Abstract
Approximately half of all cancer patients receive radiation therapy, which is conventionally delivered in relatively small doses (1.8 to 2 Gy) per daily fraction over 1–2 months. Stereotactic body radiation therapy (SBRT), in which a high daily radiation dose is delivered in 1 to 5 fractions, has improved local control rates for several cancers. However, despite the wide-spread adoption of SBRT in the clinic, controversy surrounds the mechanism by which SBRT enhances local control. Some studies suggest that high doses of radiation (≥10 Gy) trigger tumor endothelial cell death, resulting in indirect killing of tumor cells through nutrient depletion. On the other hand, mathematical models predict that the high radiation dose per fraction used in SBRT increases direct tumor cell killing suggesting that disruption of the tumor vasculature is not a critical mediator of tumor cure. Here, we review the application of genetically engineered mouse models to radiosensitize tumor cells or endothelial cells in order to dissect the role of these cellular targets in mediating the response of primary tumors to high-dose radiotherapy in vivo. These studies demonstrate a role for endothelial cell death in mediating tumor growth delay, but not local control following SBRT.
Keywords: ATM, radiation therapy, SBRT, endothelial cell, mouse model
Introduction
Stereotactic body radiation therapy (SBRT) is now routinely used in the clinic to deliver large doses of daily radiation in a small number of fractions to a very precise target volume (1). This type of radiation delivery was first developed for the treatment of brain tumors in the early 1950s (2) and was not shown to be feasible in the setting of extracranial tumors until the 1990s (3, 4). With advances in imaging and medical physics, non-small cell lung cancer patients with inoperable tumors were among the first patients to be treated with SBRT (5). Remarkably, local control rates reached approximately 80–90% across several clinical trials (6–9), significantly higher than historical rates achieved with conventional, low dose per fraction radiotherapy. Therefore, SBRT is now being employed for the treatment of a wide variety of cancers, including non-small cell lung cancer, hepatocellular carcinoma, and oligometastatic disease at a variety of sites, with impressive rates of local control (10–15). Despite the efficacy of SBRT in the clinic, controversy surrounds the mechanism by which high-dose radiotherapy leads to tumor eradication. Two competing models on the mechanistic basis for improved efficacy with SBRT predominate: (1) functional impairment of tumor vasculature results in indirect cell death by killing tumor cells that would otherwise not have died from radiation or (2) higher radiation dose per fraction increases the biologically effective dose which leads to more direct tumor cell death. By understanding the mechanism of improved local control by SBRT, new therapeutic approaches with targeted agents can be designed to enhance the therapeutic ratio.
Indirect Tumor Cell Killing Hypothesis
Data supporting the first model were recently reviewed by Song and colleagues (16). They argue that radiation-induced tumor cell death alone is insufficient to explain the increased rates of local control achieved with SBRT as radiation doses are not large enough to kill every tumor cell (17–23). For example, in vitro survival studies using human tumor cell lines suggest that single radiation doses between 22 and 36 Gy are required to eradicate the number of cells estimated to be present in a 3 cm tumor (24). Although single doses of 30 Gy and higher are used in the clinic to achieve local control (25, 26), the in vitro survival studies found that the curative radiation dose increased by a factor of approximately three when 20% of the tumor cells were assumed to be hypoxic as hypoxic tumor cells are more resistant to radiation (24). As the calculated radiation dose for in vitro cell killing exceeded the SBRT dose used in the clinic, the investigators concluded that the efficacy of SBRT required indirect in addition to direct tumor cell death (27). Further supporting this model, the number of surviving tumor cells in a fibrosarcoma xenograft several days after high-dose radiotherapy was significantly less than the number of cells immediately following radiation exposure, suggesting that indirect killing mediates a second wave of tumor cell death after irradiation (21).
Early studies using tumor allografts revealed that low doses of radiation (< 5 Gy) caused only a temporary impairment in vascular function, while irradiation with 10 Gy triggered a persistent decrease in circulating blood volume in tumors (28, 29). A connection between such vascular dysfunction and indirect tumor cell death has been well described (30–33). Garcia-Barros et al. assessed endothelial cell death in xenograft tumors along a range of radiation doses, but reported endothelial cell death only at doses greater than 8 Gy (34), suggesting that radiation doses associated with conventional radiotherapy are not capable of killing vascular endothelial cells. In this study, tumors transplanted into mice lacking acid sphingomyelinase were resistant to endothelial cell apoptosis and had a shorter growth delay following high-dose radiotherapy when compared to tumors implanted into wild type mice (34). The authors concluded that a decrease in endothelial cell death caused resistance to SBRT, although this finding was later challenged (35, 36). Recently, these investigators further characterized the response of transplanted tumors in acid sphingomyelinase knock out mice and proposed a new mechanism, which did not rely on endothelial cell death, to explain the indirect cell death associated with SBRT. They argued that high single dose radiotherapy triggers ischemia/reperfusion injury that interferes with homologous recombination, thereby preventing the repair of radiation damage in tumor cells (37). Regardless of the mechanism of indirect cell death, these studies indicate that high-dose radiotherapy (≥10 Gy) can cause indirect tumor cell death and suggest that this “new biology” could explain increased efficacy of SBRT.
Proponents of the indirect cell death hypothesis contend that the linear quadratic model, which is commonly used to model radiation-induced cell death, does not accurately estimate tumor cell killing at high doses per fraction. It is argued that this mathematical model overestimates the amount of cell death caused directly by radiation-induced DNA damage because it does not account for indirect cell death mediated by the immune system or vascular dysfunction (38). Incorporation of a secondary tumor cell killing mechanism into the model results in a downward bend of the dose response curve at radiation doses greater than 10 Gy (27). Thus, the adapted model predicts that SBRT causes more cell death than would be estimated by the standard linear quadratic model, which may explain the increased rates of local control with SBRT.
