Abstract
Transglutaminase 2 (TG2) is a ubiquitous but enigmatic mammalian protein to which a number of biological functions have been ascribed but not definitively proven. As a member of the transglutaminase family, TG2 can catalyze deamidation or alternatively transamidation of selected Gln residues in proteins and peptides. It is also known to harbor other enzymatic properties, including protein disulfide isomerase, GTP-dependent signal transduction, and ATP dependent protein kinase activity. Given its multifunctional chemistry, it is unsurprising that a long list of proteins from the mammalian proteome have been identified as substrates and/or binding partners; however, the biological relevance of none of these protein-protein interactions has been clarified as yet. Remarkably, the most definitive insights into the biology of TG2 stem from its pathophysiological role in gluten peptide deamidation in celiac disease. Meanwhile our understanding of TG2 chemistry has been leveraged to engineer a spectrum of inhibitors and other molecular probes of TG2 biology in vivo. This review summarizes our current knowledge of the enzymology and regulation of human TG2 with a focus on its physiological substrates as well as tool molecules whose engineering was inspired by their identities.
Keywords: transglutaminase, post-translational modifications, chemical biology
Introduction
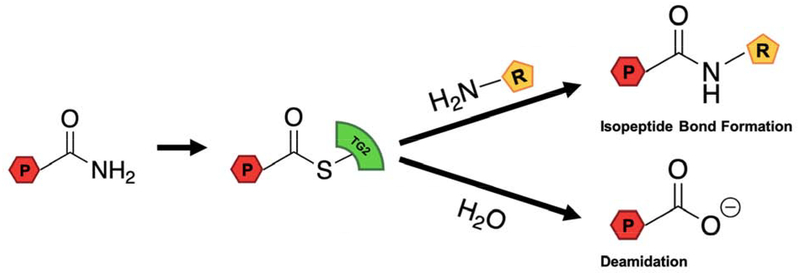
Transglutaminase 2 (TG2) is one of nine members of the mammalian transglutaminase family [1]. All but one of these proteins harbor a cysteine protease-like active site that catalyzes the deamidation or alternatively transamidation of selected Gln residues in their protein and peptide substrates (Figure 1). Additionally, TG2 is known to harbor other unrelated catalytic properties, including GTPase activity coupled to transmembrane signal transduction [2], protein disulfide isomerase activity [3], and ATP-dependent kinase activity [4].
Figure 1. TG2 catalyzed transamidation and deamidation reactions.
P represents a Gln-containing peptide or protein, and R represents a small molecule or protein containing a primary amine.
The biological role of TG2 has, for the most part, remained enigmatic for more than 50 years since this protein was originally discovered. Regrettably, genetics has had little to say thus far about its role in mammalian development or physiology, principally because TG2-knockout mice have no overt phenotype [5]. Meanwhile, TG2 is also thought to play a role in the pathogenesis of many unrelated human diseases including celiac disease [6], certain cancers [7], fibrosis of various organs [8], and some neurodegenerative disorders [9], although with the notable exception of celiac disease [10], definitive evidence for a causative role of TG2 is lacking in any of these disease states.
The inability of genetic approaches to shine light on TG2 biology places a heavy emphasis on chemical biological ones. The promise of chemical biology lies in its ability to: (i) reveal physiological substrates of TG2, and to structurally characterize the resulting post-translational modifications; (ii) identify tight-binding protein partners of TG2, and to define their protein-protein interfaces; and perhaps most importantly (iii) shine light on the biological relevance of these myriad covalent and non-covalent interactions. This review is therefore focused on our current knowledge of TG2 substrates and its interacting partners, as well as the types of molecular tools they have inspired that are targeted at interrogating TG2 biology, eventually all the way into humans.
