Abstract
Background:
Oral candidiasis is a common fungal infection caused by Candida albicans. In recent years, Candida species have shown resistance against many synthetic drugs, which has highlighted the need for novel antifungal drugs with fewer side effects for effective management of candidiasis. Several studies have suggested that some plant species possess promising antimicrobial compounds. Piper betle and Ocimum sanctum Linn are such common medicinal plants that show strong antimicrobial activity by the release of secondary metabolites. However, the effect of these plants on oral candidiasis has not been studied and hence, the present study aimed to evaluate the antifungal activity of these plant extracts on the subcultures of C. albicans and compared with a standard drug, fluconazole.
Materials and Methods:
Subcultures of C. albicans obtained from oral thrush patients were used in the present study. Ethanolic and ethyl acetate extracts of P. betle (betel) and O. sanctum L. (tulsi) leaves were prepared by cold extraction method. The anticandidal activity and minimum inhibitory concentration (MIC) were evaluated using disc diffusion method and microbroth dilution method, respectively. Values were compared with the standard drug fluconazole.
Results:
Both the extracts exhibited anticandidal activity on the subcultures of C. albicans. The ethyl acetate extract of mature betel leaf showed a maximum zone of inhibition (26 mm) when compared with tulsi and fluconazole (13 mm). Betel leaf extract showed better MIC values (125 μg/ml) than tulsi (2000 μg/ml). However, these values were high when compared with those of fluconazole (62.5 μg/ml).
Conclusion:
Ethyl acetate extract of mature betel leaf exhibited good anticandidal activity than that of tulsi and fluconazole.
Keywords: Antifungal, Candida albicans, fluconazole, Ocimum sanctum, Piper betle
INTRODUCTION
Infectious diseases continue to have a significant impact on the health of communities around the world. Oral candidiasis is a common infectious fungal disease of the oral cavity caused by an overgrowth of Candida species, with the most common being Candida albicans.[1] The incidence of infection has increased dramatically over the past three decades, causing an increase in morbidity and mortality in immunocompromised patients.[2] Secreted aspartyl proteinase, the most crucial extracellular enzyme produced by C. albicans, plays a vital role in invading, colonizing and causing damage to the host tissue.[3]
In the modern world, multiple drug resistance has developed against many microbial infections including candidiasis due to the haphazard use of commercial antimicrobial drugs in the treatment of infectious diseases. In addition to this problem, antibiotics are often associated with adverse effects on the host including hypersensitivity, immunosuppression and allergic reactions.[4] This situation forced scientists to search for novel antimicrobial agents that would be less toxic and more effective. Interestingly, several medicinal plants have been extensively investigated to find novel bioactive compounds.[5] Moreover, several studies have suggested that many plant species possess promising antimicrobial compounds.[6,7,8,9]
Piper betle (betel leaf) is one such medicinal plant widely used as a mouth freshener after meal among Asians. This plant is extensively grown in Bangladesh, India, Sri Lanka and other Southeast Asian countries.[10] Previous studies on the betel leaf, root and whole extract showed a robust antimicrobial activity by the release of secondary metabolites.[11,12] Hydroxychavicol, a major phenolic component of betel leaves, is reported to have activity against Candida spp.[13] These plant extracts are also used to cure urinary tract infections, cervicitis vaginitis, gastrointestinal disorders and skin infections such as herpes simplex virus type-1.[10,14] The plant extract can also act as an antioxidant, chemopreventive, anti-inflammatory, antiplatelet and antithrombotic agent.[15]
Ocimum sanctum Linn is another medicinal plant having numerous medicinal properties.[16,17] O. sanctum L., commonly known as “Holy Basil” in English and “Tulsi” in Hindi and Sanskrit, is a bushy plant with a unique fragrance found in the semitropical and tropical regions of the world including India.[18] O. sanctum (tulsi) has been studied on a significant scale and has shown a plethora of biological and pharmacological activities benefiting humans since ages. Tulsi has been widely accepted to possess antioxidant, anticancer, antidiabetic, antimicrobial, antifungal, hepatoprotective, analgesic, antifertility, anti-inflammatory, antiulcer and antihypertensive actions.[19,20,21,22,23,24] In addition, it has been recommended for the treatment of various diseases such as bronchial asthma, malaria, diarrhea, dysentery, skin diseases, chronic fever and insect bite.[25,26]
However, literature on the anticandidal activity of betel and Tulsi on oral candidiasis has not been reported earlier. Therefore, the present study was conceptualized as the initial step to comprehensively detail the anticandidal potential of betel and Tulsi leaf extract by assessing the inhibition zones with agar well diffusion method against C. albicans, which is the most predominant pathogen of oral candidiasis. The findings were then compared with those obtained with fluconazole, a drug used commonly as a therapeutic agent for the treatment of candidiasis, as a standard.
