Abstract
Objective:
To compare therapeutic outcomes between hepatic resection (HR) and radiofrequency ablation (RFA) for small hepatic masses diagnosed as hepatocellular carcinoma (HCC) on pre-treatment imaging study.
Methods:
Our institutional review board approved this retrospective study, and informed consent was waived. Patients with a single (≤3 cm) mass diagnosed as HCC on pre-treatment imaging study between January 2008 and December 2009 who underwent HR (n = 145) or RFA (n = 178) were included. Recurrence-free survival (RFS) and overall survival (OS) were assessed. In the HR group, the false-positive rate for imaging diagnosis was calculated. For the RFA group, the local tumor progression rate was calculated.
Results:
RFS rates at 5 years were 59.3% for the HR group and 32.2% for the RFA group. OS rates at 5 years were 85.4% for the HR group and 76.8% for the RFA group. In the RFA group, cumulative local tumor progression rates were 8.3 and 20.2% at 1 and 3 years. Treatment modality was not an independent prognostic factor for either RFS or OS on multivariate analysis. The false-positive rate for HCC diagnosis based on imaging criteria was 4.8% in the HR group.
Conclusion:
The imaging criteria for diagnosis of HCC have a high positive predictive value. Multivariate analysis showed that RFS and OS rates were not significantly different between HR and RFA for small hepatic masses diagnosed as HCC on pre-treatment imaging.
Advances in knowledge:
Treatment modality (hepatic resection vs RFA) was not an independent prognostic factor for both RFS and OS for small masses (≤3 cm) diagnosed as hepatocellular carcinoma on pre-treatment imaging.
Introduction
Many studies have compared therapeutic outcomes of hepatic resection (HR) and radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC). RFA is comparable to HR in terms of long-term survival in patients with early-stage HCC based on the results of previous studies.1–5
However, many retrospective studies that compared therapeutic outcomes between HR and RFA for HCC have problematic patient selection.6–10 While patients with pathologically confirmed HCC after surgery comprised the HR group, patients with HCC diagnosed via imaging without pathologic confirmation were included in the RFA group. These non-uniform inclusion criteria may cause various problems. First, false-positive diagnosis of HCC can only be included in the RFA group. The most widely used criteria for imaging diagnosis of HCC are enhancement on arterial phase and wash-out on portal venous or delayed-phase multiphasic contrast-enhanced CT or MRI.1 Although the criteria showed a relatively high positive predictive value, there were still false positives.11,12 Second, HCC with various imaging findings can be included in the HR group, whereas HCC with typical imaging findings can be included in the RFA group. Several studies have reported that therapeutic outcomes can vary according to imaging findings of HCC,13,14 so this method of patient selection may affect therapeutic outcomes after HR and RFA.
To minimize this problem in patient selection, the same inclusion criteria must be applied to both HR and RFA groups. In real clinical circumstances, hepatic masses in high risk patients are diagnosed as HCC if they show typical imaging findings, and treatment is initiated without tissue confirmation. Under these clinical circumstances, only masses that were diagnosed as HCC on imaging studies before treatment should be included to apply the same inclusion criteria to both HR and RFA groups. Therefore, we compared therapeutic outcomes between HR and RFA for small hepatic masses that were diagnosed as HCC on pre-treatment imaging.
Patients and methods
The institutional review board of Samsung medical center approved this retrospective study, and the need for informed consent was waived.
