Abstract
Objective:
MRI provides clear visualization of spinal cord, tumor, and bone for patient positioning and verification during MRI-guided radiotherapy (MRI-RT). Therefore, we wished to evaluate spine stereotactic ablative radiotherapy (SABR) feasibility with MRI-RT. Given dosimetric limitations of first generation Co-60 MRI-RT, we then evaluated improvements by newer linear accelerator (linac) MRI-RT.
Methods:
Nine spinal metastases were treated with Co-60 MRI-RT. Seven received a single 16 Gy fraction, and two received three fractions totaling 24 or 30 Gy. After replanning with linac MRI-RT software, comparisons of organ at risk and dose spillage objectives between Co-60 and linac plans were performed.
Results:
Spinal cord and cauda equina dose constraints were met in all Co-60 cases. Treatments were delivered successfully with real-time imaging during treatment and no treatment-related toxicities. While limits for dose spillage into surrounding soft tissues were not achieved due to the limitations of the Co-60 system, this could be corrected with linac MRI-RT delivery.
Conclusions:
MRI-RT SABR of spinal metastases is feasible with Co-60 MRI-RT. Dose delivery is improved by linac MRI-RT.
Advances in knowledge:
This is the first report of MRI-RT for SABR of spinal metastases. The enhanced visualization of anatomy by MRI may facilitate RT dose escalation for spine SABR.
Background
Spinal metastases occur in up to 40% of all cancer patients.1 Radiation therapy (RT) is a longstanding palliative treatment for the alleviation of pain, prevention of tumor progression, and response to malignant cord compression. Conventionally fractionated radiotherapy provides limited response, which has led to use of spinal stereotactic ablative radiotherapy (SABR) in attempts to improve pain control and local control, especially for radioresistant malignancies.2–4
Image guidance techniques at the time of treatment have historically been limited to X-rays such as two-dimensional MV or kV projections or cone beam computed tomography (CBCT). While these techniques delineate the bony spine, soft tissues such as the spinal cord cannot be identified. In addition, these techniques combined with RT do not image in real-time. These limitations could be dangerous since small errors in setup or patient motion early in treatment can lead to overdose of organs at risk (OARs).5
The first generation of clinically used MRI-guided radiotherapy (MRI-RT) device (Viewray Inc., Cleveland, OH) is currently utilized by multiple institutions. The device combines three rotating Co-60 sources with a 0.35 T MRI system and a single clinically useful pulse sequence based on balanced steady state free precession (bSSFP).6 The bSSFP sequence provides fast, high resolution, high signal-to-noise ratio imaging with strong T2 weighting as configured on the device, and it is commonly used in body radiology.7 Therefore, MRI-RT allows for high contrast setup visualization at the time of treatment including spinal cord and cauda equina in addition to other nearby organs at risk such as moveable bowel that can be present anterior or lateral to the vertebral body (VB) depending on spinal level.8 Further, MRI-RT provides near real-time imaging and gating during radiotherapy, so that any patient motion is detected in less than a second and treatment automatically held for repositioning if necessary.
The RT plan quality of the first generation MRI-RT device is limited by Co-60 physical penumbra and 1 cm MLC width.9,10 Indeed, a dosimetric study of this device concluded “For spine SABR, the tri-Co-60 IMRT is inappropriate owing to the large penumbra, large leaf width and low dose rate of the ViewRay system.”10 Given the potential benefits for spine SABR of MRI-based treatment setup and real-time image guidance, we wished to verify the conclusions of the prior study. Once we treated spine SABR patients safely with MRI-RT, we sought to verify that the more recent linear accelerator (linac) MRI-RT system would deliver higher quality plans sufficient for modern spine SABR as per Radiation Therapy Oncology Group (RTOG) or NRG Oncology protocols.11
Methods and materials
Nine consecutive spinal metastases were treated with the Co-60 MRI-RT system at the University of Miami from May 2016 to January 2017. Seven were treated with a single 16 Gy fraction (T3, T4, L1-L2, L3, L3, L3-L4, and L5-S1) and two cases received 30 Gy (T4) or 24 Gy (L3) in three fractions. In all nine cases, the VB was involved and at least the entire VB received the prescription dose. Cases treated to the VB alone (one subsite) include L3, L1-L2, L5-S1 (one fraction), and L3 (three fractions). Cases L3 (one fraction) and T4 (three fractions) were treated to the VB and one pedicle (two subsites). Cases T3, T5 and L3-L4 (one fraction) were treated to the VB, one pedicle and one transverse process and lamina (three subsites). Normalization for each of the Co-60 MRI-RT SABR cases was physician-dependent based on individualized constraints or spillage priorities for each case.