MATERIALS AND METHODS
Plant collection and preparation of extracts
Fresh leaves of young and mature betel and Tulsi were collected from the nursery. All the leaves were washed with tap water followed by distilled water and then shade dried. The dried leaves were grounded using a mortar and a pestle into a fine powder. Plant extracts were prepared by cold extraction method using solvents such as ethanol and ethyl acetate. 25 g of each powdered sample was soaked in 20 ml of the respective solvents and kept in dark for 3–5 days so that secondary metabolites diffuse out into the solvents. It was then filtered with Whatman filter paper No. 2, and the filtrate was collected. The ethanolic and ethyl acetate extracts were concentrated at 40°C using a rotary evaporator (Stuart RE 300, Mumbai, India) at a speed of 25 rpm.[27] The residue was collected and stored at −20°C until further use. Three concentrations (100, 500 and 1000 μg/ml) of the extracts were prepared.
Collection of Candida albicans isolates
Five clinical isolates of C. albicans were collected from patients of our institute who were suffering from oral thrush by using sterile cotton swabs. The swabs were aseptically streaked on Sabouraud's dextrose agar plates. Suspended yeast colonies were subcultured for identification purpose. The plates were incubated at 37°C for 24 h, and the resultant colonies were maintained at 4°C for short-term storage. Identity was reconfirmed by germ tube test.
Anticandidal assay using disc diffusion method
The fungal strains were maintained on potato dextrose agar medium (Hi-Media, Mumbai, India). A loopful of culture from the slant was inoculated into the medium and incubated at 28°C for 48–72 h, and 0.1 ml of this culture was evenly spread on the plates containing respective media. Sterile discs of Whatman No. 1 filter paper of about 6-mm diameter were impregnated on the surface of the media. Different concentrations of both extracts were prepared and applied on the discs and incubated for 48 h at 28°C. The results were recorded by measuring the inhibition zone around the discs[28] [Figures 1–3].
Figure 1.
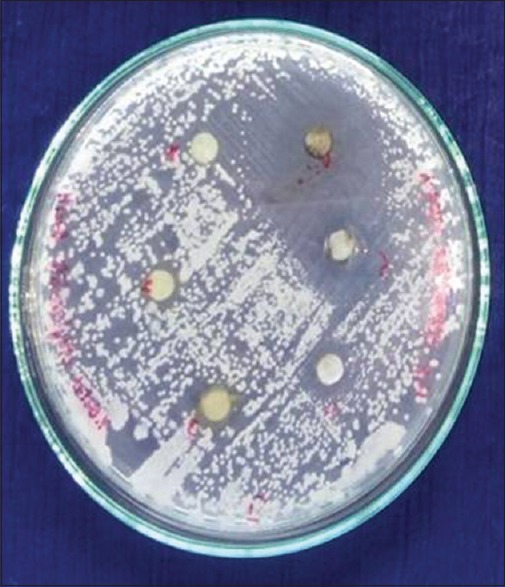
Ethanolic and ethyl acetate extracts of young betle leaf showing zone of inhibition
Figure 3.
