Abstract
Objective:
This study explored the value of serial 18-fludeoxyglucose-positron emission tomography (18F-FDG-PET/CT) in predicting disease-free survival (DFS) in locally advanced rectal cancer (LARC) treated with neoadjuvant chemoradiation (NCRT) and surgery.
Methods:
We prospectively studied 46 patients with LARC who underwent NCRT and surgery. 18F-FDG-PET/CT scans were performed at three time-points before surgery (pre-NCRT-PET1, during NCRT-PET2 and following completion of NCRT-PET3). The following semi-quantitative PET parameters were analysed at each time point: maximum standardized uptake value (SUVmax), SUVmean, metabolic tumour volume (MTV) and tumour lesion glycolysis (TLG). Absolute and percentage changes in these parameters were analysed between time points. Statistical analysis consisted of median tests, Cox regression and Kaplan–Meier analysis for DFS.
Results:
The median follow-up time was 24 months. A reduction in PET parameters showed statistically significant differences for patients with recurrence compared to those without; percentage changes in MTV between PET1 and PET3 (cut-off: 87%, p = 0.023), percentage changes in TLG between PET1 and PET3 (cut-off: 94%, p = 0.02) and absolute change in MTV PET1 and PET2 (cut-off: 10.25, p = 0.001).
An absolute reduction in MTV between PET1 and PET3 (p=0.013), a percentage reduction in TLG between PET1 and PET2 (p=0.021), SUVmax and SUVmean at PET2 (p = 0.01, p = 0.027 respectively)were also prognostic indicators of recurrence.
MTV percentage change between PET1 and PET2 and SUVmean percentage change between PET1 and PET3 were also trending towards significance (p = 0.052, p = 0.053 respectively).
Conclusion:
Serial 18F-FDG-PET/CT is a potentially reliable non-invasive method to predict recurrence in patients with LARC. Volumetric parameters were the best predictors. This could allow risk-stratification in patients who may benefit from conservative management.
Advances in knowledge:
This paper will add to the literature in risk-stratifying patients with LARC based on prognosis, using 18F-FDG-PET/CT. This may improve patient outcomes by selecting suitable candidates for conservative management.
Introduction
In 2015, colorectal cancer was the third most diagnosed cancer among males and second amongst females, with 1.65 million new cases diagnosed globally.1 Locally advanced rectal cancer (LARC) is defined as a rectal cancer that has invaded through the muscularis propria into pericolorectal tissue, or with regional lymph node involvement in the absence of distant metastatic disease– staged as T3-T4, N0 or any T stage with regional lymph node involvement and M02.
Positron emission tomography with 2-deoxy-2-[fluorine-18]flu-D-glucose integrated with computed tomography (18F-FDG-PET/CT) has emerged as an invaluable imaging tool in oncology.3 FDG is a glucose analogue that preferentially accumulates in highly metabolically active cells, such as tumour cells.4 These metabolic abnormalities often precede morphological changes, highlighting the benefit of PET over conventional imaging, especially in the early assessment of treatment response.5
Currently, a multimodality treatment approach is recommended for LARC with neoadjuvant chemoradiation (NCRT) followed by radical surgery and adjuvant chemotherapy.6 NCRT has been shown to downstage and downsize the tumour, reduce the risk of local recurrence, facilitate sphincter preservation and improve survival rates.7 However, this approach is not effective for all patients and recurrence rates for LARC remain high. Patients with a clinical response to NCRT may also be able to avoid the morbidity and toxicity associated with surgery and adjuvant therapy by opting for conservative management, or a “watch and wait approach”.8 If we can accurately prognosticate in the early stages of treatment, there is potential to adapt treatment accordingly thus personalizing therapy.
The current standard for evaluation of tumour response to NCRT is through histopathological analysis following surgery, classified according to the tumour regression grade (TRG).2 However, this is determined post-operatively, where there is no longer any scope for conservative management.
Currently, anatomy-based imaging modalities are preferred for assessing tumour response to NCRT. Pathological complete response assessment using diffusion weighted magnetic resonance imaging (DWMRI) was shown to have a pooled pre-NCRT and post-NCRT accuracy of 68 and 74% respectively, using apparent diffusion coefficient (ADC).9 There is emerging evidence, however, suggesting a benefit in performing 18F-FDG-PET/CT where cellular changes can be more predictive of response to NRCT compared to morphologic imaging. Rymer et al found post-NCRT maximum standardized uptake value (SUVmax) with specificity and sensitivity ranging from 66.7 to 85.7% and 60.9 to 88.9%, respectively, and suggested there is potential benefit in performing 18F-FDG-PET/CT.10
While the use of 18F-FDG-PET/CT in the setting of LARC is not currently routine clinical practice, there is evidence supporting its potential application in assessing treatment response and prognosis.6,9,11 In this paper, we assessed whether functional parameters on serial 18F-FDG-PET/CT in LARC patients undergoing NCRT can predict disease-free survival (DFS).
Methods
Patient characteristics
46 patients in the South West Sydney Local Health District (Sydney, New South Wales, Australia) with LARC were prospectively enrolled from March 2014 to October 2016. Patients were included in the study on the basis of: age over 18 years, a diagnosis with a Stage II or III rectal adenocarcinoma, defined as T3-T4 and/or node positive disease (N1–2), without distant metastatic disease (M0), no evidence of metastatic disease on CT chest/abdomen/pelvis and undergoing treatment regimen consisting of NCRT. Patients were excluded if they were found to have any other malignancies or inflammatory bowel disease. 16 patients were excluded from the consecutively acquired series as per the exclusion criteria, and concerns regarding adherence to study protocol. Local ethics approval was obtained.
