Abstract
Background
Vitamin D is an important steroid that can regulate bone metabolism including osteoclast (OC) differentiation. Transient receptor potential cation channel subfamily V member 5 (TRPV5), is a calcium channel protein involved in OC differentiation. However, the impact of vitamin D on TRPV5 expression during OC differentiation is not clear.
Objectives
To determine if 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates the expression of TRPV5 during OC differentiation.
Methods
Bone marrow mononuclear macrophage (BMMs) were induced to differentiate into OC with or without treatment with 10 nM 1,25(OH)2D3. The expression levels of vitamin D receptor (VDR) and TRPV5 were examined. The expression of several OC markers, including tartrate resistant acid phosphatase (TRAP), carbonic anhydrase II (Ca II), cathepsin K (CTSK), and vacuolar-type H+-ATPase (V-ATPase) were also detected.
Results
We found that the VDR was expressed in murine bone marrow-derived macrophages at the early stage of OC differentiation. TRPV5 expression was increased during OC differentiation, which was down-regulated by 1,25(OH)2D3 after a prolonged exposure. The 1,25(OH)2D3 and TRPV5 inhibitors inhibited OC differentiation.
Conclusions
1,25(OH)2D3 can inhibit TRPV5 expression as well as TRPV5 inhibitors during OC differentiation. This suggests that 1,25(OH)2D3 may suppress OC differentiation by inhibiting TRPV5 expression.
Keywords: Osteoclast; 1,25(OH)2D3; TRPV5; RANKL; Ca2+
1. Background
Osteoclasts (OC) are multinucleated cells that resorb bone in vivo (1). Its differentiation is induced by two important factors, e.g. macrophage colony-stimulating factor (M-CSF) and the receptor activator of nuclear factor-κB ligand (RANKL) (2). Both factors can induce OC differentiation in vivo and in vitro (3-5). The OC precursors (OCP) are first differentiated from bone marrow mononuclear macrophages (BMMs) by M-CSF. These cells fuse to form tartrate-resistant acid phosphatase (TRAP) positive multinucleated cells in the presence of RANKL (6). Mice deficient of RANKL or its receptor RANK develop severe bone sclerosis, further indicating that the RANKL-RANK pathway is essential for OC differentiation (7, 8).
Vitamin D can be produced by 7-dehydrocholesterol of the skin under ultraviolet light exposure and also be consumed directly from the food. Vitamin D is processed by the kidneys after liver processing to obtain the main active form 1, 25-(OH)2D3. The active form of 1, 25-(OH)2D3 binds to the serum vitamin D binding protein (DBP) and is then transported to the target tissue/organ through the blood circulation. The important biological functions of 1, 25-(OH)2D3 is acting through a soluble receptor protein called vitamin D receptor (VDR). Active 1,25(OH)2D3 binds to its vitamin D receptor (VDR) and regulates the physiological activity of osteoblasts (OB) by enhancing RANKL secretion, which results in enhancing the differentiation of OC (9-11). On the other hand, 1,25(OH)2D3 can also inhibit c-Fms and RANK expression in OCP and therefore inhibit OC differentiation (12). The expression of nuclear factor of activated T cells cytoplasmic 1 (NFATc1) could also be inhibited in the OC differentiation process by 1,25(OH)2D3 treatment. Thus, it is imperative to study the mechanism of regulation 1,25(OH)2D3 on OC differentiation.
The Ca2+ ion is an intracellular secondary messenger and has important physiological functions on a wide variety of cells types. It can be uptaken into the cytoplasm through a calcium channel expressed on plasma membrane. On the contrary, Ca2+ moves across the basolateral membrane and then into blood circulation via Na+/Ca2+ exchanger and plasma membrane calcium pumps (PMCA) such as PMCA1b (13-15). Transient receptor potential (TRP) cation channel subfamily vanilloid member 5 (TRPV5) constitutes a calcium influx pathway in a wide range of epithelial cell types (16). As one of the most selective calcium ion TRP channels, its mRNA is abundantly expressed in bone and OC of humans and rodents (17). Moreover, Ca2+ oscillation can be observed in RANKL-treated BMMs during OC differentiation (18). It has been thought that TRPV5 is involved in OC differentiation. However, whether 1,25(OH)2D3 regulates OC differentiation by affecting TRPV5 expression has not been studied.
