Abstract
Objectives:
Anatomic changes may occur during chemoradiation treatment for lung cancers, requiring adaptive replanning. Here we characterize these cases.
Methods:
We retrospectively studied lung cancer cases that underwent resimulation and adaptive replanning during 1/2016–3/2019. We compared first and second CT-simulation regarding tumor location, timing of change, tumor volume, anatomical alteration and change in simulation technique. We also compared dosimetric parameters between the plans, recorded local control, and overall survival outcomes.
Results:
Out of 281 patients, 58 underwent replanning (20.6%). Histology included small cell (22.4%) and non-small cell (77.6%). Stage III was in 91.4%. Mean radiation dose of 59.4 Gray (Gy) (range 50-66Gy).
Tumor location was peribronchial in 53.5%. Timing of replanning was in the first, second and final third of the treatment course in 26%, 43% and 31% respectively. Changes in gross tumor volume were observed in 74%; mean gross tumor volume was 276.7cc vs 192.7 cc (first vs second simulation, p = 0.001). Anatomical changes were identified in 35.4% including pleural fluid accumulation, atelectasis or pneumothorax alteration. Change in simulation technique was performed in 25.9%, including breath-hold or continuous positive airway pressure.
Changes in dosimetric parameters when the same technique was used: lung V20Gy 26% (standard deviation, SD 7.6) vs 25.3% (SD 6.6) (p = 0.36), mean lung dose 15.1 Gy (SD 3.7) vs 14.7Gy (SD 3.3) (p = 0.23), heart V40Gy 10.2% (SD13) vs 7.2% (SD 9.8) (p = 0.037). When simulation technique changed: lung V20Gy 30.8% (SD 8.2) vs 27.3% (SD 8) (p = 0.012), mean lung dose 17.3 Gy (SD 4.4) vs 15.3 Gy (SD 3.8) (p = 0.007), heart V40Gy 11.1% (SD 14.7) vs 6.5% (SD 6.7) (p = 0.014).
2 year local control was 60.7% (95% confidence interval, 34.5–79.2%), and median overall survival was 19.7 months.
Conclusion:
Adaptive replanning of radiation was performed in a fifth of locally advanced lung cancer patients. In most cases tumor volume decreased, or atelectasis resolved, causing mediastinal shifts, which, if unidentified and left uncorrected, may have led to local failure and increased toxicity. The heart V40Gy was reduced significantly in all cases, but significant reduction in lung doses was evident only if simulation technique was altered.
Advances in knowledge:
In locally advanced lung cancer image-guidance with cone beam CT can detect significant mediastinal shifts and gross tumor volume changes that raise the need for adaptive replanning. Image guidance-triggered adaptive replanning should be added to the armament of advanced radiation treatment planning in locally advanced lung cancer.
Introduction
Intensity modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) are technologic advances in thoracic radiation that deliver conformal dose distributions to the tumor with steep dose gradients. They have been shown to reduce pulmonary, esophageal and cardiac radiotherapy-related toxicity in lung cancer patients, compared to three-dimensional-conformal radiotherapy (3DCRT).1,2 Since dose distributions with IMRT/VMAT are tightly sculptured around the planning target volume (PTV), it is imperative that the tumor will be in the expected position: even if the patient is positioned according to skin and bone anatomy, the tumor may be missed, when changes in internal thoracic anatomy occur. This may reduce the local control rate, and increase side-effects due to normal tissues over dose. Hence, image-guided radiotherapy (IGRT) techniques are required to ensure accurate patient setup. Cone-beam computed tomography (CBCT) is one of the IGRT modalities available. By virtue of the soft tissue contrast of CBCT, it is used for monitoring of the set-up of the patient immediately before treatment delivery, and thus may reduce setup-error and may permit margin-reduction compared to bone match using KV/KV images.3
Frequent volumetric IGRT using CBCT, as is used in Chaim Sheba Medical Center, allows also monitoring of lung anatomic changes and tumor regression or progression that may occur throughout the six weeks of radiotherapy course.
In our institute, if anatomic changes were detected by CBCT during chemoradiation to lung cancers, we would suggest repeat simulation and replanning.
This case series sought to characterize the reasons for repeat simulation and adaptive replanning in locally advanced lung cancer.
Methods and materials
After institutional review board approval, we conducted a review of clinical records for patients with locally advanced lung cancer treated with chemoradiation during the period 1/2016–3/2019. We included in this cohort patients that were treated with thoracic radiation to a dose of at least 50 Gray (Gy) in standard fractionation. Both small cell and non-small cell lung cancer histologies were included, whether treated with definitive or neoadjuvant intent. Exclusion criteria included stereotactic body radiation therapy (SBRT), and adjuvant indications. Within this population, we identified cases that underwent repeat simulation and adaptive replanning during the course of treatment. For every case, two sets of simulations and treatment plans were available.
Contouring and planning details have been described previously.4 In both simulations, the same mediastinal lymph node stations were contoured, conversely, the primary tumor gross tumor volume (GTV) was contoured based upon the new imaging findings.