Direct Tumor Cell Killing Hypothesis
The second model rejects the need for a “new biology” to explain the efficacy of SBRT and argues that improved outcomes after SBRT simply reflect increased direct tumor cell death from the higher radiation dose (39, 40). Experimental evidence supporting this model comes from Budach et al., who investigated the radiation dose required to cure 50% of tumors implanted into either nude or severe combined immunodeficiency (SCID) mice. Although SCID mice are three-fold more radiosensitive than nude mice due to a mutation in DNA-dependent protein kinase (DNA-PK) that impairs DNA repair, no difference was detected in the single dose of radiation needed to achieve local control in a panel of human tumors (41). These data indicate that the radiosensitivity of tumor stromal cells, such as endothelial cells, are not critical regulators of tumor cure. This study supports the direct cell killing hypothesis, which asserts that stromal cell death is not a critical mediator of local control. Importantly, the direct tumor cell killing hypothesis does not imply that stromal cells lack any role in tumor response to radiation therapy. Indeed, using doses of radiation that were not sufficient for tumor cure, Budach and colleagues observed increased growth delay of some xenografts in radiosensitive SCID hosts (41). Other studies combining molecularly targeted drugs with radiation therapy have also demonstrated that an increase in tumor growth delay does not always translate to an enhanced rate of local control with a curative radiation dose (42, 43). These studies highlight the importance of characterizing the impact of stromal cell death directly on local control.
Furthermore, local control for patients with non-small cell lung cancer treated with conventional radiotherapy or SBRT is enhanced with an increase in the biologically effective dose (BED) (44). BED provides a mathematical framework to adjust for dose fractionation to compare different radiation therapy prescriptions (45). Correcting the human lung cancer dataset for BED resulted in comparable rates of local control between the conventional RT and SBRT arms (39). These data suggest that the higher BED associated with SBRT may completely explain the increased rates of local control, thus eliminating the need for indirect tumor cell killing to explain the efficacy of SBRT.
Proponents of the direct tumor cell killing model also argue that the linear-quadratic model accurately predicts radiation-induced cell killing at high, single fraction doses (46). Data collected in irradiated rat spinal cord, mouse skin, and mouse small intestine in vivo fit the linear-quadratic model over a wide range of doses (47–49). Likewise, local control data in patients with primary lung cancer or brain metastases irradiated with SBRT were better fit by the linear-quadratic model than any adapted model that factored in indirect tumor cell death at high doses (50). Therefore, these results suggest that the linear quadratic model should not be replaced with an adapted model that incorporates increased cell death at high doses and they argue against the need for a “new biology” to explain the efficacy of SBRT.
Using Primary Mouse Models to Dissect the Cellular Target of Radiotherapy
To address the controversy regarding the cellular target that mediates the efficacy of SBRT, we utilized genetically engineered mouse models of cancer. Although transplanted tumor models are relatively fast and inexpensive for radiation therapy studies, experiments with xenografts are often unable to predict clinical outcomes (51–53). Genetically engineered mouse models (GEMMs), by contrast, require additional time and expense to develop but enable the study of autochthonous tumors that arise in their native microenvironment with an intact immune system (51). These primary tumors may more accurately model the role of stromal cells in human cancer compared to transplanted tumors (54, 55). Indeed, patterns of tumor vascularization and hypoxia differ significantly between spontaneous and transplanted models (51, 56, 57). Therefore, GEMMs may better recapitulate the response of human cancer to treatment in the clinic (58–60). Like all model systems, GEMMs have limitations, including the limited number of genetic alterations and decreased heterogeneity. Nevertheless, the intact stromal cell compartment and similarity with human cancer are critical considerations when investigating the impact of the tumor microenvironment on response to radiation therapy.
Dual Recombinase Technology
The utilization of the Cre/loxP system to mediate site-specific recombination in the 1980s (61, 62) revolutionized the field of cancer biology by enabling the generation of sophisticated GEMMs of cancer. Cre is a bacteriophage P1-derived enzyme that recombines a pair of DNA sequences termed loxP sites. These sites are often engineered into genomic DNA in order to flank a target or “floxed” gene so that the flanked region will be deleted in the presence of Cre. In a similar manner, the Flp-FRT system, adopted from Saccharomyces cerevisiae in the mid-1990s, employs the recombinase activity of flippase (Flp) to delete genomic targets flanked by Flp recombinase target (FRT) sites, referred to as “FRTed” regions (63). The recombinase activity of Cre and Flp can be controlled by a variety of mechanisms, including viral delivery and expression under the control of a cell-type specific promoter (64). Thus, Cre and Flp can temporally and spatially restrict recombination to study tumor development and response to radiation therapy (65). By combining Cre and Flp in a GEMM, the sophisticated interactions between tumor cells and the supporting stroma can be dissected by using one recombinase for tumor initiation and the second recombinase to genetically manipulate a specific stromal compartment (66, 67). Recently, we applied this dual recombinase technology to investigate the role of endothelial cells in mediating the response of primary tumors to SBRT (68–70).