2. Objectives
Here, the study was to confirm the effects of vitamin D on OC differentiation from mouse BMMs, and whether vitamin D could regulate TRPV5 expression during OC differentiation.
3. Methods
3.1. Animals
For this study, BALB/c mice (5 - 6-week-old, male, weight 20 to 25 g) of clean grade were purchased from Comparative Medical Center of Yangzhou University, were sacrificed by euthanasia.
3.2. Osteoclastogenesis
Bone marrow cells were isolated from long bones (including femur and humerus). The cells were then treated with red blood cell lysis buffer (Sigma, USA) to remove erythrocyte and cultured overnight in α-minimum essential medium (α-MEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco). Floating cells, which contained BMMs, were collected and resuspended at the density of rated amount per mL. The cells (plated at concentration of 2 × 105 cells/well and 6 × 105 cells/well in 24-well and 12-well plates respectively) were cultured with α-MEM supplement with 10% FBS and were treated by M-CSF (10 ng/mL) and RANKL (50 ng/mL) (Peprotech, USA)-induced form OC as previously reported (19). After OC formation, the cells were treated by different concentrations of ruthenium red (RuR, 500 nmol/L) and ECO (5 nmol/L) with or without 1,25(OH)2D3 treatments., A well-known marker of OC, TRAP, was stained by leukocyte acid phosphatase kit (Sigma, USA). TRAP-positive cells with > 3 nuclei were considered OC.
3.3. Immunofluorescence Staining
VDR immunofluorescent staining was performed as reported previously (20-22). The BMMs were plated at concentration of 2 × 105 cells/well in 24-well plates) incubated with α-MEM supplement with 10% FBS for 5 days, and added M-CSF (10 ng/mL) and RANKL (50 ng/mL)-induced form OC. Cells on the chamber slides were rinsed by phosphate-buffered saline (PBS) gently. After fixing with 4% paraformaldehyde at room temperature for 10 min, cells were treated by 0.5% Triton X-100 for 20 minutes. Cells were then washed twice in PBS and blocked by 5% bovine serum albumin (BSA) for 20 minutes, followed by incubation with anti-VDR antibody (Santa Cruz Biotechnology, USA) at 4°C overnight. After washing by PBS, anti-mouse IgG conjugated with fluorescein isothiocyanate (FITC, Cell Signaling Technology, USA) and was incubated for 2 hours. Nuclei were visualized by staining with 2 µg.mL-1 4',6-diamidino-2-phenylindole (DAPI, Sigma, USA) for 15 minutes. The images were captured by DM2500 Fluorescence Microscope (Leica, Germany).
3.4. Western Blot
The total cellular proteins in each group were extracted by lysing with radioimmunoprecipitation assay buffer containing protease inhibitor (Sigma, USA). Protein concentration was measured by BCA Protein Assay Kit (Beyotime, China) and adjusted to the equal concentrations. Samples were boiled in SDS sample buffer for 10 minutes. For immunoblotting, each sample (containing 20 - 30 µg proteins) was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 8% or 10% sodium dodecyl sulfate polyacrylamide gel and transferred onto nitrocellulose membranes using wet transfer technology. For blocking, the membranes were incubated in Tris-buffered saline with 5% dry nonfat milk and 0.1% Tween-20 at room temperature for 1 hour. After washing by Tris-buffered saline with 0.1% Tween-20 (TBST), the membranes were probed with anti-TRPV5 antibody (Abcam, UK), anti-Vacuolar H+-ATPase (V-ATPase) antibody (Santa Cruz Biotechnology, USA), anti-TRAP, anti-Cathepsin K (CTSK) antibody (Abcam, UK), and anti-Carbonic anhydrase II (CA II) antibody (Abcam, UK). β-actin detected by β-actin antibody (Abcam, UK) was considered as a loading control. The expression of these proteins was visualized by a chemiluminescence kit (Merck Millipore, USA) after incubating with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse IgG (Cell Signaling Technology, USA) by electro chemiluminescence (ECL) Tanon 5200 detection system (Shanghai, China). The density of the bands was determined using an Image Lab software (Bio-Rad Laboratories, USA).
