Abstract
Introduction:
One of the major aspects of tumor cell invasion and metastasis is the interaction between cancer cells and the extracellular matrix component. The invasion of epithelial tumor cells into the underlying connective tissue stroma causes dynamic changes in its microenvironment, which can be seen as radical changes in the stroma. The characteristics of the stroma in relation to the invading malignant epithelial cells and the interdependence between the stroma and the tumor cells are always a matter of discussion and interest.
Aim:
The aim of this study is to predict the biological behavior of oral squamous cell carcinoma (OSCC) by evaluating stromal desmoplasia and its possible use as important prognostic indicators.
Objective:
To assess the desmoplastic reaction in varying histopathological grades of OSCC.
Materials and Methods:
The study included a total of 30 histopathologically prediagnosed cases of OSCC (well, moderate and poorly differentiated grades of OSCC). Picrosirius red stain in conjunction with polarizing microscope was used to evaluate the stromal desmoplastic reaction.
Results:
The results of the study revealed that, in the initial grades, cancer invasion induces a desmoplastic reaction, whereas in the later stages, there is degradation of the stroma, thereby facilitating tumor invasion.
Conclusion:
The study would emphasize the importance of stromal desmoplasia as a prognostic indicator and may help to reflect the biological diversity of oral cancer and predict the clinical outcomes.
Keywords: Desmoplasia, polarizing microscope, squamous cell carcinoma
INTRODUCTION
Oral cancer is rapidly becoming a global health concern.[1] More than 90% of all oral cancers are squamous cell carcinomas.[2]
Squamous cell carcinomas are malignant epithelial neoplasms showing atypically arranged epithelial cells with varying degrees of differentiation. These oncogenetically mutated epithelial cells invade through the basement membrane causing changes in its microenvironment, which can profoundly be seen as changes in the stroma.[3]
The morphological signs of cancer-associated stromal alterations are desmoplasia, angiogenesis and inflammatory cell infiltration.[4] The collagenous tissue which is the chief component of the stroma plays a vital role during the evolution and progression of carcinoma. Desmoplasia is the result of increased synthesis of extracellular matrix proteins and collagen by stromal cells. It is considered to be a reaction and response of the host tissue against invasive cancer cells.[5]
The biological relevance of the desmoplastic response to cancer is still unclear. Incorporation of the methods to detect, quantify and evaluate the nature of collagen, into a prognostic system may help to reflect the biologic diversity of oral cancer and predict clinical outcomes.
Hence, the aim of the present study was to predict the biological behavior of oral squamous cell carcinoma (OSCC) by evaluating stromal desmoplasia and their possible role as an important prognostic indicator.
MATERIALS AND METHODS
A retrospective analysis was conducted using 30 histologically prediagnosed cases of varying grades of squamous cell carcinoma. Squamous cell carcinoma of buccal mucosa was considered for the study. Formalin-fixed and paraffin-embedded soft-tissue samples of squamous cell carcinoma were retrieved from the archives of the Department of Oral Pathology and Microbiology. Incisional biopsy samples with an adequate size of tissue were selected.
The hematoxylin and eosin-stained retrospective cases were classified by Broder's grading system. The study sample included 11 cases of well-differentiated grade, 11 cases of moderately differentiated grade and 8 cases of poorly differentiated grades.
Tissue sections of about 4 μ were cut and stained with picro-sirius red (PSR) stain using standard protocols. Blinding of the picrosirius stained slides was done. Stained sections were examined using polarizing microscope under high-power magnification. The polarizing colors of collagen fibers in the immediate vicinity of the tumor islands were analyzed. The color of collagen fiber birefringence was analyzed in five randomly selected areas in each case, and findings were recorded. The color of collagen observed was recorded in three categories as proposed by Aparna and Charu [Table 1].[6] Of the five different fields, the predominant polarizing color was taken into consideration while deciding the nature of stroma for that particular case. Accordingly, the nature of stroma was recorded for each case.
Table 1.