Enzymology and regulation of human TG2
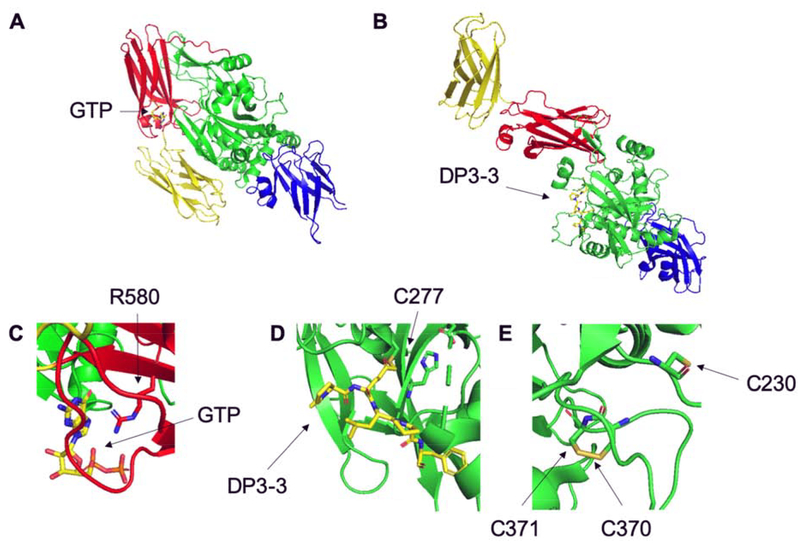
X-ray crystallographic analysis has been an invaluable source of insights into TG2 structure-function relationships. The overall protein is comprised of four domains, an N-terminal β-sandwich, an α/β-catalytic domain, and two C-terminal β-barrels (Figure 2) [11]. Human TG2 has been crystallized in its GDP- and GTP-bound form (PDB IDs: 1KV3, 4PYG), in complex with ATP (PDB ID: 3LY6), and with alternative inhibitors bound to its transamidase site within the α/β-catalytic domain (PDB IDs: 2Q3Z, 3S3J, 3S3P, 3S3S) [11–14]. Importantly however, although transglutaminase activity requires multiple Ca2+ ions, no available X-ray structure has revealed the presence of bound Ca2+. As such, the Ca2+ binding sites have been primarily inferred by comparison to other structurally characterized mammalian transglutaminases and via site-directed mutagenesis [15].
Figure 2. Crystal structures of open and closed conformations of TG2.
TG2 is comprised of the N-terminal β-sandwich (blue), the α/β-catalytic domain (green), and two C-terminal β-barrels (red and yellow, respectively). (A) “Closed” GTP-bound conformation of TG2 (1KV3). (B) “Open” DP3–3 bound conformation of TG2 (2Q3Z). (C) Space-filling model of GTP interacting with R580 in the closed conformation. (D) Space-filling model of DP3–3 interacting with C277 in the open conformation. (E) Space-filling model of the PDI-reactive, redox-regulated cysteines C230, C370, and C371.
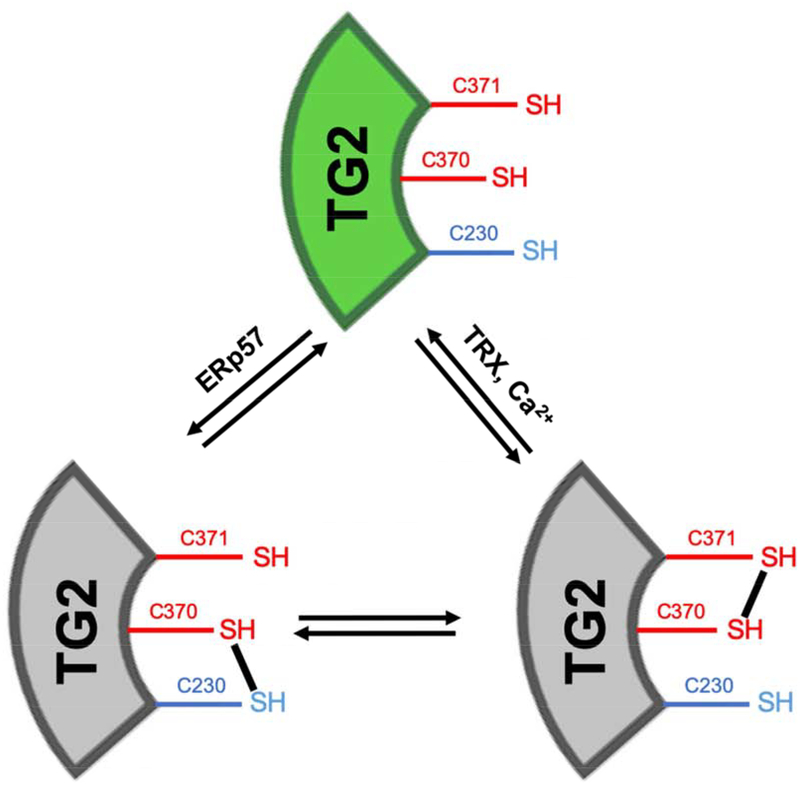
In addition to defining each of catalytic sites of human TG2, the above-mentioned X-ray structures have led to mechanistic proposals for conformation-dependent regulation of TG2 activity. Specifically, two distinct modes of regulating its transamidase activity have been put forward. On one hand, a large conformational change (illustrated in Figure 2) is associated with interconversion between the inactive GTP-bound and the active Ca2+-bound forms of TG2; this transition is presumably important in the cytosol, where GTP is an abundant metabolite. On the other hand, a subtler conformational change limited to the α/β-catalytic domain is responsible for redox-dependent reversible activation of the transamidase activity of TG2 (Figure 3) [16]; this mode of regulation is presumably relevant to extracellular TG2, and involves at least two protein cofactors, thioredoxin (TRX) and ERp57 [17,18]. To the extent other proteins can functionally replace either TRX or ERp57 in this on-off transition, they would qualify as substrates for the protein disulfide isomerase activity of TG2. Whereas formal kinetic comparisons remain to be conducted, it is however likely that TG2 has considerably lower specificity for these putative protein substrates than it does for TRX or ERp57. Notably, TG2 is the only mammalian transglutaminase to harbor the conserved Cys-triad shown in Figures 2 and 3, suggesting that not only is redox regulation unique to this isozyme but also that it evolved relatively recently. (TG2 homologs in other vertebrates appear to lack this structure feature.)