3.5. Statistical Analysis
Statistical analysis was performed with a Student t test by using the SPSS software (version 19.0; IBM SPSS, Armonk, NY, USA). All data were displayed as mean ± standard deviation. P-values less than 0.05 and 0.01 represent a significant or very significant difference, respectively.
4. Results
4.1. OCP Express VDR
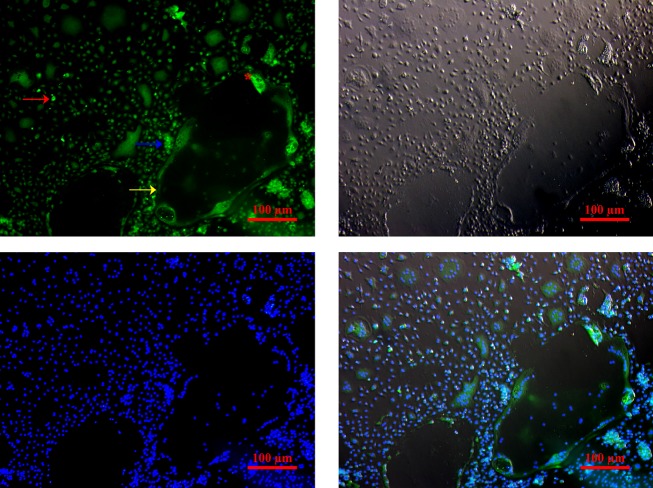
We first conducted immunofluorescence staining to examine VDR expression in the cultured cells. As shown in Figure 1, VDR expression was detected on day 5 in cells-treated with M-CSF and RANKL (Figure 1). VDR signals were present in the nuclei of OCPs (red arrow, and and mature OC (yellow arrow, Figure 1A). The intensity of VDR signals was particularly high in aggregated mononucleated OCPs (blue arrow, Figure 1A). In mature OC, the VDR signals were weaker and mainly present in the ruffled border (red asterisk, Figure 1A). These results suggest that 1,25(OH)2D3 can influence OC differentiation through VDR in particular in those mononucleated cells with high levels of VDR expression.
Figure 1. VDR detection by immunofluorescence. Expression of VDR (green) was observed in mononuclear cells (red arrow), especially in the cell aggregates (blue arrow). In mature OC (yellow arrow), VDR distribution can be observed in ruffled border (red asterisk). Counterstain of nuclei was carried out with DAPI (blue). Objective 200 ×; bars = 100 µm.
4.2. Vitamin D Suppresses TRPV5 Expression
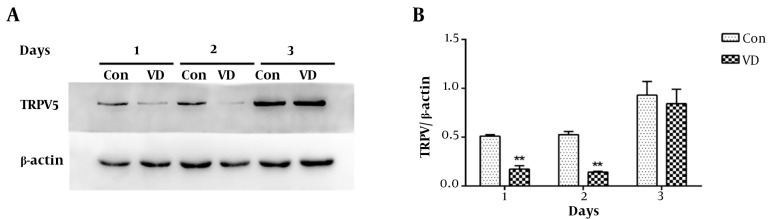
To examine the effect of 1,25(OH)2D3 on TRPV5 expression, BMMs cells cultured for 3 days were then treated by 10 ng/mL M-CSF plus with 50 ng/mL RANKL (control group) with or without 10 ng/mL M-CSF, 50 ng/mL RANKL plus 10 nM 1,25(OH)2D3 for another 1, 2, and 3 days. Protein expression of TRPV5 (~90 kDa) was detected by Western Blot. The TRPV5 expression can be detected in both groups, and its expression was up-regulated with OC differentiation. Significantly reduced expression of TRPV5 was observed in the first two days of treatment with 1,25(OH)2D3 (10 nM) (p < 0.01) but not in Day 3 (Figure 2), implying that 1,25(OH)2D3 can inhibit TRPV5 at the early stage of OC differentiation.