Patients
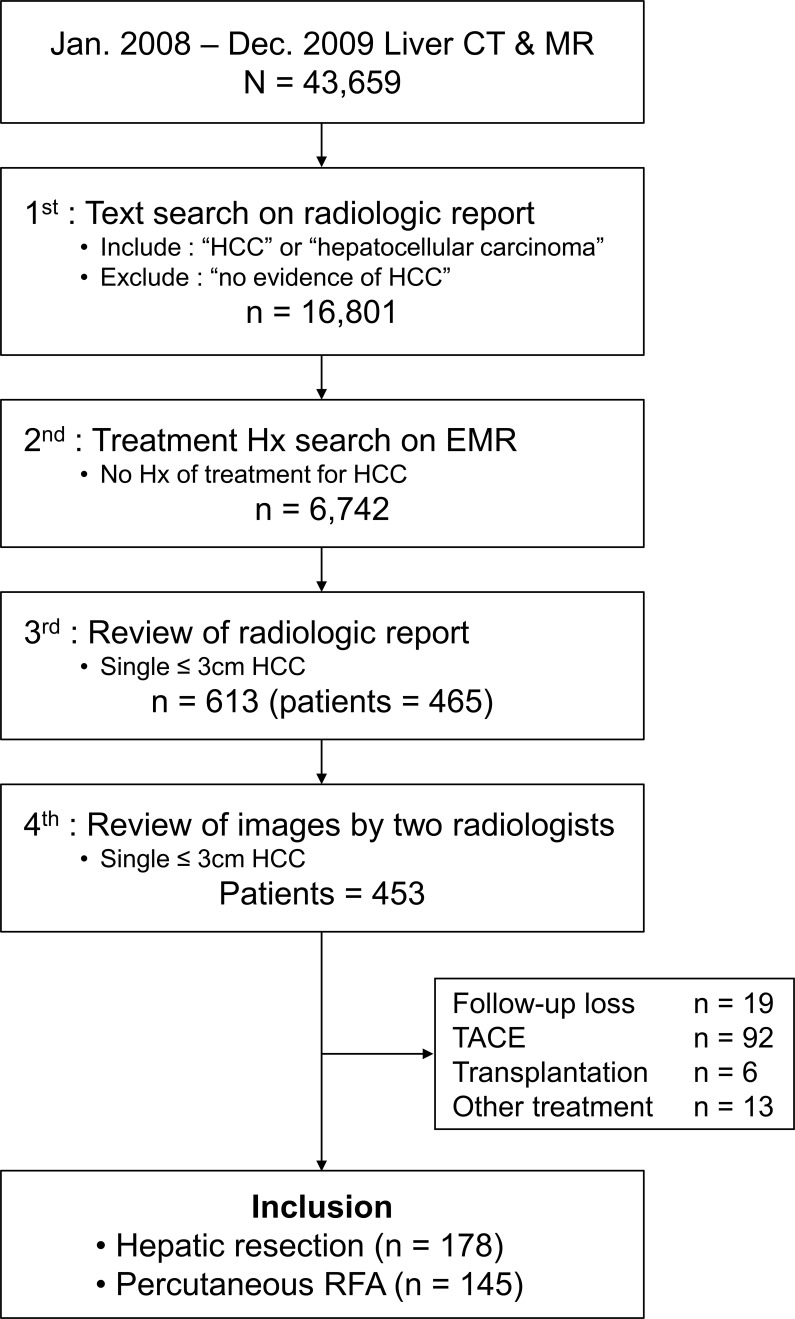
A total of 43,659 liver CTs or MRIs were taken at Samsung medical center between January 2008 and December 2009. Stepwise search was conducted to find patients who had a hepatic mass (≤3 cm) diagnosed as HCC on pre-treatment imaging study. First, a computerized text search was performed with radiologic reports. The following terms were searched for: “HCC” or “hepatocellular carcinoma.” Then, reports with the term “no evidence of HCC” were excluded. Second, a computerized search of our electronic medical record system was conducted to identify if a previous history of treatment for HCC existed. Patients who had prior treatment history for HCC were excluded at this stage. Third, radiologic reports were reviewed by a radiologist to identify patients with a single ≤3 cm mass in the liver diagnosed as HCC. Fourth, two radiologists reviewed images to confirm whether the mass showed enhancement on arterial phase and wash-out on portal venous or delayed phase. Finally, 453 treatment-naïve patients with a single ≤3 cm mass that showed typical imaging findings of HCC on imaging study were identified. Among 453 patients, 145 patients who underwent HR and 178 patients who underwent RFA as primary treatment were included in this study (Figure 1). Among 323 patients who underwent HR or RFA as primary treatment, 319 patients (98.8%) underwent multiphase liver CTs. A total of 313 (96.9%) of 323 patients underwent contrast-enhanced liver MRIs using either extracellular fluid agent (n = 17) or hepatobiliary-specific agents (n = 296).