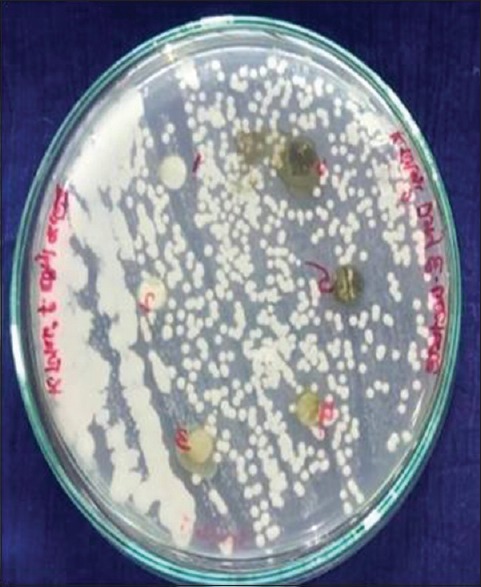
Ethanolic and ethyl acetate extract of tulsi leaf showing zone of inhibition
Figure 2.
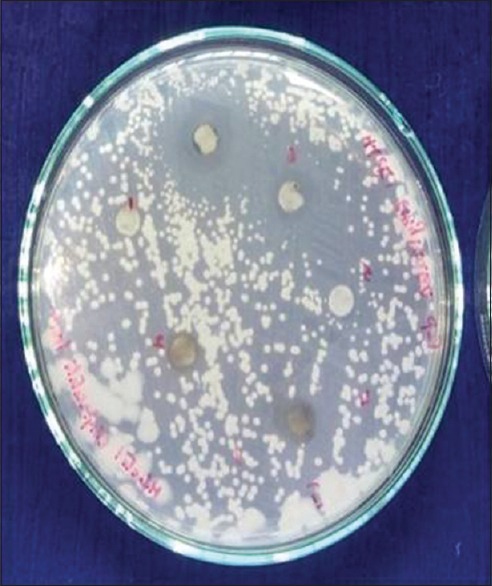
Ethanolic and ethyl acetate extracts of mature betle leaf showing zone of inhibition
Determination of minimum inhibitory concentration
Minimum inhibitory concentration (MIC) is the lowest concentration of an antimicrobial agent that appears to inhibit the growth of the microbes. The lower the concentration required, the higher is the efficacy. MIC was determined by a microbroth dilution method according to the Clinical and Laboratory Standards Institute guidelines. An extract concentration of 0.1–2 mg/ml was evaluated. Specifically, 0.1 ml of standardized inoculum (1–2 × 107 colony-forming unit/ml) was added to each test tube. The tubes were incubated aerobically at 28°C for 48–72 h. Two controls were maintained for each test sample. The lowest concentration (highest dilution) of the extract that produced no visible signs of microbial growth (no turbidity) when compared with the control tubes was regarded as the MIC.[29]
RESULTS
The anticandidal activity of ethanolic and ethyl acetate extracts of betel and tulsi leaves along with standard drug fluconazole was analyzed in the present study, and the results are tabulated [Tables 1–3]. The zone of inhibition increased in a dose-dependent manner. Among the three concentrations (100, 500 and 1000 μg/ml) used, the maximum inhibitory zone was observed at 1000 μg/ml in the ethyl acetate extract of mature leaves (26 mm), followed by tulsi and fluconazole (13 mm). This was twice as better in betel leaf than the other two.
Table 1.
Anticandidial activity of betel leaf extract
| Concentration (μg/ml) | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| Ethanolic extract | Ethyl acetate extract | |||
| Young | Mature | Young | Mature | |
| 100 | - | 5 | 10 | - |
| 500 | 8 | 12 | 22 | 17 |
| 1000 | 15 | 22 | 22 | 26 |
Table 3.