18F-FDG-PET/CT scanning schedule
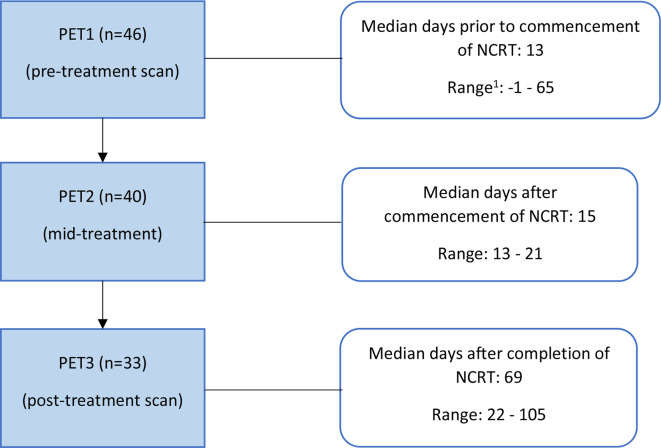
Patients underwent serial 18F-FDG-PET/CT scanning at three time points as outlined in Figure 1: the staging scan performed prior to the commencement of NCRT (PET1), mid-treatment scan performed in the third week of NCRT (PET2), and the post-treatment scan one week prior to the scheduled surgery (PET3).
Figure 1.
Serial scanning at specific time points throughout NCRT. 1patient underwent PET1, one dayfollowing the start of NCRT and excluded for any PET1 analysis. NCRT, neoadjuvant chemoradiation; PET, positron emission tomography.
Treatment details
All patients received neoadjuvant treatment with long course radiotherapy and all, but one patient received concurrent chemotherapy. The treatment protocol is as follows: 50.4 Gy in 28 fractions of radiation with concurrent 5-fluorouracil (225 mg/m2/day continuous infusion) or Capecitabine (825 mg/m2 bd on days of radiation). All but two patients underwent curative surgery. Following surgery, histopathological analysis of the resected tissue was carried out to determine TRG according to AJCC guidelines.2
Follow-up
Median follow-up was 24 months (range: 4–43 months). Recurrence was determined through imaging and when clinically indicated, tissue confirmation. Time to recurrence was calculated from PET1 (indicative of time of diagnosis) to first investigative evidence of recurrence. Overall survival was not analyzed due to the limited events that occurred.
18F-FDG-PET/CT acquisition
GE Discovery-710 PET-CT was used to acquire the PET studies. Patients received 4.1 MBq/kg of FDG after at least 4 h of fasting. All scans were performed on the same scanner with the same acquisition and reconstruction protocols. PET scans were acquired in three-dimensional (3D) mode with an acquisition time of 1.5–2.5 min per bed position, corrected for the patient’s weight, from mid-brain to proximal femora. Median FDG uptake time was 60 minutes (range: 54–85 mins, SD: 3 mins). Attenuation correction was obtained using low dose CT: 64-slice GE CT, using helical mode without the use of contrast medium. CT images were acquired at 3.75–5 mm slice thickness and reconstructed to a transaxial matrix size of 512 × 512. The current (30–40 mAs) and voltage (120–140 kV) was adjusted based on patient’s weight. PET images were reconstructed according to the GE VUE Point FX (Time of Flight) algorithm into a 256 × 256 matrix size with a slice thickness of 3.75–4.0 mm.
18F-FDG-PET/CT analysis
Semi-quantitative analysis was performed on an Advantage Workstation (GE Healthcare) using the PET-VCAR (Volume Computer-Assisted Reading) software (v. 1.0). The SUVmax was derived by selecting the most intensely avid area of uptake at the primary tumour and lymph nodes. The metabolic tumour volume (MTV) is the volume of interest (VOI) with abnormal FDG accumulation derived via two methods; applying a fixed SUV threshold of 2.5, and a threshold of 42% of SUVmax.12 The contouring margins were automatically derived and the VOI was then checked and manually adjusted by two experienced nuclear medicine physicians on three axes (axial, coronal and sagittal) to ensure accurate inclusion of primary tumour and exclusion of adjacent normal structures. Image analysis was performed independently by each observer and any discrepancy was resolved by consensus. Observers were blinded to all other information, and analyses was performed retrospectively. The tumour lesion glycolysis (TLG) represented the volume and intensity of an FDG avid area calculated according to the formula: TLG = SUVmean x MTV. Changes between serial scans were calculated as absolute and percentage changes.
Statistical analysis
The Kruskal–Wallis median test was used to analyze the continuous PET variables with recurrence status. Cox proportional hazard models were then performed to determine the effect of demographic factors (age, gender), clinical factors (body mass index, tumour stage, TRG and nodal status) factors on recurrence. TRG was categorized as partial and no response (TRG3 and TRG4) or complete and near-complete response (TRG0 and TRG1). Univariate Cox proportional hazard models were used to determine the relationship between PET parameters and recurrence. Estimated hazard ratios and its 95% confidence intervals (CIs) were presented. The Cox proportion hazard assumption for each categorical variable was not violated.
Survival curves were then generated to determine the predictive value of PET parameters for DFS using Kaplan–Meir estimates and their significance determined by log-rank tests. Cut-offs for PET parameters were determined using receiver operating characteristic (ROC) curves; derived from the value providing the best combined sensitivity and specificity. Statistical significance was defined as p < 0.05. Statistical analysis was performed using IBM SPSS statistics, v. 21.0.