Dose calculations were performed using the analytical anisotropic algorithm (AAA) in the Eclipse (Varian Medical Systems, Palo Alto, CA) treatment planning system. Radiation in both cohorts had a planned prescription goal of ≥95% of the treatment dose was prescribed volumetrically to >95% of the planning target volume (PTV), unless limited by organ at risk. Dose limitations used were lung volume receiving 20 Gy and above (V20) to be less than 35%; mean lung dose less than 20 Gy; spine dose max of up to 50 Gy; heart V40 less than 35%.
Treatment delivery verification was performed according to the matched images between the daily CBCT and the initial CT simulation. The initial match was to the main carina; subsequently the primary tumor was assessed to ensure that it is inside the PTV.
Reasons for replanning
In cases where significant misalignments of the target were detected, the treating physician would decide if the case required repeat simulation and replanning. Significant changes in tumor volume also were considered for replanning.
There were also cases for which this situation was not foreseen in advance, and became evident only after completing the plan optimization. For example, if dose to organ at risk were exceedingly high. In these cases, deep inspiration breath hold with or without continuous positive airway pressure (CPAP) were required.
In this study, the radiation dose and the treatment techniques were recorded as well as the total treatment duration and the timing of the change from the initial plan to the second plan. GTV of the primary cancer was measured and compared to the second simulation. Change in GTV was determined if there was an increase or decrease of greater than 20% compared to the initial volume. Lung volumes were measured and compared. The tumor location was recorded and defined “peribronchial” if the tumor was causing any distal atelectasis. Anatomical changes were recorded by comparing the first CT simulation to the second.
Dosimetric parameters that were analyzed included lung V20 (percent of volume of the lung receiving 20 Gy or above), mean lung dose (MLD) (the mean radiation dose to the lung, in Gy), heart V40 (percent of heart volume receiving 40 Gy), PTV D95% (PTV; volume covered by 95% of the dose) and spine maximal dose in Gy. These parameters were compared between first and second plans.
In cases that underwent completion surgery after chemoradiation, we recorded the pathologic regression according to the American College of Pathology guidelines.5
Finally, overall survival (OS) was calculated from initiation of treatment till death (recorded from the hospital electronic medical records data or from the national registry) or censored at last follow-up. Disease free survival (DFS) and local control (LC) were determined similarly, based on progression of disease on CT or PET/CT reports (performed as per standard-of-care every 3 months in the first 2 years and every 3–6 months after the second year).
Statistical analysis
Survival data were expressed using Kaplan–Meier estimation, with July 2019 being the date for data censoring. Wilcoxon signed-rank test was used for non-parametric comparisons, performed separately for the cases with the same simulation technique and for the cases with altered simulation technique. χ2 and Fisher exact test were used for contingency between variables. Analyses were performed using STATA v. 13 (StataCorp. 2013. Stata Statistical Software: Release 13.Texas).
Results
281 patients with locally advanced lung cancer were identified, of which 58 cases underwent repeat simulation and replanning (20.6%). Of these, 74.2% were male with mean age 65.3 years (Table 1). The histologies were non-small cell lung cancer in 75.9% and small cell lung cancer or neuroendocrine in 22.4%. Stage 3a-3b comprised 91.4% of patients. 90% of patients received concomitant platinum-based chemotherapy. Tumor location was peribronchial in 31/58 (53.5%).
Table 1.
Patients characteristics, disease and treatment details
| Parameters | |
|---|---|
| Total study cohort N (%) | 58 (100) |
| Age N (%) | |
| Mean (years) , (Range) | 65.3 (41–81) |
| Sex N (%) | |
| Male | 43 (74.2%) |
| Female | 15 (25.8%) |
| Histology N (%) | |
| NSCLC | 44 (75.9%) |
| SCLC /NE | 13 (22.4%) |
| Sarcomatoid | 1 (1.7%) |
| Stage N (%) | |
| II | 3 (5.2%) |
| III | 53 (91.4%) |
| IV | 2 (3.4%) |
| Chemotherapy N (%) | |
| Cisplatin-Vinorelbine | 2 (3.4%) |
| Carboplatin-Paclitaxel | 29 (50%) |
| Etoposide- Cisplatin | 17 (29.3%) |
| Pemetraxed-Cisplatin | 2 (3.4%) |
| Cisplatin | 2 (3.4%) |
| Non | 6 (10.3%) |
| Radiation therapy | |
| Radiation Dose ; Mean (SD) | 59.45 Gray (4.8) |
| Total Number of Fractions ; Mean (SD) | 29 (3.5) |
| Number of Fractions In First Plan; Mean (SD) | 14.9 (6.8); range 2–25 |
| Duration of radiation treatment; Mean (SD) | 42.3 days (11.6) |
| Planning technique N (%) | |
| 3 Dimensional conformal | 12 (20.7%) |
| IMRT/VMAT | 34 (58.6%) |
| Hybrid | 12 (20.7%) |
| IGRT CBCT N (%) | |
| Daily | 45 (77.6%) |
| Weekly | 13 (22.4%) |
| Timing of second plan N (%) | |
| First third | 15 (26%) |
| Second third | 25 (43%) |
| Last third | 18 (31%) |
| Tumor Locations peribronchial (with atelectasis) N (%) | |
| Yes | 31 (53. 5%) |
| No | 27 (45.5%) |
| Changes In GTV Volume (More Than 20%) | |
| Decrease ; (Mean% From Initial GTV); Mean volume,±SD (ml)) | 35 (60.3%) ; (−40%; −124 ml,±SD 121) |
| Increase; (Mean % From Initial GTV); Mean volume ± SD (ml) | 8 (13.8%) ; (+89%;+65 ml,SD 50.8) |
| No Change ( ± 20%) (Mean % From Initial GTV); Mean volume ± SD (ml) | 15 (25.9) ; (−4% ; 20.8 ml,±SD 0.13) |
| Other Anatomical Changes Detected In CBCT N (%) | |
| Yes | 20 (34.5%) |
| No | 38 (65%) |
| Need to Improve Technique of Simulation N (%) | |
| Yes | 14 (24.2%) |
| No | 44 (75.8%) |
| Surgery after chemo-radiation N (%) | |
| Yes | 15 (25.9%) |
| No | 43 (74.1%) |
| Pathological Response (Total 15) N (%) | |
| Complete Response, no viable tumor cells | 8 (53.3%) |
| <10% Residual viable tumor cells | 5 (33.3%) |
| >10% Residual viable tumor cells | 2 (13.3%) |
CBCT, cone beam CT; GTV, gross tumor volume; IGRT, image guided radiation therapy; IMRT, intensity modulated radiation therapy; SCLC /NE, small cell lung cancer/neuroendocrine; SD, standard deviation; VMAT, volumetric modulated arc therapy.
