Abstract
A 43-year-old male patient reported with a swelling in the left submandibular region of 3–4 months’ duration. The soft fluctuant swelling was painless and cystic on palpation. The excised submandibular gland was submitted for histopathological examination, which showed the presence of a bluish, thin-walled cystic cavity containing gelatinous straw-colored fluid. H and E-stained sections showed thin cystic lining with papillary projections of salivary glandular cells and multiple cystic spaces. Immunohistochemistry was recommended to differentiate between acinic cell carcinoma of papillary-cystic variant and low-grade mucoepidermoid carcinoma.
Keywords: Acinic cell carcinoma papillary-cystic variant, mucoepidermoid carcinoma, submandibular salivary gland
INTRODUCTION
Submandibular swelling is a common clinical disorder of the maxillofacial region that may be one of the manifestations of several pathologic disorders including cyst or tumor. Salivary gland tumors are uncommon, and salivary gland malignancies are even rarer.[1] Slow-growing soft and fluctuant swelling in the submandibular region warrants a clinical diagnosis of benign lesion. The diversity of the diseases varies mainly with the localization of the glands. Bacterial and viral infections, sialolithiasis, systemic diseases such as Sjögren's syndrome and Mikulicz's disease, granulomatous diseases such as tuberculosis and actinomycosis, cystic lesions and tumoral lesions can all be noted as miscellaneous diseases affecting the salivary glands. As tumors are most frequent lesions of the parotid glands, sialolithiasis and inflammatory diseases are common features of the submandibular glands.[2] The propensity of malignancy increases as the size of the gland decreases; 25% of the parotid gland neoplasms, 43% of the submandibular gland and 82% of the minor salivary gland neoplasms are reported as malignant in the literature.[3]
CASE REPORT
A 43-year-old male patient reported with a swelling in the left submandibular gland region. The swelling was soft, fluctuant and painless and the patient gave a history of 6–8 months’ duration. There was no change in size during meals and remained asymptomatic other than the obvious facial asymmetry due to the evident swelling of approximately 6 cm × 4 cm in size. The clinical diagnosis was sialocele (cyst of the submandibular salivary gland) and Cysticercus cellulosae. The submandibular salivary gland was excised and submitted for histopathology.
Gross features
Gross appearance of the received specimen was brownish in color of 7 cm × 6 cm × 6 cm in size. Lower border of the specimen showed a fluctuant swelling of bluish color. On incising the fluctuant swelling, a straw-colored gelatinous fluid of approximately 2–3 ml in volume was expulsed and cystic cavity was exposed. A soft whitish nodular projection was present within the cystic cavity. Different parts of the excised specimen were subjected to histopathologic examination [Figures 1 and 2].
Figure 1.
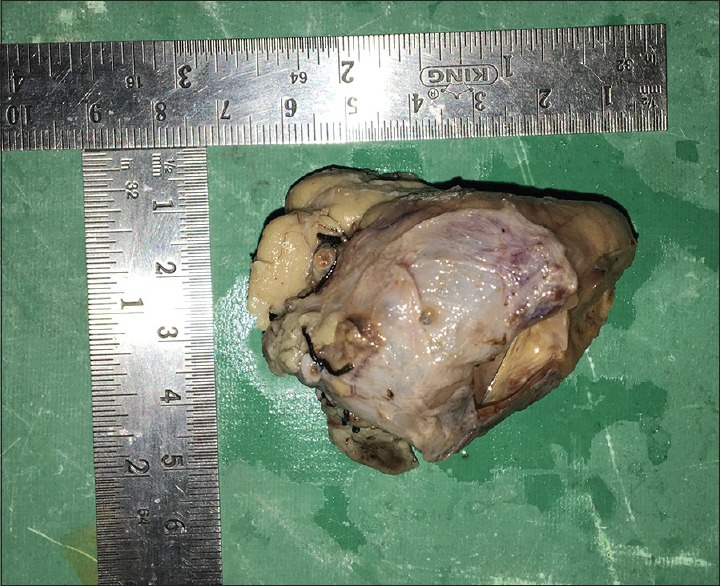
Approximate size of the specimen 7 cm × 6 cm × 6 cm
Figure 2.