Results
Patient population
46 patients were included in the study. Staging was based on AJCC seventh Edition and determined through a combination of clinical evaluation and imaging.2 Table 1 illustrates patient characteristics.
Table 1.
Patient characteristics
| No. of patients | 46 |
| Age (mean ± SD) | 62.52 ± 11.02 |
| Gender (%) | |
| Male | 34 (74) |
| Female | 12 (26) |
| Tumour Stage (%) | |
| T2N1 | 2 (4.5) |
| T2N2 | 1 (2) |
| T3N0 | 7 (15) |
| T3N1 | 12 (26) |
| T3N2 | 19 (41.5) |
| T4N1 | 1 (2) |
| T4N2 | 4 (9) |
| PET scan participationa (%) | |
| PET1 | 46 (100) |
| PET2 | 40 (87) |
| PET3 | 33(72) |
| Chemotherapy (%) | |
| 5-fluorouracil | 10 (22) |
| Capecitabine | 35 (76) |
| Noneb | 1 (2) |
| Surgery | |
| Anterior resection | 31 (67.5) |
| Abdominoperineal resection | 12 (26) |
| Hartmann’s procedure | 1 (2) |
| No surgeryc | 2 (4.5) |
| TRGd (%) | |
| 0 | 6 (13) |
| 1 | 14 (30.5) |
| 2 | 18 (39) |
| 3 | 6 (13) |
| NAe | 2 (4.5) |
SD, standard deviation;TRG, tumour regression grade.
Not all patients underwent all three scheduled scans.
One patient was included in a clinical trial where they were administered aspirin.
2 patients elected not to undergo surgery.
According to AJCC classification.2
Histopathological analysis was unable to be obtained as two patients did not undergo surgery.
At the time of data census, nine patients had recurred, two had died because of metastatic disease and one died as a result of surgical complication. Median DFS was 26.5 months and the recurrence rate was 19.6% in the overall population.
PET parameters
All patients demonstrated an increased FDG uptake at the primary tumour site at PET1 with a median SUVmax of 17.6 (range: 8.3–45.7). A general downward trend of FDG uptake was observed between the staging scan and post-treatment scan with the median SUVmax at PET3 being 6.4 (range: 2–15.1), with a median percentage decrease in SUVmax from PET1 to PET3 of 70%.
All volumetric PET parameters, that were found to be significant, were calculated using a SUV threshold of 2.5. SUVmean which was found to be significant on Kaplan–Meier analysis was calculated using a SUV threshold of 42% of SUVmax.
Comparative analyses of median PET parameters
The PET parameters at serial time points were analyzed and correlated with recurrence. Table 2 outlines PET parameters utilizing a SUV threshold of 2.5 analyzed against disease recurrence.
Table 2.
PET parameters between recurrence and non-recurrence patients
| All patients median (min-max) | Non-recurrence median (min-max) | Recurrence median (min-max) |
p-value (Kruskal–Wallis test) |
|
|---|---|---|---|---|
| SUVmax | ||||
| PET1 | 17.6 (8.3–45.7) | 17.9 (8.3–45.7) | 15.3 (9.8–27.8) | 0.966 |
| PET2 | 10.5 (4.7–34) | 10.15 (4.7–34) | 13.5 (8.2–18.3) | 0.140 |
| PET3 | 6.4 (2–15.1) | 5.6 (2–15.1) | 7.15 (3.1–11.8) | 0.284 |
| PET1-2 abs | 7.15 (-2.5–19.8) | 7.3 (-2.5–19.8) | 2.9 (-1.3–12.3) | 0.317 |
| PET1-2 % | 37 (-17–73) | 39 (-17–73) | 25 (-11–52) | 0.142 |
| PET1-3 abs | 11.45 (2.7–33.3) | 13.2 (3.4–33.3) | 7.25 (2.7–20.60) | 0.258 |
| PET1-3 % | 69 (19–87) | 72 (19–87) | 51 (28–74) | 0.085 |
| SUVmeana | ||||
| PET1 | 6.45 (3.6–11.5) | 6.6 (3.6–11.5) | 5.6 (4.4–8.8) | 0.729 |
| PET2 | 4.5 (3.1–8.5) | 4.4 (3.1–8.5) | 4.9 (3.6–6.5) | 0.089 |
| PET3 | 3.6 (1.4–5.2) | 3.4 (1.4–5.2) | 3.75 (2.2–4.5) | 0.322 |
| PET1-2 abs | 1.8 (-0.3–5.4) | 2 (0.2–5.4) | 0.8 (-0.3–3.3) | 0.125 |
| PET1-2 % | 28 (-5–57) | 30 (4–57) | 16 (-5–38) | 0.113 |
| PET1-3 abs | 3.2 (0.6–7.3) | 3.5 (1–7.3) | 2.4 (0.6–5.2) | 0.286 |
| PET1-3 % | 49 (14–76) | 51 (21–76) | 40 (14–59) | 0.145 |
| MTVa | ||||
| PET1 | 48.95 (8.6–201.9) | 49.7 (8.6–176) | 29.6 (12.1–201.9) | 0.570 |
| PET2 | 20.8 (5–243.9) | 20.7 (5–119) | 23.4 (6.2–243.9) | 0.549 |
| PET3 | 5.9 (0–127.2) | 4.05 (0–55.4) | 8.8 (0–127.2) | 0.252 |
| PET1-2 abs | 21.95 (-42–89.5) | 25.1 (-18.5–89.5) | 7.4 (-42–37.3) | 0.077 |
| PET1-2 % | 43 (-44–84) | 49 (-44–75) | 28 (-21–52) | 0.033 |
| PET1-3 abs | 36.55 (6.8–164.6) | 42.8 (10.9–164.6) | 17.6 (6.8–56.5) | 0.037 |
| PET1-3 % | 92 (25–100) | 94 (40–100) | 70 (25–100) | 0.061 |
| TLG a | ||||
| PET1 | 341.17 (30.96–1601.6) | 357.84 (30.96–1601.6) | 167.09 (58.08–1393.11) | 0.865 |
| PET2 | 100.62 (19.5–1585.35) | 95.2 (19.5–904.4) | 106.2 (22.32–15885.35) | 0.339 |
| PET3 | 20.06 (0–559.68) | 13.2 (0–232.68) | 34.38 (0–559.68) | 0.235 |
| PET1-2 abs | 213.38 (-192.24–697.2) | 225.53 (-85.3–697.2) | 49.28 (-192.24–404.3) | 0.077 |
| PET1-2 % | 59 (-36–89) | 60 (-36–87) | 35 (-14–70) | 0.053 |
| PET1-3 abs | 270.19 (34.84–1400.88) | 291.04 (58.85–1400.88) | 142.95 (34.84–539.4) | 0.223 |
| PET1-3 % | 95 (39–100) | 96 (62–100) | 81 (39–100) | 0.086 |
MTV, metabolic tumour volume; PET, positron emission tomography; PETx-y %, percentage reduction; PETx-y abs, absolute reduction; SUV, standardized uptake value; TLG, total lesion glycolysis.