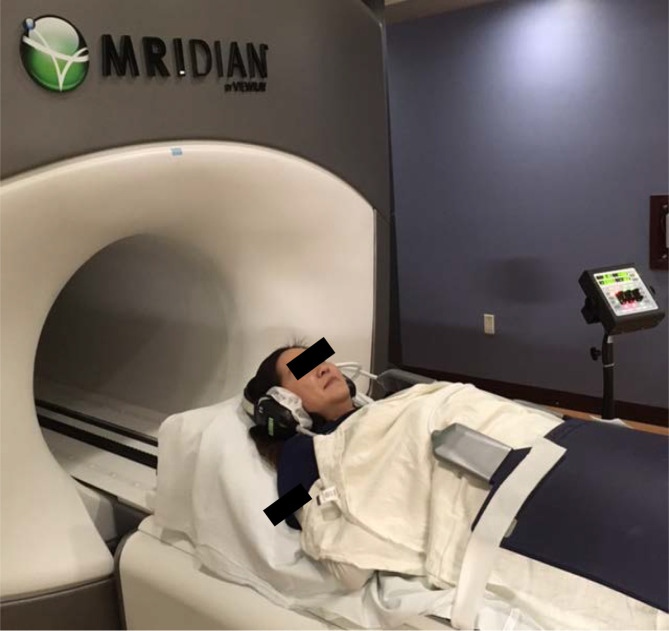
Patients were simulated on the Co-60 MRI-RT system and CT simulator with supine positioning without immobilization except a wing board for patients treated to thoracic locations. MRI coils were wrapped around the surface of the patient at the treated spinal level (Figure 1). Real-time image guidance at time of treatment was performed using either of the two vendor provided options. Specifically, single sagittal slice MRI imaging at the center of the VB with bSSFP at 4 Hz or three sagittal MRI images positioned at the edges and center of the VB, each imaged at 2 Hz with 1–2 mm gating boundary in the anteroposterior and craniocaudal axes.
Figure 1.
Example setup for a lumbar spinal metastasis treated with MRI-guided SABR. The vendor supplied flexible MRI coil array (black) is seen on the right overlying the abdomen. SABR, stereotactic ablative radiotherapy
Target contours were based on the International Spine Radiosurgery Consortium Consensus in which the vertebra is divided into six anatomical sectors.12 The clinical target volume (CTV) was the sum of the gross tumor volume (GTV) plus the neighboring anatomical sectors that could harbor subclinical disease. There was no planning target volume (PTV) expansion. Extraosseus disease extension was contoured based on a combination of the simulation MRI on the Co-60 MRI-RT system, diagnostic MRI, and simulation CT.
Treatment planning was performed with the ViewRay Planning platform and Monte Carlo optimization algorithms. Highest priorities were assigned to the target and penalizing dose regions outside the target in discrete intervals. Beam characteristics of the respective deliveries were considered in assessing penalties in the regions outside the target. There are two major differences between Co-60 and 6 MV linac delivery incorporated into the planning system. Co-60 has a wider physical penumbra and uses three Co-60 sources with three independent multileaf collimators with 1 cm leaf width at isocenter, with the smallest achievable segment size of 2 x 10 mm. The 6 MV linac uses a 4 mm leaf width at isocenter with the smallest achievable segment size of 2 x 4mm.
