Abstract
Escherichia coli encoding colibactin (clb), cytolethal distending toxin (cdt), and hemolysin-associated cytotoxic necrotizing factor (cnf) are associated with various intestinal and extra-intestinal diseases in humans and animals. Small mammal pets are not evaluated for genotoxin-encoding E. coli. Thus, the prevalence of such strains is unknown. The objective of this study was to isolate and characterize genotoxin-encoding E. coli from healthy and ill small mammal pets examined at a veterinary clinic and at two animal adoption centers. E. coli isolates were cultured from fecal samples and biochemically characterized. A total of 65 animals, including mice, rats, rabbits, guinea pigs, and hedgehogs, were screened. Twenty-six E. coli isolates were obtained from 24 animals. Twelve of the 26 isolates (46.2%) were PCR-positive for the pks genes clbA and clbQ. Two isolates (7.7%) were PCR-positive for cnf. All isolates were PCR-negative for cdt. All genotoxin-encoding isolates belonged to the pathogen-associated phylogenetic group B2. Representative genotoxin-encoding isolates had serotypes previously associated with clinical disease in humans and animals. Isolates encoding pks or cnf induced megalocytosis and cytotoxicity to HeLa cells in vitro. Although most isolates were obtained from healthy pets, two guinea pigs with diarrhea had pks-positive isolates cultured from their feces. Whole genome sequencing on four representative isolates confirmed the presence of pks and cnf genes and identified other virulence factors associated with pathogenicity in animals and humans. Our results suggest that small mammalian pets may serve as a reservoir for potentially pathogenic E. coli and implicate a zoonotic risk.
Keywords: colibactin, pks, cnf, cytotoxin, E. coli, pets, rodents, hedgehogs, rabbits
Introduction
Escherichia coli are a diverse group of gram-negative, facultatively anaerobic, rod-shaped bacteria of the family Enterobacteriaceae. Commensal strains are normally present in the lower intestinal tract of healthy humans and animals, but pathogenic enteric and extra-intestinal strains also exist, and have been associated with a wide range of harmful conditions, including septicemia, meningitis, hemorrhagic colitis, hemolytic-uremic syndrome (HUS), renal injury, and colorectal cancer.
E. coli can be categorized by phylogenetic groups (A, B1, B2, and D) for epidemiological characterization(Lee et al., 2018). Strains belonging to groups B2 and D are typically pathogenic, while A and B1 strains are usually avirulent(Patricia et al., 2006). Furthermore, pathogenic E. coli have been defined by multiple enteric pathotypes, including enterotoxigenic E. coli (ETEC), enterohemorrhagic E. coli (EHEC), enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAEC), Shiga-toxin producing enteroaggregative E. coli (STEAEC), diffusely adhering E. coli (DAEC), adherent-invasive E. coli (AIEC), and enteroinvasive E. coli (EIEC).
Pathogenic E. coli strains encode virulence genes and pathogenicity islands that promote bacterial invasion and colonization, evasion of host defenses, and damage to eukaryotic host cells. Virulence factors genes including genotoxin colibactin (pks), cytotoxic necrotizing factor (cnf) and cytolethal distending toxin (cdt) have been shown to induce intestinal and extra-intestinal diseases by E. coli belonging to phylogenetic groups B2 and D.
Colibactin, a hybrid peptide-polyketide genotoxin, is encoded by a cluster of clb genes within a 54-kb genomic island called pks, and is found in 30 to 40% of phylogenetic group B2 E. coli strains(Putze et al., 2009). Colibactin causes DNA double-stranded breaks, chromosome instability, cell cycle arrest, and cellular senescence. E. coli encoding clb genes are associated with bacterial meningitis, septicemia, genitourinary tract infections, gastrointestinal inflammation and colorectal tumors in humans and rodents(Nougayrede et al., 2006; Buc et al., 2013; Mccarthy et al., 2015; Garcia et al., 2016; Bakthavatchalu et al., 2018). Colibactin-derived DNA adducts form in human cells and mice colonized with pks+ E. coli. DNA alkylation induced by colibactin has been demonstrated in vivo, further supporting its connection in cancer development or progression.(Wilson et al., 2019)
Cytotoxic necrotizing factor (CNF) is a 115 kDa cyclomodulin protein that is expressed by approximately 40% of UPEC isolates and up to 30% of diarrheal E. coli isolates(Hofman et al., 2000). Necrotoxigenic E. coli (NTEC) express either the cnf1 or cnf2 homolog, encoded on the chromosome or a plasmid, respectively(Falbo et al., 1993). Both classes of CNF toxins cause cell cycle alterations, cytoskeletal changes such as micropinocytosis, megalocytosis, and multinucleation, as well as enlargement, multinucleation and rounding of HeLa cells(Rosadi et al., 2016). CNF-harboring E. coli have been associated with urinary tract infections (UTIs), septicemia and enteritis in humans, diarrhea in pigs, septicemia and diarrhea in calves and lambs, as well as healthy animals such macaques(De Rycke et al., 1990; Martin et al., 2009; Feng et al., 2017).
Cytolethal distending toxin (CDT) is a heterotrimeric AB-type DNAse genotoxin that causes irreversible G1 or G2 cell cycle arrest, megalocytosis, and apoptosis(Jinadasa et al., 2011). The CDT gene cluster consists of three adjacent genes called cdtA, cdtB and cdtC, which are found on the chromosome, and EcolCdtB-III, encoded by an operon located on a large conjugative plasmid(Jinadasa et al., 2011). Various EPECs expressing CDT have been isolated from patients with gastrointestinal infections, UTIs, and sepsis, as well as from healthy cattle and swine(Pandey et al., 2003; Hinenoya et al., 2014).
Previously, our laboratory has detected potentially pathogenic E. coli isolates encoding these cytotoxins in laboratory animals, including mice, rats, and macaques(Garcia et al., 2016; Feng et al., 2017; Kurnick et al., 2019). However, the prevalence of specific E. coli strains in small mammal pets is unknown. Furthermore, these E. coli strains may pose a zoonotic risk to pet owners and others exposed to small mammals, such as veterinary health care professionals. In this study, we hypothesized that small mammal pets would harbor E. coli strains encoding known virulence factors such as pks, cnf, and cdt and therefore may have pathogenic and zoonotic potential.
Materials and Methods
Animals
A total of 65 animals were included in this study. Forty of the animals were client-owned pets seen at a private practice, and the remaining 25 were animals available for adoption at two non-profit animal adoption centers in Massachusetts. Client-owned pets included healthy animals presenting for wellness exams as well as those presenting for various illnesses, including non-specific clinical signs. Adoption center animals were examined and apparently healthy but their previous medical histories were unknown. Animals included 36 guinea pigs, 9 rats, 12 rabbits, 4 hamsters, 2 mice, and 2 hedgehogs. Animals ranged in age from 9 weeks to 8 years and included both males and females. A physical examination and same-day fecal sample collection were performed for each animal by a veterinarian (NF) between 2016-2018. Authorization to collect fecal samples was obtained from pet owners as well as from the director of the adoption centers. Additionally, a 4-year-old female Dunkin-Hartley laboratory guinea pig housed at an academic research institution was evaluated after having acute weight loss, polyuria/polydipsia and diarrhea.
Fecal samples, bacterial culture, and biochemical characterization
Fecal pellets were placed in freeze media (Brucella broth with 20% glycerol) immediately after collection and stored frozen at −80 °C until further use. Samples were then thawed at room temperature, homogenized and plated onto Remel MacConkey lactose agar (media selective for gram-negative bacteria and differential for lactose fermentation). A four-quadrant isolation streak was performed, and plates were incubated at 37 °C (5% CO2) overnight. To maximize the probability of obtaining all E. coli isolates, the swab and remaining fecal slurry for each sample were placed into a tube containing sterile gram-negative (BD, Sparks, MD, USA) broth, which was subcultured after 24 hour incubation onto another MacConkey agar plate.