Figure 2. TRPV5 expression down-regulated by 1,25(OH)2D3 (VD). A, TRPV5 protein expression after treatment with VD was analyzed by western blotting. B, Quantitative analysis of TRPV5 protein expression after treatment with VD. Data means average ± SD (n = 9 each). Compared to the control group, double asterisk means P < 0.01.
4.3. 1,25(OH)2D3 and TRPV5 Inhibitors Inhibit OC Differentiation
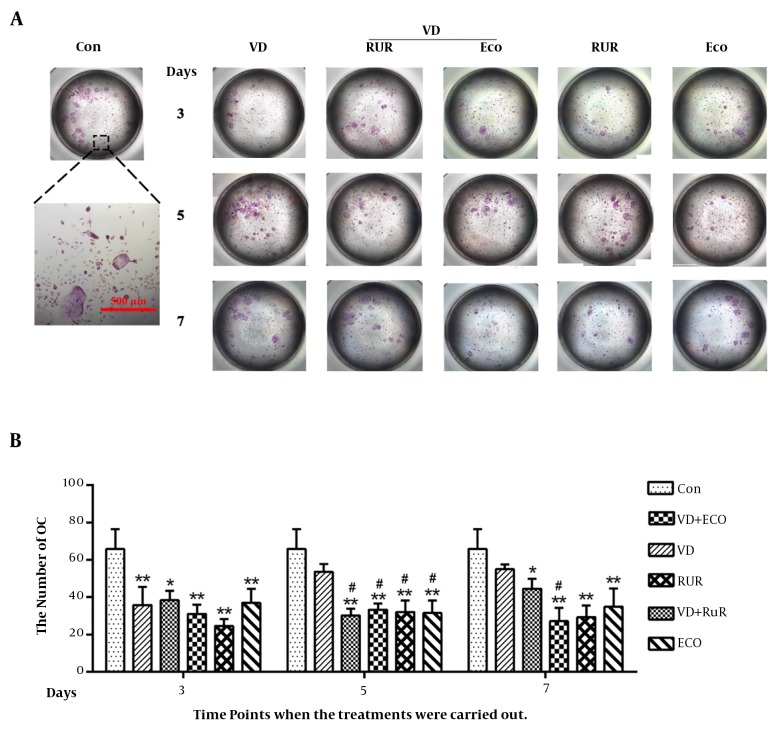
We next investigated the effects of 1,25(OH)2D3 and TRPV5 inhibitors on OC differentiation. 1,25(OH)2D3 and TRPV5 inhibitors were added into the culture medium, when BMMs cultured with 10 ng/mL M-CSF and 50 ng/mL RANKL for 3, 5 or 7 days. After incubation, TRAP staining was performed. As shown in Figure 3A, 1,25(OH)2D3 (10 nM) significantly inhibited osteoclastogenesis when added into the medium on day 3 (35.80 ± 19.32/well) (P < 0.01). However, 1,25(OH)2D3 did not affect OC formation when added into medium on day 5 (53.60 ± 9.48/well) or day 7 (55.00 ± 4.42/well) (P > 0.05). Two TRPV5 inhibitors, RUR and ECO, significantly inhibited osteoclastogenesis (P < 0.01) at any time (Figure 3B). Compared to 1,25(OH)2D3 group, both TRPV5 inhibitors equally further inhibited OC differentiation when they were added into the medium on day 5 (not on day 3) (P > 0.05) (Figure 3B).