We focused on the doses received in the spinal cord, cauda equina, and other OARs as a measure of plan safety and on dose spillage as a measure of plan quality. Dosimetric objectives were derived from protocols RTOG 063111 for single fraction and NRG BR00113 for three fraction treatments. The OAR constraints for single fraction cases were for the spinal cord V10Gy <0.35 cc, V14Gy <0.03 cc, for the partial cord V10Gy <10% and for the cauda equina V16Gy <0.03 cc. The OAR constraints for three fraction treatments were spinal cord V22.5Gy <0.03 cc and V13Gy <1.2 cc, and cauda equina V25.5Gy <0.03 cc and V21.9Gy <5 cc. The dosimetric parameters used to assess dose spillage were acceptable deviations on the RTOG 0631 and BR001 protocols: volume of tissue receiving 105% or more of the prescription dose outside of the PTV (Vol > 105% out of PTV, target <3 cc), volume of tissue receiving 115% or more of the prescription dose outside of the PTV (Vol > 115% out of PTV, target 0cc) and conformity index ≤1.5. PTV coverage by prescription isodose was >80% for all plans.
Linac-based MRI-guided SABR plans were generated for the treated Co-60 SABR cases using vendor provided linac-based MRI-RT radiation planning software. The linac-based cases were optimized and then normalized to the same prescription dose coverage as the original case. We then compared OAR constraints and spillage parameters as above.
Results
MRI-Cobalt 60 treatments
The spinal cord and cauda equina were clearly visualized for all patients during daily treatment setup. An example case of a Co-60 MRI-RT SABR of a L1 spinal metastasis is shown in Figure 2. No gating events were observed since all patients were observed to remain immobile despite a median beam-on time of 26.5 min (range: 18.9–61).
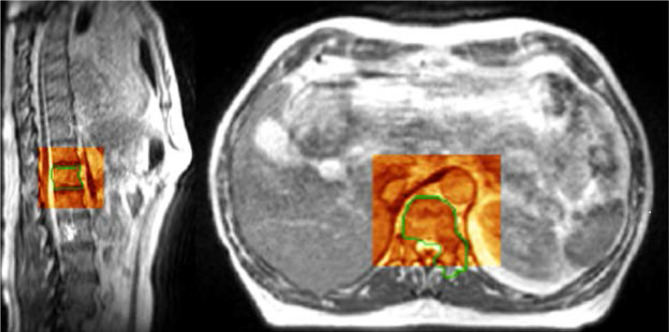
Figure 2.
Example slices (sagittal and axial) from 3D setup volume at time of treatment for Co-60 MRI-RT SABR of a L1 spinal metastasis. The grayscale image represents the MRI simulation image. The red shaded box demonstrates the aligned setup imaging of the day. A green outline delineates the PTV which includes the vertebral body and left pedicle. The bright cerebral spinal fluid, spinal cord, and bony anatomy are clearly visualized in the spinal canal on the sagittal image. On the axial image, the tip of the spinal cord and cauda equina are observed posteriorly in the dural sac. 3D,three-dimensional; SABR, stereotactic ablativeradiotherapy; PTV, planning target volume.
The spinal cord and cauda equina dose constraints were met in all Co-60 cases (Tables 1 and 2). Other OARs such as kidneys, bowel etc were also met per RTOG 0631 or NRG BR001 limits. However, dose spillage indices could not be met per protocol specifications due to the limitations of the Co-60 system. The median conformity index was 1.63 (range: 1.4–2.27) and homogeneity index was 1.25 (range: 1.09–1.47). Plans were considered safe for delivery because the dose spillage was into perispinal musculature, fat, and great vessels which are well within the radiation tolerances of the doses used here.