Bacterial colonies displaying positive lactose fermentation (LF+) were identified on MacConkey agar by their characteristic bright pink coloration. The LF+ colonies were isolated with sterile loops and plated on Remel sheep blood agar plates. Plates were incubated at 37 °C (5% CO2) overnight. E. coli isolates were identified by their grey to dark grey coloration and smooth margins. Isolates were evaluated for beta hemolysis. Cultures were further characterized by API® 20E (Biomérieux, Cambridge, MA, USA). A loop of each of the 26 E. coli isolates grown overnight on sheep blood agar plates was placed in 400 μl of sterile phosphate-buffered saline (PBS) in a sterile Eppendorf tube, centrifuged at 12,000 rpm for 5 minutes and the resulting bacterial pellets were stored at −20 °C until DNA extraction was performed.
E. coli control strains
Control E. coli strains NC101 (pks+/cnf−/cdt−), 06-2830 (cnf+), a laboratory rat isolate (pks+/cnf+/cdt−), V27 (pks+/cnf−/cdt+), and K12 (pks−/cnf−/cdt−) were used for conventional PCR, phylogenetic grouping PCR, and HeLa cell cytotoxicity assays. NC101, a commensal mouse strain, was acquired from Christian Jobin’s lab(Feng et al., 2017). 06-2830 was acquired from Martin et al. from a clinically normal macaque(Martin et al., 2009). The laboratory rat isolate was acquired from Kurnick et al. (Kurnick et al., 2019). V27, isolated from a human urosepsis patient, was acquired from the E. coli Reference Center(Feng et al., 2017). K12 was acquired from David B. Shauer’s collection: catalog samples DBS 83.
DNA extraction, PCR amplification, and sequencing
The High Pure PCR Template Preparation Kit (Roche Molecular Biochemicals, Indianapolis, IN, USA) was used for bacterial DNA extraction from pelleted bacteria in phosphate buffered saline (PBS). The commercial kit illustra™ puReTaq Ready-to-Go™ PCR Beads (GE Healthcare, Buckinghamshire, UK) was used for PCR. Two sets of primers (clbA and clbQ) were used to identify pks genes(Feng et al., 2017). Multiplex PCR was used to amplify cnf and cdt genes. Five sets of primers (for svg, chuA, yjaA, uidA, and TspE4.C2 genes) were used in multiplex PCR to determine the phylogroup of each isolate(Feng et al., 2017). The phylogenetic groups were determined by the PCR gel pattern. PCR primers were designed to amplify the 3’end of wildtype clbJ (clbJF: 5’ CAA-CGC-TCA-GCG-TAT-TCA-GGT-G /clbJF: 5’ ACT-CCC-TAC-GCT-GTC-GAG-CTG-TT) and the 5’ end of wildtype clbK (clbKF: 5’ GTT-CAC-CAG-CTT-GCT-CAA-TCA-AT /clbKR: 5’ GTG-GCT-CCT-TGA-GTT-TGA-ACG-CT) using 1 ng of template DNA (PCR program: 95 °C for 2 minutes; 35 cycles of 95 °C for 1 minutes, 55 °C for 1 minutes, and 72 °C for 1.5 minutes; 72 °C for 5 minutes) using Taq polymerase from the Expand™ High Fidelity PCR System kit (Roche Molecular Biochemicals). The hybrid clbJ-K gene was amplified using primers clbJF/clbKR with 0.5 ng of template DNA (PCR program: 95 °C for 2 minutes; 35 cycles of 95 °C for 1 minutes, 55 °C for 0.5 minutes, and 72 °C for 1.5 minutes; 72 °C for 5 minutes) using Taq polymerase from the Expand™ High Fidelity PCR System kit (Roche Molecular Biochemicals).
Serotyping
Fourteen E. coli isolates (10 pks+/cnf−/cdt−, 2 pks+/cnf+/cdt−, and 2 pks−/cnf−/cdt−) from clinic patients and animals at adoption centers were submitted to the E. coli Reference Center at Penn State University for serotype testing, including O and H typing and PCR analyses for heat-labile toxin (elt), heat-stabile toxin (estA and estB), Shiga-type toxin 1 and 2 (stx1 and stx2), intimin gamma (eae) and cytotoxic necrotizing factor 1 and 2 (cnf1 and cnf2).
Cell culture assay for colibactin cytotoxicity
Twelve pks+ E. coli isolates were evaluated. The cytotoxicity assay was performed as previously described with modifications(Feng et al., 2017). HeLa S3 cells (ATCC CCL2.2) were grown and maintained in Eagle’s Minimum Essential Medium (EMEM, Gibco, Grand Island, NY, USA) containing 10% fetal calf serum (FCS, Gibco) and 1% antibiotic-antimycotic (Gibco) at 37 °C with 5% CO2. 5 × 103 cells were seeded onto 96-well cell culture plates and incubated at 37 °C degrees with 5% CO2 for 24 h. Overnight liquid cultures of E. coli strains were grown in LB (Luria-Bertani, BD) broth for 2 hours at 37 °C and then adjusted to O.D. 600 nm of 0.0001, 0.0005, 0.0025 and 0.01 in 1% FCS EMEM media, corresponding to a multiplicity of infection (MOI; the number of bacteria per cell at the onset of infection) of 1, 5, 25, and 100, respectively. Following inoculation, plates were centrifuged at 200 g for 10 min to facilitate bacteria interaction and then incubated at 37 °C with 5% CO2 for 4 h. Cells were then washed with EMEM and replaced with EMEM containing 10% FCS and 200 ug/mL gentamicin (Gibco). Following 72 h incubation, plates were stained with Diff-quick stain (Thermo Fisher Scientific, Kalamazoo, MI, USA). Cells were then evaluated microscopically for confluence and morphological changes. Images were captured with a Zeiss Axiovert-10 microscope using Image Pro-Plus software version 7.0 at 20x magnification.
Cell culture for sonicate cytotoxicity
Overnight cultures of E. coli strains were pelleted by centrifugation at 12,000 rpm for 5 min. The pellets were washed in 1 ml of PBS and pelleted again by centrifugation at 12,000 rpm for 5 min. Pellets were resuspended in 2 ml of PBS and then sonicated on ice using the follow protocol: amplitude: 35; power: 7 W; 30 s intervals for a total of 5 min with 1 min breaks between intervals. Sonicate samples were centrifuged at 12,000 rpm for 10 min at 4 °C to remove large debris and then filter-sterilized through 0.2 μm filters. Total protein was quantified using the BCA assay (Thermo Scientific, Rockford, IL, USA). 5 × 103 HeLa cells were seeded onto 96-well cell culture plates and incubated at 37 °C with 5% CO2 for 24 h. Cells were treated with 5 μg of total protein of crude bacterial sonicate for 72 h. Plates were stained and cells were microscopically analyzed for confluence and morphological changes as described above.
Draft genome sequencing and comparative analysis
The draft genomes of four representative E. coli isolates (rat S3; hedgehogs S4 and S5; and A5; guinea pig S12) were sequenced. Genomic DNA was isolated using the High Pure PCR Template Preparation Kit (Roche Molecular Biochemicals, Indianapolis, IN, USA) following the manufacturer’s protocol for bacterial cell samples. DNA libraries were using the QIAseq FX DNA Library Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s protocol for 500 bp fragments. DNA libraries were sequenced using 2 × 300 bp paired-end reads by Illumina MiSeq by the MIT BioMicro Center. Raw sequenced reads were decontaminated of adapter sequences and quality trimmed to a Phred quality score (Q) ≥ 10 using BBDuk from the BBMap package version 38.34 (http://sourceforge.net/projects/bbmap/). Decontaminated reads were then assembled into contigs with SPAdes followed by genome annotation with RAST, both services hosted by PATRIC(Wattam et al., 2017). Sequences encoding putative virulence factor and antibiotic-resistance genes were identified using PathogenFinder 1.1(Cosentino et al., 2013) and VirulenceFinder 2.0 and ResFinder 3.1 using the 90% identity and 60% minimum length threshold parameters, as described previously(Feng et al., 2017). Syntenic relationships of pks genes and the hemolysin-cnf1 operon between genomes were determined with SimpleSynteny, as described previously(Feng et al., 2017). The “Similar Genome Finder” tool, hosted by PATRIC(Wattam et al., 2017), was used to identify E. coli genomes that were related to the pet E. coli isolate genomes. Sequence have been deposited in GenBank under the following accession numbers SKFO00000000, SKFM00000000, SKFN00000000, and SKFL00000000 for isolates S3, S4, S5, and S12, respectively.
