Abstract
Tumor budding (TB) is a histopathologically evident feature that represents a scattered pattern of invasion consisting of isolated single tumor epithelial cells or tumor cells in small clusters (up to 5 cells) seen primarily at the invasive front dispersed within the stroma for variable distance. Presence of TB has been linked with lymph node metastasis, recurrence, distant metastasis and reduced survival in numerous cancers including oral squamous cell carcinoma (OSCC). Tumor buds are also considered as histopathological markers of epithelial–mesenchymal transition which is a molecular process implicated as a hallmark for invasion and metastasis. This review gives an overview of the current evidence regarding TB assessment in OSCC and its future prospects.
Keywords: Assessment, epithelial–mesenchymal transition, histopathological marker, lymph node metastases, oral squamous cell carcinoma, prognosis, tumor budding
INTRODUCTION
About 90% of oral malignancies are oral squamous cell carcinoma (OSCC) and it has been stated to be the sixth to eighth common cancer worldwide, the occurrence of which is reported to vary among different geographic regions. In India, it has been categorized as the third most frequent malignancy and has reached dangerous trends due to the extensively prevalent tobacco and areca nut habits.[1,2]
The prognosis is usually not very predictable as the clinical course is typically aggressive characterized by frequent locoregional relapses and more than 60% of the cases have cervical lymph node metastasis (LNM) at presentation.[3] In most cases, the management protocol usually consists of surgery with radical neck dissection; postoperatively, radiotherapy is recommended. In spite of the massive strides seen in the research related to the diagnostic modalities and management aspects of OSCC, the mortality rates are still dismally low with 5-year survival rate being <50%, questioning the existing approaches of prognostic appraisals.[2,3]
The conventional method for determining the prognosis and stratification of the patients into suitable management schemes is based on the tumor–node–metastasis (TNM) staging system.[3] However, the uncertainty of being dependent on TNM solitarily has been proven by quite a few reports that suggest that early-stage tumors, i.e., T1 and T2 tumors may show lymph node metastases and demonstrate aggressive behavior leading to mortality.[3,4] Various histological markers that have been shown to have prognostic impact including tumor differentiation, thickness of the tumor, pattern of invasion, depth of invasion, lymphovascular emboli, perineural invasion, regional LNM and extracapsular spread in the lymph nodes are usually evaluated routinely and stated in the histopathology reports.[3,4] In addition, enormous amount of molecular studies have been done in OSCC for the identification of biomarkers that can predict the prognostic outcomes for OSCC; however, none of them have shown convincing results with lot of ambiguity seen in the results and there is insufficient evidence regarding their usefulness which precludes their use in routine practice.[3] Hence, the quest for a more dependable and consistent prognostic parameter is still tangible. The elusive parameter if identified would allow improved categorization of the patients, based on the aggressive behavior of the tumor and ultimately offer a guide for more effective and personalized therapeutic options. One such important prognostic parameter widely described in several cancers, but has been most comprehensively researched in colorectal carcinomas, is tumor budding (TB).
TUMOR BUDDING
TB is a histopathologically evident feature that represents a scattered pattern of invasion consisting of isolated single tumor epithelial cells or tumor cells in small clusters (up to 5 cells) seen primarily at the invasive front dispersed within the stroma for variable distance[5] [Figure 1].
Figure 1.
