Abstract
Despite decades of efforts, non-invasive sensitive detection of small malignant brain tumors still remains challenging. Here we report a dual-modality 124I-labeled gold nanostar (124I-GNS) probe for sensitive brain tumor imaging with positron emission tomography (PET) and subcellular tracking with two-photon photoluminescence (TPL) and electron microscopy (EM). Experiment results showed that the developed nanoprobe has potential to reach sub-millimeter intracranial brain tumor detection using PET scan, which is superior to any currently available non-invasive imaging modality. Microscopic examination using TPL and EM further confirmed that systemically administered GNS nanoparticles permeated the brain tumor leaky vasculature and accumulated inside brain tumor cells following systemic administration. Selective brain tumor targeting by enhanced permeability and retention (EPR) effect and ultrasensitive imaging render 124I-GNS nanoprobe promise for future brain tumor-related preclinical and translational applications.
Keywords: Brain tumor detection, PET/CT, Optical imaging, Gold nanostar probe
Introduction
Despite significant advances in neuro-oncology, early detection of malignant brain tumors remains one of the most formidable challenges in oncology [1–4]. Even with the highest first year cost (> $120,000), the prognosis for patients of glioblastoma (GBM), the most common and aggressive primary brain cancer, is dismal and the median survival is only 15 months after comprehensive treatments including surgery, radiation therapy and chemotherapy [5–11]. Sensitive tumor imaging may result in earlier detection, improved ability to determine extent of disease, and better treatment planning, which could improve patients’ outcome as tumor size has been found to be an important prognostic factor [12–14]. Magnetic resonance imaging (MRI) is an imaging modality effective for soft tissue examination and has been widely used as the clinical diagnostic method for a suspected brain tumor and computed tomography (CT) is usually used only for patients who cannot undergo MRI scan [15–16]. While MRI remains the most commonly applied imaging method for clinical screening, the current detection limit of 3 mm hinders its use for early detection of small brain tumors in patients [17–20]. Positron emission tomography (PET) is a quantitative and highly sensitive imaging method with detection threshold as low as 10−10 to 10−12 M for positron emitters [21–22], Fluorodeoxyglucose (18F) (18F-FDG), which has been utilized to differentiate tumor and normal tissue based on their glucose uptake difference has allowed detection of tumor size less than 1 mm in an animal model with a preclinical PET scanner and 18F-FDG tracer [23–25]. However, traditional 18F-FDG PET is not ideal for brain tumor detection because brain tumors tend to be isometabolic or even hypometabolic compared to normal brain tissue [26–29]. Therefore, there is an urgent unmet need to develop novel methods for sensitive brain tumor imaging aimed to improve early detection, treatment guidance and therapeutic response monitoring.
Nanomedicine, which integrates nanotechnology and medicine, provides promising platforms for novel cancer detection and therapy [30–34]. Nanoparticles with optimized size and surface properties can preferably accumulate in tumors through leaky vasculature, known as the enhanced permeability and retention (EPR) effect [35–37]. Custom-designed nanoprobes permit a wide range of detection scales, from whole body diagnostic scanning to subcellular microscopic imaging. Exploiting their unique surface plasmon resonance property, gold nanoparticles have been applied for optical imaging and photothermotherapy [38–44]. A triple-modality gold nanosphere probe has been reported for brain tumor imaging using MRI, photoacoustic and Raman spectroscopy for both pre-operative detection and intraoperative tumor margin delineation [45]. Despite advances, sub-millimeter brain tumor detection by noninvasive imaging has not been demonstrated to date.
Our group has developed a surfactant-free method to synthesize star-shaped gold nanoparticles, known as gold nanostars (GNS) [46–48]. In contrast to other gold nanoparticles, GNS not only have a tunable plasmonic peak in the near infrared (NIR) ‘tissue optical window’ range but also contain multiple sharp tips generating a “lightening rod” effect that enhances local surface plasmon dramatically. The synthesized GNS with tip-enhanced plasmonics, exhibit extremely high two-photon cross section (4 × 106 Goeppert-Mayer (GM) units), several orders of magnitude higher than organic fluorophores (102–103 GM) and quantum dots (104–105 GM), allowing sensitive tracking of single GNS nanoparticle using high-resolution two-photon photoluminescence (TPL) imaging [49–50]. In previous preclinical studies, GNS have been used as multimodality nanoprobes for cancer imaging and treatment, showing their potential for biomedical applications [51–52]. However, GNS nanoprobe has not been combined with PET imaging to detect brain tumor sensitively.
In this study, we developed a dual-modality 124I-GNS nanoprobe for sensitive brain tumor detection using PET imaging as well as high-resolution nanoparticle tracking using optical and electron microscopy. PET imaging of 124I-GNS achieved detection limit down to 0.5 mm in an orthotopic brain tumor mouse model. After intravenous (IV) injection, GNS nanoparticles were identified to accumulate within brain tumor cells with optical imaging and electron microscopy. No significant toxicity was observed up to 6 months after systemic administration. The developed biocompatible GNS nanoprobe warrants further studies for future brain tumor management.
Methods
GNS nanoprobe development and characterization
Gold nanostars were synthesized using the surfactant-free method developed previously in our laboratory [48], The synthesized GNS were functionalized with PEG (M.W. 6000) to improve in vivo stability. 5 mg of GNS nanoparticles (27 pmol) were radiolabeled by incubation with 1.5 mCi (50 pmol) of 124I (IBA Molecular, Richmond, VA, USA) in 0.5 ml of 20 mM NaOH aqueous solution for 30 min at room temperature. The 124I-labeled GNS were purified by centrifugation at 6000 g for 10 min. Supernate was removed and the pellet was resuspended in PBS solution. The purification process was repeated three times. Transmission electron microscopy (TEM) was performed under 160 kV voltage with a Tecnai G2 Twin (FEI, Hillsboro, OR, USA) microscope and VIS-NIR spectra were obtained using a UV-3600 (Shimadzu, Kyoto, Japan) instrument. The nanoparticle concentration and hydrodynamic size were measured by nanoparticle tracking analysis (NTA) with Nanosight 500 (Malvern, Worcestershire, UK). Gold mass was quantified using inductively coupled plasma-mass spectroscopy (ICP-MS) with a Varian 820 mass spectrometer (Varian, Palo Alto, CA, USA). Thermogravimetric analysis (TGA) was performed with a Q50 instrument (TA Instruments, New Castle, DE, USA) to calculate PEG chain number per nanoparticle. For TGA analysis, GNS coated with PEG (M.W. 6000) were concentrated by centrifugation and the pellets were dried at 60°C overnight for TGA analysis. Samples (~10 mg) were first heated to 110°C and held isothermally for 10 min to remove moisture. Then the sample temperature was increased to 700°C and then held isothermally for 15 min to complete all thermal decomposition. The ramp rate was set to be 10°C per min.
Development of intracranial brain tumor model
Mouse neural stem cells (NSCs) were generated and harvested as described in the previous study [53]. Retroviral particles for transgene delivery were generated using HEK293FT cells. HEK293FT cells were split to ~80% confluency, and cells were transfected 24 hours later with pGag/Pol (12 μg), pVSV/G (5 μg), and expression vector (MIGR1- PDGFB and MIGR1-luciferase separately, 7 μg) using Lipofectamine 2000 in 7 ml of OPTI-MEM. 8 hours later, transfection media was replaced with full growth media. 16 hours after addition of full growth media, full growth media was replaced with NSC proliferation media (containing EGF and Stem Cell Technologies NSC proliferation supplement). 24 hours after incubating in NSC proliferation media, conditioned supernatant was collected, spun at 3000 rpm to remove debris, and used to infect NSCs. 80,000 NSCs were seeded in a 24 well dish in 450 μl NSC growth media, and 50 μl of viral particle-containing supernatant was added to the well for 36 hours. After this incubation, NSCs were resuspended and washed to remove viral particles, and NSCs were then split into fresh NSC growth media.