Figure 1.
Inclusion flow chart. HCC, hepatocellularcarcinoma; RFA, radiofrequency ablation.
Treatment of HCC and follow-up
Treatment modality was selected after considering liver function reserve, tumor location, patient age and surgical risk. The general inclusion criteria for hepatic resection at our institution were as follows: (a) single tumor or oligonodular tumors within a monosegment of the liver, (b) indocyanine green retention rate less than 20% at 15 min, (c) serum total bilirubin level less than 1.5 mg dl−1, (d) no severe portal hypertension, and (e) no gross ascites. Inclusion criteria for percutaneous RF ablation were as follows: (a) single tumor (≤5 cm in greatest dimension) or multiple nodular tumors (three or fewer, each ≤3 cm in greatest dimension), (b) Child-Pugh class A or B disease, (c) no portal vein tumor thrombosis or extrahepatic metastasis, (d) prothrombin time ratio greater than 50%, and (e) platelet count greater than 50,000/mm3 (50 × 109/L).
Hepatic resection was performed by one of two surgeons who had more than 10 years of experience in hepatobiliary surgery by the end of the study. RF ablation was performed by one of four interventional radiologists who had more than 6 years of experience in RF ablation by the end of the study. After treatment, follow-up multiphase liver CT or MRI was performed every 3 months during the first 2 years, and every 4–6 months thereafter according to the risk of recurrence for both HR and RFA groups.
Data acquisition
Baseline characteristics of patients were obtained by reviewing the electronic medical records of our institution. For the HR group, pathologic reports were reviewed and the final diagnosis for hepatic masses was obtained. To compare therapeutic outcomes between the two groups, recurrence-free survival (RFS) and overall survival (OS) were calculated. RFS was defined as the interval from date of treatment to one of the following events: intrahepatic recurrence, extrahepatic recurrence, or death. OS was defined as the interval from date of treatment until death. If patients underwent liver transplantation, they were considered censored at the time of liver transplantation. Complications were stratified according to the Clavien classification of post-operative complications, and complications of Grade II or higher were considered major.15 Cumulative local tumor progression (LTP) rate was evaluated for the RFA group. LTP was defined as the appearance of new tumor foci at the margin of the ablation zone after at least one contrast-enhanced follow-up study had demonstrated absence of viable tumor.16
Statistical analysis
For the HR group, the positive predictive value of imaging criteria for diagnosis of HCC was calculated. Continuous data were evaluated using two-sample t tests or Mann–Whitney tests depending on the assumption of normality. Categorical variables were analyzed using χ2 tests or Fisher’s exact tests. Cumulative LTP, RFS, and OS rates were estimated using the Kaplan–Meier method. Prognostic factors for RFS and OS were assessed using Cox regression models. Proportional hazard (PH) assumption for Cox proportional hazard model was tested by Schoenfeld’s method. For variables with violation of the PH assumption, time-dependent Cox regression was applied. When time dependence was not significant, Cox proportional hazard model was applied. Possible risk factors with p-values of 0.1 or less at univariate analyses were entered into multivariate Cox proportional hazard models. All statistical analyses were performed using statistical software (PASW statistical software, v. 18.0; SPSS, Chicago, IL). p-values < 0.05 were considered indicative of a significant difference.