Figure 3. Suppression of TRPV5 inhibits OC formation. A, TRAP staining confirmed that 1,25(OH)2D3 (VD), similar to TRPV5 inhibitors (RuR and ECO), inhibited OC differentiation. B, Quantitative analysis of OC number after different treatment by VD, RuR, ECO, VD + RuR or VD + ECO. Data means average ± SD (n = 9 each). Compared to the control group, asterisk means P < 0.05, double asterisk means P < 0.01. Compared to the VD group, octothorpe represents P < 0.05.
4.4. Suppression of TRPV-5 Inhibits Protein Expression in OC
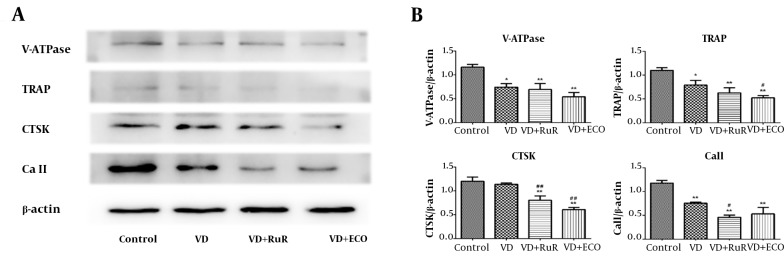
We further studied the effect of 1,25(OH)2D3 and TRPV5 on the expression of OC marker proteins including V-ATPase, CTSK, TRAP and Ca II by using Western blot (Figure 4A). Firstly, the effects of different concentrations of RuR and ECO were tested, showing no significant differences on these treatment doses (Supplementary File Appendix 1). BMMs cultured with 10 ng/mL M-CSF plus with 50 ng/mL RANKL were treated by 1,25(OH)2D3 and TRPV5 inhibitors. In addition, 1,25(OH)2D3 significantly suppresses the expression of V-ATPase, TRAP and Ca II. Compared to the 1,25(OH)2D3 group, the expression of CTSK and Ca II was further inhibited by RUR; whereas the expression of TRAP and CTSK was further suppressed by ECO (Figure 4B).
Figure 4. Suppression of TRPV5 inhibits OC marker proteins expression. A, V-ATPase, TRAP, CTSK and Ca II expression after treatment by 1,25(OH)2D3 (VD), VD+RUR or VD + ECO were analyzed by western blotting. B, Quantitative analysis of V-ATPase, TRAP, CTSK and Ca II after treatment by 1,25(OH)2D3 (VD), VD+RUR or VD + ECO. Data means average ± SD (n = 9 each). Compared to the control group, * P < 0.05, ** P < 0.01, compared to the VD group, # P < 0.05, ## P < 0.01.
5. Discussion
Vitamin D is required for normal osseous development and maintenance of bone architecture integrity. Vitamin D, usually acquired from food and skin (23), is metabolized into an active form 1,25(OH)2D3 once it passes through the liver and kidney (24). The active form of 1,25(OH)2D3 then reaches its target tissues and organs by binding the serum DBP during circulation (25). Active 1,25(OH)2D3 binds to VDR with high affinity and selectivity. In this study, we detected the expression of VDR in BMM-derived mononuclear cells. Its expression levels decreased in mature OC. This result is consistent with the observation of low VDR expression in OC of bone tissue in patients with Paget’s disease (26), suggesting that 1,25(OH)2D3 may act on VDR-rich OCP and regulate their differentiation.
Active 1,25(OH)2D3 exerts its biological effects in its target organs such as the small intestine, bones, kidneys and parathyroid gland where it participates in Ca2+ homeostasis (27). The first characterized channel in calcium transmembrane transport, TRPV5, is involved in renal Ca2+ reabsorption (28). Furthermore, 1,25-(OH)2D3 (29) may also cooperate with PTH (30), estrogen (31), testosterone (32) and other hormones to regulate Ca2+ homeostasis through TRPV5. Our study showed that TRPV5 expression was dramatically increased in OC, which is consistent with the results reported by van der Eerden et al. (17). Additionally, our data showed that TRPV5 was significantly inhibited by 1,25(OH)2D3 at the early stage (day 1 and 2) during OC differentiation possibly by inhibiting expression of TRPV5. Similarly, Allard et al. (12, 33) also reported that 1,25(OH)2D3 significantly inhibits OC differentiation at the early stage of cell differentiation.