Radiosensitization of Specific Cell Types within a Tumor
As DNA damage is a key cause of cell lethality following exposure to ionizing radiation (71), blocking DNA repair mechanisms may increase the number of unresolved DNA double-stranded breaks thus enhancing radiation-induced cell death. Ataxia telangiectasia mutated (ATM) is a protein kinase that regulates homologous recombination and cell cycle arrest through the phosphorylation of a large number of downstream targets, including p53, MRE11, RAD50, BRCA1, and CHK2 (72–75). Additionally, patients with inherited homozygous mutations in ATM, human cell lines lacking functional ATM, and Atm knockout mice are hypersensitive to radiation (76–78). Therefore, genetic deletion of Atm in either primary tumor or endothelial cells can be utilized to radiosensitize specific cell populations in order to define the roles of these cell types during tumor response to radiotherapy. To specifically assess the impact of vascular damage on tumor response to radiation, we employed dual recombinase technology to delete floxed alleles of Atm specifically in endothelial cells. In this model, viral delivery of Flp recombinase initiated tumorigenesis by deleting FRTed alleles of the tumor suppressor p53 and drove expression of the mutated oncogene KrasG12D by deleting an upstream FRTed STOP cassette at the endogenous promoter (79). Cre recombinase was not used to initiate the primary tumor in this system, but instead was expressed under the control of the VE-Cadherin promoter to direct Cre expression to endothelial cells in order to delete floxed Atm alleles specifically in the vasculature without affecting Atm gene expression in the primary tumor cells (Table 1). To specifically radiosensitize tumor cells, we used Cre-loxP technology to simultaneously initiate tumorigenesis and modulate expression of Atm exclusively within the tumor cells. Cre expression in tumor initiating cells activated expression of oncogenic KrasG12D by deleting a floxed STOP cassette and deleted floxed alleles of the tumor suppressor p53 in addition to Atm. Because Atm was deleted in the tumor initiating cells, which gave rise to the primary tumor, this genetic approach specifically enhanced the radiosensitivity of the tumor cells (Table 1).
Table 1.
Genetic changes within primary tumor cells and tumor vasculature
| VE-Cadherin-Cre; AtmfL/FL | Viral Cre + AtmFL/FL | |||
|---|---|---|---|---|
| Tumor Cell | Endothelial Cell | Tumor Cell | Endothelial Cell | |
| No Cre Recombinase | Wild Type | N/A | N/A | Wild Type |
| Cre Recombinase | No effect | Atm deleted | Atm deleted | No effect |
| Resulting Radiosensitivity | Wild Type | Radiosensitive | Radiosensitive | Wild Type |
Primary Sarcomas
A GEMM of primary soft tissue sarcoma (79–81) was the first setting in which we employed dual recombinase technology to examine the impact of endothelial or tumor cell radiosensitization on tumor response to SBRT. In this primary mouse model, an SBRT dose of 20 Gy caused vascular injury as measured by dual energy micro-Computed Tomography (micro-CT) and histology (82). To characterize whether endothelial cells are critical targets of SBRT, we used adenoviral delivery of Flp to initiate sarcomagenesis in FRT-STOP-FRT (FSF)-KrasG12D; p53FRT/FRT; VE-Cadherin-Cre; AtmFL/+ (KPVAFL/+) or AtmFL/FL (KPVAFL/FL) mice (69). Thus, primary sarcomas expressing oncogenic KrasG12D with both alleles of p53 deleted were compared, with one cohort lacking expression of both alleles of Atm in endothelial cells (KPVAFL/FL) and the other retaining expression of one wild type allele of Atm in endothelial cells (KPVAFL/+). As expected, loss of ATM signaling in the vasculature enhanced endothelial cell death 24 hours post irradiation with 20 Gy. Consistent with results in transplanted tumor models, radiosensitization of the endothelial cell compartment of primary sarcomas resulted in vascular dysfunction, as indicated by a decrease in perfusion after radiation exposure. Furthermore, an increase in the total amount of cell death in KPVAFL/FL tumors suggested that functional changes to the tumor vasculature triggered indirect killing of adjacent tumor cells.
To evaluate the impact of enhanced vascular dysfunction after SBRT, tumor-bearing mice were treated with a single 20 Gy dose of focal irradiation (69). Growth delay to a volume tripling endpoint in tumors lacking expression of Atm in the vasculature was significantly longer, when compared to control tumors that retained expression of one allele of Atm. These results in a primary sarcoma model support the indirect tumor cell hypothesis by demonstrating that an increase in the number of dying tumor cells after SBRT leads to an increase in growth delay. However, these data are not sufficient to conclude that the increased indirect tumor cell death caused by vascular dysfunction contributes to the efficacy of SBRT to achieve local control, which is a more relevant clinical endpoint. In order to assess the role of endothelial cell radiosensitivity on primary tumor eradication by SBRT, sarcomas in KPVAFL/+ and KPVAFL/FL mice were treated with a curative single dose of 50 Gy (68). Although 50 Gy was sufficient to cure approximately 10% of the sarcomas, mice bearing tumors with enhanced vascular radiosensitivity did not achieve a higher rate of local control. Why was the rate of local failure the same in tumors with enhanced indirect tumor cell killing? One potential explanation is that different tumor cells have different susceptibilities to indirect cell killing. Just as hypoxic tumor cells are resistant to radiation therapy, cells that have adapted to survive in the hypoxic microenvironment far from tumor vasculature may also be resistant to indirect cell death caused by vascular dysfunction. In this scenario, the radiation dose required to kill every hypoxic tumor cell with the capacity to cause local recurrence would not be affected by increased indirect tumor cell death adjacent to blood vessels. Regardless of the explanation, these findings support a role for endothelial cell death in sarcoma growth delay following SBRT, but not in local control following high single dose irradiation.
As a positive control for the ability to modulate rates of tumor eradication with SBRT, we also deleted Atm specifically in tumor parenchymal cells. Pax7-CreER; LoxP-STOP-LoxP (LSL)-KrasG12D; p53FL/FL; AtmFL/+ (P7KPAFL/+) and AtmFL/FL (P7KPAFL/FL) mice were injected into the gastrocnemius muscle with 4-hydroxy-tamoxifen to activate Cre recombinase to initiate sarcomagenesis and delete Atm within the same cell population (68). Deletion of both floxed alleles of Atm (P7KPAFL/FL) within the tumor cells of the primary sarcoma resulted in enhanced radiosensitivity. This radiosensitivity translated to a significantly improved tumor response to 50 Gy compared to tumors with deletion of one floxed allele of Atm (P7KPAFL/+), as measured by the time to tumor volume tripling and rate of local control. Collectively, these findings in a primary sarcoma mouse model demonstrate that tumor cell death, rather than endothelial cell death, is a critical mediator of achieving local control following SBRT, which supports the direct cell killing hypothesis.