Figure 3. Redox regulation of TG2.
TG2 cofactors TRX and ERp57 reversibly regulate TG2 in a redox-dependent manner, and act as “on” and “off” switches for the active enzyme (green). As TG2 is natively inactive (gray), TRX is able to reduce the vicinal disulfide bond between C370-C371 to activate TG2, and ERp57 can oxidize the disulfide between C230 and C370 to catalytically inactivate the enzyme.
Sub-cellular localization of TG2
TG2 is expressed in most or all organs in the mammalian body [19–20]. It is predominantly a cytosolic protein, but can also be exported out of the cell or localized to specific compartments such as the nucleus and mitochondrion [21–22]. The mechanisms for export or sub-cellular localization of TG2 are not understood, although several non-canonical models have been proposed for these transport processes [23–25]. Importantly, because all of these environments are Ca2+-poor, GTP-rich, and/or oxidative, a vast majority of mammalian TG2 is maintained in an inactive state under ordinary homeostatic conditions. This regulatory feature of the enzyme further complicates our understanding of its biological function(s).
Physiological Substrates of TG2
A key to understanding TG2 biology lies in our ability to identify its natural substrates. However, notwithstanding the long list of potential protein substrates that have been identified through in vitro or in situ experimentation (e.g., http://genomics.dote.hu/wiki/index.php/Main_Page) [26], the recognition of very few of these substrates by TG2 has been verified in intact mammals. In this section we highlight some substrates that have been discovered or confirmed in vivo.
Beta-crystallin, a major structural protein of the eye lens, is an archetypal TG2 substrate. Its target Gln residue is localized to a peptide sequence denoted A25 (TVQQEL) [27], and is crosslinked to a Lys residue near the C-terminus of the same protein. However, because TG2-knockout mice have no gross visual defects, the role of this post-translational modification is unclear.
Fibronectin is a high-MW glycoprotein that is abundant in the extracellular matrix of most solid organs as well as in blood plasma. It oligomerizes via disulfide bonding into a scaffolding structure, thereby presenting binding sites for a host of other extracellular matrix substances such as heparin, collagen, fibrin, and integrin. The 220 kDa fibronectin monomer harbors multiple Gln residues susceptible to TG2 modification, including sites within its N-terminal collagen/fibrin-binding domain, its central (RGD-containing) integrin-binding domain, and its C-terminal glycosaminoglycan-binding domain [28]. In addition, its N-terminal domain also harbors a high-affinity non-covalent docking site for TG2 [29]. Remarkably, the biogenic amine serotonin can serve as an effective nucleophile in TG2-catalyzed modification of fibronectin; this post-translational modification of fibronectin appears to be a biomarker of pulmonary hypertension in humans as well as cellular and animal models of the disease [30–31], although its pathogenic relevance remains to be elucidated.
Like fibronectin, a number of other proteins comprising the extracellular matrix have been shown to harbor TG2-reactive sites. Amongst these, TG2-catalyzed crosslinking of the aminopropeptide of type III collagen onto the mature collagen fibril [32] and the oligomerization of osteonectin in the matrix of differentiating cartilage [33] represent especially intriguing examples, notwithstanding very limited insight into their biological relevance.
A potentially important but poorly characterized post-translational modification catalyzed by TG2 occurs during the activation of transforming growth factor-β (TGF-β). This homodimeric mammalian growth factor, which signals via a receptor tyrosine kinase, is a potent inhibitor of epithelial cell growth while simultaneously stimulating fibroblast proliferation. It is constitutively secreted by many cell types, albeit as an inactive precursor that includes a cleaved but noncovalently associated propeptide. In turn, the propeptide is disulfide-bonded to another protein called the latent TGF-β binding protein (LTBP). TG2 catalyzes an early step in the activation of TGF-β by crosslinking LTBP to the extracellular matrix [34]. Whereas the molecular logic for how this post-translational modification ultimately results in TGF-β activation remains unclear, further analysis of this phenomenon may shine light into the role of TG2 in early mammalian development. Indeed, comparative analysis of TGF-β activation in wild-type and TG2-knockout mice promises to be a fruitful avenue for investigating the biological role of extracellular TG2.