Calculated using an SUV threshold of 2.5
The reduction in MTV between serial scans were significant indicators of recurrence. The absolute median reduction between PET1 and PET3 was 43.15 in patients with no recurrence compared to 17.6 patients who recurred (p = 0.037). The percentage reduction between PET1 and PET2 was 49% and 28%, in patients with no recurrence and recurrence respectively (p = 0.033). The TLG % change between PET1 and PET2 was also trending towards significance with a median reduction of 35% in patients who recurred compared to 60% in patients without recurrence (p = 0.053).
Univariate analysis (Cox regression)
No significant relationship was found between demographic or clinical factors and recurrence. The demographic factors tested were age and gender. The clinical factors tested were body mass index, stage, TRG and nodal status.
Since no potential confounders were found to be statistically significant, univariate Cox regression analysis was then performed on PET parameters and survival outcomes.
PET parameters and recurrence
The absolute difference in MTV between PET1 and PET2 exhibited a hazard ratio of 0.962 (p = 0.024, 95% CI 0.930–0.9995). The MTV percentage change between PET1 and PET3 had a hazard ratio of 0.967 (p = 0.017, 95% CI 0.940–0.994). Furthermore, the percentage difference in TLG between PET1 and PET3 resulted in a hazard ratio of 0.957 (p = 0.002, 95% CI 0.922–0.993).
Volumetric parameters on PET2 and PET3 were also significant indicators of recurrence on regression analysis albeit being insignificant on survival analysis. MTV showed a hazard ratio of 1.015 (p = 0.014, 95% CI 1.003–1.027) and 1.026 (p = 0.024, 95% CI 1.003–1.048) on PET2 and PET3 respectively. TLG exhibited a hazard ratio of 1.002 (p = 0.028, 95% CI 1.001–1.004) and 1.005 (p = 0.036, 95% CI 1.000–1.010) on PET2 and PET3 respectively.
PET parameters and DFS
ROC curves were constructed for PET parameters. The optimal cut-off was determined as the value providing the best combined specificity and sensitivity. The results for volumetric parameters, calculated using a SUV threshold of 2.5, against DFS are outlined in Table 3. Two parameters were significant indicators of recurrence on ROC curve analysis: MTV percentage reduction between PET1 and PET2 (AUC = 0.753, p = 0.035) and absolute MTV reduction between PET1 and PET3 (AUC = 0.750, p = 0.037).
Table 3.