Results
Baseline characteristics of patients and tumors are summarized in Table 1. The median follow-up period was 8.1 years (range, 0.1–10.1 years) in the HR group and 7.4 years (range, 0.1–9.9 years) in the RFA group. The HR group was significantly younger, had higher α-fetoprotein level, higher platelet count, higher serum albumin level, and lower prothrombin time. In the HR group, the proportion of patients with hepatitis B virus was higher. Mean tumor size was significantly larger in the HR group.
Table 1.
Baseline patient characteristics
| Variable | Hepatic resection (n = 145) | RF ablation (n = 178) | p-value |
|---|---|---|---|
| Mean age (y) | 53.3 ± 10.0 | 56.75 ± 9.5 | 0.002 |
| Sex (M / F) | 108/37 | 145/33 | 0.130 |
| Etiology | 0.049 | ||
| HBV | 123 | 131 | |
| HCV | 12 | 27 | |
| NBNC | 10 | 20 | |
| Tumor size (cm) | 2.1 ± 0.6 | 1.8 ± 0.5 | <0.001 |
| Child-Pugh classification (A/B) | 131/14 | 156/22 | 0.442 |
| α-feto protein (ng/mL) | 376.5 ± 1199.0 | 102.2 ± 226.3 | 0.004 |
| Platelet count (x103/mm3) | 147.9 ± 50.4 | 105.3 ± 51.1 | <0.001 |
| Alanine aminotransferase (IU/L) | 40.9 ± 27.8 | 40.8 ± 29.0 | 0.980 |
| Total bilirubin (mg/dL) | 0.9 ± 0.5 | 0.9 ± 0.6 | 0.228 |
| Albumin (g/dL) | 4.1 ± 0.4 | 3.8 ± 0.5 | <0.001 |
| Prothrombin time (INR) | 1.1 ± 0.1 | 1.2 ± 0.1 | <0.001 |
HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio.; NBNC, non B non-C;RF, radiofrequency.
Data represent number of patients with percentages in parentheses or the mean ± standard deviation.
Therapeutic outcomes
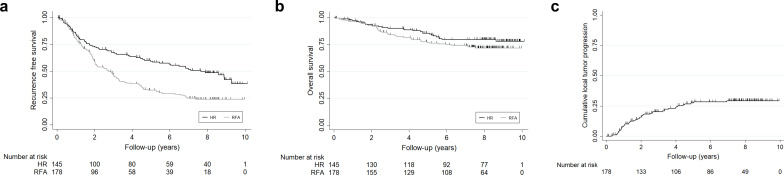
RFS rates at 1, 3, and 5 years were 85.8%, 67.4%, and 59.3%, respectively, for the HR group and 81.4%, 46.8%, and 32.2% for the RFA group, respectively. OS rates at 1, 3, and 5 years were 97.2%, 90.0%, and 85.4%, respectively, for the HR group and 96.6%, 83.9%, and 76.8% for the RFA group, respectively. For the RFA group, cumulative LTP rates were 8.3%, 20.2%, and 27.4% at 1, 3, and 5 years, respectively (Figure 2).
Figure 2.
Therapeutic outcomes of the hepatic resection group and radiofrequency ablation group. (a) Recurrence-free survival rate. (b) Overall survival rate. (c) Cumulative LTP rate in RFA group.
Analysis of risk factors
Independent prognostic factors for RFS were age [p = 0.007; hazard ratio, 1.022; 95% confidence interval (CI), 1.006, 1.038] and platelet count (p = 0.014; hazard ratio, 0.996; 95% CI, 0.992, 0.999) on multivariate analysis (Table 2). Independent prognostic factors for OS were age (p = 0.043; hazard ratio, 1.029; 95% CI, 1.001, 1.057) and prothrombin time (p = 0.023; hazard ratio, 22.355; 95% CI, 1.550, 322.500) on multivariate analysis (Table 3). On univariate analysis, the RFS rate was better in the HR group. However, treatment modality (HR vs RFA) was not an independent prognostic factor for either RFS or OS on multivariate analysis.