In preparation for intracranial injection, mouse NSCs were dissociated into a single cell suspension and re-suspended at a concentration of 300,000 cells per 5 μl. NOD scid gamma (NSG) mice were positioned into a Stoelting digital stereotaxic frame with a non-rebreathing nose cone with a flow of isoflurane at 2–3% to maintain sedation throughout the procedure (about 5 min). Their heads were immobilized with mandibular cuffs and after the animals were secured, both lack of consciousness and normal breathing were confirmed. Cholorhexidine was used to clean the incision site three times and then a 0.5-in midline incision was made on the top of the head. Bregma was located and the syringe was then digitally guided 2 mm right of the bregma and 3 mm deep. The injection of 300,000 cells in 5 μl was then performed over several seconds. When the needle was removed, bone wax was applied and the skin was pulled together and sealed with skin glue. Two to three drops of 0.25% bupivacaine were applied along the length of the incision as additional analgesia. The animals were then placed in a clean cage and monitored daily for two weeks following the procedure, and assessed for signs of distress. The tumors took approximately two months to develop. This tumor model is representative of grade 2 and grade 3 astrocytoma as well as secondary glioblastoma [54–55].
Bioluminescent imaging
Following the orthotopic intracranial transplantation procedure, an IVIS Lumina XR in vivo imaging system (Perkin Elmer, Waltham, MA USA) was used to monitor the tumor burden of animals in preparation for further PET/CT imaging. Prior to IVIS imaging, mice were anesthetized with isoflurane in an induction chamber at 4–5% with oxygen flowing at 1–1.5 liters per minute until lack of consciousness was ensured by a toe pinch and injected subcutaneously with 0.1 ml of D-luciferin potassium salt (Gold Bio, St. Louis, MO) at 30 mg/ml (dissolved in PBS). Mice were then placed on a heated stage with an isoflurance anesthesia nose cone system and, after eight minutes, images were acquired and analyzed with the Living Image 4.3.1 software to evaluate the bioluminescence signal of the tumor cells.
PET/CT and optical imaging
PET/CT imaging was performed using a Siemens Inveon small animal PET/CT scanner (Siemens Medical Systems, Knoxville, TN USA). Two mice with brain tumors were IV injected with 124I-GNS (~100 μCi) through the tail vein via a catheter. 10-minute PET scans were started immediately after injection, followed by a CT scan. Follow-up PET/CT scans were obtained 4, 24, 48, and 120 h post injection. In addition, one mouse with a high bioluminescence signal was imaged with both 18F-FDG and 124I-GNS. The mouse was administered with 100 μCi of 18F-FDG through tail vein and then anesthetized using isoflurane inhalation 50 min post injection and remained under anesthesia for the duration of the scan. Following a CT positioning scan, the mouse had a 10-min PET emission scan. One day later, 124I-GNS (~100 μCi) was IV injected through tail vein. PET/CT scans were performed 0, 4, 24 and 48 h after GNS injection. Mouse brain was harvested after PET/CT scan and fixed with formalin for histopathology and optical imaging. TPL imaging was performed with a FV 1000 multiphon microscope (Olympus, Tokyo, Japan) with a Ti: Sapphire fsec laser and 25x/1.05 NA water objective. The laser is tunable in the range of 680–1080 nm and has 80 MHz repetition rate and 140 fsec pulse width. H&E imaging was performed using an Axio Imager widefield microscope (Carl Zeiss, Oberkochen, Germany) coupled with an Axiocam 506 color camera.
Toxicity study and histopathology examination
To evaluate the potential toxicity of the GNS probe, the WST-8 assay was performed using Cell Counting Kit 8 from Dojindo Molecular Laboratories Inc. (Kumamoto, Japan) on NSCs. Those NSCs were plated one day prior to GNS administration. After the addition of varying concentrations of GNS, cells were incubated at 37°C with 5% CO2 in Stem Cell Technologies’ NeuroCult™ Proliferation Kit with 10% Proliferation Supplement and 2% EGF for 3 d. On the third day, 10 μl of CCK-8 solution was added to the cell culture media and incubated for an additional 6 h. The plate was then transferred to the TECAN Infinite Pro Microplate Reader (Switzerland) to determine the absorbance of the treated and untreated cells. The absorbance was measured at 450 nm with a reference wavelength of 650 nm.
For long term in vivo toxicity evaluation, 12 BALB/c mice were randomly divided into three groups with PBS (control), 20 mg/kg or 80 mg/kg GNS dose IV injected through tail vein. Mouse body weight and behavior was monitored weekly until the end of the study. 6 months after GNS IV injection, mice were sacrificed and blood and tissue samples were harvested for examination. Tissue samples for H&E and immunohistochemistry (IHC) examinations were fixed with formalin for 24 h, washed in ethanol for a minimum of 24 h, paraffin embedded, and then sectioned at a thickness of 5 μm. The H&E and IHC staining procedures were performed by Pathology Research Histology and Immunohistochemistry Laboratory at Duke University Medical Center and Molecular Pathology Core at Univeristy of Florida. Blood chemistry analysis was performed by the Veterinary Diagnostic Lab (VDL) at Duke University Medical Center. Samples were processed by VDL and the Heska Dri Chem 7000 Chemistry Analyzer (3760 Rocky Mountain Ave Loveland, Colorado USA) was used for analysis of BUN, creatinine, calcium, protein, albumin, globulin, ALT, AST, ALP and bilirubin.
For brain TEM imaging, a mouse with brain tumor was IV injected GNS (20 mg/kg) through tail vein. One day after injection, the mouse was sacrificed and brain was harvested and then put into 4% formaldehyde + 2% glutaraldehyde fixative. The sample preparation and TEM imaging was performed by Research Electroscopy Facility of Pathology Department at Duke University Medical Center.
Statistical analysis
Statistical analyses on blood chemistry and body weight were performed using MATLAB (version 8.4). Multiple one-way ANOVAs were performed comparing the effect of gold nanoparticle dosage (PBS control, 500 μg, and 2 mg) on blood chemistry parameters - BUN, creatinine, calcium, total protein, albumin, globulin, ALT (GPT), AST (GOT), ALP and bilirubin. Statistical analysis on body weight was performed using a mixed-model ANOVA to assess the effect of GNS dosage on body weight over time. The MATLAB script for the mixed-model ANOVA is publicly available at http://www.mathworks.com/matlabcentral/fileexchange/27080-mixed--between-within-subjects--anova. For all comparisons, the data were considered statistically significant for P < 0.05 (95% confidence interval).
Ethics statement
All animal studies were approved by the Institutional Animal Care and Use Committee of Duke University, an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), International. The application protocol numbers covering these experiments were A109-10-04, A072-13-03 and A037-16-02. Pain and distress was minimized with analgesics and euthanasia was performed for mice that met humane endpoints, as specified in these protocols.
Results
GNS dual-modality nanoprobe development and characterization
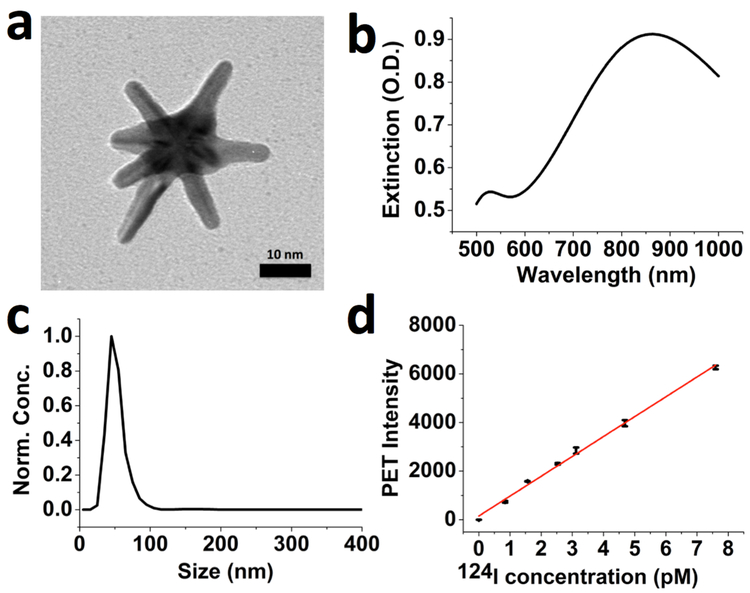
GNS nanoparticles (Figure 1a) were synthesized using a surfactant-free method and then functionalized with thiolated polyethylene glycol (PEG) polymer (M.W. 6000) to improve in vivo stability and circulation half-life. The coated PEG polymer accounted for 8.5 ± 0.1% of total weight and the number of PEG chains per GNS nanoprobe was calculated to be 2,840 ± 32. The PEGylated GNS nanoprobe has surface plasmon resonance (SPR) in the near-infrared region (Figure 1b) and a mean hydrodynamic size of 48 nm (Figure 1c). GNS nanoparticles were labeled with 124I through strong I-Au chemical bonding [56–57] with >98% labeling efficiency after 30 min incubation at room temperature. The stability of radiolabeled GNS was examined in both phosphate buffered saline (PBS) and plasma with anti-clotting heparin. Experimental results showed that 97.2 ± 0.2% (PBS) and 97.7 ± 0.4% (plasma) of 124I remained on the GNS after 7-day incubation at 37 °C. The detection sensitivity for the 124I loaded on GNS nanoprobe using PET was evaluated in PBS; the limit of detection (LOD) was calculated to be 0.5 pM from the regression line (Figure 1d). In addition, the spatial resolution was also evaluated and a phantom as small as 1 mm in diameter could be detected by PET scan (Figure S1).