Comet assay for DNA damage
The genotoxic activity of E. coli isolate S3 was investigated using single-cell gel electrophoresis (comet assay) following the manufacturer’s instructions (Enzo Life Sciences, Inc, Farmingdale, NY, USA). 5×105 HeLa cell cultures were infected as described above at MOI of 25 to 200. The cells were washed 4 h after inoculation and incubated overnight in EMEM media containing 200 μg/ml gentamicin and 10% FBS at 37 °C under a 5% CO2 atmosphere. HeLa cells were collected by gently scraping with a cell scraper, pelleted, and then resuspended in PBS to a concentration of 1×105 cells/ml. 25 μl of cells were mixed with 250 μl of low-melting-point agarose and then 75 μl were applied to microscope slides. After cell-agarose mixture solidified, slides were incubated in lysis buffer at 4 °C for 1 hr and then incubated in alkaline solution (pH >13) at room temperature for 1 hr. Slides were immersed in alkaline electrophoresis buffer (pH >13) for 1 h at 4 °C with an electric field applied at 1 V/cm and ~300 mA. The slides were then incubated in 70% ethanol for 5 minutes. After slides were air dried, 100 μl of a 1:10,000 dilution of SybrGreen were applied directly to the slide and incubated for 30 minutes in the dark. Slides were viewed by the Zeiss Axioskop 2 Plus microscope (Zeiss, Germany) with the FITC filter at 10x magnification.
γ-H2AX assay for DNA damage
The genotoxic activity of E. coli isolate S3 was further investigated using the γ-H2AX assay. 1×105 HeLa cells were plated in NuncLab-Tek II 8-well chamber slides (Thermo Fisher Scientific) for 24 h. HeLa cell cultures were infected as described above at MOI of 500. The cells were washed 1 h after inoculation and incubated overnight in EMEM media containing 200 μg/ml gentamicin and 10% FBS at 37 °C under a 5% CO2 atmosphere. Cells were fixed in 4% paraformaldehyde for 15 minutes at room temperature and washed 3 times with PBS. Cells were then incubated in blocking buffer (3% BSA, 0.3% Triton X-100 in TBS) for 1 h at room temperature. Following blocking, cells were incubated with monoclonal rabbit anti-γ-H2AX antibody (1:200 dilution; Cell Signaling, Danvers, MA, USA) in antibody dilution buffer (1% BSA, 0.3% Triton X-100 in TBS) overnight at 4 °C. Cells were then washed 3 times with TBS-0.01% Tween 20 (TBST) and incubated with Alexa Fluor 488-conjugated goat anti-rabbit F(ab′)2 fragment (Cell Signaling) (1:1000) in antibody dilution buffer (1% BSA, 0.3% Triton X-100 in TBS) for 1 h at room temperature. Cells were again washed 3 times with TBST. The chamber wells were removed and the slides were mounted with Prolong Gold antifade reagent with DAPI (Cell Signaling). After coverslips were applied, slides were dried overnight at room temperature in the dark. Cells were observed using the Zeiss Axioskop 2 Plus microscope (Zeiss, Germany) at 40x magnification.
Statistical Analysis
Data were analyzed using Webtool (https://astatsa.com/FisherTest/) to calculate Fisher exact tests for pks and cnf genotype, phylogenetic group, and API code based on PCR analysis and microbiologic characterization. A p-value < 0.05 was considered to be statistically significant.
Results
Microbiological Characterization
Twenty-six E. coli isolates were cultured from fecal samples from 24 out of 65 (36.9%) small mammals. Nine E. coli isolates were cultured from 36 guinea pigs (25%), 7 E. coli isolates were cultured from 9 rats (77.8%), one E. coli isolate was cultured from 12 rabbits (8.3%), 3 E. coli isolates were cultured from 4 hamsters (75%), 2 E. coli isolates were cultured from 2 mice (100%), and 2 E. coli isolates were cultured from 2 hedgehogs (100%). All animal species surveyed had at least one E. coli isolate cultured from a fecal sample. Two animals (one rat and one guinea pig) harbored two unique E. coli isolates as determined by distinct colony morphology and API codes. The profile characteristics of representative E. coli isolates from small mammal pets are summarized in Table 2 (see Supplemental Table 1 for complete description of all E. coli isolates in this study). The most common API code was 5144572, observed in 19/26 (73%) isolates. Four of the isolates had API code 5144552. The remaining three isolates had API codes 5144532, 5144172 or 5144512. Among all isolates, there was no correlation between API code and pks or cnf genotype. Two of the 26 isolates demonstrated beta hemolysis, both of which were obtained from healthy hedgehogs.
Table 2.
Profile characteristics of representative E. coli isolates from small mammal pets.
| Animal ID |
E. coli Isolate ID |
Species | Age | Sex | Clinical History |
Animal Source |
API Code |
Serotype Profile |
Phylogenetic Group PCR Result |
pks PCR Result |
cnf PCR Result |
β-Hemolytic Activity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1a | S1 | Rat | 1.5 yr | Spayed Female | Apparently healthy | Private owner | 5144532 | O7:H7 | B2 | Pos. | Neg. | Neg. |
| A2 | S2 | Rat | 2 yr | Male | Upper respiratory infection, dyspnea | Private owner | 5144512 | O83:H15 | B2 | Pos. | Neg. | Neg. |
| A3a | S3 | Rat | 1 yr | Castrated Male | Apparently healthy | Private owner | 5144572 | O7:H7 | B2 | Pos. | Neg. | Neg. |
| A4 | S4 | Hedgehog | 1.5 yr | Female | Apparently healthy | Private owner | 5144572 | O6:H+ | B2 | Pos. | Pos. | Pos. |
| A5 | S5 | Hedgehog | 5 m | Female | Apparently healthy | Private owner | 5144552 | O2:H1 | B2 | Pos. | Pos. | Pos. |
| A6 | S6 | Guinea pig | 6 yr | Male | Diarrhea, dental disease (malocc lusion) | Private owner | 5144572 | O8:H10 | B2 | Pos. | Neg. | Neg. |
| A7b | S7 | Guinea pig | 6 m | Female | Apparently healthy | Adoption center | 5144572 | O8:H− | B2 | Pos. | Neg. | Neg. |
| A8b | S8 | Guinea pig | 6 m | Female | Apparently healthy | Adoption center | 5144572 | O8:H− | B2 | Pos. | Neg. | Neg. |
| A9 | S9 | Guinea pig | 1.5 yr | Female | Apparently healthy | Adoption center | 5144572 | O8:H10 | B2 | Pos. | Neg. | Neg. |