Table 2.
Univariate and multivariate analysis of prognostic factors for recurrence free survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | Hazard ratio | p-value | Hazard ratio | p-value |
| Group [RFA] | 0.531 (0.394, 0.715) | <0.001 | 0.812 (0.572, 1.153) | 0.244 |
| Age | 1.024 (1.009, 1.038) | 0.001 | 1.022 (1.006, 1.038) | 0.007 |
| Sex [male] | 0.757 (0.530, 1.081) | 0.125 | ||
| Etiology [hepatitis B virus]* | 0.618 | |||
| Hepatitis C virus | 1.384 (0.850, 2.253) | 0.272 | ||
| NBNC | 0.962 (0.541, 1.712) | 1.000 | ||
| Tumor size | 0.957 (0.736, 1.244) | 0.740 | ||
| Child-Pugh score | 1.181 (0.974, 1.432) | 0.090 | 0.833 (0.634, 1.093) | 0.188 |
| α-fetoprotein† | 1.016 (0.861, 1.199) | 0.852 | ||
| Platelet count | 0.993 (0.991, 0.996) | <0.001 | 0.996 (0.992, 0.999) | 0.014 |
| Alanine aminotransferase | 1.002 (0.997, 1.006) | 0.448 | ||
| Total bilirubin | 1.140 (0.853, 1.522) | 0.376 | ||
| Albumin | 0.559 (0.420, 0.745) | <0.001 | 0.661 (0.432, 1.010) | 0.056 |
| Prothrombin time (INR) | 8.875 (2.797, 28.164) | <0.001 | 1.949 (0.301, 12.617) | 0.484 |
RFA, radiofrequency ablation; NBNC, non-B non-C; INR, international normalized ratio.
Cox proportional hazards model was used for univariate and multivariate analysis. The reference category for each categorical variable is in the square brackets in first column.
Bonferroni correction was used owing to multiple comparisons.
Log-transformation was used for analysis of α-fetoprotein concentration. Numbers in parentheses represent the 95% confidence interval.
Table 3.
Univariate and multivariate analysis of prognostic factors for overall survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | Hazard ratio | p-value | Hazard ratio | p-value |
| Group [RFA] | 0.705 (0.440, 1.131) | 0.147 | ||
| Age | 1.032 (1.008, 1.056) | 0.008 | 1.029 (1.001, 1.057) | 0.043 |
| Sex [male] | 0.598 (0.315, 1.135) | 0.116 | ||
| Aetiology [hepatitis B virus]* | 0.114 | 1.000 | ||
| Hepatitis C virus | 1.979 (1.004, 3.899) | 0.048 | 1.373 (0.640, 2.947) | 0.704 |
| NBNC | 1.547 (0.687, 3.485) | 0.458 | 1.238 (0.522, 2.937) | 1.000 |
| Tumor size | 1.547 (1.016, 2.356) | 0.042 | 1.588 (1.035, 2.437) | 0.340 |
| Child-Pugh score | 1.429 (1.110, 1.841) | 0.006 | 0.906 (0.609, 1.346) | 0.624 |
| α-fetoprotein† | 1.042 (0.792, 1.372) | 0.767 | ||
| Platelet count | 0.994 90.989, 0.998) | 0.008 | 0.999 (0.993, 1.004) | 0.604 |
| Alanine aminotransferase | 0.996 (0.987, 1.004) | 0.329 | ||
| Total bilirubin | 1.384 (0.904, 2.119) | 0.135 | ||
| Albumin | 0.419 (0.266, 0.661) | <0.001 | 0.679 (0.340, 1.357) | 0.273 |
| Prothrombin time (INR) | 38.244 (6.678, 219.001) | <0.001 | 22.355 (1.550, 322.500) | 0.023 |
RFA, radiofrequency ablation; NBNC, non-B non-C; INR, international normalized ratio.