Table 1.
MRI-RT for spinal bone metastases in one fraction
| PTV | PTV Subsites | 16 Gy PTV Coverage |
S.Cord V14Gy (<0.03 cc) |
P.Cord 10% (<10 Gy) |
C.Equina V16Gy (<0.03 cc) |
Vol > 105% out of PTV (<3 cc) | Vol > 115% out of PTV (0.0cc) | Conformity index (<1.5) | Homogeneity index (<2) |
|---|---|---|---|---|---|---|---|---|---|
| Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | ||
| T3 | 3 | 80–80% | 0.0cc 0.0cc | 1.68–0.1% | N/A N/A | 22.8 cc 0.18 cc | 1.9 cc 0.0 cc | 2.27 0.80 | 1.32 1.83 |
| T5 | 3 | 80–80% | 0.0cc 0.0cc | 2.68–0.1% | N/A N/A | 22.8 cc 0.62 cc | 1.9 cc 0.0 cc | 2.11 1.10 | 1.28 1.62 |
| L1-L2 | 1 | 95–95% | N/A N/A | N/A N/A | 0.0 cc 0.0 cc | 26.9 cc 2.8 cc | 0.1 cc 0.0 cc | 1.63 1.07 | 1.19 1.55 |
| L3 | 2 | 95–95% | N/A N/A | N/A N/A | 0.0 cc 0.0 cc | 25.0 cc 2.3 cc | 8.2 cc 0.0 cc | 1.44 1.06 | 1.35 1.29 |
| L3 | 1 | 90–90% | N/A N/A | N/A N/A | 0.0 cc 0.0 cc | 12.4 cc 1.3 cc | 0.3 cc 0.0 cc | 1.57 1.03 | 1.18 1.18 |
| L3-L4 | 3 | 80–80% | N/A N/A | N/A N/A | 0.0 cc 0.0 cc | 11.4 cc 3.0 cc | 0.0 cc 0.0 cc | 1.17 1.00 | 1.10 1.27 |
| L5-S1 | 1 | 97–97% | N/A N/A | N/A N/A | 0.0 cc 0.0 cc | 26.9 cc 1.4 cc | 0.0 cc 0.0 cc | 1.63 1.50 | 1.09 1.6 |
C.Equina, cauda equina; Co-60, Cobalt-60; MRI-RT, magnetic resonance image-guided radiotherapy; OAR, organ at risk; PTV, planning target volume; SABR, stereotactic ablative radiotherapy; S.Cord, spinal cord; VXGy, volume of OAR receiving at least X Gy; fx, fraction.
The treated patients on Co-60 MRI-RT were replanned with the linac MRI-RT system to identify whether second generation MRI-RT with linac treatments would improve dose delivery. For spine SABR cases treated with Co-60 MRI-RT in one fraction, OAR objectives (spinal cord and cauda equina) were met based on protocol objectives. However, dose spillage (three right columns in the table) into peri-spinal musculature and fat are higher than desired. Objectives are given in parentheses (e.g. conformity index should be 1.5 or less).
Co-60 MRI-RT delivered plan vs. Linac-based MRI-RT replanning showing OAR constraints and dose spillages with objectives based on RTOG 0631.
Table 2.