| A9 | S14 | Guinea pig | 1.5 yr | Female | Apparently healthy | Adoption center | 5144552 | O180:H14 | B1-like | Neg. | Neg. | Neg. |
| A10c | S10 | Guinea pig | 9 w | Female | Apparently healthy | Private owner | 5144572 | O8:H10 | B2 | Pos. | Neg. | Neg. |
| A11c | S11 | Guinea pig | 9 w | Female | Upper respiratory infection, minor skin wound | Private owner | 5144572 | O8:H10 | B2 | Pos. | Neg. | Neg. |
| A12 | S12 | Guinea pig | 1 yr | Male | Hemorrhagic diarrhea | Private owner | 5144572 | O8:H− | B2 | Pos. | Neg. | Neg. |
| A13 | S13 | Hamster | 2 yr | Male | Facial tumor | Private owner | 5144572 | O43:H2 | B1-like | Neg. | Neg. | Neg. |
a, b, c are cohoused animal pairs, respectively
Identification of pks, cnf and cdt genes
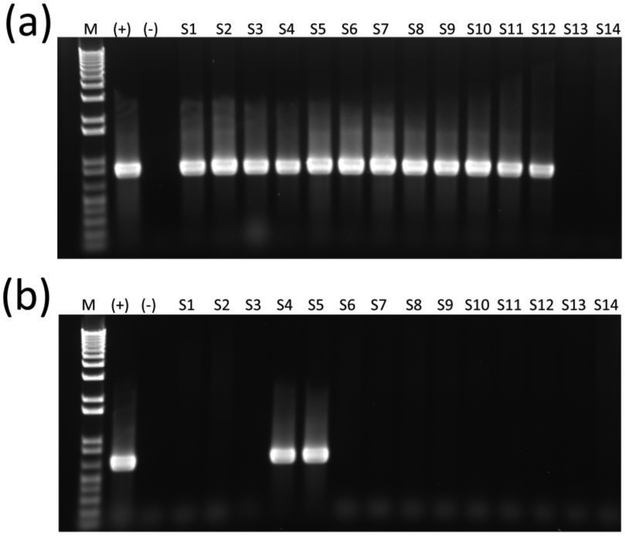
Conventional PCR for pks genes clbA and clbQ (Figure 1a) and multiplex PCR for cnf (Figure 1b) and cdt were performed to screen for genotoxin genetic elements. The distribution of genotoxin frequency by pet species is shown in Table 1. Twelve of the 26 isolates (46%) were positive for pks, and 2 of the 12 pks+ isolates (7.7 %) were also positive for cnf (Table 1). None of the isolates were positive for cdt, and cnf was never present without pks (Table 1). The two pks+/cnf+ isolates were both obtained from two pet hedgehogs from separate owners, both apparently healthy. One was a 5-month-old intact female, and the other a 1.5-year old intact female. The remaining pks+ E. coli isolates were obtained from two separately housed, apparently healthy pet rats, one of which was a 6-month-old intact male, and the other a 1.5-year-old spayed female. One was a 2-year-old intact male rat with an upper respiratory infection. Three apparently healthy, 6-month-old intact female adoption center guinea pigs had E. coli isolates, two of which were housed together and were possibly littermates; two 9-week-old intact pet female guinea pigs (littermates that were housed together), one apparently healthy and one with a mild upper respiratory infection and a minor bite wound inflicted by its littermate; one 6-year-old intact male pet guinea pig with severe diarrhea and dental disease; and one 1-year-old intact pet male guinea pig with severe hemorrhagic diarrhea. The laboratory guinea pig with acute weight loss, polyuria/polydipsia and diarrhea had E. coli isolated on fecal culture; however, the isolate was negative for all three genotoxins.
Figure 1.
Amplification of clbQ (a) and cnf (b) genes in E. coli isolates from small mammal pets. Lanes 1 through 14 are representative E. coli isolates. (+) designates pks and cnf positive controls. (−) designates negative controls. Lane M designates 1 kb+ molecular marker.
Table 1.
Distribution of genotoxin frequency by pet species.
| Total | Guinea pigs |
Rats | Rabbits | Hamsters | Hedgehogs | Mice | |
|---|---|---|---|---|---|---|---|
| Total pks+ E. coli | 12/26 (46.2%) | 7/10 (70%) | 3/8 (37.5%) | 0/1 (0%) | 0/3 (0%) | 2/2 (100%) | 0/2 (0%) |
| Total cnf+ E. coli | 2/26 (7.7%) | 0/10 (0%) | 0/8 (0%) | 0/1 (0%) | 0/3 (0%) | 2/2 (100%) | 0/2 (0%) |
| pks−/cnf− | 14/26 (53.8%) | 3/10 (30%) | 5/8 (62.5%) | 1/1 (100%) | 3/3 (100%) | 0/2 (0%) | 2/2 (100%) |
| pks+/cnf− | 10/26 (38.5%) | 7/10 (70%) | 3/8 (37.5%) | 0/1 (0%) | 0/3 (0%) | 0/2 (0%) | 0/2 (0%) |
| pks−/cnf+ | 0/26 (0%) | 0/10 (0%) | 0/8 (0%) | 0/1 (0%) | 0/3 (0%) | 0/2 (0%) | 0/2 (0%) |
| pks+/cnf+ | 2/26 (7.7%) | 0/10 (0%) | 0/8 (0%) | 0/1 (0%) | 0/3 (0%) | 2/2 (100%) | 0/2 (0%) |
Phylogenetic Analysis
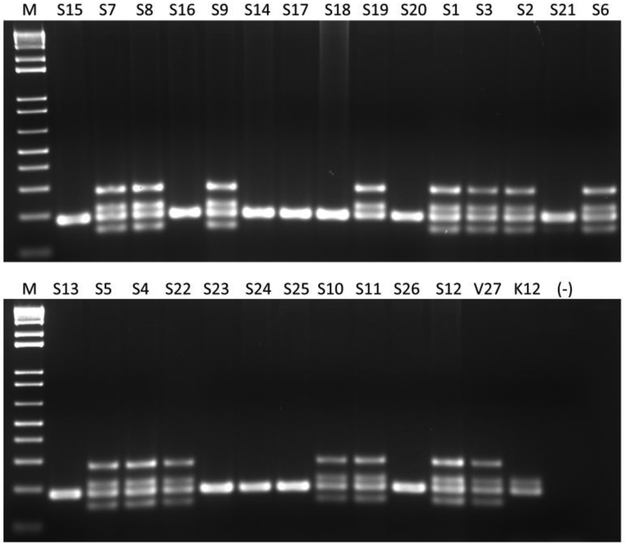
Phylogenetic group was determined based on the amplification pattern of multiplex PCR for svg, chuA, yjaA, uidA, and TspE4.C2 genes (Figure 2). All 12 genotoxin-positive isolates were members of pathogen-associated phylogroup B2 (Table 2). Of the 14 isolates that were negative for all the genotoxins screened, two were members of phylogroup B2, and one was a member of phylogroup B1. Of the remaining 11 isolates, which were genotoxin negative, only the uidA gene (186 bp) was amplified and thus had an undetermined phylogenetic group that was B1-like (see Supplemental Table 1). A Fisher’s exact probability test determined a statistically significant association between phylogenetic group and pks genotype among isolates (p < 0.001). There was not a statistically significant association between phylogenetic group and cnf genotype.
Figure 2.
Phylogenetic group determination of E. coli isolates from small mammal pets. Amplification of multiplex PCR of chuA (279 bp), yjaA (211 bp), uidA (186 bp), TspE4.C2 (152 bp) genes for phylogenetic group determination. Lanes 1 through 26 represent E. coli isolates obtained from small mammal pets. V27 is a positive control for phylogenetic group B2, and K12 is a positive control for phylogenetic group A. (−) designates negative controls. Lane M designates 1 kb+ molecular marker.
Serotyping
Fourteen representative E. coli isolates were serotyped (Table 2). pks+ E. coli isolated from guinea pigs were either O8:H− (found in triplicate) or O8:H10 (identified in quadruplicate) serotypes. The serotype of two E. coli isolates cultured from clinically ill guinea pigs, one with hemorrhagic diarrhea (animal A12) and one with non-hemorrhagic diarrhea (animal A6), were O8:H− and O8:H10, respectively. The pks+ E. coli isolates from rats were either O7:H7 or O83:H15 serotype. The remaining O8:H− and O8:H10 isolates originated from guinea pigs that were clinically healthy or with issues not considered to be related to pathogenic E. coli.
The second most common serotype was O7:H7, which was obtained from pks+ rats, found in duplicate. Serotypes of E. coli isolates obtained from cohoused animals (S1 and S2; S5 and S6; and S8 and S9) were identical. The two pks+/cnf+ isolates, both obtained from two separately pet hedgehogs, were of different serotypes (O6:H+ and O2:H1). All of 14 E. coli isolates that were serotyped as well as the single E. coli isolate from the diarrheic laboratory guinea pig (serotype O39:H21) were negative for elt, estA, estB, stx1, stx2, eae and cnf2 genes.