Hybrid – combination of IMRT and 3D Conformal.
Radiation planning and dosing
Mean radiation dose was 59.43 Gy (SD 4.8, range 50–66 Gy), in 29 fractions (SD 3.5). Planning technique was 3DCRT in 12/58 (20.7%), VMAT or IMRT in 34/58 (58.6%) and hybrid (combination of 3DCRT and IMRT) in 12/58 (20.7%). CBCT was performed daily in 77.6% of cases. In the rest it was performed at least once a week.
Timing of replanning was in the first, second or final thirds of treatment in 26, 43 and 31% respectively. Mean duration of treatment with the first plan was 14.9 days (SD 6.9 range 2–25) and mean dose with first plan was 30.5 Gy (SD 14, range 4–50). Mean time interval from start-to-end of radiation therapy was 42.3 days (SD 11.6).
The reason for early replanning (during the first third of treatment) were enlarging tumor (4/7 cases) or need for change in simulation technique. Of the 14 cases with change in simulation technique, 8 occurred in the first third of treatment (57%) with the new plan started after mean of 6.9 fractions (range 3–10). In the middle and last thirds, the prevalent reason was shrinking of the tumor, occurring in 32/47 (68%) of cases. This was more likely to occur in the middle and late phases of the treatment compared to the early third (p = 0.049). The cohort was grouped into three categories according to changes observed:
Change in GTV of primary tumor (>20% compared with initial GTV) were observed in 43/58 (74.1%). GTV decreased in volume in 35/58 (60.3%) and increased in 8/58 (13.8%) (Table 1 for further details). In cases where the same simulation technique was used, the mean GTV in first and second scan were 276.7 cc (SD 253.8) vs 192.7 cc (SD 180) (p = 0.001) (Table 2a). Figure 1 shows an example of GTV regression.
Significant anatomical changes: were detected in 20/58 (34.5%) including resolution of atelectasis in 14/58 (24%), pleural fluid accumulation in 2/58 (3.5%), new atelectasis in 3/58 (5%) and emergence or absorption of pneumothorax in 2/58 (3.5%). We noted that some cases had overlapping changes, i.e. the GTV changed and anatomical alteration occurred in the same patient (as can be observed in Figure 1). The proportion of patients with change in GTV did not differ by change in anatomy (p = 0.6). The proportion of patients with anatomical changes did not differ by location of the primary tumor (p = 0.27). Figure 2a–c show examples of peribronchially located tumors causing lung atelectasis that resolved during the radiation treatment course. Figure 2d shows fused images between the simulation CT and the CBCT, with mediastinal shift. Repeat simulation of this patient revealed a large pneumothorax that was decompressed with immediate insertion of chest tube (Figure 2e).
A change in simulation technique was recorded in 14/58 (24.2%) including breath-hold in 5/58 (8.6%) or use of CPAP to expand the normal lung in 9/58 (15.5%). Figure 3a presents an example of breath-hold, and Figure 3b the use of CPAP.
Table 2.