ROC curve analysis of PET parameters using a SUV threshold of 2.5 against DFS
| Parameter | Optimal Cut-off | Sensitivity (%) | 1-Specificity (%) | AUC | 95% CI (upper and lower) | P value |
|---|---|---|---|---|---|---|
| PET 2 | ||||||
| SUVmax | 10.35 | 87.5 | 37.5 | 0.698 | 0.509–0.887 | 0.098 |
| SUVmean | 4.7 | 75 | 25 | 0.706 | 0.497–0.914 | 0.086 |
| TLG | 99.75 | 75 | 37.5 | 0.594 | 0.366–0.821 | 0.433 |
| MTV | 22.1 | 62.5 | 37.5 | 0.536 | 0.294–0.779 | 0.761 |
| PET 3 | ||||||
| SUVmax | 5.65 | 75 | 37.5 | 0.643 | 0.439–0.848 | 0.231 |
| SUVmean | 3.35 | 87.5 | 58.3 | 0.633 | 0.423–0.842 | 0.267 |
| TLG | 34.525 | 62.5 | 29.2 | 0.651 | 0.434–0.868 | 0.207 |
| MTV | 8.95 | 62.5 | 29.2 | 0.646 | 0.427–0.864 | 0.223 |
| PET 1–2 absolute reduction | ||||||
| SUVmax | 3.6 | 62.5 | 20.8 | 0.641 | 0.417–0.864 | 0.240 |
| SUVmean | 1.3 | 62.5 | 29.2 | 0.643 | 0.410–0.876 | 0.231 |
| TLG | 195.72 | 75 | 33.3 | 0.667 | 0.467–0.866 | 0.164 |
| MTV | 10.25 | 75 | 16.7 | 0.698 | 0.486–0.910 | 0.098 |
| PET 1–2 relative reduction (%) | ||||||
| SUVmax | 25.185 | 62.5 | 29.2 | 0.693 | 0.502–0.883 | 0.107 |
| SUVmean | 25.5 | 62.5 | 41.7 | 0.648 | 0.430–0.866 | 0.215 |
| TLG | 46 | 62.5 | 25 | 0.703 | 0.509–0.897 | 0.09 |
| MTV | 30.5 | 62.5 | 20.8 | 0.753 | 0.574–0.931 | 0.035 |
| PET 1–3 absolute reduction | ||||||
| SUVmax | 10.15 | 75 | 33.3 | 0.635 | 0.400–0.871 | 0.258 |
| SUVmean | 3.35 | 75 | 50 | 0.635 | 0.377–0.873 | 0.296 |
| TLG | 175.66 | 62.5 | 25 | 0.646 | 0.441–0.851 | 0.223 |
| MTV | 24.75 | 75 | 25 | 0.750 | 0.559–0.941 | 0.037 |
| PET 1–3 relative reduction (%) | ||||||
| SUVmax | 69 | 75 | 41.7 | 0.706 | 0.517–0.895 | 0.086 |
| SUVmean | 42.5 | 62.5 | 33.3 | 0.674 | 0.476–0.873 | 0.145 |
| TLG | 94 | 75 | 33.3 | 0.703 | 0.485–0.922 | 0.09 |
| MTV | 87 | 75 | 29.2 | 0.721 | 0.497–0.945 | 0.064 |
AUC, area under curve; CI, confidence interval; MTV, metabolic tumour volume; PET, positron emission tomography; SUV, standardized uptake value; TLG, total lesion glycolysis.
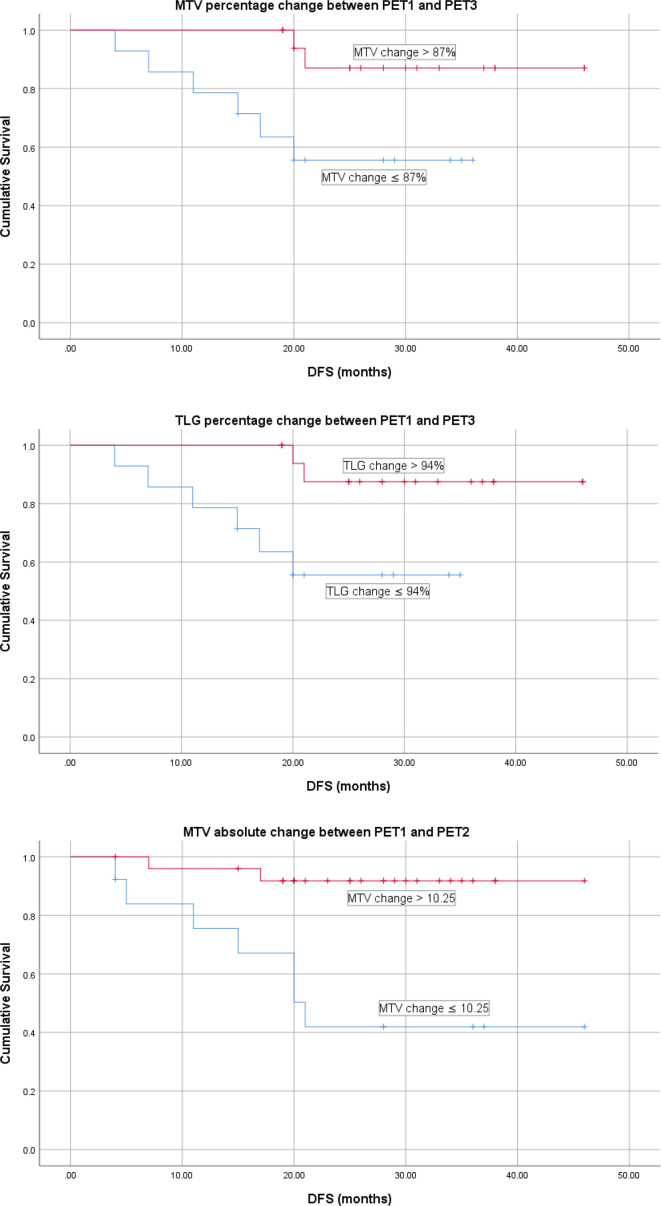
Kaplan–Meier curves (log-rank tests) were then constructed using the optimal cut-offs for each parameter as determined by ROC curve analysis. The parameters that were found to be predictive of DFS on both regression and Kaplan–Meier analysis were (Table 4, Figure 2): MTV percentage reduction between PET1 and PET3 (sensitivity = 75%, specificity = 67%, p = 0.023), TLG percentage reduction between PET1 and PET3 (sensitivity = 75%, specificity = 67%, p = 0.02) and MTV absolute reduction between PET1 and PET2 (sensitivity = 78%, specificity = 80%, p = 0.001).
Table 4.