Primary Lung Cancer
In the clinic, SBRT is routinely used to treat inoperable non-small cell lung cancer because of the high rate of local control (4). To study the role of endothelial cell death in SBRT for lung cancer, we utilized a sophisticated GEMM of non-small cell lung cancer to radiosensitize the tumor vasculature (79, 83). An adenovirus expressing Flp recombinase was administered intranasally to KPVAFL/+ and KPVAFL/FL mice to initiate tumorigenesis in the lung epithelium, while the Cre-driver, VE-Cadherin, mediated recombination of Atm in the vasculature (70). Upon detection of lung tumors by micro-CT imaging, mice were treated with a single 15 Gy dose of whole thorax irradiation and individual tumors were monitored every two weeks by micro-CT to evaluate tumor growth. As expected, an increase in tumor endothelial cell death as well as total cell death was observed in KPVAFL/FL mice 24 hours post radiation exposure, which supports the occurrence of indirect tumor cell death. Despite the enhanced radiosensitivity of the tumor vasculature and indirect tumor cell death, no significant difference in tumor growth delay was detected 2 to 6 weeks after 15 Gy in tumors lacking Atm expression in the vasculature as compared to tumors retaining one wild type allele of Atm. A small, though not statistically significant, decrease in tumor volume was detected in KPVAFL/FL tumors 8 weeks after irradiation. Overall, this study suggested that endothelial cell death has only a modest effect on the response of primary lung tumors to SBRT.
To investigate the impact of tumor parenchymal cell radiosensitization on the lung tumor response to SBRT, lung tumors were initiated in LSL-KrasG12D; p53FL/FL; AtmFL/+ (KPAFL/+) and AtmFL/FL (KPAFL/FL) mice (70). In this model, inhalation of a lentivirus expressing Cre facilitated the recombination of Atm specifically in lung tumor-initiating cells. Disruption of ATM signaling enhanced lung cancer cell radiosensitivity in a colony survival assay in vitro. Radiosensitization of lung tumor cells in KPAFL/FL mice translated to a significant decrease in tumor volume 6 and 8 weeks after 15 Gy irradiation to the whole thorax. Taken together, these results support a model in which tumor cells play a larger role than endothelial cells in regulating the response of primary lung cancers to SBRT and provide further evidence for the direct tumor cell killing hypothesis to explain the efficacy of SBRT.
Discussion
The large dose per fraction (≥10 Gy) radiotherapy schedules used to treat cancer with SBRT have increased local control rates for several diseases when compared to 2 Gy daily fractions delivered with conventional radiotherapy (10, 11, 84–86). However, biologists and physicists continue to debate the mechanism by which SBRT improves tumor response to radiation therapy. Many investigators have shown that radiation can induce proliferative defects in endothelial cells, thereby triggering endothelial cell death, increased vascular permeability, and indirect tumor cell death (34, 69, 70, 87–90). The controversy surrounding the mechanism of SBRT revolves around whether such functional changes to the vasculature and the accompanying indirect tumor cell death are sufficient to enhance tumor eradication. While some investigators argue that indirect cell killing caused by vascular impairment can regulate tumor cure in response to radiation (34, 91, 92), others have employed mathematical modeling to counter that the level of endothelial cell death does not accurately predict clinical outcomes and therefore increased dose per fraction simply kills more tumor cells directly (41, 93, 94). These divergent views may be reconciled by strictly limiting the conclusions of transplanted tumor models to the endpoints studied: indirect tumor cell death does occur as a consequence of vascular injury following single high-dose radiotherapy, and this increases tumor growth delay but not local control. Indeed, others have previously demonstrated that prolonged tumor growth delay using a radiotherapy regimen with a targeted agent does not always translate into increased tumor cure when a curative dose of radiotherapy is delivered (42, 43). This conclusion is consistent with the results of Budach and colleagues studying tumors transplanted into nude and SCID mice (41) as well as our results using GEMMs of sarcoma (68, 69) and lung cancer (70) following SBRT. Furthermore, this discrepancy between growth delay endpoints and tumor cure may explain why some clinical trials of radiation therapy and targeted agents don’t recapitulate preclinical data (95).