Categories of collagen based on color noted after picrosirius staining
| Category of collagen | Colour of collagen |
|---|---|
| Category 1 | Reddish, reddish orange |
| Category 2 | Yellowish, orange, yellowish orange |
| Category 3 | Greenish yellow, greenish |
RESULTS
The nature of collagen was analyzed based on the birefringence exhibited by the fibers under polarizing microscope [Figures 1–3]. Of the 11 cases of well-differentiated squamous cell carcinoma, majority of the cases, i.e., 81.8% showed reddish-orange birefringence and 18.2% showed yellowish-orange birefringence. In moderately differentiated squamous cell carcinoma cases, 63.6% showed yellowish-orange birefringence, 18.2% showed reddish orange and another 18.2% showed greenish yellow. Seventy-five percent of poorly differentiated carcinoma cases showed greenish yellow and 25% showed yellowish-orange birefringence. Comparison between the natures of collagen in different grades of OSCC showed that the difference was statistically highly significant (P ≤ 0.001) between well, moderately and poorly differentiated OSCC [Table 2]. The comparison was made based on color change of collagen in different grades of OSCC [Graph 1].
Figure 1.
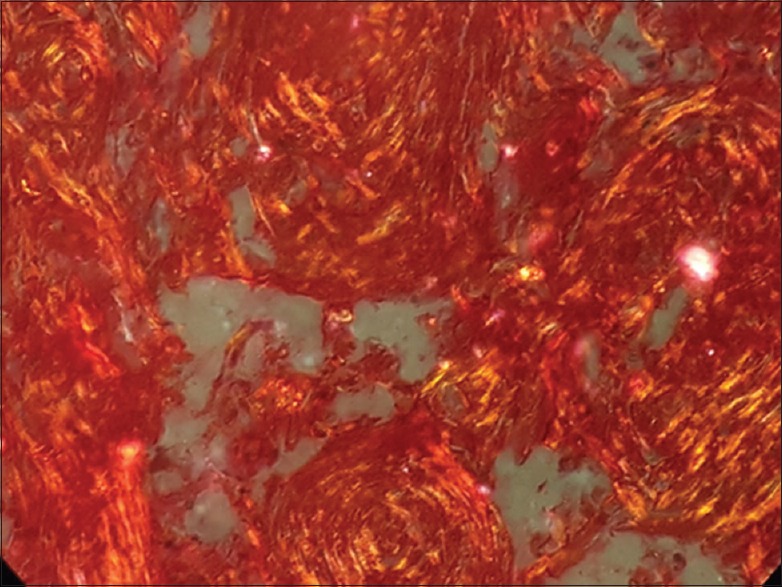
Photomicrograph of well differentiated squamous cell carcinoma showing collagen fibers with reddish orange birefringence under polarizing microscope (×40)
Figure 3.

Photomicrograph of poorly differentiated squamous cell carcinoma showing collagen fibers with greenish yellow birefringence under polarizing microscope (×40)
Table 2.
Comparison between predominant color exhibited by collagen fibers in various grades of oral squamous cell carcinoma
| Colour | Number of cases (%) | |||
|---|---|---|---|---|
| Well-differentiated OSCC | Moderately differentiated SCC | Poorly differentiated SCC | Total | |
| Greenish yellow | 0 | 2 (18.2) | 6 (75) | 8 (26.7) |
| Reddish orange | 9 (81.8) | 2 (18.2) | 0 | 11 (36.7) |
| Yellowish orange | 2 (18.2) | 7 (63.6) | 2 (25) | 11 (36.7) |
| χ2 | 23.817 | P | <0.001** | |
**Statistically highly significant (P<0.001). OSCC: Oral squamous cell carcinoma
Graph 1.
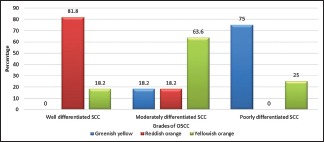
The prominent color of collagen fibers seen in different grades of oral squamous cell carcinoma
Figure 2.