Predictive value of significant volumetric PET parameters for DFS
| MTV relative change from PET1 to PET3 | ||||
| Recurrence | No recurrence | Total | 2 year recurrence free survival | |
| MTV Δ ≤ 87% | 6 | 8 | 14 | 43% |
| MTV Δ > 87% | 2 | 16 | 18 | 89% |
| TLG relative change from PET1 to PET3 | ||||
| Recurrence | No recurrence | 2 year recurrence free survival | 2 year recurrence free survival | |
| TLG Δ ≤ 94% | 6 | 8 | 14 | 43% |
| TLG Δ > 94% | 2 | 16 | 18 | 89% |
| MTV absolute change from PET1 to PET2 | ||||
| Recurrence | No recurrence | Total | 2 year recurrence free survival | |
| MTV Δ ≤ 10.25 | 7 | 6 | 13 | 46% |
| MTV Δ > 10.25 | 2 | 24 | 26 | 92% |
Δ, change; MTV, metabolic tumour volume; PET, positron emission tomography;TLG, total lesion glycolysis.
Calculated using a SUV threshold of 2.5.
Figure 2.
DFS for PET parameters significant on Cox regression and Kaplan–Meier analysis. ¹Calculated using a SUV threshold of 42% of SUVmax. DFS, disease-free survival; MTV, metabolic tumour volume; PET, positron emission tomography; TLG, tumour lesion glycolysis.
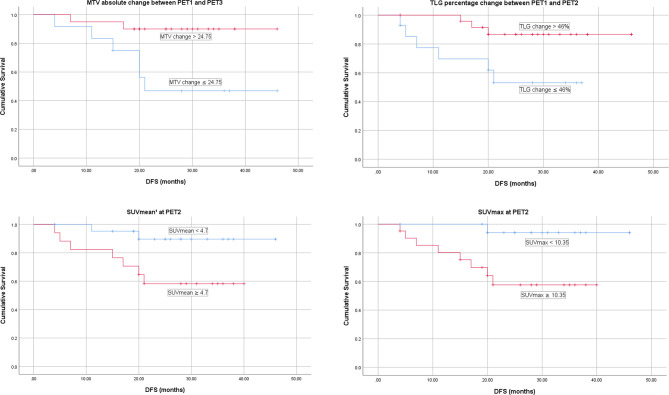
Additionally, several parameters produced statistically significant differences in DFS despite being insignificant on regression analysis (Figure 3): MTV absolute change between PET1 and PET3 using a cut-off of 24.75 (p = 0.013), TLG relative change between PET1 and PET2 utilizing a cut-off of 46% (p = 0.021), SUVmax at PET2 using a cut-off of 10.35 (p = 0.01) and SUVmean at PET2 (using a SUV threshold of 42% of SUVmax) with a cut-off of 4.75 (p = 0.027).
Figure 3.
DFS for PET parameters significant on Kaplan–Meier analysis without significance on Cox regression analysis. ¹Calculated using a SUV threshold of 42% of SUVmax. DFS, disease-free survival; MTV, metabolic tumour volume; PET, positron emission tomography; TLG, tumour lesion glycolysis.
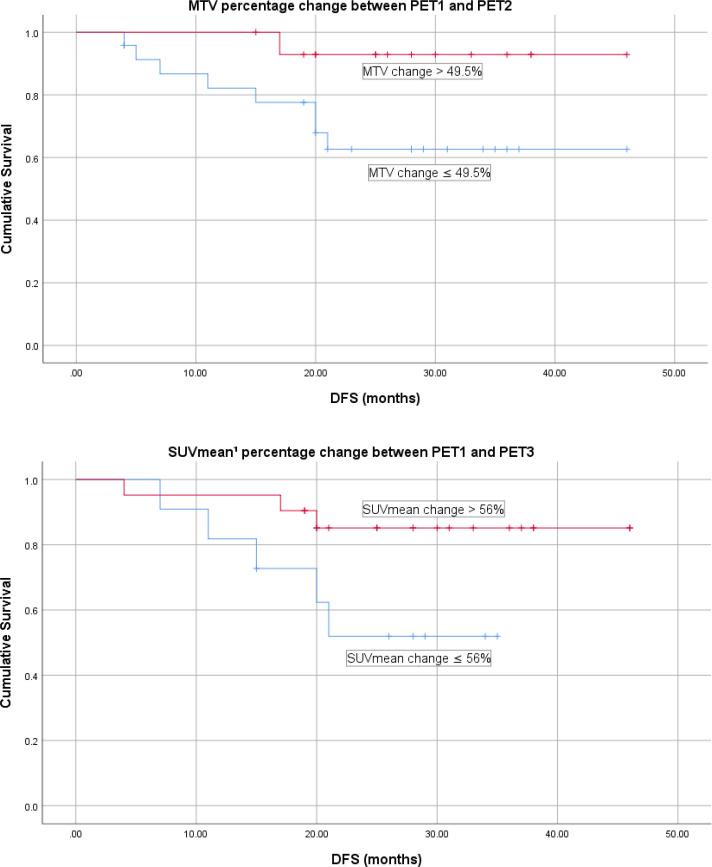
Two parameters were also found to be trending towards significance on Kaplan–Meier analysis in predicting DFS: MTV percentage change between PET1 and PET2 using 49.5% (p = 0.052) as a cut-off and SUVmean percentage change between PET1 and PET3 (calculated using a SUV threshold of 42% of SUVmax) using a cut-off of 56% (p-0.053) (Figure 4).
Figure 4.
DFS for PET parameters trending towards significance on Kaplan–Meier analysis. ¹Calculated using a SUV threshold of 42% of SUVmax. DFS, disease-free survival; MTV, metabolic tumour volume; PET, positron emission tomography; TLG, tumour lesion glycolysis.