Cox proportional hazards model was used for univariate and multivariate analysis. The reference category for each categorical variable is in the square brackets in first column.
Bonferroni correction was used owing to multiple comparisons.
Log-transformation was used for analysis of α-fetoprotein concentration. Numbers in parentheses represent the 95% confidence interval.
False-positive patients
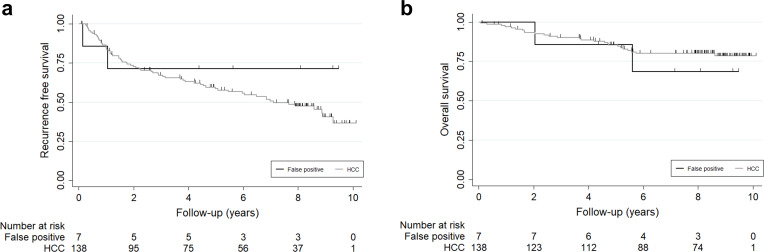
False-positive imaging diagnosis of HCC (final pathologic diagnosis was not HCC) was confirmed in seven patients (4.8%) of the HR group, including combined HCC and cholangiocarcinoma (n = 4), cholangiocarcinoma (n = 2), and focal nodular hyperplasia (n = 1). Both RFS and OS rates were not significantly different between the HCC group and false-positive patients in the HR group (p = 0.357 and p = 0.582) (Figure 3).
Figure 3.
Comparison of recurrence-free survival and overall survival rates between false-positive patients and HCC patients in the HR group. (a) Recurrence-free survival rate. (b) Overall survival rate. HCC, hepatocellularcarcinoma; HR, hepatic resection
Complications
Major complications occurred in two patients (1.4%) in the HR group. The complications were Grade V, death due to hepatic failure (n = 2). In the RFA group, major complications occurred in two patients (1.1%). Both complications were Grade III, including liver abscess (n = 1) and colon thermal injury with perforation (n = 1).
Discussion
In our study, therapeutic outcomes of HR and RFA were compared for small hepatic tumors diagnosed as HCC on pre-treatment imaging study. Treatment modality was not a significant prognostic factor for RFS and OS. In addition, imaging criteria for diagnosis of HCC had a high positive predictive value. To compare therapeutic outcomes between treatment modalities, the target groups should be the same. However, patient inclusion criteria for HR and RFA were not the same in many previous retrospective studies that compared the two treatment modalities for HCC because biopsy is usually not recommended if imaging findings are typical for HCC. In our study, the same inclusion criteria were applied to both HR and RFA groups to solve this problem. Inclusion criteria were ≤3 cm hepatic masses with typical imaging findings of HCC on pretreatment imaging study. As a result, both HR and RFA groups were expected to have the same disease population. In this aspect, our study is valuable compared to previous studies.
The European Association for the Study of the Liver criteria for the imaging diagnosis of HCC was used in this study, including enhancement on arterial phase and wash-out on portal venous or delayed phase of multiphase liver CT or MRI.1 In the HR group, seven patients had diseases other than HCC on post-operative pathologic results, representing false positive cases. This result indicates that the positive predictive value of imaging study for diagnosis of HCC was as high as 95.2%. Although results differ slightly from study to study, the positive predictive values of imaging criteria for diagnosis of HCC in previous studies were comparable to that of our study.11,12,17,18 The diagnoses of the seven false-positive patients were combined HCC and cholangiocarcinoma, cholangiocarcinoma, and focal nodular hyperplasia. Various diseases have been reported to show similar imaging findings to HCC and and false-positive cases in our study are also part of these diseases.6,19
In terms of OS, treatment modality was not a significant prognostic factor. Previous studies that compared therapeutic outcomes between HR and RFA for small HCCs reported that there was not a significant difference in OS. Our study applied different inclusion criteria from previous studies. Nevertheless, our results were not different from those of previous studies. This is possibly due to the following reasons. First, the false-positive rate of imaging diagnosis was as low as 4.8%. Therefore, the effect of these cases on the results may not be significant. Second, false-positive cases included both malignant tumors and benign disease. Cholangiocarcinoma and combined HCC and cholangiocarcinoma have a worse prognosis than HCC.20–23 Conversely, focal nodular hyperplasia is a benign lesion with a better prognosis than HCC. It is possible that the influence of malignant tumors and benign nodules on OS offset each other.