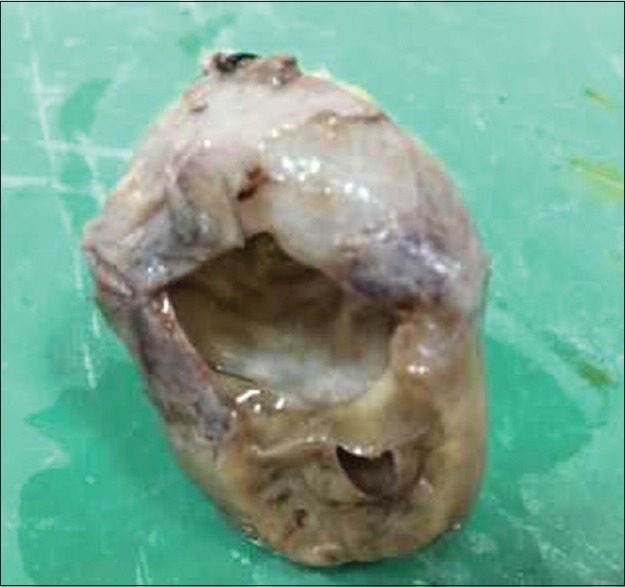
Cystic cavity at the base of the excised specimen
Histopathological features
Histopathologic sections showed seromucous salivary glandular acini with areas of proliferating cells and cyst lining composed of flattened cuboidal cells. Cystic cavity was lined by flattened cuboidal cells with budding in areas and a papillary projection of acinar cells containing vacuoles and darkly stained nucleus. Fine granules were seen in the cytoplasm of the cells. Whitish loose material obtained from within the cyst cavity showed sheets of cuboidal salivary glandular cells with islands of mucous cells. Cystic cavities were present within the proliferating mass. Minimal cellular atypism and uniformity of cells were evident. A single lymph node received along with the resected specimen showed germinal centers and reactive hyperplasia. Focal area of epidermoid cell infiltration was suspected.
The overall impression was that of submandibular gland sialocele with evidence of:
Acinic cell carcinoma of papillary-cystic variant
Low-grade mucoepidermoid carcinoma (MEC).
Immunohistochemical staining was advised for further confirmation [Figures 3–5].
Figure 3.
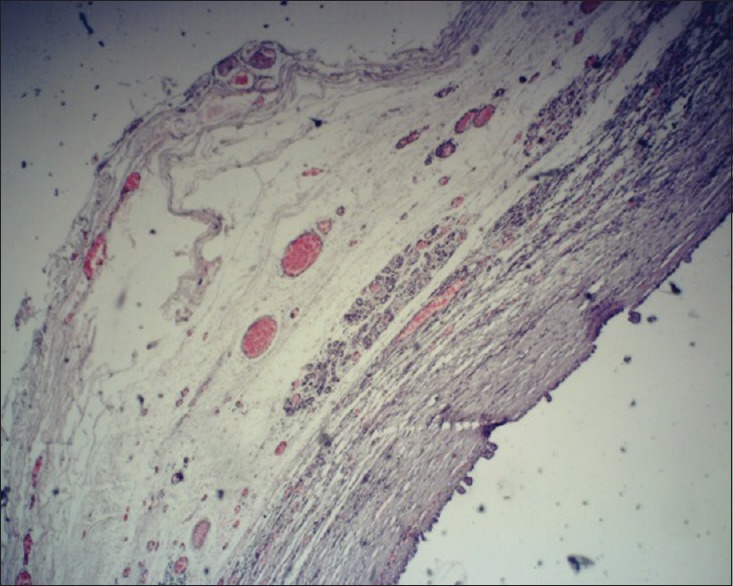
Cystic cavity lined by cuboidal cells with intraluminal budding (×4)
Figure 5.