Discussion
The current practice in assessing response to NCRT is from histopathological analysis, with its associated limitations.2 Conventional imaging is also limited, as tumour response does not necessarily lead to morphological changes.13 Habr-Gama et al stratified patients according to clinical response to NRCT and reported that a “wait and see” approach in patients who were responders did not have an increased risk of recurrence or mortality, compared with surgical intervention.14 Hence, an accurate, non-invasive method to assess response to NRCT could potentially personalize treatment, i.e. escalation or de-escalation.
Our study adds to the growing literature surrounding the utility of 18F-FDG-PET/CT in assessing treatment response and prognosis, and its potential to risk-stratify patients pre-operatively. The parameters we found to be the best prognosticators for DFS were: percentage changes in MTV and TLG between PET1 and PET3. These findings suggest the potential use of volumetric PET parameters as the primary focus in predicting DFS in LARC. This is consistent with previous studies correlating PET volumetry and survival.15–17 Volumetry may prognosticate better than SUVmax alone, as it takes into consideration the heterogenous nature of a tumour, and more accurately reflects tumour burden.18,19
Despite the evidence for volumetry, the cut-off values within studies vary. Kim utilized a percentage reduction in TLG of 47.73 compared with Guillem’s 69.5%, differing from our cut-off of 94%.16,17 Factors affecting these results may include the timing of the PET scans, the treatment protocols and the differences in PET acquisition and analysis. These variabilities may also explain the different cut-off values for SUVmax, where we found limited utility in prognosticating LARC.
The change in SUVmax is also reported to have a correlation with long-term outcomes. Guillem and Sorenson found that a percentage reduction between PET1 and PET3 of 62.5 and 66% respectively, was predictive of DFS.16,20 In our study, however, we found no correlation between change in SUVmax and DFS. This may be explained by the small sample size and the limited number of patients who had PET3 (33 out of the 46).
We found that a high SUVmean at PET2 predicted a worse DFS. Patients categorised as having a high SUVmean showed a 3 year recurrence rate of 41% whereas the low SUVmean group had a significantly lower rate of recurrence of 9% (p = 0.0027). While the timing of PET two is more consistent, the timing of PET3 is more variable due to surgical scheduling which was a limitation in our study. A short time interval between NCRT and PET3 can lead to false-positive PET findings due to inflammation, therefore, PET2 may be a more suitable timepoint for PET analysis.21 This potentially allows more accurate prognostication and early implementation of a risk-stratified strategy and treatment modification.
Parameters using a SUV threshold of 2.5 were found to be more significant in predicting long term outcomes compared to parameters using a SUV threshold of 42% of the SUVmax. We hypothesize that this is because an SUV of 2.5 as a threshold incorporates a larger volume of the tumour burden especially in extensive and more FDG-avid tumours compared to a percentage of the SUVmax. Hence, changes between PET parameters at different time points were accentuated.
As previously discussed, we acknowledge the limitations of our study. While there was variability in the FDG uptake time, the uptake times of subsequent scans were matched as close to the initial PET scan as feasible. Apart from one patient with an FDG uptake time of 85 min, all other patients were within 10 min of the target 60 min as per protocol. Ideally, we would have liked to ensure consistent timings in all aspects of management including NCRT, scanning and surgery. The follow-up period of our study was relatively short and it may take 5 years to detect 80% of recurrences.22 Many patients were also lost to follow up during data census where our follow-up time varied from 4 to 43 months. Furthermore, in our statistical analysis, the cut-offs were determined by the best possible combination of specificity and sensitivity, leading to some degree of confirmation bias. We did not analyze any additional parameters, such as SUV normalized to lean body mass (SUL) or perform textural analysis which could be an area of interest for further research. The intra- and interobserver variability was not formally assessed, although the PET data was primarily obtained by semi-automated software and the differences may be small.
Although 18F-FDG-PET/CT is not yet incorporated into guidelines for the assessment of response to NCRT, we have shown that an increased reduction in volumetric parameters and lower SUV values at specific time points are predictive of improved survival. We are also analyzing the PET and DWMRI data for this cohort of patients to compare and determine the incremental benefit of either modality in assessing pathological response. Preliminary results suggests postNCRT ADC can potentially prognosticate pathological response.23
Due to the nature of our study and its inherent limitations, a multicentre study with more stringent control over 18F-FDG-PET/CT timing and acquisition protocols would allow us to draw firmer conclusions regarding the novel use of 18F-FDG-PET/CT in assessing response to NCRT and prognosis.
Conclusion
This study supports the potential clinical utility of 18F-FDG-PET/CT, in predicting DFS in LARC. Changes in PET volumetry appear to be the best predictors of DFS.
Footnotes
Contributors: Sumal Fernando: data curation, formal analysis, writing – original draft; Michael Lin: data curation, writing – review & editing; Shanley Chong: data analysis and interpretation; Karen Wong: writing – review & editing; Emilia Ip: writing – review & editing; Wei Chua: writing – review & editing; Weng Ng: writing – review & editing; Peter Lin: writing – review & editing; Stephanie Lim: conceptualisation, writing – review & editing.
Ethics approval: This study has been approved by the South Western Sydney Local Health District Human Research and Ethics Committee (HREC). Reference HREC/13/LPOOL/158, local project number 13/ 097a, 13/097b, 13/097c, Sub-study Rectal Cancer.
Contributor Information
Sumal Fernando, Email: z5059126@unsw.edu.au.
Michael Lin, Email: michael.lin@sswahs.nsw.gov.au.
Trang Thanh Pham, Email: Trang.Pham@health.nsw.gov.au.
Shanley Chong, Email: Shanley.Chong@health.nsw.gov.au.