Figure 1.
Characterization of gold star nanoparticles. (a) Transmission electron microscopy (TEM) image of synthesized GNS nanoparticle with a 10-nm scale bar. (b) VIS-NIR extinction spectrum of 0.2 nM PEGylated GNS aqueous solution. (c) Hydrodynamic size distribution of PEGylated GNS nanoprobes measured by nanoparticle tracking analysis (NTA) method. (d) PET sensitivity evaluation for 124I-GNS. Data points with error bar in black color were experiment results and the red line was linearly fitted from experimental data points. The standard error of the regression was calculated to be 135.68. The fitted equation is y = 817.64x + 151.35 with the adjusted R2 = 0.997. Error bar shows the standard deviation (n=3).
PET-optical imaging for brain tumor detection
For in vivo PET imaging studies, the orthotopic brain tumor animal model was generated by intracranial injection of mouse neural stem cells (NSCs) with isocitrate dehydrogenase-1 (IDH-1), p53 homozougly deleted and human platelet derived growth factor subunit B (PDGFB) overexpressed. The NSCs were also transfected with luciferase to allow for bioluminescent detection. The time from injection to tumor formation averaged approximately 60 days. Tumor development was qualitatively assessed by luciferase bioluminescence imaging prior to PET/CT (Figure S2).
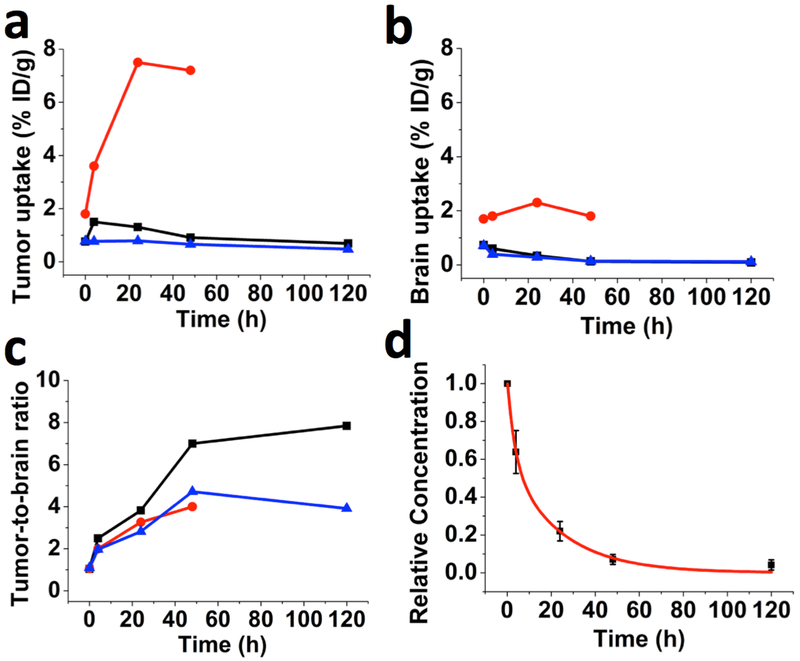
PET/CT scans were acquired at 10 min, 4 h, 24 h, 48 h and 120 h after IV injection of 124I-GNS nanoprobes. Figure. 2 shows the GNS uptake in tumor (Figure 2a), uptake in normal brain (Figure 2b), and tumor-to-normal brain PET signal ratio (T/N) (Figure 2c) measured from PET/CT scans at different time points. The highest T/N was found to be 7.8 at 120 h for Mouse 1 and the largest tumor uptake was 7.5% ID/g at 24 h for Mouse 2. The optimized time point for brain tumor detection with 124I-GNS seems to be 48 h based on consideration of 124I decay half life (4.2 days) and T/N ratio (Figure 2c). The GNS pharmacokinetics was investigated with a two-compartment model using GNS concentration in heart measured from PET/CT scans at 5 time points (Figure 2d). The terminal blood clearance half-life (T1/2) for the PEGylated GNS nanoparticles was calculated to be 16.3 h and the adjusted R2 for the fitted curve was 0.991. Typical PET/CT scans for brain at various time points are shown in Figure 3. At 10 min, there was no discernable uptake difference between tumor and normal brain; however, beginning at 4 h, the tumor uptake was higher than that for normal brain with the contrast ratio between tumor and normal brain increasing over time. The T/N ratio was measured to be 1.0, 2.5, 3.8, 7 and 7.8 at 10 min, 4 h, 24 h, 48 h and 120 h, respectively (Figure 2c). The increased T/N ratio at 48 and 120 h compared to 24 h was consistent with the decreased normal brain background signal due to GNS clearance from the blood.
Figure 2.
GNS uptake in tumor and normal brain tissues measured by PET/CT. The maximum PET signal was measured for brain tumor and its contralateral normal brain tissue. (a) 124I-GNS brain tumor uptake, (b) 124I-GNS normal brain uptake, and (c) tumor-to-normal brain PET signal ratios measured at different time points for 3 mice (Mouse 1, black; Mouse 2, red; Mouse 3, blue) with orthotopic brain tumors. Quantitative results are shown in the Table S2. (d) Nanoprobe relative concentration in blood was measured from dynamic PET/CT scans in the heart at 10 min, 4 h, 24 h, 48 h and 120 h after IV injection and normalized to the concentration value at 10 min. The nanoprobe relative concentrations (black color) are fit to a two-compartment pharmacokinetic model (Adjusted R2 = 0.991) and the fitted curve is in red color. The error bars indicate standard deviations (n=3). Please note that mouse 2 (red) was sacrificed at 48 h time point due to brain tumor symptoms. The high 124I-GNS uptake for mouse 2 (red) might be due to large tumor size (Figure. S3), which results in increased vasculature leakage.
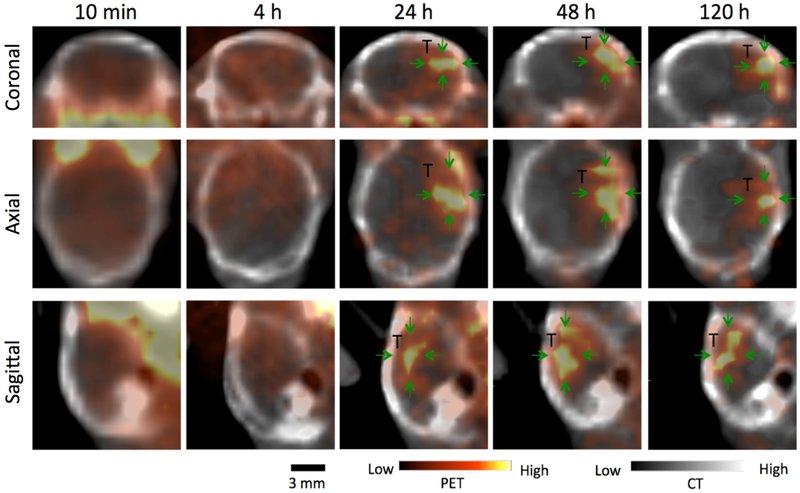
Figure 3.