Non-physiological substrates of TG2
Two classes of non-physiological substrates of TG2 warrant attention. The first includes peptides derived from dietary gluten. More than two decades ago, the Sollid and Koning laboratories identified Gln-rich peptides from wheat gluten that were regioselectively deamidated by TG2; the resulting deamidated gluten peptides elicited an inflammatory response from T cells derived from small intestinal biopsies of celiac disease patients in an HLA-DQ2 or -DQ8 restricted manner [35,36]. Since then, the list of gluten-derived epitopes whose T cell reactivity is strongly enhanced by selective deamidation by TG2 has grown immensely [37]. A particularly vivid example is the case of the proteolytically resistant 33-residue peptide from α2-gliadin in wheat [38]. In addition to being one of the most potent elicitors of disease-specific T cell response in celiac disease patients, this 33-mer contains multiple copies of the pentameric sequence PQLPY that remains to this date as one of the best-known substrates of human TG2 [12,39,40]. The discovery that certain gluten peptides are excellent TG2 substrates has prompted extensive investigation of the substrate specificity of this enzyme. The most favorable substrates appear to harbor a reactive Gln within a Q-X-P motif, whereas sequences containing Q-P, Q-G, Q-X-X-P, or Q-X-X-G motifs are not recognized (X denotes any amino acid) [41]. As discussed below, these insights have also been leveraged in the design of peptidic sensors and inhibitors of TG2.
Beyond gluten peptides, directed evolution strategies have been effectively deployed by several investigators to identify peptide substrates of TG2 harboring reactive Gln residues. For example, M13 phage display libraries have led to the discovery of minimal preferred substrates of not only TG2 (HQSYVDPWMLDH) but also related mammalian transglutaminases including TG1, TG3, TG6, TG7 and Factor XIII [42–46]. In an independent study, a random 7-mer peptide library yielded GQQQTPY, GLQQASV and WQTPMNS as preferred substrates of TG2 [47]. Other researchers have deployed mRNA display systems to identify RLQQP as the preferred TG2 substrate [48]. Together, these results provide a strong rationale for the observation that certain P/Q-rich sequences from dietary gluten are specifically recognized by human TG2 in the context of celiac disease pathogenesis.
Inhibitors as probes of TG2 biology
Our understanding of the mechanisms and molecular recognition features of the distinct active sites in TG2 has inspired the engineering of a variety of active site-directed inhibitors. Several excellent recent reviews have discussed TG2 inhibition and the design of TG2 inhibitors per se [49–53]. Here we focus on a few tool molecules that have found widespread use as probes of this biologically mysterious mammalian protein.
Monodansyl cadaverine (MDC) [54] and 5-biotinamido pentylamine (5BP) [55] are arguably the most widely used probes of TG2, which utilizes these simple amines as nucleophiles in the transamidase reaction (Figure 1). 5BP is widely used in chromogenic TG2 assays, based on the ability of streptavidin conjugates to recognize its biotin substituent with high specificity. Indeed, 5BP can even be injected into animals as a probe of TG2 activity in vivo [56]. In contrast, MDC is predominantly used as a competitive inhibitor of the protein crosslinking activity of TG2. The primary limitation of both probes lies in the relative non-specificity of TG2 for amine substrates. As such, these amines must be present at relatively high concentrations (~100 μM) in order to detect or inhibit TG2 activity in cells and tissues.
In contrast to amine probes of TG2 activity, peptide harboring Gln mimics have the potential to show considerably higher specificity for TG2. The best-known example is 6-diazo-5-oxo-L-norleucine (DON) [57]. This warhead can be incorporated into peptides as short as 5-mers (e.g., DP3–3) to generate ligands that bind irreversibly to TG2 with nanomolar affinity [12]. DON-containing peptides have also been used to engineer positron emission tomography (PET) probes of TG2 [58]. However, the difficulties associated with scalable synthesis of DON-containing peptides limits their utility, as it involves the dangerous Arndt-Eistert reaction. New synthetic routes have the potential to alleviate this problem (Zhuang, et al., manuscript in preparation). Alternatively, “clickable” TG2 inhibitors can also be deployed to probe in situ TG2 activity in biological samples [59].
Last but not least, non-hydrolyzable GTP analogs (e.g., 5’-guanylyl imidodiphosphate) have also been used as probes of TG2 activity [60–61]. Because of the tight coupling between the GTP binding site and the transamidase/deamidase active site of TG2, these ligands act as competitive inhibitors of both the GTPase and transglutaminase activities of this enzyme.