Comparison for volumes and dosimetric parameters between first and second scan
| 2a: No change in simulation technique | |||
|---|---|---|---|
| Mean (SD) (min–max) | p-value | ||
| GTV (cc) | First scan | 276.7(253.8) (4.8–933) | 0.001 |
| Second scan | 192.7 (180) (2.4–733) | ||
| Lung volume (cc) | First scan | 3700 (1085) (1791–6540) | 0.064 |
| Second scan | 3833 (1046) (1313–6145) | ||
| PTV D95 (%) | First scan | 94.99 (4.04) (84–99.7) | 0.38 |
| Second scan | 94.52 (4.9) (80–99.7) | ||
| Lung V20 (%) | First scan | 26 (7.6) (0.8–39) | 0.36 |
| Second scan | 25.3 (6.6) (9.7–37) | ||
| Mean lung dose (Gy) | First scan | 15.1 (3.7) (3–22.5) | 0.23 |
| Second scan | 14.7 (3.3)5–21 | ||
| Heart V40 (%) | First scan | 10.2 (13) (0–64) | 0.037 |
| Second scan | 7.2 (9.8) (0–45) | ||
| Spine maximal dose (Gy) | First scan | 39 (8.4) (19.6–50.7) | 0.72 |
| Second scan | 39.2 (8.8) (21-52) | ||
| 2b: With change in simulation technique | |||
| Mean (SD) (min-max) | p-value | ||
| GTV (cc) | First scan | 165.8 (161) (9.3–480) | 0.13 |
| Second scan | 131 (126) (3.3–349) | ||
| Lung volume (cc) | First scan | 2835.8 (1062) (1613–5300) | 0.001 |
| Second scan | 4466 (1250)(2567–6838) | ||
| PTV D95 (%) | First scan | 92.2 (4.9) (84-98) | 0.72 |
| Second scan | 93.3 (6.5) (79-99) | ||
| Lung V20 (%) | First scan | 30.8 (8.2) (8–37.5) | 0.012 |
| Second scan | 27.3 (8) (4–36.5) | ||
| Mean lung dose (Gy) | First scan | 17.3 (4.4) (5.6–22.6) | 0.007 |
| Second scan | 15.3 (3.8) (3–18.8 | ||
| Heart V40 (%) | First scan | 11.1 (14.7) (0–51) | 0.014 |
| Second scan | 6.5 (6.7) (0–19) | ||
| Spine maximal dose (Gy) | First scan | 46.6 (8.4 (31.7–60) | 0.028 |
| Second scan | 39.4 (7.9) (26.8–50.3) | ||
GTV, Gross tumor volume; Gy, radiation units in Gray; LungV20, lung volume receiving above 20 Gy; PTV, Planning Target Volume; SD, standard deviation.
D95 volume covered by 95% of the dose; Heart V 40-heart volume receiving dose above 40 Gy. Dose if plan was to the full prescribed dose with the current scan. For p-value Wilcoxon signed-rank test was used.
Figure 1.
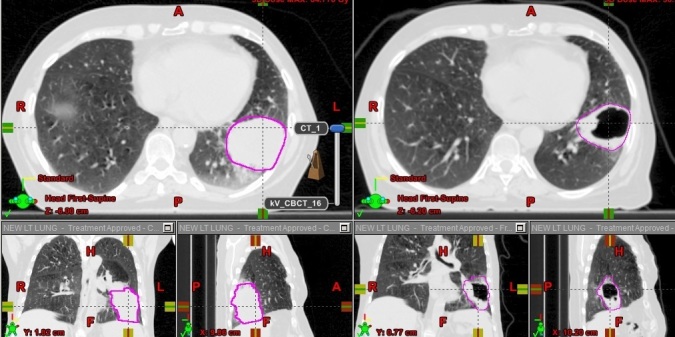
An example of GTV regression. GTV, gross tumor volume.
Figure 2.
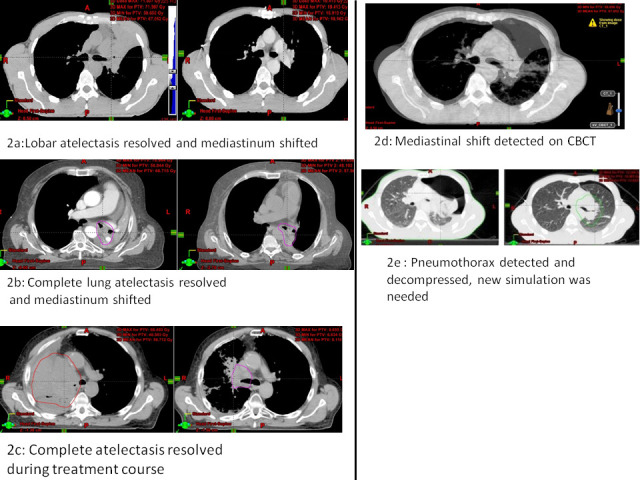
(a–c) Examples of peribronchially located tumors causing lung atelectasis that resolved during the radiation treatment course. (d) Fused images between the simulation CT and the CBCT, with mediastinal shift. (e) Repeat simulation revealed a large pneumothorax that was decompressed with immediate insertion of chest tube. CBCT, cone beam CT.
Figure 3.
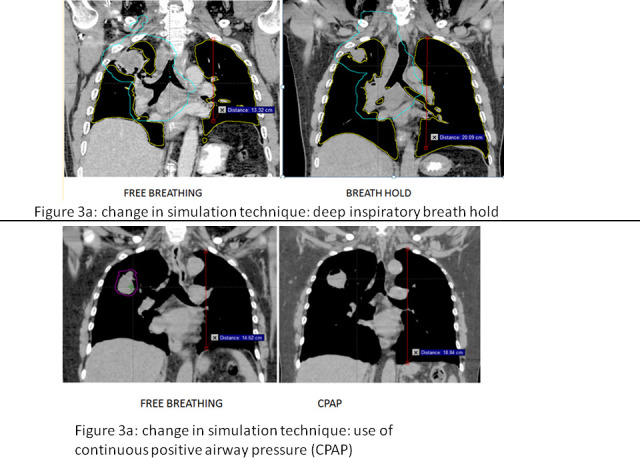
(a) Example of change in simulation technique by using breath-hold. (b) Example of change in simulation technique by using CPAP. CPAP, continuous positive airway pressure.