In vitro cytotoxicity of E. coli isolates
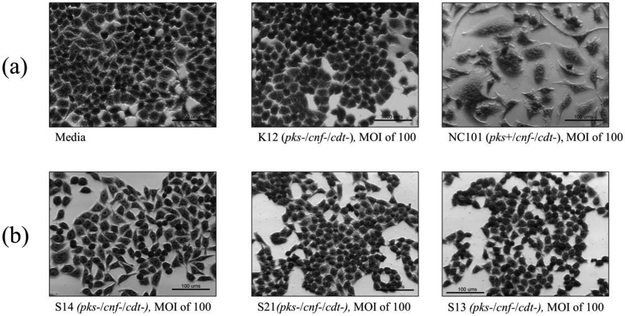
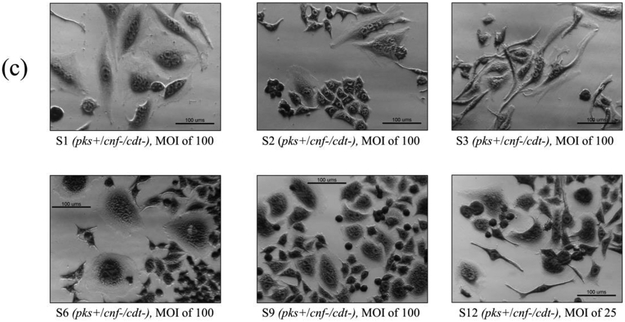
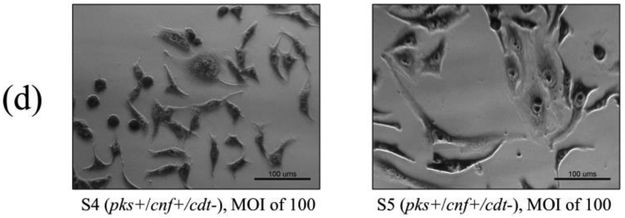
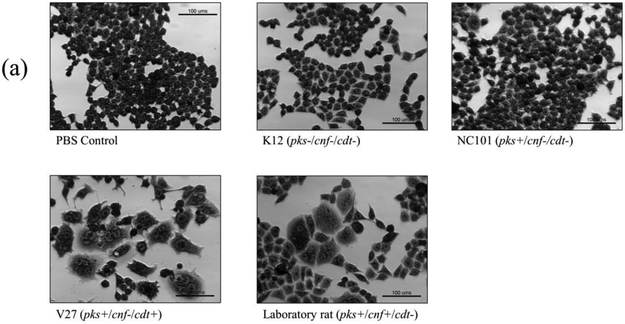
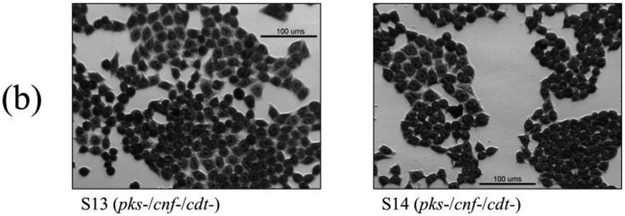
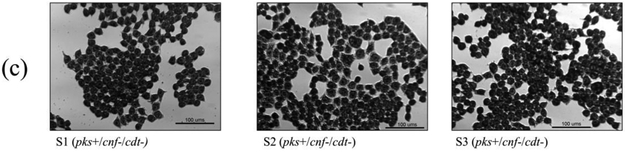
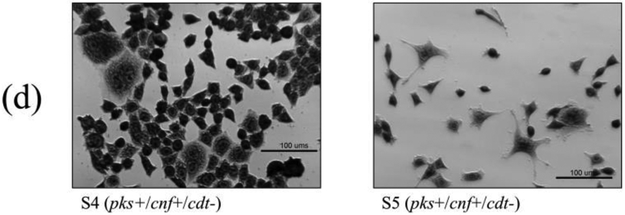
Cell culture assays were performed to determine if in vitro infection or sonicates of the 12 isolates positive for pks and/or cnf caused cytotoxicity to HeLa cells. Live bacteria were used rather than sonicate, as whole cells are required for the complete expression of colibactin. Viable pks+ E. coli isolates induced megalocytic cytotoxicity to HeLa cells, indicating contact-dependent colibactin expression (Figure 3). Unlike colibactin, CNF and CDT cytotoxic effects are only detectable in vitro using sonicate preparations. HeLa cells treated with sonicate from cnf+ E. coli isolates also displayed megalocytic cytotoxicity, which are characteristic of this cytotoxin (Figure 4). E. coli isolates PCR-negative for pks or cdt did not induce cytotoxicity. The cnf+ isolates (S4 and S5) appeared more cytotoxic compared to the cnf+ control.
Figure 3.
In vitro infection of HeLa cells with live E. coli isolates, including controls (a), pks−/cnf−/cdt− isolates (b), pks+/cnf−/cdt− isolates (c), and pks+/cnf+/cdt− isolates (d). HeLa cells were treated with E. coli at a multiplicity of infection (MOI) 25 or 100 for 4 h, followed by a 72 h incubation in gentamicin-containing media. Cells infected with the 12 isolates encoding pks (seen in c and d) displayed megalocytosis (enlargement of the cell body and nucleus) similar to the pks positive control. No cytotoxicity was observed for cells treated with K12, media, or representative E. coli isolates negative for pks, cnf and cdt. K12 was used as a non-pathogenic E. coli control. NC101 was used as a pks positive control for colibactin-induced cytotoxicity. Media was used as a negative control for uninfected cells.
Figure 4.
Cell culture assay for cytotoxicity using E. coli sonicate preparations. HeLa cells were treated with 5 μg total protein of E. coli sonicate for 72 h from controls (a), pks−/cnf−/cdt− isolates S13 and S14 (b), pks+/cnf−/cdt− isolates S1, S2, and S3 (c), and pks+/cnf+/cdt− isolates S4 and S5 (d). Cells treated with sonicate prepared from isolates PCR-positive for cnf displayed megalocytosis similar to the E. coli V27 (cdt positive control) and laboratory rat (cnf positive control) isolates. No cytotoxicity was observed for cells treated with sonicate prepared from isolates PCR-negative for cnf and cdt, including control strains NC101 and K12. PBS was used as a vehicle control.
Draft genome sequencing and comparative analysis
Whole genome sequences of four E. coli strains isolated from a pet rat (S3, pks+/cnf−) two hedgehogs (S4 and S5, both pks+/cnf+), and one guinea pig (S12, pks+/cnf−) were obtained for identification of virulence factor genes and comparative analysis with other genotoxin-encoding E. coli from humans and laboratory rodents. As summarized in Table 3, the genomes of the E. coli isolated from pets had comparable sizes, G+C% content, and number of gene annotations were representative of E. coli genomes.
Table 3.
Genome statistics and virulence factor profile.