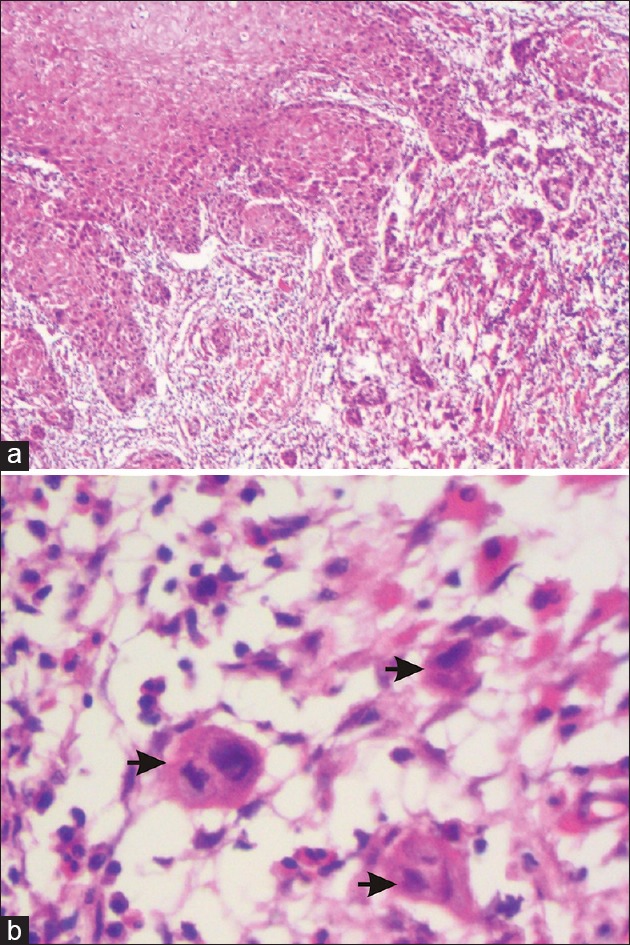
(a) Shows the tumor buds proliferating into the underlying connective tissue at the invasive front of oral squamous cell carcinoma. (b) High power view of the tumor buds i.e clusters having less than 5 tumor cells (black arrows)
It was originally described as “sprouting” by Imai et al. in the 1950s, but the earliest detailed description is credited to Gabbert et al. who identified these isolated tumor cells and clusters at the invasive front in colorectal cancers.[6,7] They termed this feature as “tumor dedifferentiation” as these areas appeared to be less well differentiated exhibiting large nuclei showing loss of junctional complexes and desmosomes as evidenced in electron microscopy. It was much later that Morodomi et al. and Hase et al., who gave the terminology of “budding” because they observed that these undifferentiated cells and nests appeared to be budding out from larger tumor islands.[8,9]
TB is an important refinement in the pattern of invasion and is a morphological feature that represents an aggressive invasive phenotype, i.e., loss of cell adhesion and local invasion. Consequently, it has been related to aggressive behavior of the tumor and has shown a definitive correlation with poor prognosis. This has contributed to its popularity as it is relatively simple to use and can be assessed on routinely used hematoxylin and eosin (H&E)-stained section and does not mandate use of any other additional expensive techniques or equipment.[9,10]
High TB count has been linked with LNM, recurrence, distant metastasis and reduced survival in numerous cancers such as colorectal carcinomas, pancreatic carcinoma, esophageal carcinomas, lung carcinomas, anal carcinoma and laryngeal carcinoams.[9,10,11,12,13,14] In colorectal carcinoma, TB has been well recognized as prognostic factor for adverse outcome and has classified as an “additional prognostic factor” by the International Union against Cancer.[15]
TUMOR BUDDING IN ORAL SQUAMOUS CELL CARCINOMA
TB has been evaluated in several studies in OSCC with numerous studies evaluating this parameter in tongue SCC.[5,10,11,16,17,18,19,20,21,22,23,24,25]
Studies have shown that TB is a powerful prognosticator for LNM. TB has shown a strong correlation with LNM, and in multivariate analysis, it has emerged as an independent predictor which means that tumor bud count can be used to assess the risk of LNM.[8,11,21,25] Further higher budding intensity has been associated with LNM even in early stage and node negative OSCC (T1,2N0 M0).[10,11,17] Seki et al. evaluated TB in preoperative biopsy specimens and showed it to correlate with LNM in tongue and floor of the mouth SCC.[26,27] Pedersen et al.[8] and Angadi et al.[25] have demonstrated the use of a risk model involving tumor bud count for treatment decisions and showed that it performed markedly better than traditional parameters such as tumor stage, grade and depth of invasion in determining LNM.