Minimum inhibitory concentration of betel and tulsi leaf extracts
| Extract | MIC (μg/ml) | ||
|---|---|---|---|
| Betel leaf | Tulsi leaf | ||
| Young | Mature | ||
| Ethanol | 500 | 750 | 2000 |
| Ethyl acetate | 250 | 125 | 2000 |
MIC: Minimum inhibitory concentration
Table 2.
Anticandidial activity of tulsi leaf extract
| Concentration (μg/ml) | Zone of inhibition (mm) | |
|---|---|---|
| Ethanolic extract | Ethyl acetate extract | |
| 100 | - | - |
| 500 | 7 | - |
| 1000 | 13 | 13 |
The MIC of ethanolic and ethyl acetate extracts of betel and tulsi leaves along with standard drug fluconazole was tested, and the results are shown in Tables 3 and 4. Fluconazole showed the least MIC value (62.5 μg/ml) followed by ethyl acetate extract of betel (125 μg/ml) and tulsi (2000 μg/ml) leaves.
Table 4.
Anticandidial activity of standard drug
| Drug | Zone of inhibition (mm) | MIC (μg/ml) |
|---|---|---|
| Fluconazole | 13 | 62.5 |
MIC: Minimum inhibitory concentration
DISCUSSION
The indiscriminate use of commercial antimicrobial drugs has caused multiple drug resistance in human pathogenic microorganisms. The resistant strains of C. albicans, a contributing agent for oral candidiasis, have become a cause of major health concerns, and novel antifungal agents are required to tackle this problem. This situation forced scientists to search for new and effective antimicrobial agents to replace the current regimens.[30]
Natural products are in great demand for their extensive biological properties and bioactive components which had been proved to be useful against a large number of causative agents of diseases.[10] Studies on the natural products of plant origin such as betel and tulsi proved that the released secondary metabolites have strong antimicrobial activity.[11,12,13,19,20,21,22,23,24]
The current study investigated the anticandidal activity of two plants against the subcultures of C. albicans obtained from oral candidiasis patients. This was the first study where ethanolic and ethyl acetate extracts have been used to investigate the anticandidal activity of a plant material. According to the results obtained in the current study, the ethyl acetate extract of mature leaves of P. betle showed the maximum inhibitory zone against C. albicans (26 mm). This was twice larger than the inhibitory zone obtained from tulsi and standard drug fluconazole (13 mm).
A study by Nanayakkara et al. revealed that extracts from young betel leaf showed higher anticandidal activity than that of mature leaves. This was explained by the release of more secondary metabolites from young leaves because of the fragility of their physical defenses such as lack of a thick waxy cuticle and low cell wall rigidity.[27] Our study showed contrary results wherein mature leaves had better anticandidal activity than young leaves, which can probably be explained by the use of ethyl acetate, a solvent not used in the study by Nanayakkara et al., hence an increased release of secondary metabolites in mature leaves. However, this requires more studies to confirm the effect of ethyl acetate solvent in the increased release of secondary metabolites from betel leaf.
In the current study, the ethanolic and ethyl acetate extracts of tulsi leaves showed half the amount of inhibitory zone against C. albicans (13 mm) when compared with betel leaf and the equal amount with standard drug fluconazole. To the best of our knowledge, this was the first study to examine the anticandidal activity of betel leaf and tulsi with an approved drug. The low candidal activity of the tulsi leaf may be improved via the use of different solvents and different extraction procedures, considering the polarity of the active compounds. In addition, the purification of the active compound may lead to higher anticandidal activity.
Hydroxychavicol present in betel leaf extract and methyl chavicol and linalool in tulsi leaf extract are responsible for their anticandidal activity. They alter the cell membrane structure, resulting in the disruption of the permeability barrier of microbial membrane structure.[13,23] Phytochemical analysis in our laboratory also revealed the presence of these secondary metabolites.