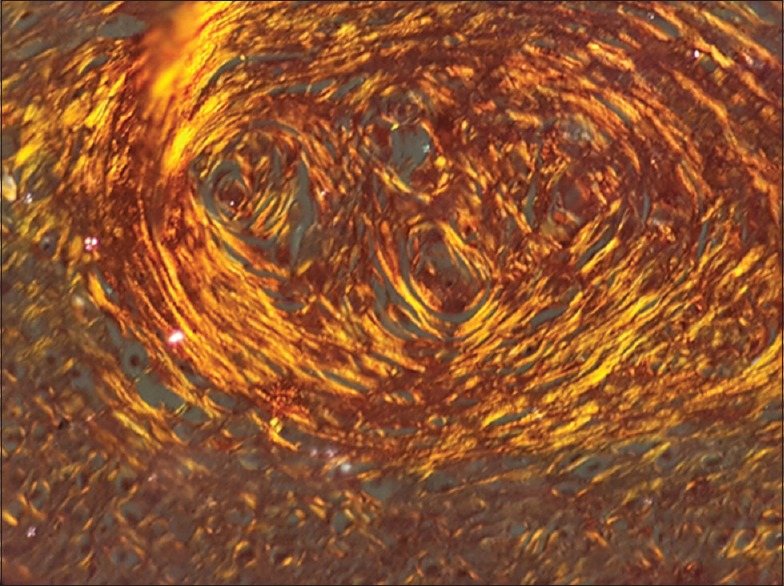
Photomicrograph of moderately differentiated squamous cell carcinoma showing collagen fibers with yellowish orange birefringence under polarizing microscope (×40)
In this study, we found that the birefringence of the collagen fiber changed from reddish-orange to greenish yellow from well to poorly differentiated OSCC. In well-differentiated OSCC, the collagen fibers displayed polarizing colors of reddish orange around the tumor islands in the majority of the fields. A gradual change in polarizing colors from yellowish orange to greenish yellow was seen in moderately differentiated OSCC. However, poorly differentiated OSCC predominantly showed greenish yellow polarizing color and weak birefringence of this color was seen surrounding the tumor islands.
DISCUSSION
Squamous cell carcinoma consists of two distinct and interdependent components-the tumor epithelial cells and the stroma, in which they are dispersed.[7] During carcinogenesis, it is not only the tumor cells that change but also the stroma.
The extracellular matrix is a complex network of macromolecules which provides structural stability to cells and tissue. Collagen is the chief abundant protein in the extracellular matrix. Changes in any component of this matrix may disturb the physiochemical properties of tissues leading to altered cellular phenotype and changes in cell-matrix interactions. Interactions between invading tumor cells and the extracellular matrix of the host are critical events, which creates an environment conducive to growth and metastasis of tumor cells. There is expression of cell surface receptors for the synthesis of extracellular matrix molecules on the surface of malignant tumor cells.[8] The matrix produced by the transformed cells differs from that produced by the normal cells as the invading tumor cells induce an abundant collagenous or desmoplastic stroma.[9] Collagen is considered as the main barrier to be cleared away during invasion, thus making room for the infiltrating cell mass.
Collagen molecules contain basic amino acids, which gives a strong reaction with acidic dyes such as PSR. Sirius red dye reacts with collagen and increases the birefringence property of collagen. The birefringence color produced by polarization microscopy of PSR-stained tissue section is a result of size, alignment and packing of collagen fibers.[10] The dye has the capability to detect and differentiate between thick and thin collagen fibers.[8] The present study used PSR stain with polarizing microscopy to analyze the nature of collagen.
The color profile and intensity of birefringence determines the thickness, maturity and molecular packing of the collagen fibers. The Type I collagen fibers, which are thick, mature and densely packed show a strong birefringence exhibiting polarization colors of longer wavelength, i.e., red or orange color. A weak birefringence of green color is exhibited by thin fibrillar or immature and loosely packed collagen such as Type III or immature Type I.[11] The observation of our study revealed a gradual change in polarizing colors along with the advancing grade of OSCC from reddish-orange to yellowish-orange to greenish-yellow.