Emilia Ip, Email: emilia.ip@health.nsw.gov.au.
Karen Wong, Email: karen.wong@sswahs.nsw.gov.au.
Wei Chua, Email: wei.chua@health.nsw.gov.au.
Weng Ng, Email: Weng.Ng@health.nsw.gov.au.
Peter Lin, Email: Peter.Lin1@health.nsw.gov.au.
Stephanie Lim, Email: stephanie.lim@health.nsw.gov.au.
REFERENCES
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA oncology 2017; 3: 524–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edge SB, Compton CC. The American joint Committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–4. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 3.Gallamini A, Zwarthoed C, Borra A. Positron emission tomography (PET) in oncology. Cancers 2014; 6: 1821–89. doi: 10.3390/cancers6041821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burt BM, Humm JL, Kooby DA, Squire OD, Mastorides S, Larson SM, et al. Using positron emission tomography with [(18)F]FDG to predict tumor behavior in experimental colorectal cancer. Neoplasia 2001; 3: 189–95. doi: 10.1038/sj.neo.7900147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D. Value of (18)F-FDG PET for predicting gresponse to neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. AJR Am J Roentgenol 2015; 204: 1261–8. doi: 10.2214/AJR.14.13210 [DOI] [PubMed] [Google Scholar]
- 6.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28(suppl_4): iv22–40. doi: 10.1093/annonc/mdx224 [DOI] [PubMed] [Google Scholar]
- 7.Valentini V, Gambacorta MA, Barbaro B, Chiloiro G, Coco C, Das P, et al. International consensus guidelines on clinical target volume delineation in rectal cancer. Radiother Oncol 2016; 120: 195–201. doi: 10.1016/j.radonc.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 8.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017; 2: 501–13. doi: 10.1016/S2468-1253(17)30074-2 [DOI] [PubMed] [Google Scholar]
- 9.Joye I, Deroose CM, Vandecaveye V, Haustermans K. The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiother Oncol 2014; 113: 158–65. doi: 10.1016/j.radonc.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 10.Rymer B, Curtis NJ, Siddiqui MRS, Chand M. FDG PET/CT can assess the response of locally advanced rectal cancer to neoadjuvant chemoradiotherapy: evidence from meta-analysis and systematic review. Clin Nucl Med 2016; 41: 371–5. doi: 10.1097/RLU.0000000000001166 [DOI] [PubMed] [Google Scholar]
- 11.Maffione AM, Galeotti F, Capirci C, Colletti PM, Rubello D. When and why to use FDG PET/CT in locally advanced rectal cancer. Clin Nucl Med 2014; 39: 1–30. doi: 10.1097/RLU.0000000000000435 [DOI] [PubMed] [Google Scholar]
- 12.Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015; 42: 328–54. doi: 10.1007/s00259-014-2961-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beets-Tan RGH, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology 2004; 232: 335–46. doi: 10.1148/radiol.2322021326 [DOI] [PubMed] [Google Scholar]
- 14.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004; 240: 711. doi: 10.1097/01.sla.0000141194.27992.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuno T, Kawai K, Koyama K, Takahashi M, Ishihara S, Momose T, et al. Value of FDG-PET/CT volumetry after chemoradiotherapy in rectal cancer. Dis Colon Rectum 2018; 61: 320–7. doi: 10.1097/DCR.0000000000000959 [DOI] [PubMed] [Google Scholar]
- 16.Guillem JG, Moore HG, Akhurst T, Klimstra DS, Ruo L, Mazumdar M, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J Am Coll Surg 2004; 199: 1–7. doi: 10.1016/j.jamcollsurg.2004.02.024 [DOI] [PubMed] [Google Scholar]
- 17.Kim S-J, Chang S. Volumetric parameters changes of sequential 18F-FDG PET/CT for early prediction of recurrence and death in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy. Clin Nucl Med 2015; 40: 930–5. doi: 10.1097/RLU.0000000000000917 [DOI] [PubMed] [Google Scholar]
- 18.Chung MK, Jeong H-S, Park SG, Jang JY, Son Y-I, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 2009; 15: 5861–8. doi: 10.1158/1078-0432.CCR-08-3290 [DOI] [PubMed] [Google Scholar]
- 19.Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. the visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999; 2: 159–71. doi: 10.1016/S1095-0397(99)00016-3 [DOI] [PubMed] [Google Scholar]
- 20.Sorenson EC, Choudhry AJ, Yu JQ, Reddy SS, Denlinger CS, Meyer JE, et al. Predictive value of PET/CT for pathological complete response and survival in patients with locally advanced rectal cancer. Journal of Clinical Oncology 2017; 35(4_suppl): 697. doi: 10.1200/JCO.2017.35.4_suppl.697 [DOI] [Google Scholar]
- 21.Kubota K. From tumor biology to clinical PET: a review of positron emission tomography (PET) in oncology. Ann Nucl Med 2001; 15: 471–86. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad NR, Nagle D. Long-Term results of preoperative radiation therapy alone for stage T3 and T4 rectal cancer. Br J Surg 1997; 84: 1445–8. doi: 10.1002/bjs.1800841029 [DOI] [PubMed] [Google Scholar]
- 23.Pham T, Liney G, Wong K, Henderson C, Shin JS, Rai R, et al. PO-0792: rectal cancer: multiparametric MRI assessment of tumour heterogeneity and chemoradiotherapy response. Radiotherapy and Oncology 2018; 127: S410. doi: 10.1016/S0167-8140(18)31102-2 [DOI] [Google Scholar]