PET/CT imaging of 124I-GNS nanoprobes”” in the implanted brain tumor for Mouse 1. Top, middle and bottom rows show coronal, axial and sagittal image, respectively. Significantly higher 124I-GNS uptake in tumor (T; green arrows) compared with contralateral normal brain was observed at 24 h. PET intensity was rescaled for best contrast.
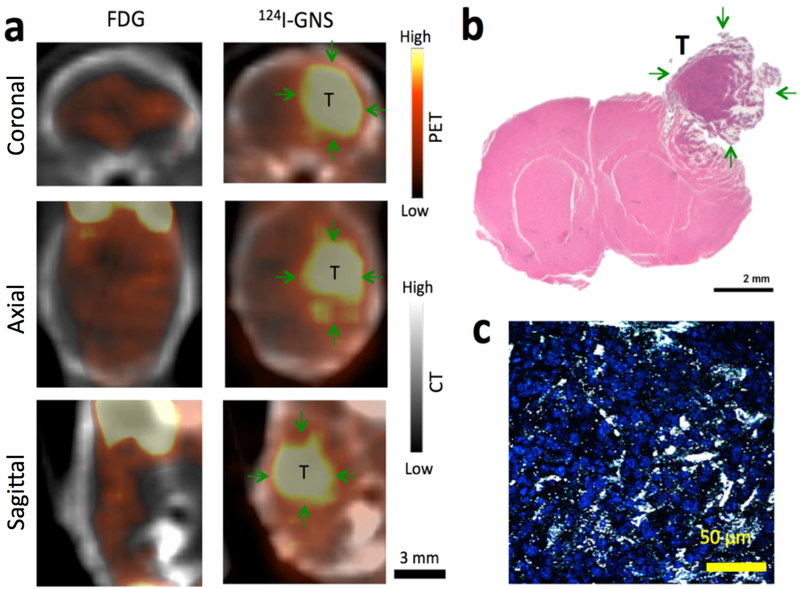
Brain tumor detection with the 124I-GNS nanoprobes was also compared with 18F-FDG in the same mouse as shown in Figure 4. The tumor region, as indicated in Figure 4a, exhibited higher GNS uptake than that in normal brain (T/N ratio = 4.0 at 48 h); on the other hand, the uptake of 18F-FDG in tumor and normal brain was almost the same (T/N ratio = 1.1 at 1 h). This comparison showed 124I-GNS could be superior to traditional 18F-FDG for brain tumor detection with PET/CT. Histopathology examination confirmed the brain tumor region (Figure 4b) in the location consistent with PET/CT scan. And 124I-GNS nanoprobes were found inside brain tumor by TPL microscopy (Figure 4c).
Figure 4.
Brain tumor detection comparison with 18F-FDG and 124I-GNS using PET/CT scan. (a) Comparison of 18F-FDG and 124I-GNS for brain tumor detection by PET imaging in the same brain tumor-bearing mouse (Mouse 2). The PET/CT scan with 18F-FDG was performed 1 h after injection. The PET/CT scan with 124I-GNS was performed 48 h after injection. The average tumor uptake of the 124I-GNS nanoprobe was 7.2 %ID/g and the T/N ratio was 4.0 while the T/N ratio for 18F-FDG was 1.1. (b) H&E histopathology examination of the brain tumor detected by PET scan; green arrows indicate tumor (T). Tumor was peeled off from brain during tissue harvest process and image was reconstructed to combine tumor with brain. (c) TPL imaging shows GNS (white spots) being inside brain tumor detected from PET scan. The brain tumor section was stained with DAPI (blue) to show cell nuclei. The image represents a 5 μm tissue section.
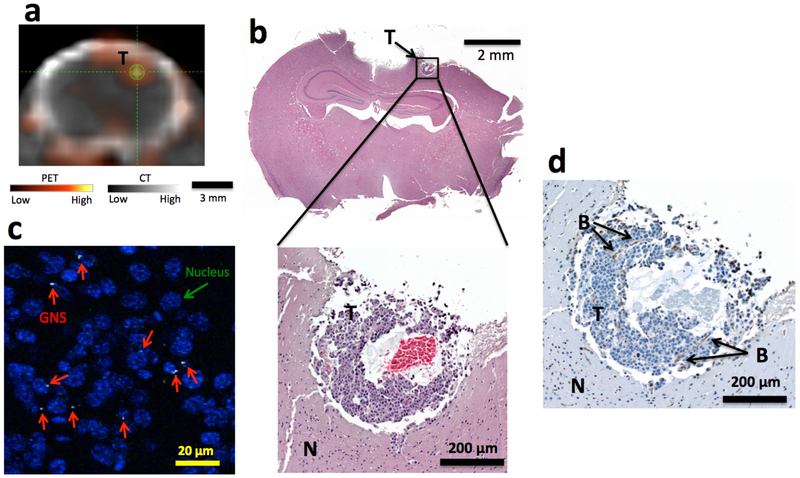
The detection size limit of 124I-GNS for brain tumor with PET/CT scan was also explored. Figure 5a shows a small focal area of increased intensity in the PET/CT scan obtained 48 h after 124I-GNS systemic injection. 124I-GNS accumulated more in the tumor than the surrounding normal brain tissue resulting in a T/N of 4.7. Histopathology showed a tumor size less than 0.5 mm in all three dimensions (Figure 5b). The red blood cells found in the tumor center could be due to hemorrhage of necrosis in the fast growing tumor. Further TPL imaging confirmed the presence of GNS inside this sub-millimeter brain tumor (Figure 5c). Some GNS nanoparticles were found to be close to tumor cell nuclei. CD 31 positive staining showed the presence of endothelial cells and at least two blood vessels inside the 0.5-mm brain tumor were identified (Figure 5d).
Figure 5.
Sub-millimeter brain tumor detection with 124I-GNS. (a) Sub-millimeter brain tumor identified on PET/CT image obtained 48 h post 124I-GNS injection in Mouse 3. The average tumor uptake was 0.66 %ID/g and the T/N was 4.7. (b) H&E histopathology examination confirmed the identified brain tumor region from PET/CT imaging. The identified tumor was less than 0.5 millimeter in size. (c). TPL imaging showed that the GNS (white spots, marked by red arrow) were inside the tumor. The tumor cell nuclei were stained with DAPI (blue). (d) CD31 immunohistochemical staining confirmed the presence of endothelial cells and the developing vasculature. Two blood vessels (B) parallel to the tumor section surface were identified. (T), tumor; (N) normal brain.
TEM imaging
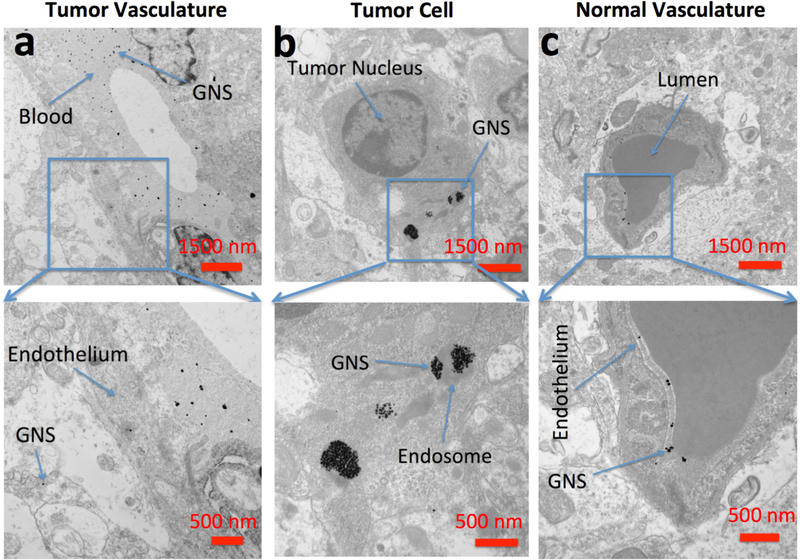
To identify the subcellular location of the GNS at nanometer resolution, TEM imaging was performed on both tumor and non-tumor regions of the brain harvested 48 h after GNS injection. A coronal section was prepared and processed for TEM imaging. Cerebral vessels were compared between the tumor region and contralateral normal brain. The brain tumor vasculature was found disrupted and became permeable to GNS. As shown in Figure 6a, GNS were found in both the tumor interstitial space and blood vessel suggesting GNS penetrate through the vessel formed inside the tumor. Figure 6b shows that the GNS diffused through the extracellular space and were localized in intracellular vesicles within brain tumor cells. In contrast, the vasculature in the normal brain part appeared intact and GNS nanoparticles were blocked by the endothelial wall, remaining inside the blood vessel (Figure 6c). Therefore, 124I-GNS can permeate tumor BBB but not normal BBB. Once permeated, 124I-GNS could enter tumor cells by endocytosis.