Tumor Cells as Critical Mediators of Single High-Dose Radiation Therapy
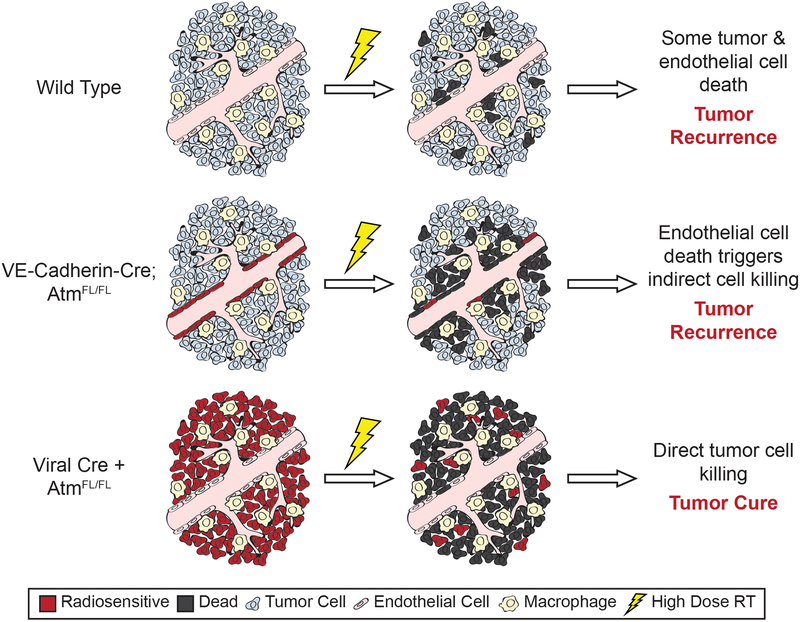
By genetically manipulating the radiosensitivity of either tumor cells with Cre-loxP technology or endothelial cells with dual recombinase technology, we generated data to address the controversy of the critical cellular target of SBRT. Importantly, SBRT triggered an increase in fractional blood volume and vascular permeability in primary soft-tissue sarcomas in a dose-dependent manner (82, 96). Radiosensitization of the endothelial cells through the deletion of Atm further disrupted AngioSense accumulation and blood flow into sarcomas, indicating an impairment of vascular function (69). Importantly, radiation-induced endothelial cell death in both the primary sarcoma and lung tumor models triggered indirect cell death of neighboring tumor cells (69, 70). Thus, primary tumors lacking Atm expression specifically in the vasculature represent powerful tools to assess whether vascular impairment can regulate growth delay and local tumor control following radiotherapy. Despite the observed increase in endothelial cell death and indirect tumor cell death in these GEMMs following irradiation with 15 Gy to 20 Gy, tumors lacking functional ATM in the vasculature displayed only a modest increase in growth delay (69, 70). Remarkably, endothelial cell radiosensitization and the resulting vascular dysfunction did not enhance local control in sarcomas treated with a curative radiation dose (69). While additional experiments with dual recombinase technology are needed to determine if these results extend beyond soft tissue sarcoma and lung cancer to other primary cancer mouse models, the available data in these two autochthonous tumor models suggest that the indirect tumor cell killing hypothesis may play a role in extending tumor growth delay and palliation with radiotherapy, but is unlikely to contribute to the impressive local control that can be achieved with SBRT (Figure 1).
Figure 1. Enhancing tumor parenchymal cell radiosensitivity preferentially promotes tumor cure.
In wild type tumors that maintain functional ATM signaling in all cellular compartments, high-dose radiotherapy induces both endothelial and tumor cell death. Deletion of both alleles of Atm in the tumor vasculature using dual recombinase technology increases the amount of endothelial and total cell death by approximately 2–3 fold. Despite the resulting vascular dysfunction and indirect tumor cell killing, the tumors still recur following high-dose radiotherapy. By contrast, radiosensitizing tumor parenchymal cells through the deletion of Atm enhances both radiation-induced growth delay and tumor cure.
In contrast, radiosensitization of the tumor cell population in the GEMMs significantly prolonged growth delay of lung tumors (70) and increased the incidence of sarcoma eradication (69). These results support the direct tumor cell killing hypothesis in which an increase in direct tumor cell death can promote local control following high dose per fraction radiotherapy (Figure 1). Taken together, these results suggest that a “new biology” mediated via endothelial cell death is not required to explain the increased rate of local control observed with SBRT.
Stromal Cells as Potential Mediators of SBRT
Although endothelial cell death may not regulate tumor eradication by SBRT, this does not rule out the possibility of other stromal cell populations regulating tumor response and cure following SBRT. For example, Brown and colleagues have reported that macrophages and endothelial progenitor cells can replenish tumor endothelium after single high-dose radiotherapy to impact the response of xenografts (97, 98). Similarly, T cells within the immune system can respond to SBRT to promote or impede transplanted tumor response to radiotherapy (99–101). In the future, dual recombinase technology can be applied to these cell populations to investigate their role in primary tumor response to SBRT.
Acknowledgements
This work was supported by the National Cancer Institute of the U.S. NIH under award R35CA197616 (D.G.K.)
DGK is a co-founder and receives research support from Xrad Therapeutics, which is developing radiosensitizers. The remaining author reports no conflict of interest.
References
- 1.Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7(1):44–54. [DOI] [PubMed] [Google Scholar]
- 2.Leksell L The stereotaxic method and radiosurgery of the brain. Acta chirurgica Scandinavica. 1951;102(4):316–9. [PubMed] [Google Scholar]
- 3.Lax I, Blomgren H, Naslund I, Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta oncologica (Stockholm, Sweden). 1994;33(6):677–83. [DOI] [PubMed] [Google Scholar]
- 4.Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34(6):861–70. [DOI] [PubMed] [Google Scholar]
- 5.Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(26):2847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. International journal of radiation oncology, biology, physics. 2005;63(5):1427–31. [DOI] [PubMed] [Google Scholar]
- 7.Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(20):3290–6. [DOI] [PubMed] [Google Scholar]
- 8.Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2008;70(3):685–92. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama. 2010;303(11):1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunven P, Blomgren H, Lax I. Radiosurgery for recurring liver metastases after hepatectomy. Hepato-gastroenterology. 2003;50(53):1201–4. [PubMed] [Google Scholar]
- 11.Schefter T, Gaspar LE, Kavanagh B, Ceronsky N, Feiner A, Stuhr K. Hypofractionated extracranial stereotactic radiotherapy for liver tumors. International Journal of Radiation Oncology • Biology • Physics.57(2):S282. [Google Scholar]
- 12.Soni PD, Palta M. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Current State and Future Opportunities. Digestive diseases and sciences. 2019;64(4):1008–15. [DOI] [PubMed] [Google Scholar]