The present study evaluated the response of stroma in different grades of OSCC and observed that as the grade of carcinoma progressed, there was an increase in thin fibers and a decrease in thick fibers. This change in thickness of fibers with differentiation of OSCC could be due to initial fibroproliferative response and in the later stages, there is abnormal collagen production and defective maturation, thereby promoting the neoplastic growth.[12]
The results of our study were consistent with the findings of Kardam et al.[7] and Kullage et al.,[13] who showed a similar color variance of the collagen fibers in different grades of OSCC.
The result of our study showed that collagen fiber is more abundant in stroma of well-differentiated squamous cell carcinoma (SCC) because of increased deposition of thick bands of densely packed collagen fibers that are Type 1 collagen. This is attributed to the initial fibroproliferative response. Various growth factors and cytokines cause the proliferation of fibroblasts and extracellular matrix, resulting in the formation of thick mature collagen. In poorly differentiated SCC, disintegration of collagen fibers is seen as thin immature Type 3 collagen fibers.
The stroma represents a reaction to myofibroblasts.[11] The neoplastic cells invading the stromal tissue evoke a fibrotic response, resulting in the release of cytokines and growth factors. Transforming growth factor beta (TGF-β) is involved in transdifferentiation of fibroblasts to myofibroblasts-producing activated myofibroblasts in the stromal microenvironment. This causes increased deposition of collagen by inhibiting the induction of nitrous oxide synthetase pathway. The survival of myofibroblasts is hence promoted by evading its apoptosis.[9] Tumor-activated myofibroblasts also prevent penetration of T-cells and macrophages within the tumor nodules, thus providing an immunological barrier.[4]
The inflammatory cells regulate the biosynthesis of collagen by the proliferation of fibroblasts, particularly by releasing lymphokines. Activated mononuclear cells produce various inhibitory and stimulatory regulatory mediators. The balanced productions of these mediators are required to regulate fibroblastic activity, for a normal fibrotic response to occur. Studies have demonstrated that activated mononuclear cells regulate fibroblast growth, fibroblast proliferation and collagen biosynthesis.[13]
The present study observed that as the grade of carcinoma progressed, packing of collagen fibers decreased and orientation of collagen fibers changed from parallel to haphazard. The disintegration of collagen fibers in poorly differentiated SCC could be attributed to:
The action of collagenase enzyme and matrix metalloproteinases (MMP) secreted by tumor cells
Uninhibited proliferation of tumor cells with the secretion of abnormal matrix[7]
Dehydration of collagen fibers[10]
Intratumorous hypoxia
Carcinoma-associated fibroblasts (CAF) – contributes to the production of altered collagen.[14]
Collagenolytic enzymes
The haphazard arrangement of collagen fibers is indicative of increased collagenolytic enzyme activity. Stromal lysis is essential for tumor growth and invasion.[10] Tumor cells produce collagenases which have the ability to degrade Type I collagen. Tumor epithelial cells produce various enzymes such as cathepsin, elastolytic and glycosaminoglycans degrading enzymes which lyse the stroma by inducing collagenolytic activities. Electron microscopic studies have shown diffuse collagenolysis and phagocytosis of intact collagen fibers in the course of carcinoma. Other collagenolytic enzymes implicated during tumor growth include lysosomal enzymes, particularly acidic cathepsin which attacks collagen fibers at nonhelical telopeptide regions.[12] The persistent secretion of these enzymes by cancer cells causes destruction of surrounding collagen, resulting in dissolution of collagen fibers or formation of immature collagen fibers, facilitating tumor growth and metastasis.[13]
Matrix metalloproteinases
MMPs belong to a family of proteases. They cause proteolysis of the extracellular matrix components, thereby paving a path for the migrating tumor cells. Most epithelial tumor cells secrete MMPs which acts on the collagen in the immediate vicinity.[7] MMPs are also synthesized by stromal cells of cancer tissue. MMP-1 causes degradation of Type I collagen. MMP-2 induces cell migration. MMP-2,3 and 7 release TGF-β. MMP-3 also causes cell apoptosis.[12] They thus exhibit a carcinogenic action.