Conclusions and Future Directions
Given the ubiquitous nature of TG2 in mammals and our relatively poor understanding of its biological roles, the most promising probes of enzymatic activity will be those that can be utilized in vivo. In this context, it is noteworthy that the first irreversible TG2 inhibitor, ZED-1227, has already entered clinical trials for the treatment of celiac disease [62]. Other inhibitors are likely to follow. Independently, there remains an acute need for a minimally invasive probe of intestinal TG2 activity that can be used not just in animals but eventually also in humans. Overall, opportunities remain bright for innovative chemical biology approaches to be harnessed in future studies of mammalian TG2.
Highlights:
X-ray crystallography has provided insights into regulatory mechanisms of TG2
Physiological substrates of TG2 remain elusive
Gluten-derived substrates of TG2 are important in celiac disease pathogenesis
Active site-directed inhibitors can be leveraged as tools to probe TG2 function in vivo
Acknowledgments
Research on TG2 in the authors’ laboratory is supported by a grant from the NIH (DK 063158).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gundemir S, Colak G, Tucholski J, Johnson GV, Transglutaminase 2: a molecular Swiss army knife, Biochim Biophys Acta 1823 (2012) 406–19. URL 10.1016/j.bbamcr.2011.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Achyuthan KE, Greenberg CS, Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity, J Biol Chem 262 (1987) 1901–6. [PubMed] [Google Scholar]
- [3].Hasegawa G, Suwa M, Ichikawa Y, Ohtsuka T, Kumagai S, Kikuchi M, Sato Y, Saito Y, A novel function of tissue-type transglutaminase: protein disulphide isomerase, Biochem J 373 (2003) 793–803. URL 10.1042/BJ20021084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mishra S, Murphy LJ, Tissue transglutaminase has intrinsic kinase activity: idenfication of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase, J Biol Chem 279 (2004) 23863–8. URL 10.1074/jbc.M311919200 [DOI] [PubMed] [Google Scholar]
- [5].Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM, Targeted inactivation of Gh/tissue transglutaminase II, J Biol Chem 276 (2001) 20673–8. URL 10.1074/jbc.M010846200 [DOI] [PubMed] [Google Scholar]
- [6].Arentz-Hansen H, Korner R, Molberg O, Quarsten H, Vader W, Kooy YM, Lundin KE, Koning F, Roepstorff O, Sollid LM, McAdam SN, The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase, J Exp Med 191 (2000) 603–12. URL 10.1084/jem.191.4.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Park KS, Kim HK, Lee JH, Choi YB, Park SY, Yang SH, Kim SY, Hong KM, Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer, J Cancer Res Clin Oncol 136 (2010) 493–502. URL 10.1007/s00432-009-0681-6 [DOI] [PubMed] [Google Scholar]
- [8].Griffin M, Smith LL, Wynne J, Changes in transglutaminase activity in an experimental model of pulmonary fibrosis induced by paraquat, Br J Exp Pathol 60 (1979) 653–61. [PMC free article] [PubMed] [Google Scholar]
- [9].Selkoe DJ, Abraham C, Ihara Y, Brain Transglutaminase: in vitro crosslinking of human neurofilament proteins into insoluble polymers, Proc Natl Acad Sci 79 (1982) 6070–4. URL 10.1073/pnas.79.19.6070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abadie V, Kim SM, Lejeune T, Palanski BA, Ernest JD, Tastet O, Voisine J, Discepolo V, Marietta EV, Fahmy M, Ciszewski C, Bouziat R, Panigrahi K, Horwath I, Zurenski MA, Lawrence I, Dumaine A, Yotova V, Grenier JC, Murray JA, Khosla C, Barreiro LB, Jabri B (2019) IL-15, gluten, and HLA-DQ8 drive tissue destruction in coeliac disease. Nature In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu S, Cerione RA, Clardy J, Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity, PNAS 99 (2002) 2743–7. URL 10.1073/pnas.042454899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pinkas DM, Strop P, Brunger AT, Khosla C, Transglutaminase 2 undergoes a large conformational change upon activation, PLoS Biol 5 (2007) e327 URL 10.1371/journal.pbio.0050327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Han BG, Cho JW, Cho YD, Jeong KC, Kim SY, Lee BI, Crystal structure of human transglutaminase 2 in complex with adenosine triphosphate, Int J Biol Macomol 47 (2010) 190–5. URL 10.1016/j.ijbiomac.2010.04.023 [DOI] [PubMed] [Google Scholar]