- 13.Robin TP, Raben D, Schefter TE. A Contemporary Update on the Role of Stereotactic Body Radiation Therapy (SBRT) for Liver Metastases in the Evolving Landscape of Oligometastatic Disease Management. Semin Radiat Oncol. 2018;28(4):288–94. [DOI] [PubMed] [Google Scholar]
- 14.Kobiela J, Spychalski P, Marvaso G, Ciardo D, Dell’Acqua V, Kraja F, et al. Ablative stereotactic radiotherapy for oligometastatic colorectal cancer: Systematic review. Critical reviews in oncology/hematology. 2018;129:91–101. [DOI] [PubMed] [Google Scholar]
- 15.Laufer I, Bilsky MH. Advances in the treatment of metastatic spine tumors: the future is not what it used to be. Journal of neurosurgery Spine. 2019;30(3):299–307. [DOI] [PubMed] [Google Scholar]
- 16.Song CW, Glatstein E, Marks LB, Emami B, Grimm J, Sperduto PW, et al. Biological Principles of Stereotactic Body Radiation Therapy (SBRT) and Stereotactic Radiation Surgery (SRS): Indirect Cell Death. Int J Radiat Oncol Biol Phys. 2019. [DOI] [PubMed] [Google Scholar]
- 17.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiation research. 2012;177(3):311–27. [DOI] [PubMed] [Google Scholar]
- 18.Song CW, Cho LC, Yuan J, Dusenbery KE, Griffin RJ, Levitt SH. Radiobiology of stereotactic body radiation therapy/stereotactic radiosurgery and the linear-quadratic model. International journal of radiation oncology, biology, physics. 2013;87(1):18–9. [DOI] [PubMed] [Google Scholar]
- 19.Song CW, Kim MS, Cho LC, Dusenbery K, Sperduto PW. Radiobiological basis of SBRT and SRS. International journal of clinical oncology. 2014;19(4):570–8. [DOI] [PubMed] [Google Scholar]
- 20.Song CW, Lee YJ, Griffin RJ, Park I, Koonce NA, Hui S, et al. Indirect Tumor Cell Death After High-Dose Hypofractionated Irradiation: Implications for Stereotactic Body Radiation Therapy and Stereotactic Radiation Surgery. International journal of radiation oncology, biology, physics. 2015;93(1):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song CW, Park I, Cho LC, Yuan J, Dusenbery KE, Griffin RJ, et al. Is indirect cell death involved in response of tumors to stereotactic radiosurgery and stereotactic body radiation therapy? International journal of radiation oncology, biology, physics. 2014;89(4):924–5. [DOI] [PubMed] [Google Scholar]
- 22.Sperduto PW, Song CW, Kirkpatrick JP, Glatstein E. A hypothesis: indirect cell death in the radiosurgery era. International journal of radiation oncology, biology, physics. 2015;91(1):11–3. [DOI] [PubMed] [Google Scholar]
- 23.Kocher M, Treuer H, Voges J, Hoevels M, Sturm V, Muller RP. Computer simulation of cytotoxic and vascular effects of radiosurgery in solid and necrotic brain metastases. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2000;54(2):149–56. [DOI] [PubMed] [Google Scholar]
- 24.Leith JT, Cook S, Chougule P, Calabresi P, Wahlberg L, Lindquist C, et al. Intrinsic and extrinsic characteristics of human tumors relevant to radiosurgery: comparative cellular radiosensitivity and hypoxic percentages. Acta neurochirurgica Supplement. 1994;62:18–27. [DOI] [PubMed] [Google Scholar]
- 25.Videtic GM, Hu C, Singh AK, Chang JY, Parker W, Olivier KR, et al. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys. 2015;93(4):757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osti MF, Agolli L, Valeriani M, Reverberi C, Bracci S, Marinelli L, et al. 30 Gy single dose stereotactic body radiation therapy (SBRT): Report on outcome in a large series of patients with lung oligometastatic disease. Lung cancer (Amsterdam, Netherlands). 2018;122:165–70. [DOI] [PubMed] [Google Scholar]
- 27.Kim MS, Kim W, Park IH, Kim HJ, Lee E, Jung JH, et al. Radiobiological mechanisms of stereotactic body radiation therapy and stereotactic radiation surgery. Radiation oncology journal. 2015;33(4):265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong HH, Song CW, Levitt SH. Early changes in the functional vasculature of Walker carcinoma 256 following irradiation. Radiology. 1973;108(2):429–34. [DOI] [PubMed] [Google Scholar]
- 29.Song CW, Levitt SH. Vascular changes in Walker 256 carcinoma of rats following X irradiation. Radiology. 1971;100(2):397–407. [DOI] [PubMed] [Google Scholar]
- 30.Lasnitzki I A quantitative analysis of the direct and indirect action of X radiation on malignant cells. The British journal of radiology. 1947;20(234):240–7. [DOI] [PubMed] [Google Scholar]
- 31.Merwin R, Algire GH, Kaplan HS. Transparent-chamber observations of the response of a transplantable mouse mammary tumor to local roentgen irradiation. Journal of the National Cancer Institute. 1950;11(3):593–627. [PubMed] [Google Scholar]
- 32.Clement JJ, Tanaka N, Song CW. Tumor reoxygenation and postirradiation vascular changes. Radiology. 1978;127(3):799–803. [DOI] [PubMed] [Google Scholar]
- 33.Clement JJ, Song CW, Levitt SH. Changes in functional vascularity and cell number following x-irradiation of a murine carcinoma. International journal of radiation oncology, biology, physics. 1976;1(7–8):671–8. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–9. [DOI] [PubMed] [Google Scholar]
- 35.Suit HD, Willers H. Comment on “Tumor response to radiotherapy regulated by endothelial cell apoptosis” (I). Science. 2003;302(5652):1894; author reply [DOI] [PubMed] [Google Scholar]
- 36.Brown M, Bristow R, Glazer P, Hill R, McBride W, McKenna G, et al. Comment on “Tumor response to radiotherapy regulated by endothelial cell apoptosis” (II). Science. 2003;302(5652):1894; author reply [DOI] [PubMed] [Google Scholar]
- 37.Bodo S, Campagne C, Thin TH, Higginson DS, Vargas HA, Hua G, et al. Single-dose radiotherapy disables tumor cell homologous recombination via ischemia/reperfusion injury. The Journal of clinical investigation. 2019;129(2):786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18(4):240–3. [DOI] [PubMed] [Google Scholar]