Further, the efficacy of these leaf extracts was tested by obtaining their MIC values. It showed that the MIC value of fluconazole (62.5 μg/ml) was twice better than those of ethyl acetate extract of mature betel leaves (125 μg/ml). In a study conducted by Ali et al.[13] using hydroxychavicol isolated from betel leaf, similar MIC values were observed against C. albicans as reflected in our study. However, a study by Makkar et al.[30] showed lower MIC values, and another study by Nanayakkara et al.[27] showed higher MIC values. Tulsi leaves showed higher MIC values (2000 μg/ml) than betel leaf and standard drug. This was the first study to find the efficacy of tulsi against C. albicans. The higher MIC values obtained from P. betle and tulsi leaves in the present study can be attributed to the use of crude extracts of the leaves when compared with the purified form of the standard drug.
CONCLUSION
The results of this study demonstrate that the ethyl acetate extract of P. betle is more effective as an anticandidal agent against C. albicans when compared with tulsi and fluconazole. This would help to replace drugs to which Candida have evolved resistance by promoting these traditional medicines. Further studies on tulsi by using different solvents would increase the efficacy of this plant product compared to standard drug.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the assistance of our lab technician Mr. Padmanabha Reddy.
REFERENCES
- 1.Akpan A, Morgan R. Oral Candidiasis. Postgrad Med J. 2002;78:455–9. doi: 10.1136/pmj.78.922.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolan K, Montgomery S, Buchheit B, Didone L, Wellington M, Krysan DJ. Antifungal activity of tamoxifen: In vitro and in vivo activities and mechanistic characterization. Antimicrob Agents Chemother. 2009;53:3337–46. doi: 10.1128/AAC.01564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–28. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharathi T, Kolanjinathan K, Saranraj P. Antimicrobial activity of solvent extracts of Ocimum sanctum, Azadirachta indica and Phyllanthus amarus against clinical Pathogens. Glob J Pharmacol. 2014;22:33–45. [Google Scholar]
- 5.Peter KV. 1st ed. 1, 2. Sawston, Cambridge: Woodhead Publishing Limited; 2004. Handbook of Herbs and Spices. [Google Scholar]
- 6.Nakamura CV, Ishida K, Faccin LC, Filho BP, Cortez DA, Rozental S, et al. In vitro activity of essential oil from Ocimum gratissimum L. Against four Candida species. Res Microbiol. 2004;155:579–86. doi: 10.1016/j.resmic.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Wannissorn B, Jarikasem S, Siriwangchai T, Thubthimthed S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia. 2005;76:233–6. doi: 10.1016/j.fitote.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Khan R, Islam B, Akram M, Shakil S, Ahmad A, Ali SM, et al. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules. 2009;14:586–97. doi: 10.3390/molecules14020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagtap SD, Deokule SS, Pawar PK, Harsulkar AM, Kuvalekar AA. Antimicrobial activity of some crude herbal drugs used for skin diseases by Pawra tribes of Nandurbar district. J Nat Prod. 2010;1:216–20. [Google Scholar]
- 10.Hoque MM, Rattila S, Shishir MA, Bari ML, Inatsu Y, Kawamoto S. Antibacterial activity of ethanol extract of betel leaf (Piper betle L.) against some food borne pathogens. Bangladesh J Microbiol. 2011;28:58–63. [Google Scholar]
- 11.Jenie BS. Antimicrobial Activity of Piper betle Linn Extract Towards Foodborne Pathogens and Food Spoilage Microorganisms, FT Annual Meeting, New Orleans, Louisiana. 2001 [Google Scholar]