Comparison of volumes and dosimetric variables between first and second plans (Table 2a, 2b)
For those patients in whom the same simulation technique was used in both simulations (Table 2a) the lung volume increased by mean 133 ml (p = 0.064), the lung V20 decreased only minimally from 26% (SD 7.6) to 25.3% (SD 6.6) (p = 0.36) and MLD decreased from 15.1 Gy (SD 3.7) to 14.7 Gy (SD 3.3) (p = 0.23). The heart V40 decreased significantly in the new plan from 10.2% (SD 13) to 7.2% (SD 9.8) (p = 0.03).
For cases when different simulation techniques were used (Table 2b) the lung volumes increased significantly from 2835.8 cc (SD 1062) to 4466 cc (SD 1250) (p = 0.001), lung V20 decreased significantly from 30.8% (SD 8.2) to 27.3% (SD 8) (p = 0.012) and the MLD decreased from 17.3 (SD 4.4) to 15.3 Gy (SD 3.8) (p = 0.007). The heart V40 decreased as well, from 11.1% (SD 14.7) to 6.5% (SD 6.7) (p = 0.014).
Surgery, DFS and OS
Surgery was performed in 15 cases (25.9%) after neoadjuvant chemoradiation. Final pathology reports indicated significant tumor regression in the majority of cases, with complete response in eight cases (53.3%), and less than 10% residual tumor in another five cases (33.3%).
Median follow-up was 17.3 months (interquartile range 25–75%, 4.08–22.8). Median OS was 19.7 months. 3 years OS was 44.4% (95% CI, 26–61.3%). Median DFS was 14.4 months, with 3 year DFS 37% (95% CI, 18–56%). The 2 year LC rate was 60.7% (95% CI, 34.5–79.2%) and 3 year LC rate 52% (95% CI 25.4–73.3%).
Discussion
In this retrospective descriptive study, a fifth of all locally advanced lung cancer patients treated with fractionated therapy required radiotherapy replanning. This finding appears consistent with other prospective studies that used weekly CT simulation for detection of changes in anatomy and GTV, with replanning rates of 25–27% of cases. In this cohort, the magnitude of GTV volume-reduction was 40%. Again, in line with other studies reporting volume-reduction of 24% to 49%, depending on the time points of measurement during the six weeks of treatment.6,7
The effects of radiotherapy on the primary tumor or adjacent lung can cause anatomical changes which require replanning to avoid incomplete tumor irradiation, or conversely, toxic effects on normal tissues. In our study, we detected anatomical changes in a third of cases that were replanned. Other authors have reported a higher rate of intrathoracic changes, in up to 83% of the patients over the course of radiotherapy treatment.8
When is the optimal time to perform a repeat simulation? Early resimulation may reduce the radiation dose to organ at risk, but if performed too early there may be a need for a third simulation, with time and resource implications. In this study, most cases were replanned in the middle third of the treatment course, allowing enough time for the new plan to be implemented. Early replanning were generally performed for purpose of improving planning dosimetric parameters (e.g. need for change in technique of simulation using breath-hold, or the novel use of CPAP in our department to expand the lungs and thus reduce the radiation dose to the normal lung tissue).9,10 Changing the plan in the last third of the radiation course occurred in 31%, where significant change developed only later in the treatment. In fact, there is evidence the GTV may continue to decrease after 50 Gy6 and some authors advocate repeat simulation and planning in the last third of treatment, allowing the effect of treatment to become evident.11,12
The duration that is taken to implement the change in radiation plan should be kept to a minimum. If the previous plan can be continued safely until the new plan is ready, we recommend continuing. However, if a break is unavoidable, due to major shifts in the mediastinum or tumor-location, these cases should be given high-priority for planning and quality assurance (QA) to resume treatment as quickly as possible. This is in accordance with the suggested “Traffic Light Protocol” from The Netherlands Cancer Institute.13 In our cohort, treatment interruptions were kept to minimum with a mean total treatment interval of 42.3 days. In cases where there are major anatomic changes, as occurred in 34.5% of patients in our group, proceeding with the previous plan may not be advised since the repositioning of the patient will not be accurate. In these cases not only normal tissue exposed to radiation increases, but equally important, the risk of geographical-miss increases significantly. These anatomical changes must be recognized as fast as possible by regular inspection of the CBCT. In our department, to mitigate these risks, our radiation therapists have undertaken specialist training to recognize the mediastinal structures and the matching parameters which are important for mediastinal set-up,14 as recommended in published data.15–17
One concern of adaptation of target volume is the risk of recurrence in the area of target-reduction (marginal relapse). In the Local Control and Toxicity of Adaptive Radiotherapy Using Weekly CT Imaging trial, marginal relapse was observed in 6% of patients.18 This incidence is considered relatively low and provides some proof of safety when adopting this treatment approach. In our cohort, LC at 2 years was 60.7%, somewhat lower than the 69.6% in the RTOG prospective trial,19 probably due to different study design, and aggressive histologies included in our cohort.