- 39.Brown JM, Brenner DJ, Carlson DJ. Dose escalation, not “new biology,” can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. International journal of radiation oncology, biology, physics. 2013;85(5):1159–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JM, Carlson DJ, Brenner DJ. The Tumor Radiobiology of SRS and SBRT: Are More Than the 5 Rs Involved? Int J Radiat Oncol Biol Phys. 2014;88(2):254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budach W, Taghian A, Freeman J, Gioioso D, Suit HD. Impact of stromal sensitivity on radiation response of tumors. J Natl Cancer Inst. 1993;85(12):988–93. [DOI] [PubMed] [Google Scholar]
- 42.Krause M, Prager J, Zhou X, Yaromina A, Dorfler A, Eicheler W, et al. EGFR-TK inhibition before radiotherapy reduces tumour volume but does not improve local control: differential response of cancer stem cells and nontumourigenic cells? Radiother Oncol. 2007;83(3):316–25. [DOI] [PubMed] [Google Scholar]
- 43.Zips D, Hessel F, Krause M, Schiefer Y, Hoinkis C, Thames HD, et al. Impact of adjuvant inhibition of vascular endothelial growth factor receptor tyrosine kinases on tumor growth delay and local tumor control after fractionated irradiation in human squamous cell carcinomas in nude mice. Int J Radiat Oncol Biol Phys. 2005;61(3):908–14. [DOI] [PubMed] [Google Scholar]
- 44.Mehta N, King CR, Agazaryan N, Steinberg M, Hua A, Lee P. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: A pooled analysis of biological equivalent dose and local control. Practical radiation oncology. 2012;2(4):288–95. [DOI] [PubMed] [Google Scholar]
- 45.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. The British journal of radiology. 1989;62(740):679–94. [DOI] [PubMed] [Google Scholar]
- 46.Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Seminars in radiation oncology. 2008;18(4):234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Kogel AJ. Chronic effects of neutrons and charged particles on spinal cord, lung, and rectum. Radiation research Supplement. 1985;8:S208–16. [PubMed] [Google Scholar]
- 48.Douglas BG, Fowler JF. The effect of multiple small doses of x rays on skin reactions in the mouse and a basic interpretation. Radiation research. 1976;66(2):401–26. [PubMed] [Google Scholar]
- 49.Peck JW, Gibbs FA. Mechanical assay of consequential and primary late radiation effects in murine small intestine: alpha/beta analysis. Radiation research. 1994;138(2):272–81. [PubMed] [Google Scholar]
- 50.Shuryak I, Carlson DJ, Brown JM, Brenner DJ. High-dose and fractionation effects in stereotactic radiation therapy: Analysis of tumor control data from 2965 patients. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015;115(3):327–34. [DOI] [PubMed] [Google Scholar]
- 51.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5(9):741–54. [DOI] [PubMed] [Google Scholar]
- 52.Singh M, Johnson L. Using genetically engineered mouse models of cancer to aid drug development: an industry perspective. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(18):5312–28. [DOI] [PubMed] [Google Scholar]
- 53.Becher OJ, Holland EC. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer research. 2006;66(7):3355–8, discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 54.Graves EE, Vilalta M, Cecic IK, Erler JT, Tran PT, Felsher D, et al. Hypoxia in models of lung cancer: implications for targeted therapeutics. Clin Cancer Res. 2010;16(19):4843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maity A, Koumenis C. Location, location, location-makes all the difference for hypoxia in lung tumors. Clin Cancer Res. 2010;16(19):4685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fenton BM, Lord EM, Paoni SF. Intravascular HBO(2) saturations, perfusion and hypoxia in spontaneous and transplanted tumor models. International journal of cancer. 2001;93(5):693–8. [DOI] [PubMed] [Google Scholar]
- 57.Field SB, Needham S, Burney IA, Maxwell RJ, Coggle JE, Griffiths JR. Differences in vascular response between primary and transplanted tumours. British journal of cancer. 1991;63(5):723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010;28(6):585–93. [DOI] [PubMed] [Google Scholar]
- 59.Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483(7391):613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh M, Murriel CL, Johnson L. Genetically engineered mouse models: closing the gap between preclinical data and trial outcomes. Cancer research. 2012;72(11):2695–700. [DOI] [PubMed] [Google Scholar]
- 61.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(14):5166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. Journal of molecular biology. 1981;150(4):467–86. [DOI] [PubMed] [Google Scholar]
- 63.Sadowski PD. The Flp Recombinase of th 2-μm Plasmid of Saccharomyces cerevisiae In: Cohn WE, Moldave K, editors. Progress in Nucleic Acid Research and Molecular Biology. 51: Academic Press; 1995. p. 53–91. [PubMed] [Google Scholar]
- 64.Castle KD, Chen M, Wisdom AJ, Kirsch DG. Genetically engineered mouse models for studying radiation biology. Translational cancer research. 2017;6(Suppl 5):S900–s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Developmental cell. 2004;6(1):7–28. [DOI] [PubMed] [Google Scholar]
- 66.Cheung PF, Neff F, Neander C, Bazarna A, Savvatakis K, Liffers ST, et al. Notch-Induced Myeloid Reprogramming in Spontaneous Pancreatic Ductal Adenocarcinoma by Dual Genetic Targeting. Cancer Res. 2018;78(17):4997–5010. [DOI] [PubMed] [Google Scholar]
- 67.Schonhuber N, Seidler B, Schuck K, Veltkamp C, Schachtler C, Zukowska M, et al. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat Med. 2014;20(11):1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moding EJ, Castle KD, Perez BA, Oh P, Min HD, Norris H, et al. Tumor cells, but not endothelial cells, mediate eradication of primary sarcomas by stereotactic body radiation therapy. Sci Transl Med. 2015;7(278):278ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moding EJ, Lee CL, Castle KD, Oh P, Mao L, Zha S, et al. Atm deletion with dual recombinase technology preferentially radiosensitizes tumor endothelium. J Clin Invest. 2014;124(8):3325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torok JA, Oh P, Castle KD, Reinsvold M, Ma Y, Luo L, et al. Deletion of Atm in Tumor but not Endothelial Cells Improves Radiation Response in a Primary Mouse Model of Lung Adenocarcinoma. Cancer Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ward JF. Some biochemical consequences of the spatial distribution of ionizing radiation-produced free radicals. Radiation research. 1981;86(2):185–95. [PubMed] [Google Scholar]