MRI-RT for spinal bone metastases in three fractions
| PTV | PTV Subsites |
Dose / PTV Coverage | S.Cord V22.5Gy (<0.03 cc) | S.Cord V13Gy (<1.2 cc) | C.Equina V25.5Gy (<0.03 cc) | C.Equina V21.9Gy (<5 cc) | Vol > 105% out of PTV (<3 cc) | Vol > 115% out Of PTV (0.0cc) |
Conformity index (<1.5) | Homogeneity Index (<2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | Co-60 Linac | ||
| T4 (three fx) | 2 | 30 Gy 30 Gy 88–88% |
0.0cc 0.0cc | 0.43cc 0.43cc | N/A N/A | N/A N/A | 12.5cc 0.4cc | 6.9cc 0.0cc | 2.1 1.01 | 1.47 1.5 |
| L3 (three fx) | 1 | 24 Gy 24 Gy 99–99% |
N/A N/A | N/A N/A | 0.0cc 0.0cc | 0.0cc 0.0cc | 8.8cc 1.0cc | 0.6cc 0.0cc | 1.40 1.12 | 1.25 1.24 |
C.Equina, cauda equina.; Co-60, Cobalt-60; MRI-RT, magnetic resonance image-guided radiotherapy; OAR, organs-at-risk; SABR, stereotactic ablative radiotherapy; S.cord, spinal cord; VXGy, volume of OAR receiving at least X Gy; fx, fraction.
Both OAR objectives (spinal cord and cauda equina) and dose spillage were met per protocol objectives for three fraction linac plans as well. Objectives are given in parentheses (e.g. conformity index should be 1.5 or less).
Co-60 MRI-RT delivered plan vs linac-based MRI-RT replanning showing OAR constraints and dose spillages with objectives based on NRG BR001 protocol.
The median follow-up was 12.3 months (range: 0–32 months). No treatment related toxicities or in-field recurrences were observed. All patients reported acute pain relief at the treated site from minor to complete, however this was not recorded in a systematic fashion. Six patients have died from their metastatic disease. Two patients are alive with complete resolution of back pain. One patient was lost to follow up shortly after radiation for a clinical trial unavailable at our institution.
MRI-linac replanning
The MRI-linac planning system was used to reoptimize the delivered Co-60-based plans with the same percentage of prescription dose coverage for each case. All OAR constraints were still met, however there was improved dose spillage (e.g. Vol >105% out of PTV less than 3 cc) meeting protocol criteria (Table 1). The median conformity index was 1.06 (range: 0.8–1.5) and significantly improved compared to the initial cobalt plans (2-tailed paired t-test p = 0.004). The median homogeneity index was 1.50 (range: 1.18–1.83) and increased compared to the initial cobalt plans (p = 0.027). Unlike the Co-60 plans, all significant hot spots in linac plans were within the GTV. One example plan demonstrating these differences between Co-60-based delivery and MRI-linac-based delivery is shown in Figure 3.
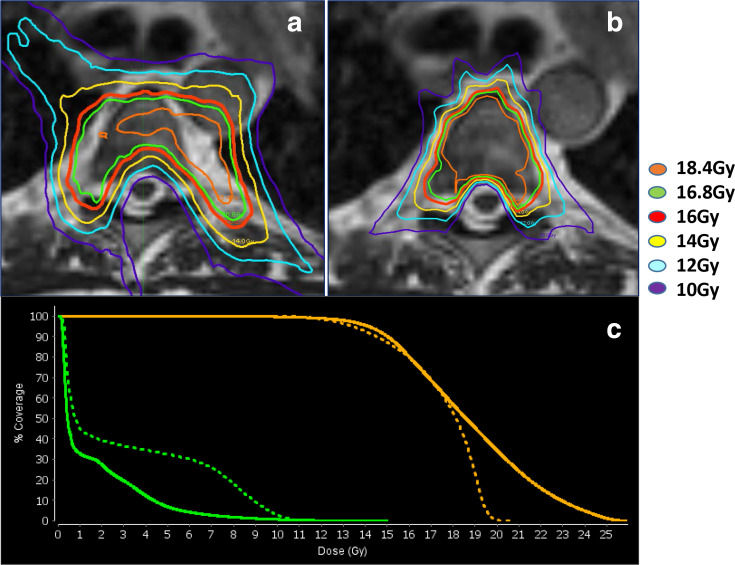
Figure 3.