Our data suggest that inhibition of OC differentiation by 1,25(OH)2D3 occurred at the early stage, while TRPV5 blockers could inhibit OC differentiation throughout the entire process. van der Eerden et al. (17) reported that OC numbers and OC areas of femoral bone in TRPV5 knockout mice were increased, whereas OC bone resorptive activity was impaired. These seemingly contradictory observations could be due to the difference of TRPV5 expression level by 1,25(OH)2D3-treated BMMs in knockout of TRPV5 mice. Alternatively, the TRPV5 gene was knocked out in all tissues and organs in TRPV5 knockout mice. Regardless, our results suggest that TRPV5 is important for OC differentiation, and that 1,25(OH)2D3 may inhibit OC differentiation by suppression of TRPV5.
Meanwhile, RuR can significantly block the TRPV5 channel activity. Since the inhibition effects of RuR on TRPV5 is actually by suppressing the electrophysiological activity of the channel. As a result, the inhibitory effect on the expression level of TPRV5 may be due to inhibition of TRPV5 electrophysiological activity and thereby hindering the differentiation and maturation-related activity of OC and bone resorption capacity. The inhibition of OC differentiation may partially inhibit the activation of RANKL-NFATc1 by TRPV5. Furthermore, RuR and Eco act as TRPV5 blockers, can inhibit the activation of NFATc1 and reduce the level of OC differentiation and bone resorption activity through calcium oscillation. Several key proteins such as Ca II, V-ATPase, CTSK and TRAP are secreted into the extracellular bone resorption site where they degrade hydroxyapatite or bone organic matter, thus functioning in OC bone resorption. These proteins are considered important markers of OC (34, 35). Indeed, our study showed that Ca II, V-ATPase, CTSK and TRAP were detected after treatment with 1,25(OH)2D3, which could be further blocked by RUR or ECO. A similar observation was made by Lin et al. (36).
In summary, our study provides evidence that VDR and TRPV5 were expressed in OCP and OC, and that TRPV5 was important in OC differentiation. Active 1,25(OH)2D3 may inhibit OC differentiation by suppressing TRPV5 expression at the early stage. The study warrants further investigation of the precise mechanism by which VDR regulates TRPV5 expression.
Footnotes
Authors’ Contribution:Study design: Jianhong Gu and Xishuai Tong. Study conduct: Jianhong Gu and Xishuai Tong. Data collection: Yang Chen and Chuang Zhang. Data analysis: Tianhong Ma. Data interpretation: Saihui Li, Wenyan Min, Yan Yuan, Xuezhong Liu and Jianchun Bian. Drafting manuscript: Jianhong Gu. Revising manuscript content: Xishuai Tong. Approving final version of manuscript: Zongping Liu takes responsibility for the integrity of the data analysis. All authors reviewed the manuscript.
Conflict of Interests:The authors declare no competing interests.
Ethical Approval:This study was approved by the Animal Care and Committee of Yangzhou University (approval ID: SYXK (Su) 2017-0044) and was carried out with the Guide for the Care and Use of Laboratory Animals by the National Research Council.
Financial Disclosure: The authors declare that there are no financial disclosures.
Funding/Support:This study was supported by National Natural Sciences Foundation of China (grant No. 31872534; 31872533; 31302154), the Natural Sciences Foundation of Jiangsu province (BK20181452) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Contributor Information
Jianhong Gu, Email: jhgu@yzu.edu.cn.
Xishuai Tong, Email: txsyzu@126.com.
