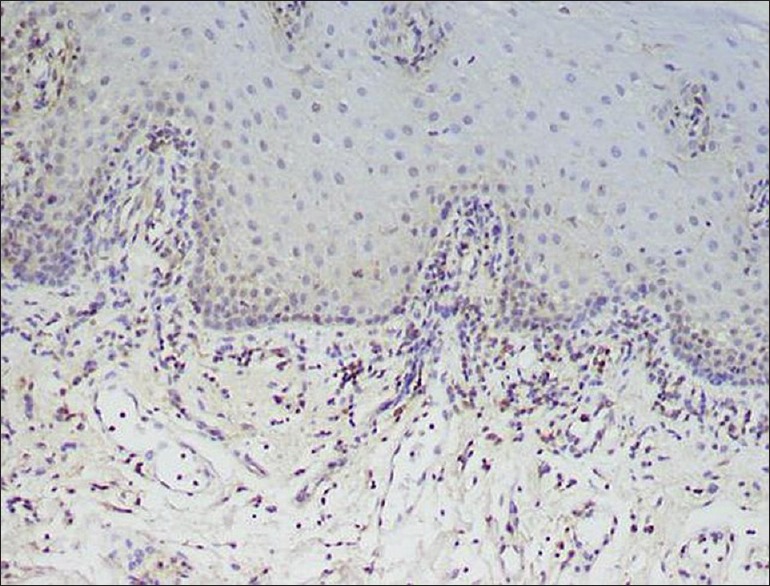
Positive expression of matrix metalloproteinases-2 within the lymphocytes in the lamina propria and sparse staining of keratinocytes within the basal layer and basement membrane (×400)
Figure 3.
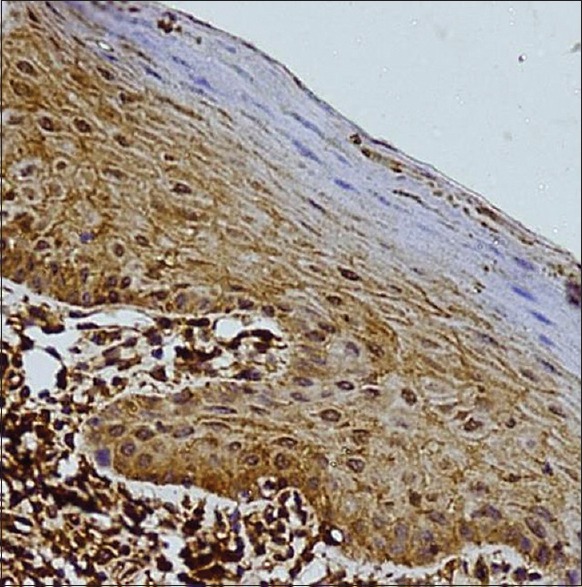
Positive expression of TIMP-2 within the lymphocytes in the lamina propria and the keratinocytes (×400)
Table 2.
Expression of matrix metalloproteinases-2 and tissue inhibitor of metalloproteinase-2 in cells of basal, suprabasal, lamina propria and deeper connective tissue in all cases of lichen planus
| Cells | MMP-2 | TIMP-2 | χ2 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1%-50% | >50% | Negative expression | Total | 1%-50% | >50% | Negative expression | Total | |||
| Basal cells | ||||||||||
| Normal | - | - | 10 | 10 | - | - | 10 | 10 | 13.44 | <0.05 |
| LP | 20 | 10 | - | 30 | 5 | 25 | 30 | |||
| Parabasal cells | ||||||||||
| Normal | - | - | 10 | 10 | - | - | 10 | 10 | 21.624 | <0.05 |
| LP | 25 | 5 | - | 30 | 6 | 24 | - | 30 | ||
| Inflammatory cells | ||||||||||
| Normal | - | - | 10 | 10 | - | - | 10 | 10 | 19.176 | <0.05 |
| LP | - | 30 | - | 30 | 16 | 14 | - | 30 | ||
| Fibroblast cells | ||||||||||
| Normal | - | - | 10 | 10 | - | - | 10 | 10 | 4.86 | <0.05 |
| LP | 25 | 5 | - | 30 | 10 | 10 | 20 | |||
| Endothelial cells | ||||||||||
| Normal | 7 | - | 3 | 10 | 8 | - | 2 | 10 | 7.67 | >0.05 |
| LP | 24 | 6 | - | 30 | 25 | 5 | - | 30 | ||
LP: Lichen planus, MMP: Matrix metalloproteinases, TIMP: Tissue inhibitor of metalloproteinase
Figure 4.