| Strain | Isolation Source |
Genome Length (bp) |
Contigs | G+C% Content |
Protein Coding Sequences (CDS) |
tRNA | rRNA | Virulence Factors Genes* |
GenBank Accession |
|---|---|---|---|---|---|---|---|---|---|
| S3 | Pet rat | 5,072,851 | 61 | 50.46 | 4,994 | 81 | 12 | astA, celb, gad, iroN, mchB, mchC, mchF, mcmA, pic, pks, vat | SKFO00000000 |
| S4 | Pet hedgehog | 5,057,752 | 79 | 50.40 | 5,000 | 80 | 11 | cnf1, iroN, iss, mcmA, pks, sfaS, vat | SKFM00000000 |
| S5 | Pet hedgehog | ,5281,892 | 170 | 50.38 | 5,366 | 85 | 11 | cnf1, gad, iha, iroN, iss, mchB, mchC, mchF, mcmA, pic, pks, sat, sfaS, vat | SKFN00000000 |
| S12 | Pet guinea pig | 4,972,660 | 102 | 50.89 | 4,924 | 79 | 10 | gad, iss, pks | SKFL00000000 |
| 1610280015 (S15) | Research rat | 5,248,403 | 58 | 47.71 | 5,078 | 79 | 8 | celb, gad, iss, pks | NHYP00000000 |
| IHE3034 | Human neonatal meningitis | 5,108,383 | 1 (complete genome) | 50.70 | 5,045 | 97 | 22 | gad, iroN, iss, pks, sfaS, vat | CP001969 |
| NC101 | Research mouse | 5,021,144 | 27 | 50.57 | 4,917 | 72 | 4 | gad, iroN, iss, pks, sfaS, vat | AEFA00000000 |
| K-12 substr. DH10B | Human non-pathogenic | 4,686,137 | 1 (complete genome) | 50.80 | 4,606 | 87 | 14 | gad, iss | CP000948 |
Gene names: astA: EAST-1 heat-stable toxin; celb: Endonuclease colicin E2; cnf1: cytotoxic necrotizing factor 1; gad: Glutamate decarboxylase; iha: adherence protein; iroN: Enterobactin siderophore receptor protein; iss: Increased serum survival; mchB: Microcin H47 part of colicin H; mchC: MchC protein; mchF: ABC transporter protein MchF; mcmA: Microcin M part of colicin H; pic: serine protease autotransporters of Enterobacteriaceae (SPATE); pks: polyketide synthetase (colibactin); sat: secreted autotransporter toxin; sfaS: S-fimbriae minor subunit; vat: vacuolating autotransporter toxin
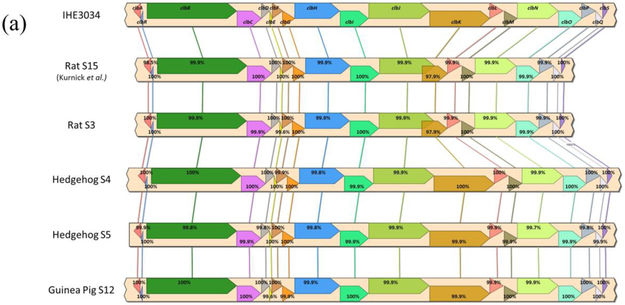
Syntenic alignments were confirmed for the pks island in all four E. coli isolates (S3, S4, S5, and S12) as well as the hly-cnf1 operon in both hedgehog E. coli isolates (Figure 5). Interestingly, the pet rat E. coli isolate (S3) appeared to have a hybrid gene for clbJ-K. The clbJ and clbK genes from this rat isolate had ~90% and ~45% sequence coverage, respectively, compared IHE3034. clbJ lacked 624 bp at the 3’ end including the stop codon, but retains two NRPS modules (Supplemental Figure 1 and 2). clbK lacked 3,540 bp at the 5’ end including a start codon and the PKS module, but retains the NRPS module and the oxidase domain (Supplemental Figure 1 and 2). The clbJ and clbK genes overlap by 1,287 bp in the genome, suggesting they are expressed as a single, continuous sequence (Supplemental Figure 1 and 2). When the clbJ start codon is used as the position of the open reading frame, the predicted sequence is translated into a 2,440 amino acid product (7,323 bp) that terminates at the clbK stop codon. This suggests the putative clbJ and clbK sequences may be transcribed and translated into a hybridized protein that contains two NPRS modules as well as an oxidase domain (Supplemental Figure 1 and 2). PCR and Sanger sequencing confirmed the clbJK hybrid gene sequence in S3, as predicted by the whole-genome sequencing results (Supplemental Figure 2 and 3). Additionally, S1 encoded the hybrid clbJ-K gene (Supplemental Figure 3). Interestingly, S1 and S3 were isolated from co-housed rats. A comet assay and γ-H2AX assay confirmed that the S3 isolate causes DNA damage to HeLa cells following in vitro infection (Supplemental Figures 4 and 5).
Figure 5.
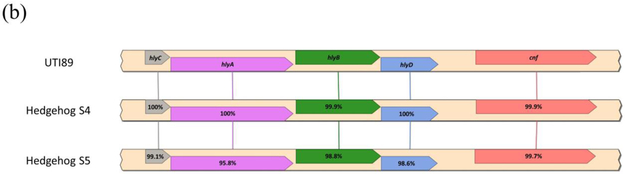
Syntenic alignment and percent gene identities of (a) pks island genes between IHE3034, laboratory rat S15 (Kurnick et al. 2019), and the novel rat, hedgehog and guinea pig E. coli isolates and (b) hemolysin-cnf operon between cnf+ UTI89 uropathogen and hedgehog E. coli isolates.
Aside from pks and cnf1, other virulence factor genes were identified in the pet E. coli isolates (Table 3). Notably, the E. coli isolates obtained from both hedgehogs encoded genes for several serine protease autotransporters of Enterobacteriaceae (SPATEs), which can elicit cytotoxicity to cell lines in vitro. The presence of these additional cytotoxin genes agreed with our in vitro cell culture results in which sonicate preparations with these pks+/cnf+ E. coli from hedgehogs appeared to induce substantially more cytotoxicity in HeLa cells compared to the positive controls.
Furthermore, all four E. coli genomes isolated from pets were enriched in gene families that correlate with pathogenicity and are predicted to be potentially pathogenic to human hosts. Both the guinea pig and hedgehog E. coli genomes were found to be most similar to E. coli isolated from urine or blood in human patients with a UTI or septicemia (Supplemental Table 2). The pet rat E. coli isolate was most similar to strains cultured from the feces of mice, primates, and humans with unreported health status (Supplemental Table 2). BLAST analysis of these similar genomes also confirmed the presence of pks and/or cnf1 genes (not shown).
Lastly, all four pet E. coli genomes encoded the mdfA gene, which is a broad-spectrum antibiotic efflux pump that contributes to multidrug resistance. The genome for the guinea pig E. coli isolate also contains aadA1, sul1, and tetA that cause resistance to aminoglycoside, sulfonamide, and tetracycline antibiotics.
Discussion
In this study, we cultured E. coli strains encoding virulence factors pks and cnf from the gastrointestinal tract of small mammal pets. Cytotoxicity activity of these genes was confirmed by in vitro cell culture assays. We observed that the API codes of the genotoxic-positive isolates were: 5144572 (9/12), 5144523 (1/12), 5144512 (1/12) and 5144552 (1/12). Cohoused animals always had isolates with identical serotypes and genotypes (S1 and S3; S7 and S8; S10 and S11; Table 2), implying that isolates may be transmitted between animals in shared environments.
Guinea pigs were the most represented species in this study due to their high availability for inclusion during veterinary or at adoption centers visits. Interestingly, while E. coli were only isolated from ~25% of the surveyed pet guinea pigs, these isolates were the most likely to encode colibactin among all E. coli isolates, representing 58% (7/12) of the total pks+ isolates identified in this study. An E. coli was isolated from the single laboratory guinea pig surveyed in this study, but it was negative for all genotoxins. In the literature, there is dearth of information regarding the prevalence and characteristics of E. coli in pet and laboratory guinea pigs. Given that guinea pigs are commonly used as in vivo models to study pathogens including E. coli(Padilla-Carlin et al., 2008), research obtained from guinea pigs naturally colonized with cytotoxin-producing E. coli could be confounded by their pathogenic potential.
Enteropathogenic and enterohemorrhagic E. coli have been frequently isolated from laboratory and petting zoo rabbits,(Garcia and Fox, 2003; Swennes et al., 2012, 2013) but in our study, only 1 out of 12 rabbits had an E. coli (pks−/cnf−/cdt−) successfully cultured, suggesting potential differences in the microbiome composition of these animal sources. Both pet hedgehogs had E. coli isolates positive for pks and cnf, which were the only isolates encoding more than one cytotoxin in this study. Previously, our lab determined that laboratory mice and rats also harbor E. coli isolates encoding pks, cnf, and/or cdt(Garcia et al., 2016; Kurnick et al., 2019). While the current study found that pet rat populations are also colonized by E. coli, only 3 out of 8 of the rat isolates were pks+. Pet mice and hamsters, while colonized with E. coli, did not have isolates positive for the genotoxins tested.