- 12.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali I, Khan FG, Suri KA, Gupta BD, Satti NK, Dutt P, et al. In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Ann Clin Microbiol Antimicrob. 2010;9:7. doi: 10.1186/1476-0711-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caceres A, Cano O, Samayoa B, Aguilar L. Plants used in Guatemala for the treatment of gastrointestinal disorders 1. Screening of 84 plants against Enterobacteria. J Ethnopharmacol. 1990;30:55–73. doi: 10.1016/0378-8741(90)90017-n. [DOI] [PubMed] [Google Scholar]
- 15.Bandaranayake BM, Panagoda GJ, Abayasekara CL. The effect of Piper betle against Candida albicans adherence to denture acrylic surfaces. Ceylon J Sci. 2018;47:153–8. [Google Scholar]
- 16.Siddiqui HH. Safety of herbal drug - san overview. Drugs News Views. 1993;1:7–10. [Google Scholar]
- 17.Cragg GM, Newman DJ. Natural product drug discovery in the next millennium. Pharm Biol. 2001;39(Suppl 1):8–17. doi: 10.1076/phbi.39.s1.8.0009. [DOI] [PubMed] [Google Scholar]
- 18.Naquvi JK, Dohare LS, Shuaib M, Ahmad IM. Chemical composition of volatile oil of Ocimum sanctum Linn. Int J Biomed Adv Res. 2012;3:129131. [Google Scholar]
- 19.Sethi J, Sood S, Seth S, Talwar A. Evaluation of hypoglycemic and antioxidant effect of Ocimum sanctum. Indian J Clin Biochem. 2004;19:152–5. doi: 10.1007/BF02894276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey G, Madhuri S. Pharmacological activities of Ocimum Sanctum (Tulsi): A review. Int J Pharm Sci Rev Res. 2010;5:61–6. [Google Scholar]
- 21.Kamyab AA, Eshraghian A. Anti-inflammatory, gastrointestinal and hepatoprotective effects of Ocimum sanctum Linn: An ancient remedy with new application. Inflamm Allergy Drug Targets. 2013;12:378–84. doi: 10.2174/1871528112666131125110017. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharyya P, Bishayee A. Ocimum sanctum linn. (Tulsi): An ethnomedicinal plant for the prevention and treatment of cancer. Anticancer Drugs. 2013;24:659–66. doi: 10.1097/CAD.0b013e328361aca1. [DOI] [PubMed] [Google Scholar]
- 23.Amber K, Aijaz A, Immaculata X, Luqman KA, Nikhat M. Anticandidal effect of Ocimum sanctum essential oil and its synergy with fluconazole and ketoconazole. Phytomedicine. 2010;17:921–5. doi: 10.1016/j.phymed.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Gopalkrishna AH, Seshagiri M, Muddaiah S, Shashidara R. In vitro antifungal activity of different components of Centratherum anthelminticum and Ocimum sanctum seed oils and their synergism against oral pathogenic fungi. J Dent Res Dent Clin Dent Prospects. 2016;10:92–8. doi: 10.15171/joddd.2016.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum linn (Tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J Physiol Pharmacol. 2005;49:125–31. [PubMed] [Google Scholar]
- 26.Pattanayak P, Behera P, Das D, Panda SK. Ocimum sanctum linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn Rev. 2010;4:95–105. doi: 10.4103/0973-7847.65323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanayakkara BS, Abayasekara CL, Panagoda GJ, Kanatiwela HM, Senanayake MR. Anti-candidal activity of Piper betle (L.), Vitex negundo (L.) and Jasminium grandiflorum (L.) Afr J Microbiol Res. 2014;8:2307–14. [Google Scholar]
- 28.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 29.CLSI document M27-A3. 3rd ed. Wayne: Clinical and Laboratory Standards Institute; 2008a. Clinical and Laboratory Standards Institute. CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. [Google Scholar]
- 30.Makkar N, Prasanna SB, Singla H. Comparative evaluation of antifungal activity of Piper betel Leaf Oil, Origanum vulgare essential oil and fluconazole suspension on Candida albicans − An in vitro study. J Indian Assoc Public Health Dent. 2017;15:89–93. [Google Scholar]