In our study, age and platelet count were significant prognostic factors for RFS. RFS was better in the HR group in our study, and the difference was significant in univariate analysis. However, treatment modality was not a significant prognostic factor for RFS in multivariate analysis although the hazard ratio was 0.812. In the baseline characteristics, the proportion of young patients with good liver function was high in the HR group. In other words, the patient’s baseline characteristics may have affected the choice of treatment modality. Although treatment modality was a significant prognostic factor for RFS in univariate analysis, the effect of treatment modality on the RFS fell below the statistically significant level in the analysis that corrected the baseline characteristics of the patients between two groups. Many studies reported that RFS was better in the HR group than the RFA group. The reason for the different results of our study from previous studies is not clear. However, some studies have reported that RFS was better with RFA in certain patient groups.24,25 In our study, the HR group was selected using different criteria from previous studies. The difference in patient inclusion criteria may be the reason for the variation in results on RFS.
There are several limitations in our study. First, this is a retrospective study and treatment modality (HR vs RFA) was not randomly selected. Therefore, there were some differences in patient and tumor characteristics between the two groups. However, multivariate analysis was performed to evaluate the effects of treatment modality on therapeutic outcomes and to adjust the effect of other factors. Second, this is a single-center study. Therapeutic outcomes can be influenced by operator experience. Therefore, care should be taken when generalizing our results.
In conclusion, imaging criteria for diagnosis of HCC have a high positive predictive value. Multivariate analysis showed that RFA and OS rates were not significantly different between HR and RFA for small hepatic masses that were diagnosed as HCC on pre-treatment imaging.
Footnotes
Funding: This was supported by Bracco Imaging Korea, Ltd.
Contributor Information
Dong Ik Cha, Email: dongik.cha@samsung.com.
Kyoung Doo Song, Email: kd3893.song@samsung.com.
Tae Wook Kang, Email: kaienes.kang@samsung.com.
Min Woo Lee, Email: mw2542.lee@samsung.com.
Hyunchul Rhim, Email: hc.rhim@samsung.com.
REFERENCES
- 1. European association for the study of the liver. electronic address eee, European association for the study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 2.Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology 2010; 51: 1284–90. doi: 10.1002/hep.23466 [DOI] [PubMed] [Google Scholar]
- 3.Wang J-H, Wang C-C, Hung C-H, Chen C-L, Lu S-N. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol 2012; 56: 412–8. doi: 10.1016/j.jhep.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 4.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57: 794–802. doi: 10.1016/j.jhep.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 5.Lee HW, Lee JM, Yoon J-H, Kim YJ, Park J-W, Park S-J, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res 2018; 94: 74–82. doi: 10.4174/astr.2018.94.2.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang B, Wu L, Lu X-Y, Xu F, Liu C-F, Shen W-F, et al. Small intrahepatic cholangiocarcinoma and hepatocellular carcinoma in cirrhotic livers may share similar enhancement patterns at multiphase dynamic MR imaging. Radiology 2016; 281: 150–7. doi: 10.1148/radiol.2016151205 [DOI] [PubMed] [Google Scholar]