In a recent meta-analysis by Almangush et al.,[11] it was evidenced that high TB intensity of more than five buds was significantly correlated with shorter disease-free survival and reduced overall survival. It has also been demonstrated that this poor prognosis associated with high tumor bud count is independent of the TNM stage. Thus, if there are two patients falling in the same TNM stage, the tumor that shows high TB index will have poorer prognosis which emphasizes the importance of this process. Further, high tumoral budding (TB) score has been strongly associated with disease-specific mortality in large series of early-stage OSCC, highlighting its prognostic value.[10,11,24,27,28]
In addition, TB has shown a strong correlation with tumor grade, tumor size, clinical stage and depth of invasion.[10,11,25]
METHODOLOGICAL CONSIDERATIONS IN THE ASSESSMENT OF TUMOR BUDDING IN ORAL SQUAMOUS CELL CARCINOMA
There are usually two methods of assessment, i.e., manual and digital method which can be done on either H&E-stained slides or on immunohistochemically stained slides for pan cytokeratin (CK).[10,11,29,30,31]
Manual and digital methods
The most common method used is the manual evaluation of TB on H&E-stained slides. Several approaches have been suggested by numerous authors with varying parameters being proposed for categorization of TB intensity ranging from absolute counts to cutoffs and use of three-tier or two-tier system for its grading.[8,10,11,32,33,34,35,36] However, in OSCC, the most accepted and widely used method is that proposed by Wang et al. in 2011.[5] In this method, the TB are defined as “isolated single cells or clusters <5 tumor cells seen at the invasive front.” Here, the section is first scanned at low power objective to identify the fields showing highest density of TB. Then, in high power, i.e., ×20 objective, the tumor buds are counted in a single field at ×200. The samples were then categorized as high intensity TB (5 or more buds) and low intensity or absent TB (<5 buds). This was also corroborated by Almangush et al. in their systematic review, who said that a cutoff of 5 buds is widely accepted for OSCC.[11]
Digital method for TB assessment has been introduced recently and uses a quantitative, semiautomatic digital image analysis algorithm using a software are called Visiopharm image analysis (Visiopharm A/S, Hoersholm, Denmark) that evaluates the tumor buds in CK stained slides. This image analysis module is based on colour thresholding applied for the brown signal emitted by the positively stained areas by chromgen used in immunohistocehemistry (DAB) and thus clearly distinguishes the stained areas from the unstained ones. They standardized that t the positively stained areas that were <950 μm2 represented cell clusters composed of less than five cells and thus were measured as tumor buds. These areas were counted to arrive at the digital tumor bud count. This method has been used by Pedersen et al.[8] and Jensen[21] et al. and have shown good correlation with prognostic factors.
Use of Hematoxylin and Eosin stained sections or cytokeratin immunohistochemistry
In most studies the scoring for tumor buds has been done in H&E stained sections.[10,11] The IJCC Recommendations given for TB appraisal in colorectal carcinoma endorses use of H&E stained sections underscoring its adaptability to routine use and worldwide utility.[29]
But there may be certain situations, where the evaluation may be difficult in H&E section and mandate use of CK Immunohistochemistry for better visualization. These include: excessive peritumoral infiltrate may make the tumor buds indistinct. There may be excessive stromal reaction and the stromal fibroblasts may appear large and atypical on high power and this may lead to problems in distinguishing them with tumor cells. Further, single cell budding are usually inconspicuous in H&E assessment.[10,11] The advantages of using CK include shorter working time, lower difficulty, greater replicability and it has produced significantly better reproducibility as well as inter and intra-observer agreement as compared to H&E. It fared especially well when used by less experienced examiners.[22,24]
Having said that, there are several studies that have shown good reproducibility even with H&E assessment of TB in OSCC. So at the present scenario, we recommend TB evaluation to be done in routine H&E stained slides whereas CK immunohistochemistry can be applied in selected cases and circumstances.[5,10,11,25]
Preoperative or postoperative biopsy for assessment of tumor budding in oral squamous cell carcinoma