Figure 6.
Electron microscopy of brain tumor 24 hours after intravenous administration of GNS (a) TEM imaging of GNS in the extracellular space of the tumor region. (b) TEM imaging of GNS in endosomes within brain tumor cells. (c) TEM imaging of GNS in normal brain vasculature. PEGylated GNS nanoparticles leak through brain tumor vasculature, diffuse into tumor extracellular space and are endocytosed inside tumor cells. In normal brain, GNS were confined inside the vasculature wall, consistent with an intact BBB. Scale bar, 1,500 nm (top row) and 500 nm (bottom row).
Toxicity evaluation
As an initial assessment of the potential toxicity of the GNS nanoprobes, the WST-8 assay was carried out using the Cell Counting Kit 8 on NSCs to quantify the proportion of NSCs that remained viable after exposure to varying concentrations of PEGylated GNS. The cells were plated and incubated for 24 h before GNS were administered. The cells were then incubated for 72 h when WST-8 was administered for a 6-h incubation at which time the plates were analyzed. The in vitro test demonstrated no significant difference in viability at all concentrations tested from 1.2 μg/ml up to 200 μg/ml (Figure. S4).
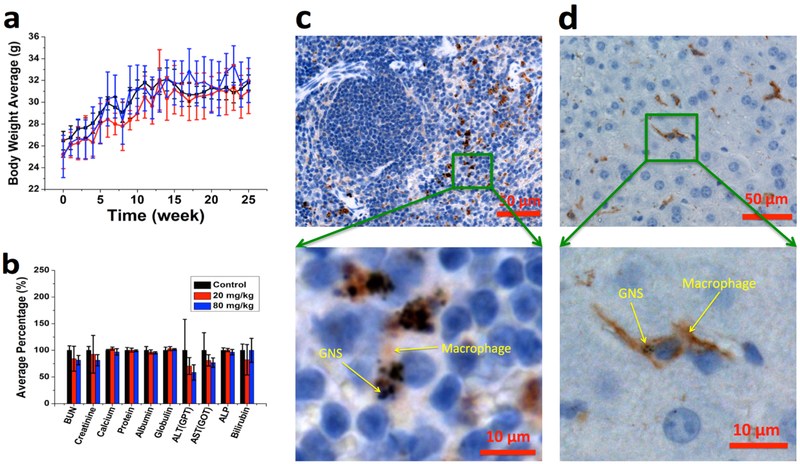
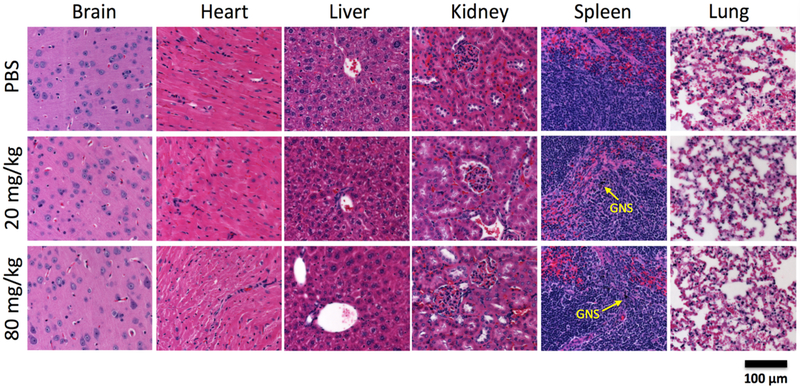
We then performed in vivo studies to investigate the potential toxicity of GNS after IV administration. Most of the GNS nanoparticles were cleared from the blood circulation by macrophages in the spleen and liver when examined one week after IV injection with a dose of 20 mg/kg (Figure S5). For the 6-month long-term toxicity study, mouse body weight was monitored weekly and there was no difference with statistical significance between the control group and groups receiving GNS doses up to 80 mg/kg (Figure 7a). All mice were carefully monitored and did not exhibit stress or any other abnormal behavior. Mice were sacrificed 6 months after GNS injection and plasma was harvested for blood chemistry evaluation that included metabolic function of kidney and liver as shown in Figure 7b. One-way ANOVA statistical analyses demonstrated no difference between control and treated groups receiving 20 mg/kg or 80 mg/kg GNS (Figure 7b). We also performed histopathological examination of hematoxylin and eosin (H&E) stained brain, heart, liver, kidney, spleen and lung in mice 6 months after GNS injection (Figure 8). No findings indicative of GNS-related toxicity were identified in mice receiving a GNS dose up to 80 mg/kg as examined by a veterinary pathologist. H&E stained slides were unremarkable at doses tested. Further immunohistochemistry (IHC) was performed to better understand cellular changes due to GNS administration with analysis focusing on the liver and spleen. IHC staining was performed for F4/80 to evaluate the co-occurrence of GNS with macrophage cells. The staining did reveal that GNS were trapped in the reticularendothelial system (RES), mainly the spleen and liver (Figures 7c and 7d), 6 months after IV injection. F4/80 staining of the spleen sample showed that GNS co-occurred with macrophages and mostly resided in the red pulp (Figure 7c) The IHC staining also confirmed the presence of GNS in kuppfer cells in the liver (Figure 7d). Overall, test groups were comparable to the control group and no deleterious effects were noted 6 months after GNS systemic administration up to 80 mg/kg dose. The full pathology report is shown in the Table S1.
Figure 7.
Toxicity study of mice after GNS IV administration. (a) Average bodyweight of animals in each cohort of 6-month toxicity study was monitored weekly; no significant difference was found between control (black) and GNS IV injection groups (20 mg/kg (red) or 80 mg/kg dose (blue)). (b) Blood chemistry test results for mice 6 months after IV injection of PBS (black), GNS with a dose of 20 mg/kg (red) and 80 mg/kg (blue). Error bar represents standard deviation (n = 4). F4/80 immunohistochemistry (IHC) staining (brown) was performed for spleen (c) and liver (d) to show macrophages (Kupffer cells). The GNS (black spots) nanoparticles were found overlaid with macrophage cells (brown).
Figure 8.
Histopathology examination of peripheral organs. Histopathology following IV administration of GNS H&E histopathology examination of brain, heart, liver, kidney, spleen and lung from mice obtained 6 months after PBS or GNS injection (20 mg/kg or 80 mg/kg dose). Scale bar, 100 μm. The GNS (black color) nanoparticles were seen inside spleen for mice in both 20 mg/kg and 80 mg/kg dose groups. H&E evaluation was unremarkable and demonstrated healthy and intact tissue.