- 7.Kang TW, Kim JM, Rhim H, Lee MW, Kim Y-sun, Lim HK, et al. Small Hepatocellular Carcinoma: Radiofrequency Ablation versus Nonanatomic Resection--Propensity Score Analyses of Long-term Outcomes. Radiology 2015; 275: 908–19. doi: 10.1148/radiol.15141483 [DOI] [PubMed] [Google Scholar]
- 8.Park EK, Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC, et al. A comparison between surgical resection and radiofrequency ablation in the treatment of hepatocellular carcinoma. Ann Surg Treat Res 2014; 87: 72–80. doi: 10.4174/astr.2014.87.2.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-Term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013; 59: 89–97. doi: 10.1016/j.jhep.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 10.Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Hayashi H, et al. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res 2013; 43: 853–64. doi: 10.1111/hepr.12035 [DOI] [PubMed] [Google Scholar]
- 11.Darnell A, Forner A, Rimola J, Reig M, García-Criado Ángeles, Ayuso C, et al. Liver imaging reporting and data system with MR imaging: evaluation in nodules 20 mm or smaller detected in cirrhosis at screening us. Radiology 2015; 275: 698–707. doi: 10.1148/radiol.15141132 [DOI] [PubMed] [Google Scholar]
- 12.Di Martino M, Marin D, Guerrisi A, Baski M, Galati F, Rossi M, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology 2010; 256: 806–16. doi: 10.1148/radiol.10091334 [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Lee JM, Kang TW, Rhim H, Kim SY, Shin YM, et al. Clinical outcomes of radiofrequency ablation for early hypovascular HCC: a multicenter retrospective study. Radiology 2018; 286: 338–49. doi: 10.1148/radiol.2017162452 [DOI] [PubMed] [Google Scholar]
- 14.Takayasu K, Arii S, Sakamoto M, Matsuyama Y, Kudo M, Ichida T, et al. Clinical implication of hypovascular hepatocellular carcinoma studied in 4,474 patients with solitary tumour equal or less than 3 cm. Liver Int 2013; 33: 762–70. doi: 10.1111/liv.12130 [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–13. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 2014; 273: 241–60. doi: 10.1148/radiol.14132958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47: 97–104. doi: 10.1002/hep.21966 [DOI] [PubMed] [Google Scholar]
- 18.Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol 2016; 41: 71–90. doi: 10.1007/s00261-015-0592-8 [DOI] [PubMed] [Google Scholar]
- 19.Kim TK, Lee E, Jang H-J. Imaging findings of mimickers of hepatocellular carcinoma. Clin Mol Hepatol 2015; 21: 326–43. doi: 10.3350/cmh.2015.21.4.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C-L, Fan S-T, Lo C-M, Ng IO-L, Lam C-M, Poon RT-P, et al. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg 2003; 138: 86–90. doi: 10.1001/archsurg.138.1.86 [DOI] [PubMed] [Google Scholar]
- 21.Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 2002; 94: 2040–6. doi: 10.1002/cncr.10392 [DOI] [PubMed] [Google Scholar]
- 22.Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg 2005; 189: 120–5. doi: 10.1016/j.amjsurg.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 23.Yoon Y-I, Hwang S, Lee Y-J, Kim K-H, Ahn C-S, Moon D-B, et al. Postresection outcomes of combined hepatocellular Carcinoma-Cholangiocarcinoma, hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Gastrointest Surg 2016; 20: 411–20. doi: 10.1007/s11605-015-3045-3 [DOI] [PubMed] [Google Scholar]
- 24.Peng Z-W, Lin X-J, Zhang Y-J, Liang H-H, Guo R-P, Shi M, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology 2012; 262: 1022–33. doi: 10.1148/radiol.11110817 [DOI] [PubMed] [Google Scholar]
- 25.Peng Z-W, Liu F-R, Ye S, Xu L, Zhang Y-J, Liang H-H, et al. Radiofrequency ablation versus open hepatic resection for elderly patients (> 65 years) with very early or early hepatocellular carcinoma. Cancer 2013; 119: 3812–20. doi: 10.1002/cncr.28293 [DOI] [PubMed] [Google Scholar]