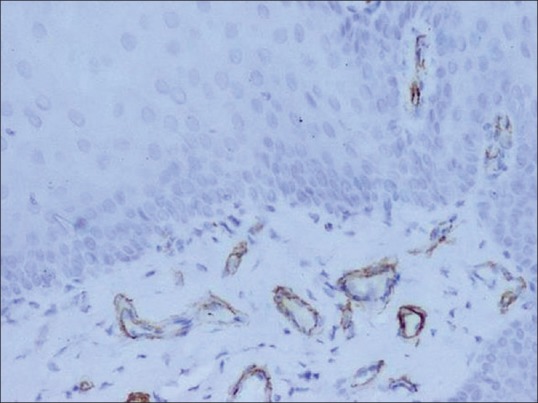
Positive expression of matrix metalloproteinases-2 within the endothelial cells in normal buccal mucosa (×200)
Figure 5.
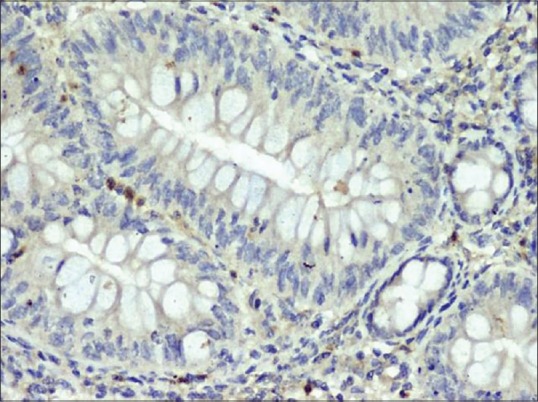
Expression of matrix metalloproteinases-2 in inflammatory bowel (×400)
DISCUSSION
OLP is a chronic inflammatory disease of unknown etiology. However, the physiopathology of this disease is very complex with involvement of both antigen-specific and antigen nonspecific mechanisms. MMPs are regarded as one of the enzyme systems that play a role in both physiological and pathological processes. Clinical features and accompanying symptoms range from asymptomatic reticular LP to erosive/ulcerated areas accompanied with pain and discomfort,[12,13] which were evident in our study. MMP-2 (gelatinase A), along with MMP-9 (gelatinase B), digests a number of ECM molecules including type IV, V and XI collagens; laminin and aggrecan core protein.[11,14] In oral squamous cell carcinoma (OSCC), there is an increased expression of MMP and is said to play an important role in tumor progression.[15] It was observed in OLP that the rate of malignancy varied, with tongue being the most common site and was more prevalent among the erosive variant.[16] We analyzed thirty case files of patients of OLP and found only six cases to be erosive type, while the rest were reticular in nature. However, during 5-year follow-up of our patients, none have progressed to OSCC though the condition appears to be waxing and waning while the treating dose of corticosteroids is being tapered.
In all cases MMP-2 expression was observed in the inflammatory cells, which was also seen in association with keratinocytes. Hence, the destruction of basement membrane with apoptosis of keratinocytes may be related to MMPs produced by the lymphocytes. It has also been observed in various cysts and tumors that increased production of MMP-2 relates to aggressiveness of the lesion.[14] TIMP-2 immuno-expression was also observed in basal and parabasal cells, (P< 0.05) in more than 50% of cells, which was significant, and this could relate to the slowing down process of destruction. Chen et al. from their study concluded that imbalance between MMPs and TIMPs may be involved in cancerization, and these markers may be useful for judging the potency of malignant transformation of OLP.[17] Similarly, in ten out of the thirty cases, basal cells expressed >50% for MMP-2, when compared to TIMP-2 this relates, these cells may not have adequate enzymatic activity to bring about the basement membrane changes i. e., degrade type IV collagen or gelatin, moreover using other biomolecules like cell adhesion molecules, nuclear proteins, transcriptional factors may be help us to understand the biological behaviour of the epithelial cells in the disease process. Among the LP variants, erosive variety is considered to be aggressive, and all the six cases of our study reported to be erosive LP had MMP-2 >50% in the inflammatory component, basal cells and parabasal layer. These cases are in the stage of remission and exacerbation with nonmalignant changes so far.
The expression of MMPs was found to be higher in OSCC and atrophic LP when compared to nonatrophic type, but Mazzarella et al. reported higher levels of MMP-9 in reticular LP than in erosive type.[18] However, the role of MMPs in the transformation of LP to OSCC is questionable because there have been reports of increased expression of MMPs in patients with chronic dermatitis with no break in the basement membrane.[19]
In case of normal epithelium, the basal and parabasal layers, inflammatory cells and fibroblasts were negative for MMP-2 and TIMP-2 expression. Seven out of the ten cases of normal mucosa showed endothelial cell positivity for MMP-2 expression (<50% cells), whereas eight out of the ten cases showed immunopositivity for TIMP-2. This could be related to the fact that for normal physiological homeostasis, a balance between the two markers plays an important role. In most of the cases, more than 50% of fibroblasts and basement membrane was positive for TIMP-2, which could relate the possibility of tissue remodeling process. Although MMP-2 is one of the common inflammatory enzymes present in almost all cases of OLP, its specific role is still disputable. These results from our study might provide a basis for considering MMP-2 as an important mediator in the pathogenesis of the disease. It would be extremely interesting to study the finest regulation of MMP-2 as a possible target for the treatment of OLP.