In most studies, TB has been evaluated in the excisional specimens that show the entire depth of the tumor tissue allowing easy evaluation of the invasive front. However, Seki et al.[26,27] have evaluated this parameter in preoperative biopsy specimens and have shown good correlation with not only postoperative tumor bud count. It has to be noted that if TB has to be evaluated in preoperative biopsy specimens, the surgeons should take a large biopsy that includes the deepest part of the tumor to visualize the invasive front clearly which may nor be feasible most of the times. A recent systematic review has shown that preoperative TB has significant prognostic value for LNM, overall survival and disease free survival. This could be beneficial especially if we can predict the aggressiveness of the tumor preoperatively and apply it for therapeutic considerations.[30]
Peritumoral budding and intratumoral budding in oral squamous cell carcinoma
TB evaluated at the invasive edge is referred to as peri-TB,[10,11] and if the tumor buds are evident within the lesion, it is referred to as intra-TB (ITB).[35] Although ITB has not been studied extensively in literature, several authors have described bud-like structures in the main tumor mass in colorectal, breast and rectal cancers to name a few.[29,35] In colorectal carcinomas particularly, it has been shown that ITB has good correlation with peri-TB, tumor grade, advanced stage, LNM and distant metastasis.[9] Studies in OSCC do not exist, but if evaluated and found prognostically useful, preoperative assessment of ITB even in shallow biopsies may serve as an important marker to be included in routine histopathology reporting.
Tumor budding and grading systems in oral squamous cell carcinoma
Almangush et al.[16] developed a budding and depth of invasion (BD) model and have shown good prognostic value for the same as compared to other commonly used grading systems. In addition, they also found good correlation between preoperative and postoperative BD scores. They suggested that it is a simple and predictive grading system for OSCC patients.
Boxberg et al.[17,18] have developed a novel three-tiered grading system combining the TB activity and the cell nest size scores. This system showed a significant prognostic impact with strong correlation associated with LNM. Further, they have shown good interobserver and intraobersver concordance for the same, suggesting it to be suitable for grading of OSCC routinely.
TUMOR BUDDING AND EPITHELIAL–MESENCHYMAL TRANSITION IN ORAL SQUAMOUS CELL CARCINOMA
TB is strongly linked with epithelial-mesenchymal transition (EMT) which is an important biologic process characterized by conversion of a highly polarized epithelial cell into a motile mesenchymal cell which is a hall mark for invasion and subsequent metastasis. EMT involves changes in cell adhesion, cell shape and gene expression and has been related to poor prognostic outcome in OSCC.[9,10,11,21,37]
Wang et al.[5] suggested that TB may represent cells undergoing EMT as they showed reduced E-cadherin expression and elevated vimentin expression in tongue squamous cell carcinoma. In addition, Jensen et al. have demonstrated enhanced expression of ZEB1 and PPRX1 genes, both renowned EMT stimulators in tumor buds using RNA sequencing.[21] They also demonstrated significantly elevated transforming growth factor (TGF)-β signaling and the genes responsive to TGFβ for EMT such as FN1, PRRX1 and COLA2. They suggested that there is a definitive association between tumor buds and activated TGF-β signaling leading to EMT.[21] In addition, identification of Mir 200 downregulation in tumor buds which is a part of reciprocal feedback loop in EMT related to ZEB1 validates these findings.[21] Thus, tumor buds can be considered as histopathological markers of EMT.[9]
OTHER MOLECULAR FINDINGS RELATED TO TUMOR BUDDING IN ORAL SQUAMOUS CELL CARCINOMA
A strong association was observed with high-intensity TB and high cell proliferation index. Hence, cell proliferation is higher in OSCC that shows budding phenomenon[38]
ALDH1, a cancer stem cell marker, was elevated in budding area as compared to areas outside that of budding. This suggests that tumor buds in OSCC have cancer stem cell-like phenotype predisposing to migratory and invasive properties[23]
Tumor microenvironment – The tumor micon evironment plays an important role in defining the aggressiveness of a cancer. It has been found in OSCC that the high density of TB was associated with enhanced expression of stromal myofibroblasts/carcinoma-associated fibroblasts which provide a conducive environment for the development of invasive phenotype in the budding tumor cells.[39] Further, a positive association between high-intensity TB and higher lamain-5 C2 expression was noted by Marangon et al.,[40] suggesting that this molecule is capable of generating a migratory cell phenotype giving a permissive environment for invasion to occur.