Example delivered Co-60 and replanned linac MRI-RT SABR plans for a T5 spinal metastasis at 16 Gy prescription dose. (a) Co-60 treatment plan isodose levels in color (legend on right) overlaid on grayscale MRI simulation. The treatment plan is not conformal due to Co-60 penumbra and MLC width. Hot spots of over 115% (18.4 Gy) are observed outside of the PTV in adjacent fat. However the spinal cord is only touching the 10 Gy isodose line. Additional OARs in the area (esophagus, aorta, trachea and lung) were contoured and maintained below TG-101 objective doses. (b) The MRI-linac SABR plan is much more conformal, with excellent coverage, and hot spots almost entirely within the PTV. (c) Co-60 (dashed line) and linac (solid line) DVH is shown for this plan for the PTV (orange) and partial cord (green). The linac plan has less low dose to the spinal cord and is hotter within the PTV target. DVH, dose–volume histogram; OAR, organ at risk; MLC, multileaf collimator; SABR, stereotactic ablative radiotherapy; PTV, planningtarget volume.
Discussion
The use of MRI-RT for spine SABR permits clear visualization of the spinal cord, cauda equina, and other OARs. Real-time image guidance is included with MRI-RT systems, which may also improve treatment safety. However, treatment on the Co-60 MRI-RT system has been controversial due to plan quality as compared to a cone beam CT-guided linear accelerator system.10 Nevertheless, we demonstrated feasibility of treatment to nine patients with MRI-RT on the Co-60 system since all constraints for OARs such as spinal cord and cauda equina were met to ensure plan safety while still meeting acceptable target coverage. We found that the MRI-linac system could combine the imaging advantage of MRI-RT with the linear accelerator plan quality that meets RTOG 0631 or NRG BR001 protocol objectives as a benchmark.
The use of MRI guidance introduces questions of spatial distortion due to magnetic field inhomogeneity. Fortunately, the position of a patient’s single or adjacent vertebral bodies on an MRI table are very close to the MRI isocenter assuming midline position of the spine, supine position, and MRI-RT isocenter position in the center of the target. For the system used in this study, 99.9% of distortions are less than 1 mm within 100 mm of isocenter.14 This accuracy compares very favorably to the geometric accuracy of cone beam CT for positioning with a 99% confidence interval of 2 mm.15
Treatment delivery times for Co-60 SABR were as long as 61 min, however MRI-linac treatment will be faster with the exact degree of improvement depending on the age of the Co-60 sources and the plan modulation. Still, any treatment time raises concern about patient motion. In the best scenario, MRI-guidance will allow for significant patient motion to be identified nearly instantly. For a rectangular target, the accuracy of the current tracking has been measured at 0.3 ± 1.1 mm.16 This is very similar to the magnitudes of intrafraction motion during spine SABR reported previously using X-ray guidance.17
However, a current limitation is that the real-time target guidance is only obtained in a single sagittal plane or in three sagittal planes simultaneously. While we observed no gating events in our experience when using a tracking boundary of 1–2 mm, tracking was only available in the anteroposterior and superoinferior axes. We felt that this was reasonable as a check for gross motion during the long Co-60 treatments. The accuracy of this approach could be improved by repeating a volumetric MRI periodically while treatment is paused (adding ~2 min per scan). Ideally, future versions of MRI-RT systems would include fast volumetric MRIs concurrent with treatment18 or would perform bSSFP with multiple orthogonal cine-MRI planes.19
Potential clinical benefits of MRI-RT for spine SABR should be explored. One suggestion might be to use MRI-RT for confidence of delivered dose in investigations of spinal cord dose constraint relaxation. Minimum dose to gross disease of 14–15 Gy in a single fraction has been associated with treatment failure,20,21 while RTOG 0631 constrains the spinal cord to a maximum dose of 14 Gy. This has led to underdosing of epidural gross tumor by many groups to treat the spinal cord safely. Nevertheless, a Phase I dose escalation study of unresectable epidural disease has demonstrated no toxicity in seven patients treated to a spinal cord 16 Gy maximum point dose.22 MRI-RT would allow for precise verification of the soft tissue setup for these studies and even permit online adaptation if there is any change in tumor size or anatomy (compression fracture, rotational setup errors, mobile OARs such as bowel in the area, etc) since the time of simulation.