Discussion
In this study, we have shown potential of detecting an intracranial brain tumor as small as 0.5 mm in diameter measured from histopathology examination with our developed 124I-GNS nanoprobe. Although the current clinical PET scanner has a spatial resolution of 4 mm, tumors with size less than 1 mm could still be detectable given the low background signal and high T/N (>2.5) [19]. Therefore, a combination of 124I-GNS and PET/CT imaging could push the brain tumor detection limit beyond the current approaches using PET/CT with 18F-FDG or MRI [13, 24]. Additionally, MRI is challenged to distinguish between recurrence and progression from inflammation and radiation necrosis. GNS based PET/CT imaging could provide an alternative and supplementary non-invasive technique to help distinguish those scenarios. High brain tumor detection sensitivity with 124I-GNS may also improve the ability to more accurately determine extent of disease and therefore affect treatment planning that includes both surgical resection and radiation therapy. Furthermore, sensitive brain tumor imaging is also important for early detection of recurrent malignant gliomas [58]. Various nanoparticle-based contrast agents for brain tumor imaging have been reported in previous studies [59–61]. For example, PET/NIRF/MRI triple functional iron oxide nanoparticles were synthesized by labeling human serum albumin (HSA) coated iron oxide nanoparticles (IONPs) with both 64Cu-DOTA and Cy5.5 [59]. The synthesized nanoparticles were found to accumulate preferably in the tumor using a subcutaneous U87MG xenograft mouse model and the tumor-to-muscle ratio was reported to be up to 8.28 by PET imaging. The developed GNS nanoprobe described here has a tumor-to-normal brain ratio up to 7.8 in the orthotopic mouse brain tumor model. These differences in tumor-to-normal ratio could be attributed to different tumor vasculature as well as microenvironment between the subcutaneous brain tumor model and the orthotopic brain tumor model. Therefore, further experiments using intracranial brain tumor models should be performed to demonstrate that the developed nanoprobe could reach high tumor-to-normal ratio for brain cancer imaging. In addition, a dual-modality manganese-doped carbon dot has been synthesized and applied for in vivo brain tumor detection with MRI and ex vivo imaging with fluorescence [60]. Experimental results show that the nano contrast agent-enhanced MRI can detect brain tumors less than 2 mm. Furthermore, a synergetic NIR-II fluorescence and NIR-I photoacoustic imaging contrast agent has been developed for orthotopic brain tumor detection with a capability of detecting mouse brain tumors that are approximately 3 mm in size [61]. The developed GNS nanoprobe in our study showed the potential to reach sub-millimeter brain tumor detection with PET imaging, which is better than previously published results. To the best of our knowledge, our approach is the most sensitive brain tumor noninvasive detection method reported to date.
High specificity of 124I-GNS for brain tumor sensitive detection originates from the EPR effect, which has been considered as one of the most important elements for cancer targeting [35]. Normal brain vasculature has a tight BBB with pore size less than 1 nm, preventing passive penetration of large molecules and nanoparticles. Therefore, a minimal amount of 124I-GNS remains in the normal brain after injected 124I-GNS nanoprobes were cleared from the blood circulation by macrophage cells, resulting in a low background signal in normal brain. On the contrary, malignant brain tumor pathogenesis would increase vasculature permeability [62]. Tumors need to develop blood vessels when they are just a few cells thick for nutrients supply and metabolic wastes clearance. The abnormally formed vasculature would be leaky and have different characteristics from normal blood vessels, including defective endothelial cells with wide fenestrations. Therefore, after IV administration, 124I-GNS nanoprobes can penetrate through leaky brain tumor vasculature, get endocytosized inside tumor cells and stay there, as demonstrated by our electron microscopy results (Figure 6). 124I has a much longer half-life (4.2 days) than alternative positron emitters such as 64Cu (12.7 h) or 18F (1.8 h) and can be used for in vivo tracking for more than one week. The high 124I-GNS T/N ratio (up to 7.8) from specific tumor accumulation by EPR effect is important for sensitive and specific brain tumor detection.
In addition to sensitive PET imaging, the dual-modality 124I-GNS nanoprobes also enable high-resolution tracking to investigate how nanoparticles selectively accumulate in brain tumors with optical imaging and electron microscopy without the need of labeling. GNS have tip-enhanced plasmonics, which provides superior TPL intensity for single particle tracking. The developed GNS nanoprobes also have high mass density and electron absorbing capability, enabling TEM imaging at a nanometer resolution. With both optical and TEM imaging, we have investigated the nanoprobe intra-tumoral distribution at high spatial resolution. Our results demonstrated that 124I-GNS nanoprobes not only crossed the disrupted BBB in brain tumor tissue and penetrated through the tumor interstitial space, but also accumulated in intracellular vesicles. The confirmation of GNS inside brain tumor cells after IV administration illustrates their potential as a therapeutic agent delivery platform for brain tumor treatment. For example, 131I is a therapeutic beta-emitting radionuclide that could be loaded on GNS with the same straightforward reaction chemistry for targeted radiotherapy. Multifunctional GNS nanoprobes with both imaging and therapeutic capabilities have promise to be used for image-guided therapy to improve brain tumor management.
A variety of radioiodination methods have been extensively investigated mainly based on nucleophilic substitution and electrophilic substitution reactions. These methods can allow stable covalent bonds between the radioactive iodine and the pharmacophore while causing minimal alteration to its biological activities. However, sophisticated radiochemistry procedures are often required including addition of oxidant, extraction and purification by chromatography which result in long preparation time and low yield. Moreover, increased radiolytic degradation was observed especially when labeling with high amount of 124I, which leads to reduced purity of the final product [63]. The iodine labeling method developed in this study utilized the strong I-Au chemical bond which enables a simple radiolabeling process with less than 1.5 h total preparation time and >98% yield. The purification can be easily accomplished by centrifugation instead of chromatography. The straightforward radiolabeling process of GNS with 124I can be easily translated to future clinical applications.
Both in vitro and in vivo toxicity studies have demonstrated the biocompatibility of GNS synthesized using our surfactant-free method. Previous studies have reported that spherical gold nanoparticles are not toxic and gold nanoshells are currently being investigated as part of a clinical trial for prostate cancer treatment () [64]. However, only a few studies have evaluated the toxicity of gold nanoparticles with other nonspherical shapes. We performed both in vitro and in vivo toxicity studies after intravenous GNS administration in mice. No signs of toxicity were observed at all doses tested with nanoparticles coated with PEG. A previous in vitro investigation reported that GNS conjugated with cell penetrating peptide TAT (trans-activating transcriptional activator from human immunodeficiency virus 1) reported no significant alteration in cell viability [52]. Likewise, we confirmed that the cell viability after in vitro administration of PEG-GNS at concentrations up to 0.2 mg/ml was also not significantly altered. Our in vivo experiments confirmed previously reported results that inorganic nanoparticles sequester in the reticuloendothelial (RES) system [65]. GNS showed significant co-occurrence with macrophage staining in the spleen and liver. But there were no histological alterations to the tissues indicating minimal effect on tissue architecture. Despite GNS accumulation in the liver, ALT, AST, ALP enzyme concentrations in blood were not significantly altered indicating that liver function remained intact. Likewise, total protein, albumin, globulin, and total bilirubin were not changed compared to control group implying normal kidney and liver function. Both creatinine and BUN, markers of glomerular filtration rate and kidney function, were also not altered and remained comparable to the control group. Therefore, our toxicity study results indicate that the GNS are biocompatible and could be further investigated for future clinical translation.
In summary, we have developed a novel gold nanoprobe (124I-GNS) for sensitive brain tumor detection and high-resolution tracking. In a murine orthotopic brain tumor model, optical and TEM imaging showed that systemically administered 124I-GNS extravasated into the brain tumor, diffused within tumor interstitium, and got endocytosed into tumor cells. 124I-GNS exhibit both high T/N ratio and sensitivity, showing potential to detect brain tumor down to 0.5 mm, which is superior to any currently available non-invasive imaging modality. Toxicity study with mice showed no major physiological impact 6 months after GNS nanoparticles systemic administration. The biocompatible GNS nanoprobe with sensitive brain tumor detection capability has promise to be used in future translational applications for brain tumor management.
Supplementary Material
Figure S1 1-mm agar gel sample with 124I-GNS (blue arrow) was prepared and then the tube was filled with 1 ml of agar gel without GNS. (a) CT scan (b) PET scan (c) an overlap of PET and CT scans. The results demonstrated PET scan could detect a sample with 1 mm in diameter. The PET/CT scan protocol was the same as that used for animal study.
Figure S2 Bioluminescence imaging was performed to monitor brain tumor growth using the IVIS Lumina XR In vivo imaging system. Red (high signal), blue (low signal). Mice were anesthetized, administered 100 μl of D-Luciferin at 30 mg/ml, and then imaged 8 min after administration.
Figure S3 The photo was taken for mouse 2 brain harvested after 48 h PET/CT scan. The tumor part has GNS accumulation and show black color. The observed tumor location is consistent with PET/CT scan as shown in Figure 4.
Figure S4 The WST-8 assay test results demonstrated the biocompatibility of GNS. Mouse neural stem cells (NSCs) were incubated with GNS at different concentrations for 3 days. Cell viability was not significantly altered due to application of GNS at doses tested. Cells exposed to GNS remained proliferative and did not show signs of cytotoxicity.