A correlated proof of safety of adaptive planning may come from the pathologic examination of specimens that were excised after chemoradiation. In this study, major pathologic regression was recorded in 86.6% of the cases that were operated. This is considerably higher than the 65% that was seen in our previous report of trimodality approach.20 However, this needs to be validated in a larger cohort.
Replanning requires considerable resources and places unexpected time pressures on radiotherapy departments’ workflow due to its typically unpredictable nature. It would be useful if we were able to foresee the need for repeat planning: are there identifiable factors that increase the probability of this occurrence? In our group, more than half the cases had primary tumor in a peribronchial location causing distal lung atelectasis. The effect of treatment in these cases usually results in some resolution of the atelectasis and thus a shift in the tumor location. Likewise, small cell lung cancers may regress significantly after chemotherapy and anatomical change can be expected. However, some changes are unpredictable, such as the development of pleural effusions, rapid regression or enlargement of the primary tumor, or change in pneumothorax after trans-thoracic biopsy. These cases are identified by the radiation therapists, with daily monitoring of the patients' CBCT before each fraction of radiation. In the future, this process may become automated: work on deformable image registration algorithms such as the Consistent Anatomy in Lung Parametric imagE Registration framework, will be able to handle large geometric changes in the thorax to facilitate accurate adaptive planning.21
In this study all cases, except two, were replanned only once. Would it be beneficial if replanning occurred more than once routinely? According to a dosimetric trial by Dial et al, incremental reductions in doses to organ at risk were seen as a function of replanning frequency. Increased frequencies of adaptation resulted in additional benefit while the magnitude of benefit decreased.22
Furthermore, we observed reduction in dose to the heart with repeated planning: the heart V40 was reduced significantly from 10.2 to 7.2% (p = 0.037) without change in simulation technique. The reason for this may be related to the change in the anatomy and the shrinkage of the tumor, displacing it away from the heart and change in planning technique from 3DCRT to VMAT. In this study, the heart V40 was chosen for measurements and comparison according to the analysis performed on the prospective RTOG 0617 trial, which was published by Chung et al.23 In their study, the volume of heart receiving 40 Gy (V40) was significantly associated with OS (p < 0.05). Other authors have reported other heart doses to be associated with OS and cardiac toxicity: Speirs et al reported that when heart volume receiving 50 Gy (heart V50) was less than 25%, the 1 year OS rates were 70.2 vs 46.8% if the V50 exceeded 25%. In their study, the heart V50 was significantly higher (20.8% vs 13.9%, p < 0.0001) in patients who suffered from cardiac toxicity.24 Wang et al presented a correlation between mean heart dose (MHD) and higher rate of cardiac events: for MHD of 20 Gy, the rate was 21% at 2 years compared to 4% in MHD if <10 Gy.25 In another study by Atkins et al, the MHD (≥10 Gy vs. <10 Gy) was also associated with a significantly increased risk of all cause mortality, but only in patients without pre-existing coronary heart disease (hazard ratio: 1.34; p = 0.014).26 The dose to the heart may be related to the nodal stations involved and if the subcarinal or multiple nodal stations are involved, as in locally advanced Stage III lung cancer, the doses to the heart might be unavoidable with photon therapy.27 Therefore, if with replanning the heart dose can be reduced, as was shown in this study, this may potentially reduce cardiac toxicity.
A potential benefit of plan adaptation may be the reduction of doses to the normal lung. Guckenberger et al reported that plan adaptation to tumor shrinkage resulted in significantly decreased mean lung doses.7 Moller et al also reported a significant decrease in lung dose (MLD decreased from 14.6 to 12.6Gy).28 In our study, the lung doses were not reduced significantly in the group that was replanned with the same simulation technique. However, significant reduction in lung dose was observed if a change was introduced to the simulation technique (breath-hold or use of CPAP). In these cases, the radical radiation doses could otherwise not be safely delivered, as the doses to the organ at risk would have exceeded their limits. This situation may occur in large tumors or if lung volumes are small. Indeed, the lung volumes increased significantly with change in simulation technique from 2835.8 cc to 4466 cc (p = 0.001) and the lung V20 as well as the heart V40 were significantly reduced. Interestingly, a study by Kataria et al demonstrated that if the initial plan was delivered with the new anatomical configuration, it would have resulted in a significantly higher doses to the lung, heart and spinal cord, while repeat planning enabled better normal tissue-sparing.11
A related important point is the increasing use of adjuvant immunotherapy for unresectable Stage III lung cancers—undoubtedly one of the most significant breakthroughs in oncologic treatments of lung cancer.29 Now, more than ever, it is important to reduce the risk of iatrogenic pneumonitis to minimum so that these patients will be able to receive the immunotherapy according to schedule without the need for steroids or treatment-breaks.
With technological advancements in automated-contouring and planning modalities such as MRI-guided adaptive planning in peribronchial located thoracic tumors30 it may be expected that frequent replanning may be feasible in the future and may be implemented in more centers.31 Certainly, if the clinical benefits of MRI-guided radiotherapy can be realized, treatment can be adapted for each fraction and in real-time, using "beam-on" imaging32 avoiding resimulation.
A limitation of this study is its single center, retrospective design, and patients' heterogeneity. However, adaptive planning in this context is still innovative and the decisions are being made locally, based on experience and available resources.