We found serotypes that were previously isolated from pks+ E. coli in laboratory animals. Serotype O7:H7 was isolated from pks+ E. coli in clinically healthy, co-housed pet rats. Previously, serotype O7:H7 was isolated from pks+ only or pks+/cdt+ E. coli in laboratory rats as well from only E. coli pks+ in rhesus macaques(Feng et al., 2017; Kurnick et al., 2019). This serotype has also been described in E. coli negative for eae and stx1 genes isolated from aymptomatic NZW and DB rabbits from commercial vendors and a petting zoo(Garcia and Fox, 2003). Additionally, Shiga toxin-producing E. coli with serotype O7:H7 have been isolated from calves in Brazil(Aidar-Ugrinovich et al., 2007).
The serotype of the pks−/cnf−/cdt− E. coli isolate from the laboratory guinea pig, O39:H21, differed from isolates obtained from pet guinea pigs. Two O8:H− strains were isolated from two asymptomatic cohoused guinea pigs. The third O8:H− isolate was cultured from a guinea pig presenting with severe hemorrhagic diarrhea. O8:H− is classified as an atypical EPEC and has been associated with diarrhea in children(Alonso et al., 2016). This serotype has also been described in eae−, stx1− E. coli isolated from an asymptomatic DB rabbit(Garcia and Fox, 2003). Four pks+ E. coli with serotype O8:H10 were isolated from four other guinea pigs, one of which had diarrhea. Since 2007, at least 9 cases of STEC O8 have been recorded within the STEC surveillance system in the Netherlands, including four cases with serotype O8:H−(Friesema et al., 2015). All of these isolates were stx2a-e-positive and stx1-, stx2f-, eae- and hly-negative. Atypical EPEC O8:H− strains have been isolated from chickens and chicken-derived products, and isolated from and characterized in children with diarrhea(Alonso et al., 2016).
Two pks+/cnf+ β-hemolytic isolates were both cultured from separately owned, clinically healthy hedgehogs, suggesting that β-hemolysin genes hlyCABD and cnf genes are linked in an operon, as previously described in healthy macaques(Feng et al., 2017). Strains positive for cnf1 reported in a previous study have also been found in the feces of healthy animals such as macaques, suggesting that primates can serve as a reservoir for these E. coli(Martin et al., 2009). Furthermore, the hedgehog-derived isolates were of different serotypes (O6:H+ and O2:H1). In multiple European and North American countries, E. coli serotype O6:H+ is commonly isolated from women with community-acquired lower UTIs(Österlund et al., 2005). The O2:H1 serotype has been characterized as an ExPEC found in human isolates causing extraintestinal infections(Cortes et al., 2010). The O2 serotype is found in avian pathogenic E. coli (APEC) and is associated with human UTIs and bacteremia(Rodriguez-Siek et al., 2005; Poolman and Wacker, 2016).
All 12 genotoxin-encoding isolates belonged to the pathogen-associated phylogenetic group B2. This statistical association of genotoxin-positive strains with B2 has also been noted in human isolates(Dubois et al., 2010; Buc et al., 2013). Interestingly, three genotoxin-negative isolates in this survey also belonged to the B2 phylogenetic group. Previous studies determined that while all genotoxin-associated E. coli isolates derived from laboratory rats and mice were always phylogenetic group B2, a small percentage of B2 isolates were also negative pks, cnf or cdt(Garcia et al., 2016; Kurnick et al., 2019). These isolates may encode additional virulence factors not screened for in this study or in the studies by Garcia et al. (Garcia et al., 2016) and Kurnick et al. (Kurnick et al., 2019) Aside from one isolate (S21) which was phylogenetic group B1, the remaining 10 isolates only amplified the uidA gene (186 bp) and thus had an undetermined phylogenetic group. However, we consider them to be more B1-like since they were genotoxin negative.
According to our genome analysis, exotic pet E. coli isolates contained other virulence factors associated with pathogenicity in animals and humans, including SPATES such as pic, sat and vat. Interestingly, the representative isolates were most similar to E. coli isolates cultured from human patients with UTIs and bacteremia than nonpathogenic commensal E. coli strains. Previously, a hybrid gene for clbJ-K was also detected in the pks island isolated for an E. coli isolate from a laboratory rat(Kurnick et al., 2019). In our study and the study by Kurnick et al.,(Kurnick et al., 2019) these E. coli isolates induced megalocytosis and cytotoxicity to HeLa cells, suggesting the hybrid clbJ-K gene did not affect the synthesis or cytopathogenic effects of colibactin in vitro. Interestingly, reports have shown the PKS module in clbK can be biochemically skipped to synthesize alternative colibactin metabolites with cytopathogenic properties(Zha et al., 2016; Trautman et al., 2017). This alternative pathway is still dependent on the NRPS modules and oxidase activity from clbJ and clbK. In agreement with these findings, the putative clbJK-hybrid gene detected in isolate S3 is predicted to contain two NRPS modules and an oxidase domain, but lacks the PKS module from clbK. This suggests that the putative clbJ-K hybrid gene may synthesize alternative colibactin metabolites, similar to the scheme described above, capable of cytopathogenic and genotoxic effects. A BLAST analysis also identified five other E. coli (GenBank: CP025703, CP007275) or Klebsiella spp. (GenBank: CP026756, CP009775, FO834906) published genomes in the nr/nt database with homologous clbJ-K hybrid gene sequences (data not shown); whether these isolates elicit cytotoxic effects has not been reported.
The results of this study implicate that certain small mammal pets may carry cyclomodulin-encoding E. coli strains or may participate in their circulation without being the primary reservoir. Colonization with these isolates may expose the host animal to pathogenic effects of their cytotoxins, and importantly these E. coli-colonized host species may pose a zoonotic risk for humans for humans.
Colibactin-producing E. coli have been commonly isolated from humans and are more prevalent in patients with colorectal tumors than controls(Buc et al., 2013). A carcinogenic effect with pks+ E. coli has also been demonstrated in laboratory animal models. A pks+ E. coli strain NC101 caused typhlitis and promoted invasive carcinoma in azoxymethane (AOM)-treated interleukin 10 knockout (C57BLIL10−/−) mice(Arthur et al., 2012). While E. coli were successfully cultured from pets in this study, our results cannot exclude the possibility that these isolates were transferred from human owners or other sources to the pets.
The potential for pets to pose a risk zoonotic humans in people exposed to them has stimulated investigations on pet-related emerging diseases as well as established zoonotic. Pertinent example are Salmonella typhimurium outbreaks linked to pet hedgehogs and guinea pigs, hamsters, mice, rats, and frozen feeder rodents(Swanson et al., 2007; Lee et al., 2008; Anderson et al., 2017; Robertson et al., 2018). Pet rats can transmit Streptobacillus moniliformis, the causative agent of rat bite fever. Human deaths due to S. moniliformis in adults and children with pet rats as well as pet store employees exposed to rats have been reported(Fox, 2015). Interestingly, the CDC has not reported outbreaks of E. coli from small mammal pets in the United States, although EHEC has been transmitted from rabbits to petting zoo visitors(Garcia et al., 2010).
Over sixty percent of U.S. households own at least one pet(Reaser et al., 2008). This statistic has risen from 56% of U.S. households in 1988. Additionally, exotic pets, which include the small mammal species surveyed in this study, are becoming more popular. At least 6.7 million households own a small mammal pet, including rodents and exotic pets surveyed in this study. Due to increasing popularity of such pets in the U.S., the incidence of associated zoonotic diseases is expected to rise. Health care professionals including veterinarians should be knowledgeable about the potential for pets to harbor genotoxin-encoding E. coli to better educate pet owners regarding proper hygiene such as hand washing and sanitation of surfaces exposed to these pets.
The limitation of the current study was the uneven and/or small sample size of each species surveyed due to the voluntary participation of animal subjects by pet owners and adoption centers. Nevertheless, this study revealed that E. coli strains encoding the genotoxins pks and cnf were isolated from the feces of both ill and healthy small mammal pets. We demonstrated that genotoxin-encoding E. coli isolates exhibit cytotoxicity in vitro, suggesting their pathogenic potential. Further studies are required to assess whether there is an association between cytotoxin-encoding E. coli isolated from pets and zoonotic or zooanthroponotic disease.