Conclusions
MRI-RT SABR for localized spinal metastases is feasible on a Co-60 MRI-RT system, with improved dosimetry on a linac MRI-RT system. MRI-RT demonstrates clear visualization of soft tissues which may provide future advantages in dose escalation trials for spine SABR. Proposed improvements in real-time MRI guidance could further enhance the safety of this technique.
Footnotes
Acknowledgements: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number K12 CA226330. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding: James Victoria, CMD is an employee of Viewray, Inc Eric A Mellon, MD, PhD has received travel funding for presentation of unrelated data from Viewray in 2016 This work was supported by the Sylvester Comprehensive Cancer Center and Department of Radiation Oncology of the Miller School of Medicine of the University of Miami
Contributor Information
Ricardo Llorente, Email: ricardo.llorente@jhsmiami.org.
Benjamin O Spieler, Email: benjamin.spieler@jhsmiami.org.
James Victoria, Email: jrvictoria@viewray.com.
Cristiane Takita, Email: ctakita@med.miami.edu.
Raphael Yechieli, Email: ryechieli@med.miami.edu.
John C Ford, Email: jcf137@med.miami.edu.
Karen Brown, Email: kmoya@med.miami.edu.
Michael A Samuels, Email: msamuels2@med.miami.edu.
Eric A Mellon, Email: eric.mellon@med.miami.edu.
REFERENCES
- 1.Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine 1990; 15: 1–4. [PubMed] [Google Scholar]
- 2.Ryu S, Jin R, Jin J-Y, Chen Q, Rock J, Anderson J, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage 2008; 35: 292–8. doi: 10.1016/j.jpainsymman.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 3.Gerszten PC, Burton SA, Ozhasoglu C, Vogel WJ, Welch WC, Baar J, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg 2005; 3: 288–95. doi: 10.3171/spi.2005.3.4.0288 [DOI] [PubMed] [Google Scholar]
- 4.Gerszten PC, Burton SA, Quinn AE, Agarwala SS, Kirkwood JM. Radiosurgery for the treatment of spinal melanoma metastases. Stereotact Funct Neurosurg 2005; 83(5-6): 213–21. doi: 10.1159/000091952 [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Shiu A, Wang C, O'Daniel J, Mahajan A, Woo S, et al. Dosimetric effect of translational and rotational errors for patients undergoing image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys 2008; 71: 1261–71. doi: 10.1016/j.ijrobp.2008.02.074 [DOI] [PubMed] [Google Scholar]
- 6.Mutic S, Dempsey JF. The ViewRay system: magnetic Resonance–Guided and controlled radiotherapy. Semin Radiat Oncol 2014; 24: 196–9. doi: 10.1016/j.semradonc.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 7.Chavhan GB, Babyn PS, Jankharia BG, Cheng H-LM, Shroff MM. Steady-State MR imaging sequences: physics, classification, and clinical applications. Radiographics 2008; 28: 1147–60. doi: 10.1148/rg.284075031 [DOI] [PubMed] [Google Scholar]
- 8.Noel CE, Parikh PJ, Spencer CR, Green OL, Hu Y, Mutic S, et al. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol 2015; 54: 1474–82. doi: 10.3109/0284186X.2015.1062541 [DOI] [PubMed] [Google Scholar]