APPLICATIONS FOR CLINICAL PRACTICE
TB evaluation in OSCC can be used as a predictor of LNM and thus used for planning surgical resection with radical neck dissection
TB evaluation in early-stage SCC and node-negative OSCC can give a clue for adverse prognosis and thus can be used for risk adjusted follow-up or to plan adjuvant therapy
TB evaluation can be done in preoperative biopsies for the determination of aggressiveness of the tumor and adequate treatment planning.
IS TUMOR BUDDING A CONTENDER FOR ROUTINE DIAGNOSTIC USE?
The recent systematic review in OSCC,[11] has shown a strong prognostic association between TB and LNM, distant metastases as well as survival. It could be adapted as a routinely assessed prognostic marker and mentioned in histopathology reports with its prognostic implication
The evaluation can be done in H&E slides; however, in situations as discussed previously, CK-IHC can be used
The Wang et al.'s methodology, i.e., a cutoff of five tumor buds in a single high-power field, can be used and propagated so that future research adds to uniform reporting and leads to early clinical translation.
FUTURE PROSPECTS
Muticentric studies evaluating the role of TB in OSCC using standardized methodologies can result in validation of the existing findings and may propel a recommendation in OSCCC similar that in colorectal cancers for clinical decision-making
Research on the use of ITB both in preoperative and postoperative tumor specimens need to be explored in OSCC
Studies on molecular profiling of tumor buds to obtain a clearer understanding of the molecular background of TB and their effect on tumor behavior is necessitated
Development of uniform guidelines for reporting of TB in pathology reports for OSCC
Incorporation of TB as a parameter in grading systems to evaluate its prognostic potential.
CONCLUSION
Tumor buds are a cluster of tumor cells that are more invasive than the other and represent an aggressive phenotype that shows an increased risk for LNM and distant metastasis. There is well-documented evidence which corroborates the fact that elevated TB activity is a harbinger of poor prognosis in OSCC. At the molecular level too, TB represents EMT which is an important biologic process implicated as a hallmark in carcinogenesis; however, additional studies may be needed for elucidation of its molecular profile. Research henceforth in TB should aim at using a standardized methodology that may lead to development of uniform guidelines in reporting and use of TB in routine pathology reporting. We are of the opinion that it is certainly time to take notice of this important phenomenon and TB seems ready to have a vital role in clinical decision-making for the management of OSCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301–8. doi: 10.1016/j.oraloncology.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Massano J, Regateiro FS, Januário G, Ferreira A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 4.Woolgar JA, Scott J, Vaughan ED, Brown JS, West CR, Rogers S. Survival, metastasis and recurrence of oral cancer in relation to pathological features. Ann R Coll Surg Engl. 1995;77:325–31. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Huang H, Huang Z, Wang A, Chen X, Huang L, et al. Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med. 2011;40:545–51. doi: 10.1111/j.1600-0714.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohike N, Coban I, Kim GE, Basturk O, Tajiri T, Krasinskas A, et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am J Surg Pathol. 2010;34:1417–24. doi: 10.1097/PAS.0b013e3181f0b05a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtsuki K, Koyama F, Tamura T, Enomoto Y, Fuji H, Mukogawa T, et al. Prognostic significance of immunohistochemical alaysis of tumor budding in colorectal carcinoma. Anticancer Res. 2008;28:1831–6. [PubMed] [Google Scholar]