Figure S5 GNS biodistribution in normal BALB/c mice one week after IV injection (20 mg/kg dose) via tail vein. Tissues were harvested and digested with aqua regia for ICP-MS to quantify gold mass. The GNS uptake is calculated as % ID/g tissue. Spleen and liver had the highest GNS uptake. Error bars indicate standard deviation (n = 5).
Table S1 Histopathological findings reported by a veterinary pathologist from the six-month toxicity study. The mice were IV injected with 0.5 mg GNS, 2 mg GNS or PBS solution as control. Six months after IV administration, mice were sacrificed and organs were harvested for histopathology examination. The scale used for histopathological analysis is: minimal, mild, moderate, marked, severe.
Table S2 GNS uptake (%ID/g) in tumor and normal brain tissues measured by PET/CT and calculated tumor-to-normal brain (T/N) PET signal ratios at five different time points (10 min, 4 h, 24 h, 48 h, 120 h).
Acknowledgements
This work was supported by the Duke Faculty Exploratory Funds. We’d like to thank Dr. Daniel Schenkman for his help on histopathology examination. In addition, we also want to thank Dr. Neil Medvitz at the Electron Microscopy Facility in the Pathology Department of Duke University.
Footnotes
The authors declare no competing financial interests.
References
- [1].Aiken R 2014. Semin Oncol 41 438–445 [DOI] [PubMed] [Google Scholar]
- [2].Bidros DS, Vogelbaum MA 2009. Neurotherapeutics 6 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. 2018. CA Cancer J. Clin 68 7–30 [DOI] [PubMed] [Google Scholar]
- [4].Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS 2015. Neuro. Oncol 17 1–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheng Y, Dai Q, Morshed RA, Fan XB, Wegscheid ML, Wainwright DA, Han Y, Zhang LJ, Auffinger B, Tobias AL, Rincon E, Thaci B, Ahmed AU, Warnke PC, He C, Lesniak MS 2014. Small. 10 5137–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, Van Den Weyngaert D, Kaendler S, Krauseneck P, Vinolas N, Villa S, Wurm RE, Maillot MHB, Spagnolli F, Kantor G, Malhaire JP, Renard L, De Witte O, Scandolaro L, Vecht CJ, Maingon P, Lutterbach J, Kobierska A, Bolla M, Souchon R, Mitine C, Tzuk-Shina T, Kuten A Haferkamp G, de Greve J, Priou F, Menten J, Rutten I, Clavere P, Malmstrom A, Jancar B, Newlands E, Pigott K, Twijnstra A, Chinot O, Reni M, Boiardi A, Fabbro M, Campone M, Bozzino J, Frenay M, Gijtenbeek J, Brandes AA, Delattre JY, Bogdahn U, De Paula U, van den Bent MJ, Hanzen C, Pavanato G, Schraub S, Pfeffer R, Soffietti R, Weller M, Kortmann RD, Taphoorn M, Torrecilla JL, Marosi C, Grisold W, Huget P, Forsyth P, Fulton D, Kirby S, Wong R, Fenton D, Fisher B, Cairncross G, Whitlock P, Belanger K, Burdette-Radoux S, Gertler S, Saunders S, Laing K, Siddiqui J, Martin LA, Gulavita S, Perry J, Mason W, Thiessen B, Pai H, Alam ZY, Eisenstat D, Mingrone W, Hofer S, Pesce G, Curschmann J, Dietrich PY, Stupp R, Mirimanoff RO, Thum P, Baumert B, Ryan G, European Org Res Treatment Canc, National Cancer Institute of Canada Clinical Trials Group. 2005. N. Engl. J. Med 352 987–996 [DOI] [PubMed] [Google Scholar]
- [7].Rutka JT, Kim B, Etame A, Diaz RJ 2014. ACS Nano 8 9716–9722 [DOI] [PubMed] [Google Scholar]
- [8].Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G 2007. Brain 130 2596–2606 [DOI] [PubMed] [Google Scholar]
- [9].Clin Davis ME. 2016. J. Oncol. Nurs 20, 2–8 [Google Scholar]
- [10].Shen C, Wang X, Zheng Z, Gao C, Chen X, Zhao S, Dai Z 2019. Int. J. Nanomed 14 101–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mariotto AB, Yabroff KB, Shao Y, Feuer EJ, Brown ML. 2011. J. Natl. Cancer Inst 103 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mohammadi AM, Schroeder JL, Angelov L, Chao ST, Murphy ES, Yu JS, Neyman G, Jia X, Suh JH, Barnett GH, Vogelbaum MA 2017. J Neurosurg 126 735–743 [DOI] [PubMed] [Google Scholar]
- [13].Dempsey MF, Condon BR, Hadley DM 2005. AJNR Am J Neuroradiol 26 770–776 [PMC free article] [PubMed] [Google Scholar]
- [14].Newton HB, Ray-Chaudhury A, Cavaliere R 2006. Top. Magn. Reson. Imag 17 127–136 [DOI] [PubMed] [Google Scholar]
- [15].Omuro A; DeAngelis LM 2013. JAMA-J Am. Med. Assoc 310 1842–1850 [DOI] [PubMed] [Google Scholar]
- [16].Huang QL, Cao X, Chai X, Wang X, Xiao C, Wang J. 2019. World Neurosurg doi: 10.1016/j.wneu.2018.12.12 [DOI] [PubMed] [Google Scholar]
- [17].Sze G, Milano E, Johnson C, Heier L 1990. AJNR Am J Neuroradiol 11 785–791 [PMC free article] [PubMed] [Google Scholar]
- [18].Schellinger PD, Meinck HM, Thron A 1999. J Neuro-Oncol 44 275–281 [DOI] [PubMed] [Google Scholar]
- [19].Erdi YE 2012. Mol. Imaging and Radionucl. Ther 21 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yuh WT, Tali ET, Nguyen HD, Simonson TM, Mayr NA, Fisher DJ 1995. Am. J. Neuroradio 16 373–380 [PMC free article] [PubMed] [Google Scholar]
- [21].Gambhir SS, Herschman HR, Cherry SR, Barrio JR, Satyamurthy N, Toyokuni T, Phelps ME, Larson SM, Balatoni J, Finn R, Sadelain M, Tjuvajev J, Blasberg R 2000. Neoplasia 2 118–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gambhir SS. Nat. Rev. Cancer. 2002;2:683. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- [23].Almuhaideb A, Papathanasiou N, Bomanji J 2011. Ann. Saudi. Med 31 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Puaux AL, Ong LC, Jin Y, Teh I, Hong M, Chow PKH, Golay X, Abastado JP 2011. Int. J. Mol. Imaging 2011 321538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim SJ, Pak K, Kim K. 2019. Abdominal Radiology doi: 10.1007/s00261-018-01891-3 [DOI] [PubMed] [Google Scholar]
- [26].Karunanithi S, Sharma P, Kumar A, Khangembam BC, Bandopadhyaya GP, Kumar R, Gupta DK, Malhotra A, Bal C 2013. Eur. J. Nucl. Med. Mol. Imaging 40 1025–1035 [DOI] [PubMed] [Google Scholar]
- [27].Delbeke D, Meyerowitz C, Lapidus RL, Maciunas RJ, Jennings MT, Moots PL, Kessler RM 1995. Radiology 195 47–52 [DOI] [PubMed] [Google Scholar]
- [28].Jost SC, Wanebo JE, Song SK, Chicoine MR, Rich KM, Woolsey TA, Lewis JS, Mach RH, Xu J, Garbow JR 2007. Neurosurgery. 60:360–370, discussion 370–361 [DOI] [PubMed] [Google Scholar]
- [29].Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, Carlin SD, La Rocca G, Lyashchenko S, Ploessl K, Rohle D, Omuro AM, Cross JR, Brennan CW, Weber WA, Holland EC, Mellinghoff IK, Kung HF, Lewis JS, Thompson CB 2015. Sci. Transl. Med 7 274ra217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Frosina G 2016. Nanomed. Nanotechnol. Biol. Med 12 1083–1093 [Google Scholar]
- [31].Cheng Y, Morshed RA, Auffinger B, Tobias AL, Lesniak MS 2014. Adv. Drug. Deliv. Rev 66 42–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meyers JD, Doane T, Burda C, Basilion JP 2013. Nanomedicine. 8 123–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye YP, Humm J, Gonen M, Kalaigian H, Schoder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS 2014. Sci. Transl. Med 6 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Youn YS, Bae YH. 2018. Adv. Drug Deliv. Rev 130 3–11 [DOI] [PubMed] [Google Scholar]