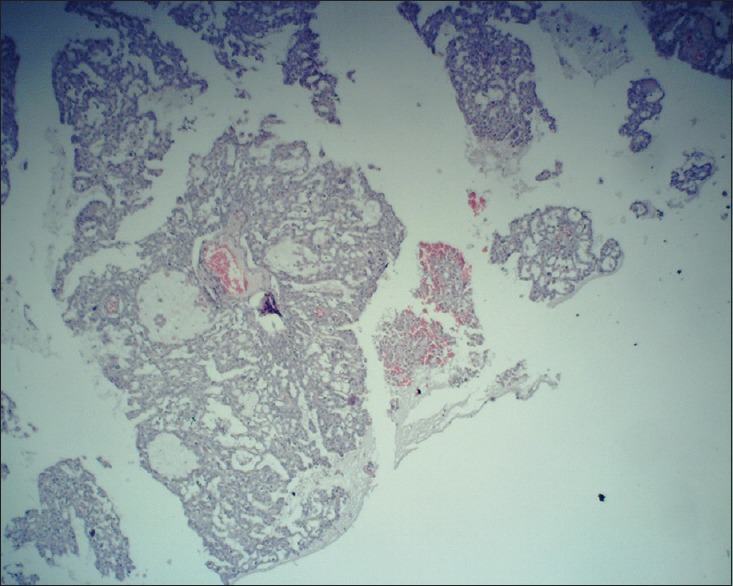
Sheets of salivary glandular cells and mucous cells with cystic cavities (×10)
Figure 4.
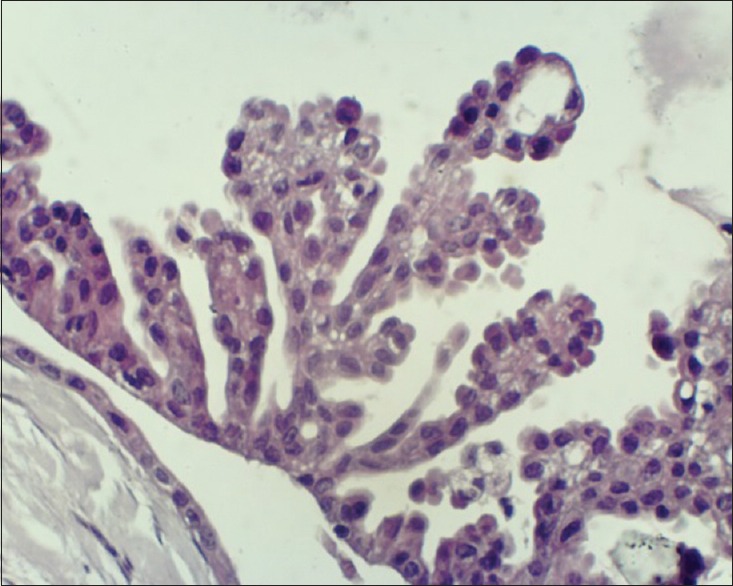
Papillary projection of cells into the cyst cavity (×40)
The squamoid cells express CK 5/6, p63 confirming MEC (low grade).
DISCUSSION
Salivary gland tumors are uncommon, but not rare. The proportion of benign and malignant tumors between the parotid, submandibular, and sublingual glands is shown in Table 1.[4]
Table 1.
Proportion of benign and malignant tumors associated with the three main salivary glands
| Benign (%) | Malignant (%) | |
|---|---|---|
| Parotid | 80 | 20 |
| Submandibular | 50 | 50 |
| Sublingual | 20 | 80 |
In the present case, the duration of the swelling, asymptomatic nature and cystic consistency of the swelling warranted a clinical diagnosis of either a cyst of the submandibular salivary gland or a benign tumor. The histopathologic appearance was such that acinic cell carcinoma of papillary-cystic variant was thought of. The cells of this tumor show serous acinar differentiation. It occurs most commonly in the parotid gland (85%–90%) and is much less common in the submandibular gland (2.7%–5%).[5] Histologically, many variants are recognized, such as solid, solid-lobular, acinar-microcystic, papillary-cystic, tubuloductal, follicular/macrocystic and dedifferentiated.[6] The term acinic cell carcinoma is defined by cytological differentiation of cells toward serous acinar cells containing periodic acid-Schiff-positive zymogen-type secretory granules.[7] The present case showed uniform appearing cuboidal cells with the presence of fine eosinophilic granules which prompted us to think of acinic cell carcinoma. The presence of cyst in the tumor with papillary projection of cells into the cyst lumen was similar to the papillary-cystic variant of the tumor where large cystic areas are seen which are lined by epithelium having papillary projections into the cystic spaces.[5] Differential diagnosis of MEC was considered as cyst formation was a major observation within the lesion. The presence of salivary glandular cells with islands of mucous cells warranted a diagnosis of low-grade MEC. Although MEC is the most common salivary gland malignancy, involvement of submandibular salivary gland is only about 13% of all cases.[8]
Immunohistochemistry (IHC) plays a limited, even though important, role in the diagnosis of salivary gland tumors, but is often useful to support the histological assessment. However, unfortunately, few tumor type-specific markers are still currently available. For these reasons, IHC should be considered a method that can be used to assist the final diagnosis, and its results themselves do not directly indicate a definitive diagnosis.[9]
It is necessary to identify serous acinar differentiation for the diagnosis of acinic cell carcinoma. A recent study reported that DOG1 staining is a marker of salivary acinar cells, and strong staining can be applied to support the diagnosis of acinic cell carcinoma.[9] DOG1 is a sensitive marker in the diagnosis of acinic cell carcinoma, p63 is sensitive in the diagnosis of MEC and the combined use of both markers is helpful in the differential diagnosis of acinic cell carcinoma versus MEC.[10] P63 is an immunohistochemical stain that can potentially aid in differentiating unusual adenoid cystic carcinoma with prominent mucin production from MEC of the salivary gland. In a study on both these tumors, the authors concluded that acinic cell carcinoma is always negative for p63 immunoreactivity whereas MEC is always positive.[11] IHC was done in the present case using the marker p63, which was positive. Thus, a confirmatory diagnosis of low-grade MEC was given.