- 8.Pedersen NJ, Jensen DH, Lelkaitis G, Kiss K, Charabi B, Specht L, et al. Construction of a pathological risk model of occult lymph node metastases for prognostication by semi-automated image analysis of tumor budding in early-stage oral squamous cell carcinoma. Oncotarget. 2017;8:18227–37. doi: 10.18632/oncotarget.15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H. Tumor Budding: The Name is EMT. Partial EMT. J Clin Med. 2016;5 doi: 10.3390/jcm5050051. pii: E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almangush A, Salo T, Hagström J, Leivo I. Tumour budding in head and neck squamous cell carcinoma-a systematic review. Histopathology. 2014;65:587–94. doi: 10.1111/his.12471. [DOI] [PubMed] [Google Scholar]
- 11.Almangush A, Pirinen M, Heikkinen I, Mäkitie AA, Salo T, Leivo I. Tumour budding in oral squamous cell carcinoma: A meta-analysis. Br J Cancer. 2018;118:577–86. doi: 10.1038/bjc.2017.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karamitopoulou E. Tumor budding cells, cancer stem cells and epithelial-mesenchymal transition-type cells in pancreatic cancer. Front Oncol. 2012;2:209. doi: 10.3389/fonc.2012.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyata H, Yoshioka A, Yamasaki M, Nushijima Y, Takiguchi S, Fujiwara Y, et al. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer. 2009;115:3324–34. doi: 10.1002/cncr.24390. [DOI] [PubMed] [Google Scholar]
- 14.Masuda R, Kijima H, Imamura N, Aruga N, Nakamura Y, Masuda D, et al. Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. Mol Med Rep. 2012;6:937–43. doi: 10.3892/mmr.2012.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: Tumor budding as oncotarget. Oncotarget. 2010;1:651–61. doi: 10.18632/oncotarget.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almangush A, Leivo I, Siponen M, Sundquist E, Mroueh R, Mäkitie AA, et al. Evaluation of the budding and depth of invasion (BD) model in oral tongue cancer biopsies. Virchows Arch. 2018;472:231–6. doi: 10.1007/s00428-017-2212-1. [DOI] [PubMed] [Google Scholar]
- 17.Boxberg M, Jesinghaus M, Dorfner C, Mogler C, Drecoll E, Warth A, et al. Tumour budding activity and cell nest size determine patient outcome in oral squamous cell carcinoma: Proposal for an adjusted grading system. Histopathology. 2017;70:1125–37. doi: 10.1111/his.13173. [DOI] [PubMed] [Google Scholar]
- 18.Boxberg M, Bollwein C, Jöhrens K, Kuhn PH, Haller B, Steiger K, et al. Novel prognostic histopathological grading system in oral squamous cell carcinoma based on tumour budding and cell nest size shows high interobserver and intraobserver concordance. J Clin Pathol. 2019;72:285–94. doi: 10.1136/jclinpath-2018-205454. [DOI] [PubMed] [Google Scholar]
- 19.Ebihara Y, Yoshida S, Nakahira M, Kogashiwa Y, Enoki Y, Kuba K, et al. Importance of tumor budding grade as independent prognostic factor for early tongue squamous cell carcinoma. Head Neck. 2019;41:1809–15. doi: 10.1002/hed.25614. [DOI] [PubMed] [Google Scholar]
- 20.Almangush A, Bello IO, Keski-Säntti H, Mäkinen LK, Kauppila JH, Pukkila M, et al. Depth of invasion, tumor budding, and worst pattern of invasion: Prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36:811–8. doi: 10.1002/hed.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen DH, Dabelsteen E, Specht L, Fiehn AM, Therkildsen MH, Jønson L, et al. Molecular profiling of tumour budding implicates TGFβ-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol. 2015;236:505–16. doi: 10.1002/path.4550. [DOI] [PubMed] [Google Scholar]
- 22.Leão PL, Marangon Junior H, Melo VV, Caixeta ÂB, Souza PE, de Aguiar MC, et al. Reproducibility, repeatability, and level of difficulty of two methods for tumor budding evaluation in oral squamous cell carcinoma. J Oral Pathol Med. 2017;46:949–55. doi: 10.1111/jop.12578. [DOI] [PubMed] [Google Scholar]