- [14].Jang TH, Lee DS, Choi K, Jeong EM, Kim IG, Kim YW, Chun JN, Jeon JH, Park HH, Crystal structure of transglutaminase 2 with GTP complex and amino acid sequence evidence of evolution of GTP binding site, PLoS One 9 (2014) e107005 URL 10.1371/journal.pone.0107005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kiraly R, Csosz E, Kurtan T, Antus S, Szigeti K, Simon-Vecsei Z, Korponay-Szabo IR, Keresztessy Z, Fesus L, Functional significance of five noncanonical Ca2+-binding sites of human transglutaminase 2 characterized by site-directed mutagenesis, FEBS J 276 (2009) 7083–96. URL 10.1111/j.1742-4658.2009.07420.x [DOI] [PubMed] [Google Scholar]
- [16].Stamnaes J, Pinkas DM, Fleckenstein B, Khosla C, Sollid LM, Redox regulation of transglutaminase activity, J Biol Chem 285 (2010) 25402–9. URL 10.1074/jbc.M109.097162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yi MC, Melkonian AV, Ousey JA, Khosla C, Endoplasmic reticulum-resident protein 57 (ERp57) oxidatively inactivates human transglutaminase 2, J Biol Chem 293 (2018) 2640–2649. URL 10.1074/jbc.RA117.001382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jin X, Stamnaes J, Kloeck C, DiRaimondo TR, Sollid LM, Khosla C, Activation of extracellular transglutaminase 2 by thioredoxin, J Biol Chem 286 (2011) 37866–37873. URL 10.1074/jbc.M111.287490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thomazy V, Fesus L, Differential expression of tissue transglutaminase in human cells. An immunohistochemical study, Cell Tissue Res 255 (1989) 215–24. URL 10.1007/bf00229084 [DOI] [PubMed] [Google Scholar]
- [20].Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, Proteomics. Tissue-based map of the human proteome, Science 347 (2015) 1260419 URL 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- [21].Lesort M, Attanavanich K, Zhang J, Johnson GV, Distinct nuclear localization and activity of tissue transglutaminase, J Biol Chem 273 (1998) 11991–4. URL 10.1074/jbc.273.20.11991 [DOI] [PubMed] [Google Scholar]
- [22].Piacentini M, D’Eletto M, Farrace MG, Rodolfo C, Nonno FD, Ippolito G, Falasca F, Characterization of distinct sub-cellular location of transglutaminase type II: changes in intracellular distribution in physiological and pathological states, Cell Tissue Res 358 (2014) 793–805. URL 10.1007/s00441-014-1990-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zemskov EA, Mikhailenko I, Hsia RC, Zaritskaya L, Belkin AM, Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes, PLoS One 6 (2011) e19414 URL 10.1371/journal.pone.0019414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Adamczyk M, Griffiths R, Dewitt S, Knauper V, Aeschlimann D, P2X7 receptor activation regulates rapid unconventional export of transglutaminase-2, J Cell Sci 128 (2015) 4615–28. URL 10.1242/jcs.175968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Furini G, Schroeder N, Huang L, Boocock D, Scarpellini A, Coveney C, Tonoli E, Ramaswamy R, Ball G, Verderio C, Johnson TS, Verderio EAM, Proteomic profiling reveals the transglutaminase-2 externalization pathway in kidneys after unilateral ureteric obstruction, J Am Soc Nephrol 29 (2018) 880–905. URL 10.1681/ASN.2017050479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Csosz E, Mesko K, Fesus L, Transdab wiki: the interactive transglutaminase substrate database on web 2.0 surface, Amino Acids 36 (2009) 615–7. URL 10.1007/s00726-008-0121-y [DOI] [PubMed] [Google Scholar]
- [27].Groenen PJ, Bioemendal H, de Jong WW, The carboxy-terminal lysine of alpha B-crystallin is an amine-donor substrate for tissue transglutaminase, Eur J Biochem 205 (1992) 671–4. URL 10.1111/j.1432-1033.1992.tb16827.x [DOI] [PubMed] [Google Scholar]
- [28].Fesus L, Metsis ML, Muszbek L, Koteliansky VE, Transglutaminase-sensitive glutamine residues of human plasma fibronectin revealed by studying its proteolytic fragments, Eur J Biochem 154 (1986) 371–374. URL 10.1111/j.1432-1033.1986.tb09407.x [DOI] [PubMed] [Google Scholar]
- [29].Radek JT, Jeong JM, Murthy SN, Ingham KC, Lorand L, Affinity of human erythrocyte transglutaminase for a 42-kDa gelatin-binding fragment of human plasma fibronectin, PNAS 90 (1993) 3152–6. URL 10.1073/pnas.90.8.3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wei L, Warburton RR, Preston IR, Roberts KE, Comhair SA, Erzurum SC, Hill NS, Fanburg BL, Serotonylated fibronectin is elevated in pulmonary hypertension, Am J Physiol Lung Cell Mol Physiol 302 (2012) 1273–9. URL 10.1152/ajplung.00082.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Penumatsa KC, Toksoz D, Warburton RR, Hilmer AJ, Liu T, Khosla C, Comhair SA, Fanburg BL, Role of hypoxia-induced transglutaminase 2 in pulmonary artery smooth muscle cell proliferation, Am J Physiol Lung Cell Mol Physiol 307 (2014) 576–85. URL 10.1152/ajplung.00162.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bowness JM, Folk JE, Timpl R, Identification of a substrate site for liver transglutaminase on the aminopropeptide of type III collagen, J Biol Chem 262 (1987) 1022–4. [PubMed] [Google Scholar]
- [33].Aeschlimann D, Kaupp O, Paulsson M, Transglutaminase-catalyzed matrix cross-linking in differentiating cartilage: identification of osteonectin as a major glutaminyl substrate, J Cell Biol 129 (1995) 881–92. URL 10.1083/jcb.129.3.881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nunes I, Gleizes PE, Metz CN, Rifkin DB, Latent transforming growth factor-beta binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-beta, J Cell Biol 136 (1997) 1151–63. URL 10.1083/jcb.136.5.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Molberg O, Mcadam SN, Koerner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjoestroem H, Sollid LM, Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease, Nat Med 4 (1998) 713–717. URL doi: 10.1038/nm0698-713 [DOI] [PubMed] [Google Scholar]
- [36].van de Wal Y, Kooy Y, van Veelen P, Pena S, Mearin L, Padadopoulos G, Koning F, Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity, J Immunol 161 (1998) 1585–8. [PubMed] [Google Scholar]
- [37].Bodd M, Kim CY, Lundin KE, Sollid LM, T-cell response to gluten in patients with HLA-DQ2.2 reveals requirement of peptide-MHC stability in celiac disease, Gastroenterology 142 (2012) 552–61. URL 10.1053/j.gastro.2011 [DOI] [PubMed] [Google Scholar]
- [38].Shan L, Molberg O, Parrow I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C, Structural basis for gluten intolerance in celiac sprue, Science 297 (2002) 2275–9. URL 10.1126/science.1074129 [DOI] [PubMed] [Google Scholar]
- [39].Piper JL, Gray GM, Khosla C, High selectivity of human tissue transglutaminase for immunoactive gliadin peptides: implications for celiac sprue, Biochemistry 41 (2002) 386–93. URL 10.1021/bi011715x [DOI] [PubMed] [Google Scholar]
- [40].Siegel M, Xia J, Khosla C, Structure-based design of alpha-amido aldehyde containing gluten peptide analogues as modulators of HLA-DQ2 and transglutaminase 2, Bioorg Med Chem 15 (2007) 6253–61. URL 10.1016/j.bmc.2007.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fleckenstein B, Molberg O, Qiao SW, Schmid DG, von der Mulbe F, Elgstoen K, Jung G, Sollid LM, Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process, J Biol Chem 277 (2002) 34109–16. URL 10.1074/jbc.M204521200 [DOI] [PubMed] [Google Scholar]
- [42].Sugimura Y, Hosono M, Wada F, Yoshimura T, Maki M, Hitomi K, Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library: identification of peptide substrates for TGASE 2 and Factor XIIIA, J Biol Chem 281 (2006) 17699–706. URL 10.1074/jbc.M513538200 [DOI] [PubMed] [Google Scholar]
- [43].Sugimura Y, Yokoyama K, Nio N, Maki M, Hitomi K, Identification of preferred substrate sequences of microbial transglutaminase from Streptomyces mobaraensis using a phage-displayed peptide library, Arch Biochem Biophys 477 (2008) 379–83. URL 10.1016/j.abb.2008.06.014 [DOI] [PubMed] [Google Scholar]
- [44].Yamane A, Fukui M, Sugimura Y, Itoh M, Alea MP, Thomas V, El Alaoui S, Akiyama M, Hitomi K, Identification of a preferred substrate peptide for transglutaminase 3 and detection of in situ activity in skin and hair follicles, FEBS J 277 (2010) 3564–74. URL 10.1111/j.1742-4658.2010.07765.x [DOI] [PubMed] [Google Scholar]
- [45].Fukui M, Kuramoto M, Yamasaki R, Shimizu Y, Itoh M, Kawamoto T, Hitomi K, Identification of a highly reactive substrate peptide for transglutaminase 6 and its use in detecting transglutaminase activity in the skin epidermis, FEBS J 280 (2013) 1420–9. URL 10.1111/febs.12133 [DOI] [PubMed] [Google Scholar]
- [46].Kuramoto K, Yamasaki R, Shimizu Y, Tatsukawa H, Hitomi K, Phage-displayed peptide library screening for preferred human substrate peptide sequences for transglutaminase 7, Arch Biochem Biophys 537 (2013) 138–43. URL 10.1016/j.abb.2013.07.010 [DOI] [PubMed] [Google Scholar]
- [47].Keresztessy Z, Csosz E, Harsfalvi J, Csomos K, Gray J, Lightowlers RN, Lakey JH, Balajthy Z, Fesus L, Phage display selection of efficient glutamine-donor substrate peptides for transglutaminase 2, Protein Sci 15 (2006) 2466–80. 10.1110/ps.051818406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lee JH, Song C, Kim DH, Park IH, Lee SG, Lee YS, Kim BG, Glutamine (Q)-peptide screening for transglutaminase reaction using mRNA display, Biotechnol Bioend 110 (2013) 353–62. URL 10.1002/bit.24622 [DOI] [PubMed] [Google Scholar]
- [49].Pietsch M, Wodtke R, Pietzsch J, Loser R, Tissue transglutaminase: an emerging target for therapy and imaging, Bioorg Med Chem Lett 23 (2013) 6528–43. URL 10.1016/j.bmcl.2013.09.060 [DOI] [PubMed] [Google Scholar]
- [50].Keillor JW, Apperley KY, Akbar A, Inhibitors of tissue transglutaminase, Trends Pharmacol Sci 36 (2015) 32–40. URL 10.1016/j.tips.2014.10.014 [DOI] [PubMed] [Google Scholar]
- [51].Keillor JW, Apperley KY, Transglutaminase inhibitors: a patent review, Expert Opin Ther Pat 26 (2016) 49–63. URL 10.1517/13543776.2016.1115836 [DOI] [PubMed] [Google Scholar]
- [52].Song M, Hwang H, Im CY, S-Y. Kim, Recent progress in the development of transglutaminase 2 (TGase2) inhibitors, J Med Chem 60 (2017) 554–567. URL 10.1021/acs.jmedchem.6b01036 [DOI] [PubMed] [Google Scholar]
- [53].Katt WP, Antonyak MA, Cerione RA, The diamond anniversary of tissue transglutaminase: a protein of many talents, Drug Discov Today 23 (2018) 575–591. URL 10.1016/j.drudis.2018.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bersten AM, Ahkong QF, Hallinan T, Nelson SJ, Lucy JA, Inhibition of the formation of myotubes in vitro by inhibitors of transglutaminase, Biochim Biophys Acta 762 (1983) 429–36. URL 10.1016/0167-4889(83)90008-3 [DOI] [PubMed] [Google Scholar]
- [55].Jeon WM, Lee KN, Birckbichler PJ, Conway E, Patterson MK, Colorimetric assay for cellular transglutaminase, Anal Biochem 182 (1989), 170–5. URL 10.1016/0003-2697(89)90737-9 [DOI] [PubMed] [Google Scholar]
- [56].Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C, Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury, PLoS One 3 (2008) e1861 URL 10.1371/journal.pone.0001861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hausch F, Halttunen T, Maki M, Khosla C, Design, synthesis, and evaluation of gluten peptide analogs as selective inhibitors of human tissue transglutaminase, Chem Biol 10 (2003) 225–31. URL 10.1016/S1074-5521(03)00045-0 [DOI] [PubMed] [Google Scholar]
- [58].van der Wildt B, Lammertsma AA, Drukarch B, Windhorst AD, Strategies towards in vivo imaging of active transglutaminase type 2 using positron emission tomography, Amino Acids 49 (2017) 585–95. URL 10.1007/s00726-016-2288-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Dafik L, Khosla C, Dihydroisoxazole analogs for labeling and visualization of catalytically active transglutaminase 2, Chem Biol 18 (2011) 58–66. URL 10.1016/j.chembiol.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lai TS, Slaughter TF, Peoples KA, Hettasch JM, Greenberg CS, Regulation of human tissue transglutaminase function by magnesium-nucleotide complexes. Identification of distinct binding sites for Mg-GTP and Mg-ATP, J Biol Chem 273 (1998) 1776–81. URL 10.1074/jbc.273.3.1776 [DOI] [PubMed] [Google Scholar]
- [61].Duval E, Case A, Stein RL, Cuny GD, Structure-activity relationship study of novel tissue transglutaminase inhibitors, Bioorg Med Chem Lett 15 (2005) 1885–9. URL 10.1016/j.bmcl.2005.02.005 [DOI] [PubMed] [Google Scholar]
- [62].Ventura MAE, Sajko K, Hils M, Pasternack R, Greinwald R, Tewes B, Schuppan D, The oral transglutaminase 2 (TG2) inhibitor Zed1227 blocks TG2 activity in a mouse model of intestinal inflammation, Gastroenterology 154 (2018) S–490. URL 10.1016/S0016-5085(18)31861-4 [DOI] [Google Scholar]