The patient underwent further surgery for lymph node dissection, considering that the diagnosis was a malignant submandibular salivary tumor and no recurrence or further complications was reported during the follow-up period of more than 1 year.
CONCLUSION
Although MEC is the most common malignancy of salivary glands, it is quite uncommon in submandibular salivary glands. The case under consideration was diagnosed clinically as a cyst of the submandibular salivary gland due to its asymptomatic and slow clinical course. Histologically, it was difficult to differentiate between papillary cystic variant of acinic cell carcinoma and low-grade MEC. Although IHC does not play a major role in the diagnosis of salivary gland tumors, p63 staining of the tissue was an adjunct in the differentiation between acinic cell carcinoma and MEC where it is positive only in MECs.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Robinson M, Hunter K, Pemberton M, Sloan P. 5th ed. Oxford: Oxford University Press; 2018. Soame's and Southam's Oral Pathology. International edition. [Google Scholar]
- 2.Yazici D, Coktu MY, Guney Z, Erkan SO, Gorgulu O, Yildirim I, et al. Differential diagnosis of submandibular gland swelling. ENT Updates. 2018;8:56–61. [Google Scholar]
- 3.Spiro RH. Salivary neoplasms: Overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177–84. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 4.Neville BW, Damm DD, Allen CM, Chi A. 4th ed. India: Elsevier, Inc.; 2016. Oral and Maxillofacial Pathology First South Asia edition. [Google Scholar]
- 5.Patel MM, Gamit BN, Patel SM, Patel MI, Gandhi SS. Acinic cell carcinoma, papillary-cystic variant: A rare case diagnosed in fine needle aspiration cytology. Int J Res Med Sci. 2018;6:1046–50. [Google Scholar]
- 6.Sivapathasundharam B. Shafer's Textbook of Oral Pathology. 8th ed. India: Elsevier, Inc.; 2016. [Google Scholar]
- 7.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: Clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82:1217–24. doi: 10.1002/(sici)1097-0142(19980401)82:7<1217::aid-cncr2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Nagao T, Sato E, Inoue R, Oshiro H, Takahashi RH, Nagai T, et al. Immunohistochemical analysis of salivary gland tumors: Application for surgical pathology practice. Acta Histochem Cytochem. 2012;45:269–82. doi: 10.1267/ahc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chênevert J, Duvvuri U, Chiosea S, Dacic S, Cieply K, Kim J, et al. DOG1: A novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol. 2012;25:919–29. doi: 10.1038/modpathol.2012.57. [DOI] [PubMed] [Google Scholar]
- 10.Abd Raboh NM, Hakim SA. Diagnostic role of DOG1 and p63 immunohistochemistry in salivary gland carcinomas. Int J Clin Exp Pathol. 2015;8:9214–22. [PMC free article] [PubMed] [Google Scholar]
- 11.Sams RN, Gnepp DR. P63 expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol. 2013;7:64–8. doi: 10.1007/s12105-012-0403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