- 23.Marangon Junior H, Melo VVM, Caixeta ÂB, Souto GR, Souza PE, de Aguiar MCF, et al. Immunolocalization of cancer stem cells marker ALDH1 and its association with tumor budding in oral squamous cell carcinoma. Head Neck Pathol. 2019;13:535–42. doi: 10.1007/s12105-018-0985-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu S, Miyazaki A, Sonoda T, Koike K, Ogi K, Kobayashi JI, et al. Tumor budding is an independent prognostic marker in early stage oral squamous cell carcinoma: With special reference to the mode of invasion and worst pattern of invasion. PLoS One. 2018;13:e0195451. doi: 10.1371/journal.pone.0195451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angadi PV, Patil PV, Hallikeri K, Mallapur MD, Hallikerimath S, Kale AD. Tumor budding is an independent prognostic factor for prediction of lymph node metastasis in oral squamous cell carcinoma. Int J Surg Pathol. 2015;23:102–10. doi: 10.1177/1066896914565022. [DOI] [PubMed] [Google Scholar]
- 26.Seki M, Sano T, Yokoo S, Oyama T. Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck. 2016;38(Suppl 1):E1582–90. doi: 10.1002/hed.24282. [DOI] [PubMed] [Google Scholar]
- 27.Seki M, Sano T, Yokoo S, Oyama T. Tumour budding evaluated in biopsy specimens is a useful predictor of prognosis in patients with cN0 early stage oral squamous cell carcinoma. Histopathology. 2017;70:869–79. doi: 10.1111/his.13144. [DOI] [PubMed] [Google Scholar]
- 28.Xie N, Wang C, Liu X, Li R, Hou J, Chen X, et al. Tumor budding correlates with occult cervical lymph node metastasis and poor prognosis in clinical early-stage tongue squamous cell carcinoma. J Oral Pathol Med. 2015;44:266–72. doi: 10.1111/jop.12242. [DOI] [PubMed] [Google Scholar]
- 29.Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299–311. doi: 10.1038/modpathol.2017.46. [DOI] [PubMed] [Google Scholar]
- 30.Almangush A, Youssef O, Pirinen M, Sundström J, Leivo I, Mäkitie AA. Does evaluation of tumour budding in diagnostic biopsies have a clinical relevance? A systematic review. Histopathology. 2019;74:536–44. doi: 10.1111/his.13793. [DOI] [PubMed] [Google Scholar]
- 31.Mäkitie AA, Almangush A, Rodrigo JP, Ferlito A, Leivo I. Hallmarks of cancer: Tumor budding as a sign of invasion and metastasis in head and neck cancer. Head Neck. 2019;41:3712–8. doi: 10.1002/hed.25872. [DOI] [PubMed] [Google Scholar]
- 32.Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004;240:832–9. doi: 10.1097/01.sla.0000143243.81014.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner RR, Li C, Compton CC. Newer pathologc assessment techniques for colorectal carcinoma. Clin Cancer Res. 2007;13:1–6876. doi: 10.1158/1078-0432.CCR-07-1151. [DOI] [PubMed] [Google Scholar]
- 34.Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127–32. doi: 10.1046/j.1365-2559.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- 35.Zlobec I, Borner M, Lugli A, Inderbitzin D. Role of intra- and peritumoral budding in the interdisciplinary management of rectal cancer patients. Int J Surg Oncol. 2012;2012:79594. doi: 10.1155/2012/795945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer. 1989;63:539–43. doi: 10.1002/1097-0142(19890201)63:3<539::aid-cncr2820630323>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 37.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marangon H, Jr, Leão PL, Melo VV, Caixeta ÂB, Souza PE, de Aguiar MC, et al. Cell proliferation is associated with intensity of tumor budding in oral squamous cell carcinoma. J Oral Pathol Med. 2018;47:128–35. doi: 10.1111/jop.12653. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Xu F, Li S, Zhong A, Meng X, Lai M. The tumor microenvironment: An irreplaceable element of tumor budding and epithelial-mesenchymal transition-mediated cancer metastasis. Cell Adh Migr. 2016;10:434–46. doi: 10.1080/19336918.2015.1129481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marangon H, Jr, Rocha VN, Leite CF, de Aguiar MC, Souza PE, Horta MC. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43:199–204. doi: 10.1111/jop.12121. [DOI] [PubMed] [Google Scholar]