Intratumorous hypoxia
Hypoxia which arises within the tumors induces genetic instability and accelerates angiogenesis. This makes the stroma edematous and unstable.[14] Studies have shown that intratumourous hypoxia arises within fibrotic foci in human malignant tumors. Intratumourous hypoxia is a driving force behind angiogenesis and significantly correlates with tumor invasion, metastasis and poor prognosis.[4]
Dehydration of collagen fibers
As carcinoma progresses, collagen is converted from mature to immature.[10] During this process, the proteoglycan content changes and dehydration occurs, thereby decreasing the diameter of collagen fibers.[12] This pathologic degradation of collagen results in disorganization and loss of polarization sensitivity.
Carcinoma associated fibroblasts
A subgroup of fibroblasts known as CAF is thought to have a critical role in cancer initiation, progression, invasion and metastasis. The CAF in tumor stroma has a diverse origin. Majority are thought to arise from normal fibroblasts. The tumor cells induce epigenetic changes in normal fibroblasts and mutate them into CAF. They also rise when epithelial cells undergo epithelial-mesenchymal transition from trans-differentiated cells such as adipocytes, pericytes or smooth muscle cells.[15] This mutated fibroblast phenotype contributes to the production of abnormal collagen. They also degrade the extracellular matrix protein by secretion of various growth factors, cytokines and chemokines, resulting in a disorganized abortive stroma.[16]
Molecular cross-talk between the neoplastic cells and stromal cells, modifies the differentiation, proliferative capacity and invasive capacity of tumor cells.[4] Epithelial-mesenchymal interactions impart mesenchymal traits to cancer cells. They can generate properties associated with malignancies, including motility, resistance to apoptosis, invasion and metastasis.[17]
Stromal changes at the invading front can be evaluated effectively with the use of PSR stain. The particular color produced by polarization microscopy of PSR-stained sections could be due to fiber size, alignment and packing, cross-linking of fibers, interstitial ground substance and water content.[12] In the present study, a shift to the longer wavelength of polarization of color was seen in tightly packed fibers. The spectrum of color change can be due to change in the thickness of collagen fibers. PSR staining under polarizing microscopy can detect the fibrotic process early, and this may help the clinician to predict the prognosis of the lesion.
In the initial grades, cancer invasion induces a desmoplastic reaction, i.e., formation of new extracellular matrix by activation of stromal cells. In progressive grades, the mechanical pressure exerted due to tumor growth and proteolysis induced by the tumor results in collagen degradation which facilitates tumor invasion.
Immunotherapeutic strategies should be targeted at preventing the interactions between cancer cells and stroma. Potential treatments targeting the stroma could include, inhibiting trans-differentiation of fibroblasts to myofibroblasts, induction of apoptosis in myofibroblasts, antiangiogenic factors and MMP inhibitors.[4] In addition, the desmoplastic response prevents the penetration of therapeutic agents into the solid tumor. Netti et al. identified collagen as a potential target of treatment to improve drug penetration.[18]
The understanding of the contribution of stroma to cancer progression is beneficial for both prognostic and therapeutic purposes.
CONCLUSION
Tumor cells have a capacity to induce stromal response, which is essential for their growth and spread. The reactive changes in the tumor stroma may reflect the biological diversity of oral cancer. PSR in conjunction with polarizing microscopy is efficient for the early detection of fibrotic changes. Incorporating this concept for assessing, the prognosis may aid the clinician to predict the clinical outcomes of oral cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.New Global Cancer Data: Globocan. 2018. [Last accessed on 2018 Nov 16]. Available from: https://www.uicc.org/globocan-2018 .