- 9.Park JM, Park S-Y, Kim HJ, Wu H-G, Carlson J, Kim J-I, et al. A comparative planning study for lung SABR between tri-Co-60 magnetic resonance image guided radiation therapy system and volumetric modulated Arc therapy. Radiother Oncol 2016; 120: 279–85. doi: 10.1016/j.radonc.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 10.Choi CH, Park S-Y, Kim J-I, Kim JH, Kim K, Carlson J, et al. Quality of tri-Co-60 MR-IGRT treatment plans in comparison with VMAT treatment plans for spine SABR. Br J Radiol 2017; 90: 20160652. doi: 10.1259/bjr.20160652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryu S, Pugh SL, Gerszten PC, Yin F-F, Timmerman RD, Hitchcock YJ, et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1-3) spine metastases: phase 2 results. Pract Radiat Oncol 2014; 4: 76–81. doi: 10.1016/j.prro.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox BW, Spratt DE, Lovelock M, Bilsky MH, Lis E, Ryu S, et al. International spine radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2012; 83: e597–605. doi: 10.1016/j.ijrobp.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 13.Al-Hallaq HA, Chmura S, Salama JK, Winter KA, Robinson CG, Pisansky TM, et al. Rationale of technical requirements for NRG-BR001: the first NCI-sponsored trial of SBRT for the treatment of multiple metastases. Pract Radiat Oncol 2016; 6: e291–8. doi: 10.1016/j.prro.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginn JS, Agazaryan N, Cao M, Baharom U, Low DA, Yang Y, et al. Characterization of spatial distortion in a 0.35 T MRI-guided radiotherapy system. Phys Med Biol 2017; 62: 4525–40. doi: 10.1088/1361-6560/aa6e1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bissonnette J-P, Moseley D, White E, Sharpe M, Purdie T, Jaffray DA, et al. Quality assurance for the geometric accuracy of cone-beam CT guidance in radiation therapy. Int J Radiat Oncol Biol Phys 2008; 71(1 Suppl): S57–61. doi: 10.1016/j.ijrobp.2007.06.086 [DOI] [PubMed] [Google Scholar]
- 16.Green OL, et al. First clinical implementation of real-time, real anatomy tracking and radiation beam control. Med Phys 2018;. [DOI] [PubMed] [Google Scholar]
- 17.Jin J-Y, Ryu S, Rock J, Faber K, Chen Q, Ajlouni M, et al. Evaluation of residual patient position variation for spinal radiosurgery using the novalis image guided system. Med Phys 2008; 35: 1087–93. doi: 10.1118/1.2839097 [DOI] [PubMed] [Google Scholar]
- 18.Raaymakers BW, Jürgenliemk-Schulz IM, Bol GH, Glitzner M, Kotte ANTJ, van Asselen B, et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol 2017; 62: L41–50. doi: 10.1088/1361-6560/aa9517 [DOI] [PubMed] [Google Scholar]
- 19.Bjerre T, Crijns S, af Rosenschöld PM, Aznar M, Specht L, Larsen R, et al. Three-Dimensional MRI-linac intra-fraction guidance using multiple orthogonal cine-MRI planes. Phys Med Biol 2013; 58: 4943–50. doi: 10.1088/0031-9155/58/14/4943 [DOI] [PubMed] [Google Scholar]
- 20.Bishop AJ, Tao R, Rebueno NC, Christensen EN, Allen PK, Wang XA, et al. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys 2015; 92: 1016–26. doi: 10.1016/j.ijrobp.2015.03.037 [DOI] [PubMed] [Google Scholar]
- 21.Lovelock DM, Zhang Z, Jackson A, Keam J, Bekelman J, Bilsky M, et al. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int J Radiat Oncol Biol Phys 2010; 77: 1282–7. doi: 10.1016/j.ijrobp.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghia AJ, Guha-Thakurta N, Hess K, Yang JN, Settle SH, Sharpe HJ, et al. Phase 1 study of spinal cord constraint relaxation with single session spine stereotactic radiosurgery in the primary management of patients with inoperable, previously unirradiated metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys 2018; 102: 1481–8. doi: 10.1016/j.ijrobp.2018.07.2023 [DOI] [PubMed] [Google Scholar]