In conclusion, image-guidance triggered-adaptive replanning should be added to the armament of radiation therapy planning for lung cancer, in addition to FDG-PET/CT registration and the advanced planning technique using VMAT. Adaptive replanning may reduce doses of radiation to the heart thus reduce the cardiac toxicity risk, but doses to the lungs could be lowered significantly only if change in simulation technique was introduced.
Some cases requiring replanning can be foreseen, especially peribronchial-located tumors that cause distal lung atelectasis and tumors expected to regress dramatically in response to chemotherapy. However, many changes are not predictable; therefore vigilant monitoring of the CBCT is recommended. Adaptive replanning may allow safer introduction of adjuvant immunotherapy and together with reduced cardiac doses, may consequently lead to improvements in survival.29
Footnotes
Acknowledgements: To Ilana Waiss for her help in data gathering.
Ethics approval: IRB approval was granted for retrospective anonimised analysis.
Zvi Symon and Yaacov Richard Lawrence have contributed equally to this study and should be considered as senior authors.
Contributor Information
Sarit Appel, Email: sarit.appel@sheba.health.gov.il, yarsar@netvision.net.il.
Jair Bar, Email: Yair.Bar@sheba.health.gov.il.
Dror Alezra, Email: Dror.Alezra@sheba.health.gov.i.
Maoz Ben-Ayun, Email: Maoz.Ben-Ayun@sheba.health.gov.il.
Nir Honig, Email: Nir.Honig@sheba.health.gov.il.
Sumit Chatterji, Email: Sumit.Chatterji@sheba.health.gov.il.
Zvi Symon, Email: Zvi.Symon@sheba.health.gov.il.
Yaacov Richard Lawrence, Email: Yaacov.Lawrence@sheba.health.gov.il.
REFERENCES
- 1.Murshed H, Liu HH, Liao Z, Barker JL, Wang X, Tucker SL, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. International Journal of radiation oncology, biology. Physics 2004; 58: 1258–67. [DOI] [PubMed] [Google Scholar]
- 2.Chang JY. Intensity-Modulated radiotherapy, not 3 dimensional conformal, is the preferred technique for treating locally advanced lung cancer. Semin Radiat Oncol 2015; 25: 110–6. doi: 10.1016/j.semradonc.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins J, Bezjak A, Hope A, Panzarella T, Li W, Cho JB, et al. Effect of image-guidance frequency on geometric accuracy and setup margins in radiotherapy for locally advanced lung cancer. International Journal of radiation oncology, biology. Physics 2011; 80: 1330–7. [DOI] [PubMed] [Google Scholar]
- 4.Appel S, Goldstein J, Perelman M, Rabin T, Urban D, Onn A, et al. Neo-Adjuvant chemo-radiation to 60 gray followed by surgery for locally advanced non-small cell lung cancer patients: evaluation of trimodality strategy. Isr Med Assoc J 2017; 19: 614–9. [PubMed] [Google Scholar]
- 5.Junker K, Langner K, Klinke F, Bosse U, Thomas M. Grading of tumor regression in non-small cell lung cancer : morphology and prognosis. Chest 2001; 120: 1584–91. doi: 10.1378/chest.120.5.1584 [DOI] [PubMed] [Google Scholar]
- 6.Fox J, Ford E, Redmond K, Zhou J, Wong J, Song DY. Quantification of tumor volume changes during radiotherapy for non-small-cell lung cancer. International Journal of radiation oncology, biology. Physics 2009; 74: 341–8. [DOI] [PubMed] [Google Scholar]
- 7.Guckenberger M, Wilbert J, Richter A, Baier K, Flentje M. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non-small cell lung cancer. International Journal of radiation oncology, biology. Physics 2011; 79: 901–8. [DOI] [PubMed] [Google Scholar]
- 8.Elsayad K, Kriz J, Reinartz G, Scobioala S, Ernst I, Haverkamp U, et al. Cone-Beam CT-guided radiotherapy in the management of lung cancer: diagnostic and therapeutic value. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft. 2016; 192: 83–91[et al].. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein JD, Lawrence YR, Appel S, Landau E, Ben-David MA, Rabin T, et al. Continuous positive airway pressure for motion management in stereotactic body radiation therapy to the lung: a controlled pilot study. International Journal of radiation oncology, biology. Physics 2015; 93: 391–9. [DOI] [PubMed] [Google Scholar]
- 10.Sadetskii I, Darras I, Appel S, Rabin T, Amit U, Weiss I, et al. Cpap improves the anatomy and dosimetry of patients undergoing Breath-Hold motion management for thoracic SBRT. Int J Radiat Oncol Biol Phys 2018; 102: e498. doi: 10.1016/j.ijrobp.2018.07.1414 [DOI] [Google Scholar]
- 11.Kataria T, Gupta D, Bisht SS, Karthikeyan N, Goyal S, Pushpan L, et al. Adaptive radiotherapy in lung cancer: dosimetric benefits and clinical outcome. Br J Radiol 2014; 87: 20130643. doi: 10.1259/bjr.20130643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong F-M, Ten Haken RK, Schipper M, Frey KA, Hayman J, Gross M, et al. Effect of Midtreatment PET/CT-Adapted radiation therapy with concurrent chemotherapy in patients with locally advanced non-small-cell lung cancer: a phase 2 clinical trial. JAMA Oncol 2017; 3: 1358–65. doi: 10.1001/jamaoncol.2017.0982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwint M, Conijn S, Schaake E, Knegjens J, Rossi M, Remeijer P, et al. Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother Oncol 2014; 113: 392–7. doi: 10.1016/j.radonc.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 14.Katzman T, Symon Z, Shelly E, Luxenburg O. Case report: A novel model for educating radiation therapists in small countries: Case study of the “Train the Trainer” initiative in Israel. Technical Innovations & Patient Support in Radiation Oncology 2018; 8: 10–12. doi: 10.1016/j.tipsro.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavoie C, Higgins J, Bissonnette JP, LW L, Sun A, Brade A, et al. Volumetric image guidance using carina vs spine as registration landmarks for conventionally fractionated lung radiotherapy. International Journal of radiation oncology, biology. Physics 2012; 84: 1086–92. [DOI] [PubMed] [Google Scholar]