- [35].Maeda H, Tsukigawa K, Fang JA 2016. Microcirculation 23 173–182 [DOI] [PubMed] [Google Scholar]
- [36].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW 2016. Nat. Rev. Mater 1 12 [Google Scholar]
- [37].Man F, Lammers T, de Rosales RTM 2018. Mol. Imaging Biol 20 683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hainfeld JF, Smilowitz HM, O’Connor MJ, Dilmanian FA, Slatkin DN 2013. Nanomedicine 8 1601–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sun XL, Huang XL, Yan XF, Wang Y, Guo JX, Jacobson O, Liu DB, Szajek LP, Zhu WL, Niu G, Kiesewetter DO, Sun XY, Chen X 2014. ACS Nano 8 8438–8446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bayazitoglu Y, Kheradmand S, Tullius TK 2013. Int J Heat Mass Transf 67 469–486 [Google Scholar]
- [41].Harmsen S, Huang R, Wall MA, Karabeber H, Samii JM, Spaliviero M, White JR, Monette S, O’Connor R, Pitter KL, Sastra SA, Saborowski M, Holland EC, Singer S, Olive KP, Lowe SW, Blasberg RG, Kircher MF 2015. Sci. Transl. Med 7 271ra7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu W, Li X, Li W, Zhang Q Bai H, Li J, Xi G. 2018. Biomaterials 163 43–54 [DOI] [PubMed] [Google Scholar]
- [43].Singh P, Pandit S, Mokkapati VRSS, Garg A, Ravikumar V, Mijakovic I. 2018. Int. J. Mol. Sci 19 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Elahi N, Kamali M, Baghersad MH. 2018. Talanta 184 537–556 [DOI] [PubMed] [Google Scholar]
- [45].Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E, Pitter K, Huang RM, Campos C, Habte F, Sinclair R, Brennan CW, Mellinghoff IK, Holland EC, Gambhir SS 2012. Nat Med 18 829–U235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu Y, Ashton JR, Moding EJ, Yuan HK, Register JK, Fales AM, Choi J, Whitley MJ, Zhao XG, Qi Y, Ma Y, Vaidyanathan G, Zalutsky MR, Kirsch DG, Badea CT, Vo-Dinh T 2015. Theranostics 5 946–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu Y, Chang Z, Yuan H, Fales AM, Vo-Dinh T 2013. Nanoscale 5 12126–12131 [DOI] [PubMed] [Google Scholar]
- [48].Yuan H, Khoury CG, Hwang H, Wilson CM, Grant GA, Vo-Dinh T 2012. Nanotechnology 23 075102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gao N, Chen Y, Li L, Guan Z, Zhao T, Zhou N, Yuan P, Yao SQ, Xu QH. 2014. J. Phys. Chem 118 13904–13911 [Google Scholar]
- [50].Yuan H, Register JK, Wang HN, Fales AM, Liu Y, Vo-Dinh T 2013. Anal. and Bioanal. Chem 405 6165–6180 [DOI] [PubMed] [Google Scholar]
- [51].Yuan H, Khoury CG, Wilson CM, Grant GA, Bennett AJ, Vo-Dinh T 2012. Nanomed.-Nanotechnol 8 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yuan H, Fales AM, Vo-Dinh T 2012. J. Am. Chem. Soc 134 11358–11361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pirozzi CJ, Carpenter AB, Waitkus MS, Wang CY, Zhu H, Hansen LJ, Chen LH, Greer PK, Feng J, Wang Y, Bock CB, Fan P, Spasojevic I, McLendon RE, Bigner DD, He Y, Yan H 2017. Mol. Cancer. Res 15 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Killela PJ, Pirozzi CJ, Healy P, Reitman ZJ, Lipp E, Rasheed BA, Yang R, Diplas B, Wang Z, Greer PK, Zhu H, Wang CY, Carpenter AB, Friedman H, Friedman AH, Keir ST, He J, He Y, McLendon RE, Herndon JE, Yan H, Bigner DD 2014. Oncotarget 5 1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Killela PJ, Pirozzi CJ, Reitman ZJ, Jones S, Rasheed BA, Lipp E, Friedman H, Friedman AH, He Y, McLendon RE, Bigner DD, Yan H 2014. Oncotarget 5 1452–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schulz A, Hargittai M 2001. Chem.-Eur. J 7 3657–3670 [DOI] [PubMed] [Google Scholar]
- [57].Shao X, Agarwal A, Rajian JR, Kotov NA, Wang X 2011. Nanotechnology 22 135102–135102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Karunanithi S, Sharma P, Kumar A,Khangembam BC, Bandopadhyaya GP, Kumar R, Gupta DK, Malhotra A, Bal C 2013. Eur. J. Nucl. Med. Mol. Imaging 40 1025–1035 [DOI] [PubMed] [Google Scholar]
- [59].Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, Li X, Chen X. 2010. Biomaterials 31 3016–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ji Z, Ai P, Shao C, Wang T, Yan C, Ye L, Gu W 2018. ACS Biomater. Sci. Eng 4 2089–2094 [DOI] [PubMed] [Google Scholar]
- [61].Sheng Z, Guo B, Hu D, Xu S, Wu W, L WH, Yao K, Jiang J, Liu C, Zheng H, Liu B 2018. Adv. Mater 30 1800766. [DOI] [PubMed] [Google Scholar]
- [62].Jain KK 2012. Nanomedicine 7 1225–1233 [DOI] [PubMed] [Google Scholar]
- [63].Guenther I, Wyer L, Knust EJ, Finn RD, Koziorowski J, Weinreich R 1998. Nucl. Med. Biol 25 359–365 [DOI] [PubMed] [Google Scholar]
- [64].Gad SC, Sharp KL, Montgomery C, Payne JD, Goodrich GP 2012. Int. J. Toxicol 31 584–594 [DOI] [PubMed] [Google Scholar]
- [65].Yu M; Zheng J 2015. ACS Nano 9 6655–6674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 1-mm agar gel sample with 124I-GNS (blue arrow) was prepared and then the tube was filled with 1 ml of agar gel without GNS. (a) CT scan (b) PET scan (c) an overlap of PET and CT scans. The results demonstrated PET scan could detect a sample with 1 mm in diameter. The PET/CT scan protocol was the same as that used for animal study.
Figure S2 Bioluminescence imaging was performed to monitor brain tumor growth using the IVIS Lumina XR In vivo imaging system. Red (high signal), blue (low signal). Mice were anesthetized, administered 100 μl of D-Luciferin at 30 mg/ml, and then imaged 8 min after administration.
Figure S3 The photo was taken for mouse 2 brain harvested after 48 h PET/CT scan. The tumor part has GNS accumulation and show black color. The observed tumor location is consistent with PET/CT scan as shown in Figure 4.
Figure S4 The WST-8 assay test results demonstrated the biocompatibility of GNS. Mouse neural stem cells (NSCs) were incubated with GNS at different concentrations for 3 days. Cell viability was not significantly altered due to application of GNS at doses tested. Cells exposed to GNS remained proliferative and did not show signs of cytotoxicity.
Figure S5 GNS biodistribution in normal BALB/c mice one week after IV injection (20 mg/kg dose) via tail vein. Tissues were harvested and digested with aqua regia for ICP-MS to quantify gold mass. The GNS uptake is calculated as % ID/g tissue. Spleen and liver had the highest GNS uptake. Error bars indicate standard deviation (n = 5).
Table S1 Histopathological findings reported by a veterinary pathologist from the six-month toxicity study. The mice were IV injected with 0.5 mg GNS, 2 mg GNS or PBS solution as control. Six months after IV administration, mice were sacrificed and organs were harvested for histopathology examination. The scale used for histopathological analysis is: minimal, mild, moderate, marked, severe.
Table S2 GNS uptake (%ID/g) in tumor and normal brain tissues measured by PET/CT and calculated tumor-to-normal brain (T/N) PET signal ratios at five different time points (10 min, 4 h, 24 h, 48 h, 120 h).