- 2.Feller L, Lemmer J. Oral squamous cell carcinoma: Epidemiology, clinical presentation and treatment. J Cancer Ther. 2012;3:263–8. [Google Scholar]
- 3.Giussani M, Merlino G, Cappelletti V, Tagliabue E, Daidone MG. Tumor-extracellular matrix interactions: Identification of tools associated with breast cancer progression. Semin Cancer Biol. 2015;35:3–10. doi: 10.1016/j.semcancer.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Sis B, Sarioglu S, Sokmen S, Sakar M, Kupelioglu A, Fuzun M. Desmoplasia measured by computer assisted image analysis: An independent prognostic marker in colorectal carcinoma. J Clin Pathol. 2005;58:32–8. doi: 10.1136/jcp.2004.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeClerck YA. Desmoplasia: A response or a niche? Cancer Discov. 2012;2:772–4. doi: 10.1158/2159-8290.CD-12-0348. [DOI] [PubMed] [Google Scholar]
- 6.Aparna V, Charu S. Evaluation of collagen in different grades of oral squamous cell carcinoma by using picrosirius red stain - A histochemical study. J Clin Diagn Res. 2010;4:3444–9. [Google Scholar]
- 7.Kardam P, Mehendiratta M, Rehani S, Kumra M, Sahay K, Jain K. Stromal fibers in oral squamous cell carcinoma: A possible new prognostic indicator? J Oral Maxillofac Pathol. 2016;20:405–12. doi: 10.4103/0973-029X.190913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan S, Vij H, Gupta V, Tyagi N. Role of histological stains in evaluation of collagen in oral squamous cell carcinoma as a diagnostic tool: A review. Univ J Dent Sci. 2016;1:85–9. [Google Scholar]
- 9.Rathore AS, Jain A, Shetty DC, Saxena E. Tumor-stromal crosstalk in oral squamous cell carcinoma: A histochemical study. Clin Cancer Investig J. 2016;5:208–12. [Google Scholar]
- 10.Gawande M, Walke AN, Patil S, Choudhary M. The assessment of role of collagen fibers in oral submucous fibrosis, oral squamous cell carcinoma and oral submucous fibrosis with oral squamous cell carcinoma by using Picrosirius red staining and polarized microscope. IOSR J Dent Med Sci. 2015;14:87–92. [Google Scholar]
- 11.Kalele KK, Managoli NA, Roopa NM, Kulkarni M, Bagul N, Kheur S. Assessment of collagen fiber nature, spatial distribution, hue and its correlation with invasion and metastasis in oral squamous cell carcinoma and surgical margins usiong Picrosirius red and polarized microscope. J Dent Res Rev. 2014;1:14–7. [Google Scholar]
- 12.Arun Gopinathan P, Kokila G, Jyothi M, Ananjan C, Pradeep L, Humaira Nazir S. Study of collagen birefringence in different grades of oral squamous cell carcinoma using picrosirius red and polarized light microscopy. Scientifica (Cairo) 2015;2015:802980. doi: 10.1155/2015/802980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kullage S, Jose M, Shanbhag VKL, Abdulla R. Qualitative analysis of connective tissue stroma in different grades of oral squamous cell carcinoma: A histochemical study. Indian J Dent Res. 2017;28:355–61. doi: 10.4103/ijdr.IJDR_683_16. [DOI] [PubMed] [Google Scholar]
- 14.Sharma R, Rehani S, Mehendiratta M, Kardam P, Kumra M, Mathias Y, et al. Architectural analysis of picrosirius red stained collagen in oral epithelial dysplasia and oral squamous cell carcinoma using polarization microscopy. J Clin Diagn Res. 2015;9:EC13–6. doi: 10.7860/JCDR/2015/13476.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bremnes RM, Dønnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, et al. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011;6:209–17. doi: 10.1097/JTO.0b013e3181f8a1bd. [DOI] [PubMed] [Google Scholar]
- 16.Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta. 2013;1832:1070–8. doi: 10.1016/j.bbadis.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCuaig R, Wu F, Dunn J, Rao S, Dahlstrom JE. The biological and clinical significance of stromal-epithelial interactions in breast cancer. Pathology. 2017;49:133–40. doi: 10.1016/j.pathol.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–503. [PubMed] [Google Scholar]