Supplementary Material
Highlights.
Escherichia coli encoding cytotoxins are increasingly associated with disease
pks+ and cnf+ E. coli were cultured from feces of healthy and diarrhetic pets
Cytotoxin-encoding isolates induced HeLa cell cytopathogenic effects in vitro
Genome sequencing identified additional virulence genes in representative isolates
Small mammal pets are a reservoir for cytotoxic E. coli and may be a zoonotic risk
Acknowledgements:
The authors would like to thank the collaborating veterinary clinic and animal adoption centers staff. The authors would also like to thank Dr. Zhongming Ge, PhD for his excellent assistance with PCR primer design for wildtype and hybrid clbJK genes.
Funding: Research reported in this publication was supported by NIH under award P30ES002109. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Conflict of interest statement: The authors declare that they have no conflicts of interest.
Data availability: Please contact corresponding author (James G. Fox) for data requests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aidar-Ugrinovich L, Blanco J, Blanco M, Blanco JE, Leomil L, Dahbi G, Mora A, Onuma DL, Silveira WD, Pestana de Castro AF, 2007. Serotypes, virulence genes, and intimin types of Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) isolated from calves in São Paulo, Brazil. Int. J. Food Microbiol 115, 297–306. doi: 10.1016/j.ijfoodmicro.2006.10.046 [DOI] [PubMed] [Google Scholar]
- Alonso MZ, Sanz ME, Irino K, Krüger A, Lucchesi PMA, Padola NL, 2016. Isolation of atypical enteropathogenic Escherichia coli from chicken and chicken-derived products. Br. Poult. Sci 57, 161–164. doi: 10.1080/00071668.2015.1135502 [DOI] [PubMed] [Google Scholar]
- Anderson TC, Marsden-Haug N, Morris JF, Culpepper W, Bessette N, Adams JK, Bidol S, Meyer S, Schmitz J, Erdman MM, Gomez TM, Barton Behravesh C, 2017. Multistate Outbreak of Human Salmonella Typhimurium Infections Linked to Pet Hedgehogs - United States, 2011-2013. Zoonoses Public Health 64, 290–298. doi: 10.1111/zph.12310 [DOI] [PubMed] [Google Scholar]
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan T-J, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C, 2012. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123. doi: 10.1126/science.1224820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakthavatchalu V, Wert KJ, Feng Y, Mannion A, Ge Z, Garcia A, Scott KE, Caron TJ, Madden CM, Jacobsen JT, Victora G, Jaenisch R, Fox JG, 2018. Cytotoxic Escherichia coli strains encoding colibactin isolated from immunocompromised mice with urosepsis and meningitis. PLoS One 13, e0194443. doi: 10.1371/journal.pone.0194443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, Bonnet R, 2013. High Prevalence of Mucosa-Associated E. coli Producing Cyclomodulin and Genotoxin in Colon Cancer. PLoS One 8. doi: 10.1371/journal.pone.0056964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes P, Blanc V, Mora A, Dahbi G, Blanco JE, Blanco M, Lopez C, Andreu A, Navarro F, Alonso MP, Bou G, Blanco J, Llagostera M, 2010. Isolation and characterization of potentially pathogenic antimicrobial-resistant Escherichia coli strains from chicken and pig farms in Spain. Appl. Environ. Microbiol 76, 2799–2805. doi: 10.1128/AEM.02421-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S, Voldby Larsen M, Moller Aarestrup F, Lund O, 2013. PathogenFinder--distinguishing friend from foe using bacterial whole genome sequence data. PLoS One 8, e77302. doi: 10.1371/journal.pone.0077302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rycke J, Gonzalez EA, Blanco J, Oswald E, Blanco M, Boivin R, 1990. Evidence for two types of cytotoxic necrotizing factor in human and animal clinical isolates of Escherichia coli. J. Clin. Microbiol 28, 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois D, Delmas J, Cady A, Robin F, Sivignon A, Oswald E, Bonnet R, 2010. Cyclomodulins in Urosepsis Strains of Escherichia coli . J. Clin. Microbiol 48, 2122–2129. doi: 10.1128/JCM.02365-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falbo V, Pace T, Picci L, Pizzi E, Caprioli A, 1993. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect. Immun 61, 4909–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Mannion A, Madden CM, Swennes AG, Townes C, Byrd C, Marini RP, Fox JG, 2017. Cytotoxic Escherichia coli strains encoding colibactin and cytotoxic necrotizing factor (CNF) colonize laboratory macaques. Gut Pathog. 9, 71. doi: 10.1186/s13099-017-0220-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JG, 2015. Diseases Transmitted by Man’s Worst Friend: the Rat. Microbiol. Spectr 3. doi: 10.1128/microbiolspec.IOL5-0015-2015 [DOI] [PubMed] [Google Scholar]
- Friesema IHM, Keijzer-Veen MG, Koppejan M, Schipper HS, van Griethuysen AJ, Heck MEOC, van Pelt W, 2015. Hemolytic Uremic Syndrome Associated with Escherichia coli O8:H19 and Shiga Toxin 2f Gene. Emerg. Infect. Dis 21, 168–169. doi: 10.3201/eid2101.140515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A, Fox JG, 2003. The rabbit as a new reservoir host of enterohemorrhagic Escherichia coli. Emerg. Infect. Dis 9, 1592–1597. doi: 10.3201/eid0912.030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A, Fox JG, Besser TE, 2010. Zoonotic enterohemorrhagic Escherichia coli: A One Health perspective. ILAR J. 51, 221–232. [DOI] [PubMed] [Google Scholar]
- Garcia A, Mannion A, Feng Y, Madden CM, Bakthavatchalu V, Shen Z, Ge Z, Fox JG, 2016. Cytotoxic Escherichia coli strains encoding colibactin colonize laboratory mice. Microbes Infect. 18, 777–786. doi: 10.1016/j.micinf.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinenoya A, Shima K, Asakura M, Nishimura K, Tsukamoto T, Ooka T, Hayashi T, Ramamurthy T, Faruque SM, Yamasaki S, 2014. Molecular characterization of cytolethal distending toxin gene-positive Escherichia coli from healthy cattle and swine in Nara, Japan. BMC Microbiol 14, 97. doi: 10.1186/1471-2180-14-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman P, LeNegrate G, Mograbi B, Hofman V, Brest P, Alliana-Schmid A, Flatau G, Boquet P, Rossi B, 2000. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J. Leukoc. Biol 68, 522–528. [PubMed] [Google Scholar]
- Jinadasa RN, Bloom SE, Weiss RS, Duhamel GE, 2011. Cytolethal distending toxin: a conserved bacterial genotoxin that blocks cell cycle progression, leading to apoptosis of a broad range of mammalian cell lineages. Microbiology 157, 1851–1875. doi: 10.1099/mic.0.049536-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnick S, Mannion A, Madden CM, Chamberlain P, Fox JG, 2019. Genotoxic Escherichia coli Strains Encoding Colibactin, Cytolethal Distending Toxin, and Cytotoxic Necrotizing Factor Colonize Laboratory Rats. Comp. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Lee Y, Ph D, 2018. Prevalence of Escherichia coli Carrying pks Islands in Bacteremia Patients 131, 271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, McReynolds JL, Fuller CC, Jones B, Herrman TJ, Byrd JA, Runyon M, 2008. Investigation and characterization of the frozen feeder rodent industry in Texas following a multi-state Salmonella Typhimurium outbreak associated with frozen vacuum-packed rodents. Zoonoses Public Health 55, 488–496. doi: 10.1111/j.1863-2378.2008.01165.x [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH, 2017. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 45, D200–D203. doi: 10.1093/nar/gkw1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HR, Taylor NS, Buckley EM, Marini RP, Patterson MM, Fox JG, 2009. Characterization of cytotoxic necrotizing factor 1-producing Escherichia coli strains from faeces of healthy macaques. J. Med. Microbiol 58, 1354–1358. doi: 10.1099/jmm.0.012088-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccarthy AJ, Martin P, Cloup E, Stabler RA, Oswald E, