Abstract
We examined the benefit of emotional support on daily health in premutation carrier mothers of adolescents and adults with fragile X syndrome (n = 114), and whether this benefit was moderated by the mother’s genetic status (FMR1 CGG repeat length). In an 8-day daily diary, maternal daily health was assessed subjectively through self-reported number of physical health symptoms and physiologically via cortisol awakening response. Multilevel lagged-day models indicated that premutation carrier mothers with midrange CGG repeats derived less health benefit from a day with high positive emotional support than those with lower or higher numbers of repeats within the premutation range. The data support the influence of both genetic and environmental influences on the health of this population.
Keywords: FMR1, fragile X syndrome, premutation, health, support, CGG repeat
Fragile X syndrome (FXS) involves the full mutation (> 200 cytosine-guanine-guanine [CGG]repeats in the 5’ untranslated region) of the fragile X gene (FMR1) and is most commonly inherited from a mother who has the premutation of the FMR1 gene (55 to 200 CGG repeats). Estimated to occur in 1 in 3,600 individuals (Hagerman et al., 2009), FXS results in a neurodevelopmental disorder associated with intellectual disability (ID) and behavior problems including inattention, hyperactivity, irritability, anxiety, and autism symptoms (Brown, 2002; Cornish, Turk, & Hagerman, 2008; Garber, Visootak, & Warran, 2008). The challenging profile of impairments and behaviors in children with FXS can create extraordinary parenting stressors (Johnston et al., 2003). On average, mothers of children with FXS report poorer psychological and physical health relative to their peers who have children without neurodevelopmental conditions (Sarimski, 1997; Smith, Greenberg, & Seltzer, 2012) or children with Down syndrome (e.g., Abbeduto et al., 2004).
Little research has focused on resiliency, or the positive adaptation of mothers in the face of child-related challenges associated with FXS. Positive emotional support (i.e., reassurance, encouragement, and understanding) has been shown to be a resiliency mechanism in the context of other stressors (e.g., Cichy, Stawski, & Almeida, 2014; Taylor, 2011; Uchino, 2006) and may foster adaptive health in mothers of children with FXS. In other populations, however, not all people similarly derive benefit from positive emotional support; instead, the extent of benefit has been shown to be shaped by both environmental (e.g., Gleason, Gleason, Iida, Shrout, & Bolger, 2008) and genetic (e.g., Chen et al., 2011) factors. The goal of the current study was to determine the association between the receipt of daily positive emotional support and physical health the next day in mothers of adolescents and adults with FXS and to explore whether these effects are moderated by the mother’s own genetic status (i.e., the number of CGG repeats). A lagged-day (i.e., previous-day positive emotional support predicting next day physical health) daily diary methodology was used to examine these associations in a time-ordered manner as they spontaneously unfolded across 8 days.
Positive Emotional Support
Several studies have documented the negative impact of the challenging profile of impairments and behaviors of adolescent or adult sons and daughters with FXS on the psychological and physical health of mothers (e.g., Abbeduto et al., 2004; Johnston et al., 2003). Yet there is considerable heterogeneity in the extent to which premutation carriers manifest health problems, so there is a need to examine sources of this heterogeneity—both with respect to vulnerability and resiliency. However, relatively little research has investigated mechanisms related to resiliency in premutation carriers, which is unfortunate as this information is needed to direct intervention. In research on the general population, subjective appraisals of the receipt of positive emotional support—defined as receiving empathy, concern, affection, love, encouragement, and acceptance (Langford, Bowsher, Maloney, & Lillis, 1997)—has been linked to resiliency in coping with a variety of types of stressors (e.g., Schaefer, Coyne, & Lazarus, 1981; Taylor, 2011; Thoits, 2011). These effects have been seen at both global (across months to year, e.g., Uchino, 2006) and daily (e.g., Gleason et al., 2008) levels. The receipt of positive emotional support has also been shown to be related to adaptive psychological and physical health in parents of children with neurodevelopmental disorders other than FXS such as autism spectrum disorder (ASD; Smith et al., 2012), ID (Gallagher & Whiteley, 2013) and attention deficit hyperactivity disorder (e.g., Lovell, Moss, & Wetherell, 2012). Positive emotional support may similarly play a role in resiliency for premutation carrier mothers of children with FXS.
CGG Repeat Length and Positive Emotional Support
The premutation expansion of the FMR1 gene (55 to 200 CGG repeats) is now recognized as being associated with its own spectrum of psychological and physical health problems depending on CGG repeat length (Hagerman & Hagerman, 2013; Roberts et al., 2016). CGG repeat length has been shown to be quantitatively associated with fragile-X-associated tremor ataxia syndrome in a linear way (e.g., Greco et al., 2006; Hessl et al., 2005). In contrast, CGG repeat length was found to have a curvilinear association with other conditions. Premutation carrier women with midrange CGG repeats were found to have an earlier age of menopause (Allen et al., 2007; Ennis, Ward, & Murray, 2006; Mailick, Hong, Greenberg, Smith, & Sherman, 2014; Spath et al., 2011; Sullivan et al., 2005), and more symptoms of depression and anxiety (Loesch et al., 2015; Roberts et al., 2009; Roberts et al., 2016) than those with a smaller or larger number of CGG repeats in the premutation range.
The molecular biological mechanism for the curvilinear association between CGG repeat and health outcomes in premutation carriers is not fully understood. Premutation carriers are known to have elevated levels of messenger ribonucleic acid (mRNA), which may be toxic (Berry-Kravis & Hall, 2011; Hoem et al., 2011). Within the premutation range, mRNA levels rise with increasing CGG repeats. As a result, those carriers with midrange numbers of CGG repeats have higher levels of mRNA than those with fewer CGG repeats within the premutation range. However, as the CGG repeat number approaches the full mutation (i.e., 200 repeats), the FMR1 gene becomes fully methylated and the level of mRNA declines. This explanation has been advanced to account for the curvilinear effect of CGG repeat length for early menopause, depressive symptoms, and anxiety.
Recent studies suggest that premutation carrier mothers’ CGG repeat length and other genetic features (e.g., activation ratio) interact with the environment to shape psychological and physical health (Hartley et al., 2012; Seltzer et al., 2012). Premutation carrier mothers with a midrange CGG length were found to be more sensitive to major life events (both positive and negative) than those with a lower or higher CGG repeat length. Compared to those with a lower or higher CGG repeat length, premutation carrier mothers with a midrange CGG length experienced more symptoms of depression and anxiety when exposed to a high number of negative life events (e.g., financial problems or problems at work), but also more symptoms of depression when exposed to a high number positive major life events (e.g., obtaining a new job or moving to a new house) in the previous year (Seltzer et al., 2012). Major life events, whether positive or negative, can be stressful and often require that individuals adapt in new ways (Miller, 2010); premutation carrier mothers with midrange CGG repeats appear to be particularly challenged by these major life stressors and transitions.
Premutation carrier mothers with midrange CGG length (as compared to lower or higher CGG length within the premutation range) were also found to evidence a lower cortisol awakening response (CAR) when exposed to a high number of negative life events in the past year (Seltzer et al., 2012). CAR is the normative increase in cortisol that occurs about 30 minutes after morning awakening and provides a biomarker of the reactivity of the hypothalamic-pituitary-adrenocortical (HPA) axis (Steptoe & Serwinski, 2016). A heightened CAR is generally seen as a normal response to stressful experiences.
However, individuals exposed to chronic stressors have been found to evidence reduced CAR in response to acute stressors. For example, maltreated children (Hart, Gunnar, & Cicchetti, 1995), adults experiencing work overload (Dahlgren, Akerstedt, & Kecklund, 2004), those who report a high lifetime exposure to stress (Lam, Sheilds, Trainor, Slavich, & Yonelinas 2018), and mothers of adults with ASD (Seltzer et al., 2010) have been found to exhibit a blunted CAR following acute stressors (i.e., a day with higher than average stress). Similarly, premutation carrier mothers of adolescents and adults with FXS are exposed to chronic parenting challenges and they have been shown to have a blunted CAR (Seltzer et al., 2012). A blunted CAR due to chronic stress exposure is posited to result from down-regulation of HPA reactivity and subsequently less secretion of cortisol in response to ongoing exposure to stressors (Gunnar & Vazquez, 2001; Miller, Chen, & Zhou, 2007). Blunted CAR is related to feeling fatigued and depressed, and to sleep disturbance (e.g., Chida & Steptoe, 2009; Wust et al., 2000).
In other populations, there is evidence that genetic status not only shapes the impact of negative environment factors, but can also shape sensitivity to positive environment factors, including positive support (e.g., Chen et al., 2011; Hostinar, Cicchetti, & Rogosch, 2014; Kaufman et al., 2006). For example, Chen and colleagues (2011) found that a single nucleotide polymorphism (rs53576) in the oxytocin receptor (OXTR) gene altered the protective effects of positive social support in the face of a stressful task; this variant has been associated with difficulties interpreting social cues and prosocial behavior (Hostinar et al., 2014), which may make it difficult for individuals to capitalize on positive social supports in their environment. It is possible that premutation carrier mothers’ CGG repeat length similarly shapes the extent to which they derive benefit from positive emotional support in their environment. The mechanism driving premutation carrier mothers with midrange CGG length to struggle with major life events (Seltzer et al., 2012), as well as their heightened risk for depressive symptoms and anxiety (e.g., Loesch et al., 2015; Roberts et al., 2009; Roberts et al., 2016) relative to those with low and high CGG length, may mean that these mothers also gain fewer advantages from positive emotional support in their everyday lives.
Current Study
The overarching goal of the current study was to understand the benefit of daily positive emotional support as it impacted the daily physical health of premutation carrier mothers of adolescents and adults with FXS, and to determine whether this benefit depended on CGG repeat length. Specifically, we examined whether CGG repeat length moderated the benefit of daily positive emotional support on next-day physical health for premutation carrier mothers. We analyzed data from an 8-day telephone daily diary completed each evening by 114 premutation carrier mothers of adolescent and adult children with FXS. On each day of the diary, premutation carrier mothers reported (1) the behavior problems manifested by their child with FXS, (2) the other daily stressors they experienced, (3) whether they received positive emotional support, and (4) the physical health symptoms they experienced that day. In addition, saliva samples were collected on days 2 through 5 of the diary study (including upon awakening and 30 minutes after awakening) to measure cortisol levels. Lagged models were used to allow us to examine the time-ordered effect of a day with versus without positive emotional support on next-day physical health.
Daily physical health was assessed subjectively via self-reported daily physical health symptoms and physiologically via CAR. Self-reported daily health symptoms (e.g., headache, muscle soreness, and constipation) is an indicator of perceived physical health, with a higher number of daily health symptoms interpreted as poorer experienced daily physical health. CAR provides a biomarker of HPA functioning (Steptoe & Serwinski, 2016), which has been shown to play a role in physical health as it is an indicator of physiological reaction to stress (Piazza, Almeida, Dimitrevia, & Kline, 2010). As noted earlier, a blunted CAR has been observed following acute stressors (or high stress days) in chronically stressed populations, including mothers of children with ASD (Seltzer et al., 2010) and premutation carrier mothers of children with FXS (Hartley et al., 2012; Seltzer et al., 2012). Thus, a lower CAR, relative to one’s own mean level, was interpreted as poorer daily health in the current study. CGG repeat length was measured through DNA analysis of blood samples, and analyzed as a curvilinear function. Daily number of behavior problems manifested by the adolescent or adult with FXS and number of other daily stressors experienced by the mother (e.g., arguments and stressors at work) were included in models to account for differences in daily stress.
The study questions were:
For premutation carrier mothers of adolescents and adults with FXS, does previous-day positive emotional support predict daily physical health from one-day-to-the-next at a within-person level?
Does CGG repeat length moderate the association between previous-day positive emotional support and daily physical heath?
In line with findings from other populations (e.g., Gallagher & Whiteley, 2013; Thoits, 2011), we hypothesized that receipt of positive emotional support would predict better next-day physical health (i.e., lower number of physical health symptoms and higher CAR) in premutation carrier mothers. Additionally, we hypothesized that premutation carrier mothers with midrange CGG repeat length would exhibit less benefit (i.e., less change in the number of physical health symptoms and CAR) following a day with positive emotional support as compared to premutation carrier mothers with lower or higher CGG repeats within the premutation range. This hypothesis was based on our own previous findings of gene by environment interactions, in which premutation carrier mothers with midrange CGG repeats derived less benefit from positive life events relative to those with low or high CGG repeats (Seltzer et al., 2012), perhaps due to greater risk for mood disorders (e.g., Roberts et al., 2009; Roberts et al., 2016).
Method
Participants
Study participants were drawn from a longitudinal study of 147 mothers of adolescent and adult children with the full mutation of FXS. Data for the present analyses were collected in 2009–2010, which was the first of the four waves of data collection (Seltzer et al., 2012). Families lived throughout the United States and one family lived in Canada. Mothers were included in this study if they were the biological mother of an adolescent or adult (aged 12+ years) child with FXS, either living in the same household or having at least weekly contact with this child. The child’s full mutation of FXS was verified by review of medical records of genetic testing. In families with multiple children with FXS, one target child was selected based on the following criteria and order: (a) the child who was ≥12 years, (b) who resided with mother, and (c) who was most severely affected child of those who had FXS. Of the 147 mothers in the study, 137 were premutation carriers (i.e., 55–200 CGG repeats), the focus of the present analysis. Of the remainder, five mothers had the full mutation of FXS (i.e., > 200 CGG repeats), three did not provide genetic testing results, and two had ambiguous genetic status. Of the 137 premutation carrier mothers, 114 completed the 8-day daily diary and were included in the present analyses. These 114 mothers did not differ from the 23 premutation carrier mothers who did not complete the 8-day daily diary in maternal education, family size, child age, partner relationship status, or target child global severity of behavior problems.
The majority of mothers had at least an associate’s degree (58.6%) and a median household income of $80,000 to $89,000 (range: < $9,999 to > $160,000). Mothers were aged 35.90 to 79.00 years at Time 1 (M = 50.30, SD = 7.52) and most were White, non-Latino (97%). On average, mothers had 2.50 children (SD = 1.21, range = 1 to 6), with 27% having more than one child with FXS. Of the 114 premutation carrier mothers, 23 (20%) were not in a partner relationship, 87 were married, and four were in a cohabiting relationship. The spouse/partner was the biological father of the son or daughter with FXS in 92% of mothers with a partner (n = 84). None of the partners had a family pedigree indicative of possible FXS, premutation, or FXS-related conditions. The target child with FXS was aged 12 to 49 years at Time 1 (M = 20.51; SD = 7.07), the majority were male (85%), had ID (83%), and lived in the family home (85%). Mothers whose son or daughter did not co-reside with them were in close and frequent contact with their son or daughter and with their care providers. A team of three research staff reviewed the daily diary reports of mothers who did not co-reside. In all cases, the daily diary reports of the son or daughter’s behavior problems were consistent with the frequency and type of problems reported to occur in a global measure.
Procedure
The 8-day daily diary was administered via nightly telephone interviews conducted by Pennsylvania State University Survey Center using the National Study of Daily Experiences protocol (NSDE; Almeida, 2005; Almeida, McGonagle, & King, 2009). On days 2 through 5 of the diary study, mothers collected saliva samples four times throughout the day—morning awakening, 30 minutes after getting out of bed, before lunch, and at bedtime. Saliva was collected using Sarstedt salivette collection devices. Salvia samples were sent in courier packages to our research office and stored at −60 °C. Mothers also had a blood sample taken at a local clinic using a preassembled kit to determine their CGG repeat size. Blood samples were sent by courier to Kimball Genetics, which conducted assays for CGG repeat length.
Measures
Sociodemographics.
Family sociodemographics were reported by mothers and included in models to control for their impact on between-person differences in initial level of daily physical health. Maternal age was coded in years. Maternal education was coded: 1 = less than high school;2 = high school graduate; 3 = some college; 4 =college graduate (4). The number of additional children with a disability in the family was defined as having other children with FXS or a related condition, a developmental disability, or a mental health condition. Partner status was reported by mothers and coded: 1 = married or co-habituating with partner; 0 = not married or co-habiting with partner. Child age was not included in models given that it was highly correlated with maternal age (r = .81, p < .001)
Daily child behavior problems and other daily stressors.
In order to control for within-person and between-person differences in daily stress, we included the number of behavior problems manifested by the target child with FXS and the number of other daily stressors experienced by mothers in our models. Behavior problems were assessed using a modified version of the Scales of Independent Behavior-Revised (SIB-R; Bruininks, Woodcock, Weatherman, & Hill, 1996) completed each day of the diary. Mothers reported on the presence (1 = present, 0 = absent) of eight types of behavior problems (self-injurious behavior, unusual or repetitive behaviors, withdrawn or inattentive behavior, behavior that is hurtful to others, property destruction, disruptive behavior, socially offensive behavior, and uncooperative behavior) during the previous 24 hours. Total number of types of behavior problems each day was used in analyses. This modified daily SIB-R has been used in previous studies (Hartley et al., 2012; Seltzer et al., 2010). On each day of the 8-day daily diary, mothers also reported on the presence (coded 1) versus absence (coded 0) of six other types of daily stressors in the past 24 hours including (a) having had an argument or disagreement, (b) avoiding having an argument or disagreement (i.e., something happened that they could have argued about but decided to let it pass), (c) stressor at work,(d) stressor at home, (e) stressor with friend or family, and (f) “other” stressors. These items were drawn from the Daily Inventory of Stressful Events (DISE; Almeida, Wethington, & Kessler, 2002) and have been previously published on (e.g., Hahn, Cichy, Small, & Almeida, 2013).
Positive emotional support.
On each day of the daily diary, mothers were asked “Did you receive any emotional support from anyone today?” Examples of positive emotional support were provided, including (a) someone listening to your problems, (b) receiving advice, or (c) receiving comfort. Positive emotional support was coded 1 = support was received and 0 = support was not received. This support could come from anyone (e.g., partner, friends, family, adult children, co-workers, etc.). This single item has been used in daily diary research in the general population (Cichy et al., 2014; Gleason et al., 2008; Iida, Seidman, Shrout, Fujita, & Bolger, 2008) and found to have strong concurrent and predictive criterion validity (Cichy et al., 2014; Gleason, Iida, Bolger, & Shrout, 2003; Gleason et al., 2008).
CGG repeat length.
As noted, mothers’ blood samples were shipped to Kimball Genetics laboratory which then assayed all samples for CGG repeat length. For the linear association, the number of CGG repeats was entered. For the curvilinear association, the quadratic function of number of CGG repeats was computed and entered. CGG was treated as a continuous variable in models; however, for post-hoc probing and graphing purposes, CGG repeat was divided into low (41% of sample; 67 to 89 CGGs), midrange (35% of sample; 90 to 105 CGGs), and high (24% of sample; 106 to 180 CGGs) repeats within the premutation range, based on cutoffs used in previous studies (e.g., Loesch et al., 2015; Mailick et al., 2014; Roberts et al., 2009).
Physical health symptoms.
On each day of the 8-day diary study, mothers reported their health symptoms using an adapted version of Larsen and Kasimatis’ (1991) symptom checklist. Mothers reported whether they had or had not experienced each of 28 health symptoms in the past 24 hours (no = 0, yes = 1). Health symptoms included headache, backache, muscle soreness, fatigue, joint pain, muscle weakness, dizziness, nausea, diarrhea, constipation, menstrual-related symptoms, and hot flashes or flushes. The summed total number of health symptoms reported to have occurred each day was used in analyses (possible range 0–28).
CAR.
Salivary cortisol samples were collected from mothers on days 2, 3, 4, and 5 of the diary study. Cortisol concentrations were quantified with luminescence immunoassay (IBL; Hamburg, Germany), and involved intraassay coefficient variations below 5% (Polk, Cohen, Doyle, Skoner, & Kirshbaum, 2005). For analysis, the salivettes were thawed and centrifuged at 3,000 rpm for 5 min. To calculate the CAR, we used area under the curve (AUC) from cortisol values upon waking to 30 min after waking. Extreme outliers (> 60 nmol/L) occurred in 13 samples (1% of total sample) and were recoded as 61 in line with recommendations (Dixson & Yuen, 1974).
Data Analysis Plan
Histograms were used to examine the distribution of key study variables and identify outliers. Pearson correlations were conducted to examine the associations among study variables and with sociodemographics (maternal age and education, partner status, household income, and presence of an additional child with a disability).
For the main analyses of this study, two multilevel models (MLMs; Bolger & Laurenceau, 2013) were conducted using Hierarchical Liner Modeling (Raudenbush, Byrk, Cheong, & Congdon,2011) to examine the two dependent variables (daily number of physical health symptoms and CAR). These analyses accounted for the within-person nested structure of the diary study, yet examined and controlled for between-person differences in intercept level of the dependent variables. MLMs were lagged to examine the temporal effect of previous-day positive emotional support on daily physical health, across the 8 days.
In light of previous findings (e.g., Loesch et al., 2015; Mailick et al., 2014; Roberts et al., 2009), our focus was on the curvilinear function of CGG repeat length. In line with recommendations (Aiken & West, 1991), the linear function of CGG repeat length was also entered in models. Level 1 variables included (a) day, (b) previous-day positive emotional support, and (c) previous-day morning awakening collection time for CAR. Level 2 variables included (a) maternal age, (b) maternal education, (c) number of additional children with a disability, (d) partner relationship status, (e) mean daily positive emotional support, and (f) CGG repeat length (linear and curvilinear). These variables were included at Level 2 to account for their between-person effects on initial daily physical health. In addition, we controlled for between-person differences and within-person fluctuation in daily level of behavior problems by the adolescent or adult with FXS and in number of other types of daily stressors experienced by mothers. Specifically, the number of previous-day behavior problems and daily stressors were included at Level 1 and the mean daily number of these variables across the 8 days were included at Level 2. Finally, to test whether the benefit of previous-day positive emotional support was moderated by CGG repeat length, the MLMs were re-run including interactions of previous-day positive emotional support x CGG repeat length (modeled as both a linear and curvilinear function) at Level 1. Level 1 continuous variables were person-centered, and Level 2 continuous variables were grand-mean centered. In the MLMs, effect size was calculated: r = sqrt [t2/(t2 + df)] and interpreted as small: r >.10, medium: r >.24 and large r >.37 (Kirk, 1996).
Results
Descriptive Findings
Daily receipt of positive emotional support, number of physical health symptoms, CAR, number of child with FXS’s behavior problems, and number of other daily stressors had a normal distribution without outliers. Table 1 provides the means, standard deviations, medians, and range for main study variables, and Table 2 presents correlations among these variables. On average, premutation carrier mothers reported receiving positive emotional support on 25% of the days (range: 0% to 100%). On average, the son or daughter with FXS had at least one episode of behavior problems on 52% of the days (range = 0% to 100%), and exhibited an average of 1.18 types of behavior problems each day. On average, mothers reported experiencing at least one other daily stressor on 55% of the days (range 1% to 100%), an average of 0.82 other daily stressors per day. The average CGG repeat length of mothers was 96.27 (SD = 20.40, range = 67 to 180). On average, premutation carrier mothers had 2.71 (SD = 2.56) daily physical health symptoms (range: 0 to 14), and reported at least one physical health symptom on 80% (SD = 0.27) of the days. The mean AUC for CAR was 17.66 (SD = 4.31, range = 1.29 to 35.41).
Table 1.
Means, Standard Deviations, Medians, and Range for Key Study Variables
| Study Variable | M (SD) | Median, Range |
|---|---|---|
| Number of Daily Stressors | 0.82 (0.56) | 0.75, 0–3.13 |
| Number of Child Behavior Problems | 1.18 (1.66) | 1.00, 0–8 |
| Positive Emotional Support | 0.25 (0.43) | 0.00, 0–1.00 |
| CGG Repeat | 96.27 (20.40) | 93.00, 67–180 |
| Number of Self-Reported Health Problems | 2.71 (2.56) | 2.00, 0–14 |
| AUC CAR | 17.66 (4.31) | 17.88, 1.29–35.41 |
Note. AUC CAR = area under the curve, cortisol awakening response; CGG = cytosine-guanine-guanine
Table 2.
Correlations Among Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Maternal Age | --- | ||||||||||
| 2. Maternal Education | .21* | --- | |||||||||
| 3. Number of Additional Children with Disability | −.08 | .02 | --- | ||||||||
| 4. Partner Status | .01 | .01 | .15 | --- | |||||||
| 5. Mean Daily Number of Child Behavior Problems | .00 | .15 | .21* | .00 | --- | ||||||
| 6. Mean Daily Number of Stressors | −.04 | .10 | −.00 | −.09 | .21* | --- | |||||
| 7. Mean Daily Positive Emotional Support | .07 | .13 | −.03 | .15 | .13 | .42** | --- | ||||
| 8. CGG Repeat - Linear | .08 | −.05 | −.04 | .08 | −.02 | −.18 | −.19* | --- | |||
| 9. CGG Repeat - Curvilinear | .05 | .06 | −.07 | .06 | −.04 | −.10 | −.14 | .69** | --- | ||
| 10. Mean Daily Number of Health Problems | .02 | −.12 | .07 | −.26* | .22* | .33** | .16+ | −.21* | −.17+ | --- | |
| 11. Mean Daily AUC CAR | −.04 | −.02 | −.21* | .06 | .03 | −.01 | .17+ | −.05 | .03 | −.05 | --- |
Note. AUC CAR= Area under the curve, cortisol awakening response; CGG = cytosine-guanine-guanine.
p < .09.
p < .05.
p < .01.
MLM Findings
Daily number of physical health symptoms.
The MLM predicting number of physical health symptoms was first conducted without the interaction terms (Table 3). In the hypothesized direction, there was a trend-level within-person effect of previous-day positive emotional support on number of health symptoms (−0.20, SE = 0.11, p = .08).
Table 3.
Multilevel Lagged Models Predicting Self-Reported Daily Number of Physical Health Symptoms (N = 114)
| Variable | Model | Model with CGG Interaction | ||
|---|---|---|---|---|
| Coefficient (SE) | Effect Size | Coefficient (SE) | Effect Size | |
| Within-Person Level 1 | ||||
| Intercept | 2.93 (0.43)** | .43 | 2.87 (0.49)** | .42 |
| Day | −0.04 (0.03) | .09 | −0.03 (0.03) | .09 |
| Previous-Day # Child Behavior Problem | 0.02 (0.05) | .03 | 0.02 (0.06) | .02 |
| Previous-Day # Stressors | 0.05 (0.07) | .05 | 0.03 (0.08) | .02 |
| Previous-Day Emotional Support | −0.20 (0.11)+ | .16 | −0.32 (0.17)* | .20 |
| X CGG Linear | ---------- | -- | 0.28 (0.19) | .12 |
| X CGG Curvilinear | ---------- | -- | −0.38 (0.18)* | .19 |
| Between-Person Level 2 | ||||
| Maternal Age | 0.02 (0.02) | .09 | 0.02 (0.02) | .11 |
| Maternal Education | −0.30 (0.11)* | .27 | −0.30 (0.10)** | .27 |
| Additional Children with Disability | 0.03 (0.26) | .01 | 0.02 (0.24) | .01 |
| Partner Status | −1.36 (0.52)* | .25 | 1.36 (0.54)* | .24 |
| Mean # Child Behavior Problem | 0.61 (0.18)** | .32 | 0.60 (0.18)* | .31 |
| Mean # Daily Stressors | 0.83 (0.39)* | .21 | 0.81 (0.38)* | .21 |
| Mean Emotional Support | −0.55 (0.77) | .07 | −0.52 (0.68) | .09 |
| CGG linear | 0.25 (0.21) | .12 | 0.28 (0.20) | .14 |
| CGG curvilinear | −0.03 (0.10) | .03 | −0.03 (0.06) | .06 |
Note. Effect size was calculated with the following equation: r = sqrt [t2/(t2 + df)]; small effect: r >.10, medium effect: r > .24 and large effect r >.37 (Kirk, 1996). df = degrees of freedom ranged from 105 to113. CGG curvilinear was computed as the square of centered CGG. We re-ran models to also include household income (coded as 1 to 14, starting at < $10K and going up by $10 to $20K intervals to $160K+) in Level 2. The pattern of effects remained the same. CGG = cytosine-guanine-guanine.
p ≤ . 09.
p ≤ .05.
p ≤ .01.
Table 3 also presents the results of the MLM predicting daily number of physical health symptoms with interaction terms included. There were significant between-person negative effects of maternal education and partner status on the intercept (i.e., initial level) number of daily physical health symptoms, such that mothers with lower levels of education and who did not have a partner had a greater number of daily physical health symptoms. These were medium size effects (r = .27 and .24). There were significant positive effects of mean daily number of behavior problems by the adolescent or adult with FXS and mean number of other daily stressors on the intercept number of daily physical health symptoms, such that mothers whose adolescent or adult child had more behavior problems and who experienced a greater number of other types of daily stressors had a greater number of daily health symptoms. These effect sizes were medium and small, respectively (r = .31 and r = .21). There were not significant between-person effects of maternal age, number of additional children with a disabilities, mean positive emotional support, or CGG repeat length (as a linear or curvilinear function) on the intercept of daily number of physical health problems.
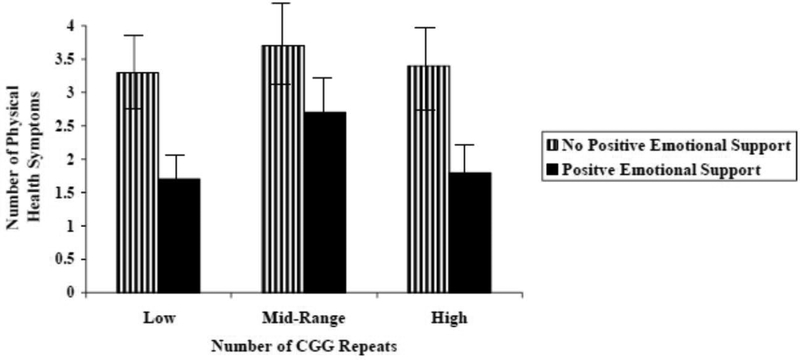
After controlling for between-person effects, CGG repeat (as a curvilinear function) significantly moderated the within-person association between previous-day positive emotional support and daily number of physical health symptoms. This was a small-sized effect (r = .19). Figure 1 shows this curvilinear moderating effect. For post-hoc probing purposes, CGG repeat was grouped into the following categories: low (67 to 89), mid (90–105), and high (106–180). As hypothesized, premutation carrier mothers with low (t = 2.27, p = .02) and high (t = 2.13, p = .03) CGG repeats evidenced fewer physical health symptoms on days following receipt of positive emotional support than following days when no emotional support was received. In contrast, for premutation carrier mothers with midrange CGG repeats, there was no significant difference in the number of physical health problems on days following receipt versus no receipt of positive emotional support (t = 1.51, p = .22). This finding suggests that premutation carrier mothers with midrange CGG repeat length benefited less from a day with positive emotional support than those with lower or higher CGG repeats.
Figure 1.
Number of self-reported physical health symptoms following a day with versus without positive emotional support for premutation carrier mothers with low (67 to 89 repeats), midrange (90 to 105 repeats), and high (106 to 180 repeats) CGG repeat length. Error bars (standard deviations) are depicted.
CAR.
The MLM predicting CAR was first conducted without the interaction terms (Table 4). In the hypothesized direction, there was a trend-level within-person effect of previous-day positive emotional support on CAR (0.52, SE = .28, p = .09). Table 4 presents the results of the MLM with the interaction terms. At a trend-level, there was a small-sized (r = .19) between-person negative effect of having additional children with a disability on the intercept (i.e., initial level) of CAR. Specifically, premutation carrier mothers who had more children with a disability had a lower initial level of CAR than did those with fewer children with a disability. There was also a trend-level small-sized (r = .17) positive effect of mean positive emotional support on the intercept (i.e., initial level) of CAR. Premutation carrier mothers who received positive emotional support on more days across the 8-day daily diary had a higher initial level of CAR than did those who received positive emotional support on fewer days. There were not significant between-person effects of maternal age, maternal education, mean number of behavior problems by the child with FXS, mean number of other daily stressors experienced by mothers, partner status, or CGG repeat length (curvilinear or linear function) on the intercept of daily number of CAR.
Table 4.
Multilevel Lagged Models Predicting Area Under the Curve (AUC) for Cortisol Awakening Response (CAR; N = 114)
| Variable | Model | Model with CGG Interaction | ||
|---|---|---|---|---|
| Coefficient (SE) | Effect Size | Coefficient (SE) | Effect Size | |
| Within-Person Level 1 | ||||
| Intercept | 17.43 (0.90)** | .87 | 17.65 (0.91)** | .88 |
| Day | −0.04 (0.08) | .05 | −0.04 (0.08) | .05 |
| Awake Cortisol Collection Time | 1.98 (0.18)** | .69 | 1.99 (0.18)** | .69 |
| Previous-Day # Child Behavior Problem | −0.06 (0.11) | .06 | −0.10 (0.10) | .06 |
| Previous-Day # Stressors | −0.20 (0.13) | .11 | −0.11 (0.16) | .04 |
| Previous-Day Emotional Support | 0.52 (0.28)+ | .16 | 0.31 (0.38) | .09 |
| X CGG Linear | ---------- | -- | −0.09 (0.57) | .08 |
| X CGG Curvilinear | ----------- | -- | 0.56 (0.28)* | .18 |
| Between-Person Level 2 | ||||
| Maternal Age | −0.05 (0.06) | .09 | −0.04 (0.06) | .08 |
| Maternal Education | −0.15 (0.20) | .08 | −0.17 (0.19) | .06 |
| Additional Children with Disability | −0.99 (0.52)+ | .18 | −0.99 (0.53)+ | .19 |
| Mean # Child Behavior Problem | 0.29 (0.45) | .07 | 0.29 (0.46) | .07 |
| Mean # Daily Stressors | 0.54 (0.78) | .07 | −0.53 (0.78) | .07 |
| Partner Status | 1.36 (0.94) | .14 | 1.24 (0.92) | .14 |
| Mean Emotional Support | 2.04 (1.23)+ | .16 | 2.51 (1.38)+ | .17 |
| CGG Linear | −0.08 (0.45) | .02 | −0.07 (0.46) | .01 |
| CGG Curvilinear | 0.03 (0.16) | .02 | 0.02 (0.16) | .01 |
Note. Effect size was calculated with the following equation: r = sqrt [t2/(t2 + df)]; small effect: r >.10, medium effect: r > .24 and large effect r >.37 (Kirk, 1996). df = degrees of freedom ranged from 106 to 113. CGG curvilinear = square of centered CGG. We re-ran models to also include household income (coded as 1 to 14, starting at < $10K and going up by $10 to $20K intervals to $160K+) in Level 2. The pattern of Level 1 effects remained the same. CGG = cytosine-guanine-guanine.
p ≤ . 09,
p ≤.05;
p ≤ .01.
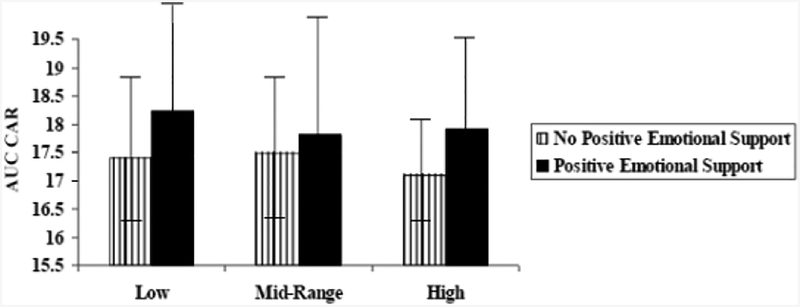
After controlling for between-person effects, CGG repeat as a curvilinear function significantly moderated the effect of previous-day positive emotional support on CAR (0.56, SE = 0.28, p = .05). As in the previous MLM for daily health symptoms, for post-hoc probing purposes, CGG repeat was grouped into the following categories: low (67 to 89), mid (90–105) and high (106–180). Premutation carrier mothers with low (t = 2.09, p = .05) and high (t = 2.19, p = .04) CGG repeats had a lower CAR on days following no positive emotional support than following a day with positive emotional support. In contrast, there was not a significant difference in CAR following a day with versus without positive emotional support for premutation carrier mothers with midrange CGG repeats (t = 1.10, p = .31). This small-sized moderation effect (r = .18) is depicted in Figure 2.
Figure 2.
Area under the curve (AUC) of cortisol awaking response (CAR) following a day with versus without positive emotional support for premutation carrier mothers with low (67 to 89 repeats), midrange (90 to 105 repeats), and high (106 to 180 repeats) CGG repeat length. Error bars (standard deviations) are depicted.
Discussion
Individuals with FXS often exhibit a challenging profile of impairments and behaviors (Cornish et al., 2008; Garber et al., 2008), which has been linked to elevated parenting stress (Johnston et al., 2003). Relatively little research has been aimed at understanding resiliency or the mechanisms that foster positive parent adaptation in the face of these child-related challenges. The current study examined the benefit of positive emotional support on daily physical health (number of physical health symptoms and CAR) in 114 premutation carrier mothers of adolescents and adults with FXS as these associations unfold in everyday life. The lagged design of our analysis (in which previous-day emotional support was used to predict next day health) guided our interpretation of the results.
Overall, findings indicated that positive emotional support is associated with resiliency—defined as adaptive physical health despite being faced with extraordinary child-related challenges—in premutation carrier mothers of adolescents and adults with FXS. However, our results showed that the benefit of positive emotional support is not the same for all premutation carrier mothers. Rather, those with midrange CGG repeat length (approximately 90 to 105 repeats) had a lower initial CAR and experienced less benefit from positive emotional support at a day-to-day level than those with lower and higher CGG repeat length within the premutation range.
In previous studies, a midrange CGG repeat length was associated with increased risk for depressive and anxiety symptoms in premutation carrier women (e.g., Loesch et al., 2015; Roberts et al., 2009; Roberts et al., 2016) even when they experience positive events (Seltzer et al., 2012). A vulnerability to mood symptoms may make premutation carrier mothers with midrange CGG repeats struggle with life transitions (even when seemingly positive) and less able to capitalize on positive emotional support in their everyday lives. Indeed, in other populations, depression and anxiety have been linked to (a) maladaptive information processing (e.g., selective attention to negative information and ignoring positive information), (b) cognitive appraisals and practices (e.g., negative beliefs about self, world, and future and rumination), and (c) interpersonal behaviors (e.g., reassurance seeking and reduced empathetic responding) that may undermine positive emotional support (e.g., by both reducing the quality of offered support and/or reducing one’s ability to gain advantages from positive support; see Beck & Bredemeier, 2016; Eysenck, 1997 Joiner, 2000; Joiner & Coyne, 1999).
Study Limitations and Strengths
There were both limitations and strengths to the current study. In terms of limitations, we used the NSDE protocol (Almeida, 2005; Almeida et al., 2009) including the single-item measure of received daily positive emotional support. This protocol and item have been widely used in research, and shown to have strong criterion validity (Cichy et al., 2014; Gleason et al., 2003; Gleason et al., 2008). However, this single item measure does not provide information about the quantity, quality, or type of received daily positive emotional support. Including such nuanced information in future studies could offer additional insight into current findings. For example, positive emotional support can be separated into distinct types such as esteem support, companionship, and caring, with some types more helpful than others (Rafaeli & Gleason, 20097). It is possible that the midrange CGG repeat length is associated with reductions in only some types of positive emotional support (e.g., esteem support) due to a heightened risk for mood disorders (e.g., Roberts et al., 2016). It is also possible that premutation carrier mothers with a midrange number of CGG repeats receive a low quantity or quality of daily positive emotional support, as having a high mean daily number of physical health symptoms may be wearing on partners, friends, and family. In the current study, we focused solely on the daily receipt of positive emotional support, not its qualitative dimensions. Other domains of social support (practical and instrumental) should also be explored in future research. Our study is also limited in that our sample was also not representative of the broader population, as it was largely White, non-Latino, and of middle socioeconomic status. In addition, all sample members had children with FXS; findings may not reflect patterns seen in premutation carrier women who do not have a child with FXS.
In terms of study strengths, a micro-longitudinal daily diary methodology allowed us to capture the time-order effect of positive emotional support on daily physical health in a natural context. Specifically, lagged-day (as opposed to same-day) models allowed us to examine how a day with relatively low or high positive emotional support was associated with next-day physical health. Physical health was assessed using both a self-reported measure (physical health symptoms) and physiological marker of HPA functioning (CAR), with converging patterns. Moreover, the number of behavior problems of the adolescent or adult child with FXS, having additional children with disabilities, and the number of daily stressors experienced by the mother were included in MLMs to account for both between-person and within-person differences in daily stress.
Implications
The current study provides evidence that positive emotional support influences next-day physical health in premutation carrier mothers. However, it is likely that positive emotional support and physical health are linked in reciprocal transactional ways; overtime, poor physical health may lead to less offered positive emotional support by others. This opposite direction of effects, and moderators of this pathway, need to be examined in future studies. In the present study, premutation carrier mother CGG repeat length was not associated with the mean number of child behavior problems, however, it possible that has meaningful associations with other aspects of child functioning and this may underlie findings in the current study. This is a topic that has received little attention in research to date.
Our findings have important implications for research and intervention planning, adding to growing evidence from other populations that positive environmental factors, such as the receipt of positive emotional support, do not benefit all individuals equally. Instead the benefit of a day with relatively high positive emotional support on the next-day physical health of premutation carrier mothers of adolescents and adults with FXS was shaped by genetic status. Although at the present time, few people are aware of their FMR1 CGG repeat number, this is likely to change as we approach the era of personalized or precision medicine (e.g., Shukla, Murali, & Brilliant, 2015), and as individuals are increasingly seeking out information about their own genetic status. Therefore, understanding how environmental factors, such as the receipt of positive emotional support, differentially affect individuals depending on their genetic profile will become increasingly relevant in health-care planning. Further research is needed to understand why positive emotional support may not have the same benefits for premutation carrier mothers with a midrange number of CGG repeats, so interventions can be tailored accordingly.
Acknowledgments
Thanks to Kimball Genetics for genetic testing and the Kirschbaum Laboratory for cortisol assays. Thanks to the National Fragile X Foundation for allowing us to share informational materials with families.
The National Institute of Child Health and Human Development provided funding to the Intellectual and Developmental Disability Research Center at the University of North Carolina (P30 HD003100-S1) to support Fragile X Research Centers. This study was based on data at the University of Wisconsin-Madison Waisman Center site (M. Mailick). Continued support was obtained from R01 HD082110 (to M. Mailick), and a core grant to the Waisman Center from the National Institute of Child Health and Human Development (U54 HD090256).
References
- Abbeduto L, Seltzer MM, Shattuck PT, Krauss MK, Orsmond GI, & Murphy MM (2004). Psychological well-being and coping in mothers of youths with autism, Down syndrome, or fragile X syndrome. American Journal on Mental Retardation, 9, 237–254. doi: [DOI] [PubMed] [Google Scholar]
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, & Sherman SL (2007). Examination of reproductive aging milestones among women who carry the FMR1 premutaiton. Human Reproduction, 22, 2142–2152. doi: 10.1093/humanrep/dem148. [DOI] [PubMed] [Google Scholar]
- Almeida DM (2005). Resilience and vulnerability to daily stressors assessed via diary methods. Current Directions in Psychological Science, 14, 64–68. doi: 10.1111/j.0963-7214.2005.00336.x [DOI] [Google Scholar]
- Almeida DM, McGonagle K, & King H (2009). Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology, 55, 220–238. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Wethington E, & Kessler RC (2002). The daily inventory of stressful events: An interview-based approach for measuring daily stressors. Assessment, 9, 41–55. doi: 10.1177/1073191102091006 [DOI] [PubMed] [Google Scholar]
- Beck AT, & Bredemeier K (2016). A unified model of depression: Integrating clinical, cognitive, biological, and evolutionary perspectives. Clinical Psychological Science, 4(4), 595–619. doi: 10.1177/2167702616628523 [DOI] [Google Scholar]
- Berry-Kravis E, & Hall DA (2011). Executive dysfunction in young FMR1 premutation carriers: Forme fruste of FXTAS or new phenotype? Neurology, 77(7), 612–613 doi: 10.1212/WNL.0b013e3182299f98 [DOI] [PubMed] [Google Scholar]
- Bolger N, & Laurenceau J-P. (2013). Intensive longitudinal methods: An introduction to diary and experience sampling research. New York, NY: Guilford. [Google Scholar]
- Brown WT (2002). The molecular biology of fragile X mutation In Hagerman R & Hagerman PJ, (Eds.). Fragile X syndrome: Diagnosis, treatment, and research (3rd ed.; pp. 110–135) Baltimore, MD: John Hopkins University Press. [Google Scholar]
- Bruininks RH, Woodcock R, Weatherman R, & Hill BK (1996). Scales of Independent Behavior-Revised. Park Allen, TX: DLM Teaching Resources. [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, & Heinrichs M (2011). Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proceedings of the National Academy of Sciences of the United States of America, 108, 19937–19942. doi: 10.1073/pnas.11130791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, & Stipoe A (2009). Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology, 80, 265–278. doi: 10.1016/j.biopsycho.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Cichy KE, Stawski RS, & Almeida DM (2014). A double-edged sword: Race, daily family support exchanges, and daily well-being. Journal of Family Issues, 35(13), 1824–1845. doi: 10.1177/0192513X13479595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Turk J, & Hagerman R (2008). The fragile X continuum: New advances and perspectives. Journal of Intellectual Disability Research, 52, 469–482. doi: 10.1111/j.1365-2788.2008.01056.x [DOI] [PubMed] [Google Scholar]
- Dahlgren A, Akerstedt T, & Kecklund G (2004). Individual differences in the diurnal cortisol response to stress. Chronobiology International, 21, 913–933. doi: 10.1081/CBI-200035937 [DOI] [PubMed] [Google Scholar]
- Dixson WJ, & Yuen KK (1974) Trimming and winsorization: A review. Statistical Papers, 15, 157–170. doi: 10.1007/BF02922904 [DOI] [Google Scholar]
- Ennis S, Ward D, & Murray A (2006). Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. European Journal of Human Genetics, 14, 253–255. doi: 101938/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW (1997). Anxiety and cognition: A unified theory. London, UK: Psychological Press. [Google Scholar]
- Gallagher S, & Whiteley J (2013). The association between stress and physical health in parents caring for children with intellectual disabilities is moderated by children’s challenging behaviours. Journal of Health Psychology, 18, 1220–1231. doi: 10.1177/1359105312464672. [DOI] [PubMed] [Google Scholar]
- Garber KB, Visootak J, & Warran ST (2008). Fragile X syndrome. European Journal of Human Genetics, 16, 666–672. doi: 10.1038/ejhg.2008.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason GE, Iida M, Bolger N, & Shrout PE(2003). Daily support equity in close relationships. Personality and Social Psychology Bulletin, 29, 1036–1045. doi: 10.1177/0146167203253473 [DOI] [PubMed] [Google Scholar]
- Gleason GEJ, Iida M, Shrout PE, & Bolger N (2008). Receiving support as a mixed blessing: Evidence for dual effects of support on psychological outcomes. Journal of Personality and Social Psychology, 94(5), 824–838. doi: 10.1037/0022-3514.94.5.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, … Hagerman PJ (2006). Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain, 129, 243–255. doi: 10.1093/brain/awh683 [DOI] [PubMed] [Google Scholar]
- Gunnar MT, & Vazquez DM (2001). Low cortisol and a flattening of the expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13, 515–538. doi: 10.1017/S0954579401003066 [DOI] [PubMed] [Google Scholar]
- Hagerman R, & Hagerman P (2013). Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurology, 12, 786–798. doi: 10.1016/S1474-4422(13)70125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, & Tranfaglia M (2009). Advances in the treatment of fragile X syndrome. Pediatrics, 123, 378–390. doi: 10.1542/peds.2008-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EA, Cichy KE, Small BJ, & Almeida DM (2013). Daily emotional and physical reactivity to stressors among widowed and married older adults. The Journals of Gerontology, 69B, 19–28. doi: 10.1093/geronb/gbt035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Gunnar M, & Cicchetti D (1995). Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Developmental and Psychopathology, 7, 11–26. doi: 10.1017/S0954579400006313 [DOI] [Google Scholar]
- Hartley SL, Seltzer MM, Hong J, Greenberg JS, Smith L, Almeida D, & Abbeduto L (2012). Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: A diathesis-stress model. International Journal of Behavioral Development, 36, 53–64. doi: 10.1177/0165025411406857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW,… Hagerman RJ (2005). Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. American Journal of Medical Genetics Part B, 139B, 115–121. doi: 10.1002/ajmg.b.30241 [DOI] [PubMed] [Google Scholar]
- Hoem G, Raske CR, Garcia-Arocena G, Tassone F, Sanchez E, Ludwig A, … Hagerman PJ (2011). CGG-repeat length threshold for FMR1 RNA pathogenesis in a cellular model for FXTAS. Human Molecular Genetics, 20, 2161–2170. doi: 10.1093/hmg/ddr101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida M, Seidman G, Shrout PE, Fujita K, & Bolger N (2008). Modeling support provision on intimate relationships. Journal of Personality and Social Psychology, 94, 460–478. doi: 10.1037/0022-3514.94.3.460 [DOI] [PubMed] [Google Scholar]
- Johnston C, Hessl D, Blasey C, Eliez S, Erba H, Dyer-Friedman J, …Reiss AL (2003). Factors associated with parenting stress in mothers of children with fragile X syndrome. Journal of Developmental and Behavioral Pediatrics, 24(4), 267–275. [DOI] [PubMed] [Google Scholar]
- Joiner TE (2000). Depression’s vicious scree: Self-propagating and erosive processes in depression chronicity. Clinical Psychology: Science and Practice, 7, 203–218. doi: 10.1093/clilpsy.7.2.203 [DOI] [Google Scholar]
- Joiner TE, & Coyne JC (1999). The interactional nature of depression: Advances in interpersonal approaches. Washington, DC: American Psychological Association. doi: 10.1037/10311-000 [DOI] [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Grasso, Lipschitz D, Houshyar S, … Gelernter J (2006). Brain-derived neurotropic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry, 59, 673–680. doi: 10.1016/j.biopsych.2005.10.026 [DOI] [PubMed] [Google Scholar]
- Kirk RE (1996). Practical significance: A concept whose time has come. Educational and Psychological Measurement, 56(5), 746–759. doi: 10.1177/0013164496056005002 [DOI] [Google Scholar]
- Lam J, Shields GS, Trainor BC, Slavich GM, & Yonelinas AP (2018). Greater lifetime stress exposure predicts blunted cortisol but heightened DHEA response to acute stress. Stress and Health, 12, 1–12. doi: 10.1002/smi.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford CPH, Bowsher J, Maloney JP, & Lillis PP (1997), Social support: A conceptual analysis. Journal of Advanced Nursing, 25, 95–100. doi: 10.1046/j.1365-2648.1997.1997025095.x [DOI] [PubMed] [Google Scholar]
- Larsen RJ, & Kasimatis M (1991). Day-to-day symptoms: Individual differences in the occurrence, duration, and emotional concomitants of minor daily illnesses. IEEE International Workshop on Performance Evaluation of Tracking and Surveillance, 59(3), 387–399. doi: 10.111/j.1467-6494.1991.tb00254.x [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Bui MQ, Hammersley E, Schneider A, Storey E, Stimpson P, … Hessl D (2015). Psychological status in female carriers of premtuation FMR1 allele showing a complex relationship with size of CGG expansion. Clinical Genetics, 87, 173–178. doi: 10.111/cage12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell B, Moss M, & Wetherell MA (2012). With a little help from my friends: Psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Research in Developmental Disabilities, 33, 682–687. doi: 10.1016/j.ridd.2011.11.014 [DOI] [PubMed] [Google Scholar]
- Mailick MR, Hong J, Greenberg J, Smith L, & Sherman S (2014). Curvilinear association of CGG repeats and age at menopause in women with FMR1 premutation expansions. American Journal of Medical Genetics Part B, 165B, 705–711. doi: 10.1002/ajmg.b.32277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi: 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Miller TW (Ed.). (2010). Handbook of stressful transitions across the lifespan. New York, NY: Springer. [Google Scholar]
- Piazza JR, Almeida DM, Dmitrieva NO, & Klein LC (2010). Frontiers in the Use of Biomarkers of Health in Research on Stress and Aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 65B, 513–525. doi: 10.1093/geronb/gbq049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk DE, Cohen S, Doyle WJ, Skoner DP, & Kirschbaum C (2005). State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology, 30, 261–272. doi: 10.1016/j.psyneuen.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Rafaeli E, & Gleason MEJ (2009). Skilled support within intimate relationships. Journal of Family Theory and Review, 1, 20–37. doi: 10.1111/j.1756-2589.2009.00003.x [DOI] [Google Scholar]
- Raudenbush SW, Byrk AS, Cheong YF, & Congdon RT (2011). HLM7: Hierarchical Linear and Nonlinear Modeling. Chicago, IL: Scientific Software International. [Google Scholar]
- Roberts JE, Bailey DB, Mankowski J, Ford A, Sideris J, Weisenfeld LA,& Golden RN (2009). Mood and anxiety disorders in females with the FMR1 premutation. American Journal of Medical Genetics Part B, 150B, 130–139. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, McCary LM, Ford AL, Golden RN, & Bailey DB Jr. (2016). Trajectory and predictors of depression and anxiety disorders in mothers with the FMR1 Premutation. Biological Psychiatry, 79, 850–857. doi: 10.1016/j.biopsych.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarimski K (1997). Behavioural phenotypes and family stress in three mental retardation syndromes. European Child and Adolescent Psychiatry, 6, 26–31. doi: 10.1007/BF00573637 [DOI] [PubMed] [Google Scholar]
- Schaefer C, Coyne JC & Lazarus RS (1981). The health-related functions of social support. Journal of Behavioral Medicine, 4, 381–406. doi: 10.1007/BF00846149 [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Barker ET, Greenberg JS, Hong J, Coe C, & Almeida D (2012). Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychology, 31(5), 612–622.doi: 10.1037/a0026528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith LE, Almeida DM, Coe C, & Stawski RS (2010). Maternal cortisol levels and behavior problems in adolescents and adults with ASD. Journal of Autism and Developmental Disorders, 40, 457–469. doi: 10.1007/s10803-009-0887-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SK, Murali NS, & Brilliant MH (2015). Personalized medicine going precise: from genomics to microbiomics. Trends in Molecular Medicine 21, 461–462. doi: 10.1016/j.molmed.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Greenberg JS, & Seltzer MM (2012). Social support and well-being at mid-life among mothers of adolescents and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42,1818–1826. doi: 10.1007/s10803-011-1420-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath MA, Feuth TB, Smits AP, Yntema HG, Braat DD, Thomas CM,… Allen EG (2011). Predictors and risk model development for menopausal age in fragile X premutation carriers. Genetics in Medicine, 13, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, & Serwinski B (2016). Cortisol awakening response In Fink G (Ed.), Stress: Concepts, Cognition, Emotion, and Behavior. London, UK: Elsevier Academic Press. [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, & Sherman SL (2005). Association of FMR1 repeat size with ovarian dysfunction. Human Reproduction, 20, 402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- Taylor SE (2011). Social support: A review In Friedman HS (Ed.), Oxford handbook of health psychology (pp. 189–214). New York, NY: Oxford University Press [Google Scholar]
- Thoits PA (2011). Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior, 52, 145–161. doi: 10.1177/0022146510395592 [DOI] [PubMed] [Google Scholar]
- Uchino BNJ (2006). Social support and health: A review of physiological processes potentially underling links to disease outcomes. Journal of Behavioral Medicine, 29, 377–387. doi: 10.1007/s10865-006-9056-5 [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, & Kirschbaum C (2000). The cortisol awakening response: Normal values and confounds. Noise and Health, 2, 79–88. doi: http://www.noiseandhealth.org/text.asp?2000/2/7/79/31739 [PubMed] [Google Scholar]