- 72.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nature reviews Molecular cell biology. 2013;14(4):197–210. [PubMed] [Google Scholar]
- 73.Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18(28):4047–54. [DOI] [PubMed] [Google Scholar]
- 74.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(19):10389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science (New York, NY). 1998;282(5395):1893–7. [DOI] [PubMed] [Google Scholar]
- 76.Paterson MC, Smith PJ. Ataxia telangiectasia: an inherited human disorder involving hypersensitivity to ionizing radiation and related DNA-damaging chemicals. Annu Rev Genet. 1979;13:291–318. [DOI] [PubMed] [Google Scholar]
- 77.Pollard JM, Gatti RA. Clinical radiation sensitivity with DNA repair disorders: an overview. International journal of radiation oncology, biology, physics. 2009;74(5):1323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86(1):159–71. [DOI] [PubMed] [Google Scholar]
- 79.Lee CL, Moding EJ, Huang X, Li Y, Woodlief LZ, Rodrigues RC, et al. Generation of primary tumors with Flp recombinase in FRT-flanked p53 mice. Disease models & mechanisms. 2012;5(3):397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kirsch DG, Dinulescu DM, Miller JB, Grimm J, Santiago PM, Young NP, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13(8):992–7. [DOI] [PubMed] [Google Scholar]
- 81.Blum JM, Ano L, Li Z, Van Mater D, Bennett BD, Sachdeva M, et al. Distinct and overlapping sarcoma subtypes initiated from muscle stem and progenitor cells. Cell Rep. 2013;5(4):933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moding EJ, Clark DP, Qi Y, Li Y, Ma Y, Ghaghada K, et al. Dual-energy micro-computed tomography imaging of radiation-induced vascular changes in primary mouse sarcomas. Int J Radiat Oncol Biol Phys. 2013;85(5):1353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65(22):10280–8. [DOI] [PubMed] [Google Scholar]
- 84.Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. International journal of radiation oncology, biology, physics. 2004;60(1):186–96. [DOI] [PubMed] [Google Scholar]
- 85.Hoyer M, Roed H, Hansen AT, Ohlhuis L, Petersen J, Nellemann H, et al. Prospective study on stereotactic radiotherapy of limited-stage non–small-cell lung cancer. International Journal of Radiation Oncology • Biology • Physics.66(4):S128–S35. [Google Scholar]
- 86.Nyman J, Johansson K-A, Hultén U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer—Mature results for medically inoperable patients. Lung Cancer.51(1):97–103. [DOI] [PubMed] [Google Scholar]
- 87.Itasaka S, Komaki R, Herbst RS, Shibuya K, Shintani T, Hunter NR, et al. Endostatin improves radioresponse and blocks tumor revascularization after radiation therapy for A431 xenografts in mice. International journal of radiation oncology, biology, physics. 2007;67(3):870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baeten CI, Castermans K, Lammering G, Hillen F, Wouters BG, Hillen HF, et al. Effects of radiotherapy and chemotherapy on angiogenesis and leukocyte infiltration in rectal cancer. International journal of radiation oncology, biology, physics. 2006;66(4):1219–27. [DOI] [PubMed] [Google Scholar]
- 89.Schwickert HC, Stiskal M, Roberts TP, van Dijke CF, Mann J, Muhler A, et al. Contrast-enhanced MR imaging assessment of tumor capillary permeability: effect of irradiation on delivery of chemotherapy. Radiology. 1996;198(3):893–8. [DOI] [PubMed] [Google Scholar]
- 90.Giustini AJ, Petryk AA, Hoopes PJ. Ionizing radiation increases systemic nanoparticle tumor accumulation. Nanomedicine. 2012;8(6):818–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia-Barros M, Thin TH, Maj J, Cordon-Cardo C, Haimovitz-Friedman A, Fuks Z, et al. Impact of stromal sensitivity on radiation response of tumors implanted in SCID hosts revisited. Cancer Res. 2010;70(20):8179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Truman JP, Garcia-Barros M, Kaag M, Hambardzumyan D, Stancevic B, Chan M, et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5(9):e12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerweck LE, Vijayappa S, Kurimasa A, Ogawa K, Chen DJ. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006;66(17):8352–5. [DOI] [PubMed] [Google Scholar]
- 94.Ogawa K, Boucher Y, Kashiwagi S, Fukumura D, Chen D, Gerweck LE. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res. 2007;67(9):4016–21. [DOI] [PubMed] [Google Scholar]
- 95.Coleman CN, Higgins GS, Brown JM, Baumann M, Kirsch DG, Willers H, et al. Improving the Predictive Value of Preclinical Studies in Support of Radiotherapy Clinical Trials. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(13):3138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ashton J, Castle K, Qi Y, Kirsch DL, West J, Badea C, Dual-Energy CT Imaging of Tumor Liposome Delivery After Gold Nanoparticle-Augmented Radiation Therapy 2018. 1782–97 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13(3):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown JM. Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy. The British journal of radiology. 2014;87(1035):20130686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63(5):1229–35. [PubMed] [Google Scholar]
- 101.Muroyama Y, Nirschl TR, Kochel CM, Lopez-Bujanda Z, Theodros D, Mao W, et al. Stereotactic Radiotherapy Increases Functionally Suppressive Regulatory T Cells in the Tumor Microenvironment. Cancer Immunol Res. 2017;5(11):992–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]