- 16.Jan N, Balik S, Hugo GD, Mukhopadhyay N, Weiss E. Interfraction displacement of primary tumor and involved lymph nodes relative to anatomic landmarks in image guided radiation therapy of locally advanced lung cancer. International Journal of radiation oncology, biology. Physics 2014; 88: 210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaake EE, Rossi MM, Buikhuisen WA, Burgers JA, Smit AA, Belderbos JS, et al. Differential motion between mediastinal lymph nodes and primary tumor in radically irradiated lung cancer patients. International Journal of radiation oncology, biology. Physics 2014; 90: 959–66. [DOI] [PubMed] [Google Scholar]
- 18.Ramella S, Fiore M, Silipigni S, Zappa MC, Jaus M, Alberti AM, et al. Local control and toxicity of adaptive radiotherapy using Weekly CT imaging: results from the LARTIA trial in stage III NSCLC. J Thorac Oncol 2017; 12: 1122–30. doi: 10.1016/j.jtho.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 19.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-Dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16: 187–99. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appel S, Bar J, Ben-Nun A, Perelman M, Alezra D, Urban D, et al. Comparative effectiveness of intensity modulated radiation therapy to 3-dimensional conformal radiation in locally advanced lung cancer: pathological and clinical outcomes. Br J Radiol 2019; 92: 20180960. doi: 10.1259/bjr.20180960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy CL, Weiss E, Christensen GE, Jan N, Hugo GD. CALIPER: a deformable image registration algorithm for large geometric changes during radiotherapy for locally advanced non-small cell lung cancer. Med Phys 2018; 45: 2498–508. doi: 10.1002/mp.12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dial C, Weiss E, Siebers JV, Hugo GD. Benefits of adaptive radiation therapy in lung cancer as a function of replanning frequency. Med Phys 2016; 43: 1787–94. doi: 10.1118/1.4943564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017; 35: 56–62. doi: 10.1200/JCO.2016.69.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speirs CK, DeWees TA, Rehman S, Molotievschi A, Velez MA, Mullen D, et al. Heart dose is an independent Dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol 2017; 12: 293–301. doi: 10.1016/j.jtho.2016.09.134 [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Eblan MJ, Deal AM, Lipner M, Zagar TM, Wang Y, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol 2017; 35: 1387–94. doi: 10.1200/JCO.2016.70.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkins KM, Rawal B, Chaunzwa TL, Lamba N, Bitterman DS, Williams CL, et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients With Lung Cancer. Journal of the American College of Cardiology 2019; 73: 2976–87. doi: 10.1016/j.jacc.2019.03.500 [DOI] [PubMed] [Google Scholar]
- 27.McNew LK, Bowen SR, Gopan O, Nyflot MJ, Patel SA, Zeng J, et al. The relationship between cardiac radiation dose and mediastinal lymph node involvement in stage III non-small cell lung cancer patients. Adv Radiat Oncol 2017; 2: 192–6. doi: 10.1016/j.adro.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Møller DS, Holt MI, Alber M, Tvilum M, Khalil AA, Knap MM, et al. Adaptive radiotherapy for advanced lung cancer ensures target coverage and decreases lung dose. Radiother Oncol 2016; 121: 32–8. doi: 10.1016/j.radonc.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 29.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379: 2342–50. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 30.Henke LE, Kashani R, Hilliard J, DeWees TA, Curcuru A, Przybysz D, et al. In silico trial of MR-Guided Midtreatment adaptive planning for Hypofractionated stereotactic radiation therapy in centrally located thoracic tumors. International Journal of radiation oncology, biology. Physics 2018; 102: 987–95. [DOI] [PubMed] [Google Scholar]
- 31.Lustberg T, van Soest J, Gooding M, Peressutti D, Aljabar P, van der Stoep J, et al. Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer. Radiotherapy and Oncology 2018; 126: 312–7. doi: 10.1016/j.radonc.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 32.Bainbridge H, Salem A, Tijssen RHN, Dubec M, Wetscherek A, Van Es C, et al. Magnetic resonance imaging in precision radiation therapy for lung cancer. Transl Lung Cancer Res 2017; 6: 689–707. doi: 10.21037/tlcr.2017.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]