Yang Chen, Email: vet.chen@qq.com.
Chuang Zhang, Email: 2215178544@qq.com.
Tianhong Ma, Email: 445735451@qq.com.
Saihui Li, Email: 1053252741@qq.com.
Wenyan Min, Email: 418432659@qq.com.
Yan Yuan, Email: yuanyan@yzu.edu.cn.
Xuezhong Liu, Email: liuxuezhong68@163.com.
Jianchun Bian, Email: bianjianchun@sina.com.
Zongping Liu, Email: liuzongping@yzu.edu.cn.
References
- 1.Takahashi N, Maeda K, Ishihara A, Uehara S, Kobayashi Y. Regulatory mechanism of osteoclastogenesis by RANKL and Wnt signals. Front Biosci (Landmark Ed). 2011;16:21–30. doi: 10.2741/3673. [DOI] [PubMed] [Google Scholar]
- 2.Van Poznak C, Sauter NP. Clinical management of osteoporosis in women with a history of breast carcinoma. Cancer. 2005;104(3):443–56. doi: 10.1002/cncr.21201. [DOI] [PubMed] [Google Scholar]
- 3.Kodama H, Yamasaki A, Nose M, Niida S, Ohgame Y, Abe M, et al. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991;173(1):269–72. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu J, Tong XS, Chen GH, Wang D, Chen Y, Yuan Y, et al. Effects of 1alpha,25-(OH)2D3 on the formation and activity of osteoclasts in RAW264.7 cells. J Steroid Biochem Mol Biol. 2015;152:25–33. doi: 10.1016/j.jsbmb.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190(12):1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P, Schwarz EM, O'Keefe RJ, Ma L, Boyce BF, Xing L. RANK signaling is not required for TNFalpha-mediated increase in CD11(hi) osteoclast precursors but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. J Bone Miner Res. 2004;19(2):207–13. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]
- 8.Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159(5):1689–99. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda S, Yoshizawa T, Nagai Y, Yamato H, Fukumoto S, Sekine K, et al. Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: studies using VDR knockout mice. Endocrinology. 1999;140(2):1005–8. doi: 10.1210/endo.140.2.6673. [DOI] [PubMed] [Google Scholar]
- 10.Haussler MR, Haussler CA, Whitfield GK, Hsieh JC, Thompson PD, Barthel TK, et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the "Fountain of Youth" to mediate healthful aging. J Steroid Biochem Mol Biol. 2010;121(1-2):88–97. doi: 10.1016/j.jsbmb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 12.Kim TH, Lee B, Kwon E, Choi CH, Sung IH, Kim Y, et al. 1,25-Dihydroxyvitamin D3 inhibits directly human osteoclastogenesis by down-regulation of the c-Fms and RANK expression. Joint Bone Spine. 2013;80(3):307–14. doi: 10.1016/j.jbspin.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276(5310):270–3. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 14.van Abel M, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ. Coordinated control of renal Ca(2+) transport proteins by parathyroid hormone. Kidney Int. 2005;68(4):1708–21. doi: 10.1111/j.1523-1755.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 15.Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31(6):253–64. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 16.van de Graaf SF, Hoenderop JG, Gkika D, Lamers D, Prenen J, Rescher U, et al. Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J. 2003;22(7):1478–87. doi: 10.1093/emboj/cdg162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, et al. The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci U S A. 2005;102(48):17507–12. doi: 10.1073/pnas.0505789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Gu JH, Chen Y, Zhao HY, Liu W, Song RL, et al. 1alpha,25-Dihydroxyvitamin D3 inhibits the differentiation and bone resorption by osteoclasts generated from Wistar rat bone marrow-derived macrophages. Exp Ther Med. 2015;10(3):1039–44. doi: 10.3892/etm.2015.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan SY, Susarla R, Canovas D, Vasilopoulou E, Ohizua O, McCabe CJ, et al. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta. 2015;36(4):403–9. doi: 10.1016/j.placenta.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Song R, Qian F, Li YP, Sheng X, Cao SX, Xu Q. Phosphatase of regenerating liver-3 localizes to cyto-membrane and is required for B16F1 melanoma cell metastasis in vitro and in vivo. PLoS One. 2009;4(2):e4450. doi: 10.1371/journal.pone.0004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamagami H, Nishioka T, Ochiai E, Fukushima K, Nomura M, Kasugai S, et al. Inhibition of osteoclastogenesis by a phosphodiesterase 4 inhibitor XT-611 through synergistic action with endogenous prostaglandin E2. Biochem Pharmacol. 2003;66(5):801–7. doi: 10.1016/s0006-2952(03)00409-x. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Vitamin D: Important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98(10):1024–7. doi: 10.1097/01.SMJ.0000140865.32054.DB. [DOI] [PubMed] [Google Scholar]
- 24.Deluca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014;3:479. doi: 10.1038/bonekey.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyan BD, Sylvia VL, McKinney N, Schwartz Z. Membrane actions of vitamin D metabolites 1alpha,25(OH)2D3 and 24R,25(OH)2D3 are retained in growth plate cartilage cells from vitamin D receptor knockout mice. J Cell Biochem. 2003;90(6):1207–23. doi: 10.1002/jcb.10716. [DOI] [PubMed] [Google Scholar]
- 26.Mee AP, Hoyland JA, Braidman IP, Freemont AJ, Davies M, Mawer EB. Demonstration of vitamin D receptor transcripts in actively resorbing osteoclasts in bone sections. Bone. 1996;18(4):295–9. doi: 10.1016/8756-3282(96)00011-7. [DOI] [PubMed] [Google Scholar]
- 27.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 28.Friedman PA. Mechanisms of renal calcium transport. Exp Nephrol. 2000;8(6):343–50. doi: 10.1159/000020688. [DOI] [PubMed] [Google Scholar]
- 29.Hoenderop JG, Muller D, Van Der Kemp AW, Hartog A, Suzuki M, Ishibashi K, et al. Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol. 2001;12(7):1342–9. doi: 10.1681/ASN.V1271342. [DOI] [PubMed] [Google Scholar]
- 30.de Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJ, et al. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol. 2009;20(8):1693–704. doi: 10.1681/ASN.2008080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irnaten M, Blanchard-Gutton N, Praetorius J, Harvey BJ. Rapid effects of 17beta-estradiol on TRPV5 epithelial Ca2+ channels in rat renal cells. Steroids. 2009;74(8):642–9. doi: 10.1016/j.steroids.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Hsu YJ, Dimke H, Schoeber JP, Hsu SC, Lin SH, Chu P, et al. Testosterone increases urinary calcium excretion and inhibits expression of renal calcium transport proteins. Kidney Int. 2010;77(7):601–8. doi: 10.1038/ki.2009.522. [DOI] [PubMed] [Google Scholar]
- 33.Allard L, Demoncheaux N, Machuca-Gayet I, Georgess D, Coury-Lucas F, Jurdic P, et al. Biphasic effects of vitamin D and FGF23 on human osteoclast biology. Calcif Tissue Int. 2015;97(1):69–79. doi: 10.1007/s00223-015-0013-6. [DOI] [PubMed] [Google Scholar]
- 34.Novack DV, Mbalaviele G. Osteoclasts-key players in skeletal health and disease. Microbiol Spectr. 2016;4(3) doi: 10.1128/microbiolspec.MCHD-0011-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao H, Liu X, Zou H, Dai N, Yao L, Zhang X, et al. Osteoprotegerin disrupts peripheral adhesive structures of osteoclasts by modulating Pyk2 and Src activities. Cell Adh Migr. 2016;10(3):299–309. doi: 10.1080/19336918.2015.1129480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J, Dong W, Zhang P, Li P, Sun H, Qi M. [Zoledronate inhibits TRPV5 and NFATc1 expression during differentiation of osteoclasts.]. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34(9):1254–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.