CONCLUSION
The expression of MMP-2 and TIMP-2 was observed in all cases of OLP. However, a clinical 5-year follow-up of the lesion revealed no progression of the disease except for chronic exacerbation and regression of these lesions. Although our study considers MMP-2 and TIMP-2 as mediators in the pathogenesis of OLP, it still remains debatable whether it has a direct role to play in the disease process or whether it is a suitable biomarker to assess the disease progression. However, future research is essential to identify various other MMPs or related molecules (proteases) that play a role in disease process like LP; to develop tailored targeted molecules which would reduce or inhibit the disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dissemond J. Oral lichen planus: An overview. J Dermatolog Treat. 2004;15:136–40. doi: 10.1080/09546630410030720. [DOI] [PubMed] [Google Scholar]
- 2.Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol. 2002;46:207–14. doi: 10.1067/mjd.2002.120452. [DOI] [PubMed] [Google Scholar]
- 3.Yardimci G, Kutlubay Z, Engin B, Tuzun Y. Precancerous lesions of oral mucosa. World J Clin Cases. 2014;2:866–72. doi: 10.12998/wjcc.v2.i12.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Waal I. Oral lichen planus and oral lichenoid lesions; a critical appraisal with emphasis on the diagnostic aspects. Med Oral Patol Oral Cir Bucal. 2009;14:E310–4. [PubMed] [Google Scholar]
- 5.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 6.Lu R, Zhang J, Sun W, Du G, Zhou G. Inflammation-related cytokines in oral lichen planus: An overview. J Oral Pathol Med. 2015;44:1–4. doi: 10.1111/jop.12142. [DOI] [PubMed] [Google Scholar]
- 7.Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi: 10.1155/2014/742826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54. [PubMed] [Google Scholar]
- 9.Stetler-Stevenson WG, Liotta LA, Kleiner DE., Jr Extracellular matrix 6: Role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993;7:1434–41. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- 10.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 11.Fregnani ER, Sobral LM, Alves FA, Soares FA, Kowalski LP, Coletta RD. Presence of myofibroblasts and expression of matrix metalloproteinase-2 (MMP-2) in ameloblastomas correlate with rupture of the osseous cortical. Pathol Oncol Res. 2009;15:231–40. doi: 10.1007/s12253-008-9110-4. [DOI] [PubMed] [Google Scholar]
- 12.Nadalin MR, Fregnani ER, Silva-Sousa YT, da Cruz Perez DE. Presence of myofibroblasts and matrix metalloproteinase 2 in radicular cysts, dentigerous cysts, and keratocystic odontogenic tumors: A comparative immunohistochemical study. J Endod. 2012;38:1363–7. doi: 10.1016/j.joen.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Covani U, Marconcini S, Giacomelli L, Sivozhelevov V, Barone A, Nicolini C, et al. Bioinformatic prediction of leader genes in human periodontitis. J Periodontol. 2008;79:1974–83. doi: 10.1902/jop.2008.080062. [DOI] [PubMed] [Google Scholar]
- 14.Henriques ÁC, Vasconcelos MG, Galvão HC, de Souza LB, de Almeida Freitas R. Comparative analysis of the immunohistochemical expression of collagen IV, MMP-9, and TIMP-2 in odontogenic cysts and tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:468–75. doi: 10.1016/j.tripleo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Krisanaprakornkit S, Iamaroon A. Epithelial-mesenchymal transition in oral squamous cell carcinoma. ISRN Oncol. 2012;2012:681469. doi: 10.5402/2012/681469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang M, Zhang W, Chen Y, He Z. Malignant transformation of oral lichen planus: A retrospective study of 23 cases. Quintessence Int. 2009;40:235–42. [PubMed] [Google Scholar]
- 17.Chen Y, Zhang W, Geng N, Tian K, Jack Windsor L. MMPs, TIMP-2, and TGF-beta1 in the cancerization of oral lichen planus. Head Neck. 2008;30:1237–45. doi: 10.1002/hed.20869. [DOI] [PubMed] [Google Scholar]
- 18.Mazzarella N, Femiano F, Gombos F, De Rosa A, Giuliano M. Matrix metalloproteinase gene expression in oral lichen planus: Erosive vs. reticular forms. J Eur Acad Dermatol Venereol. 2006;20:953–7. doi: 10.1111/j.1468-3083.2006.01693.x. [DOI] [PubMed] [Google Scholar]
- 19.Gunduz K, Demireli P, Inanir I, Nese N. Expression of matrix metalloproteinases (MMP-2, MMP-3, and MMP-9) and fibronectin in lichen planus. J Cutan Pathol. 2006;33:545–50. doi: 10.1111/j.1600-0560.2006.00456.x. [DOI] [PubMed] [Google Scholar]


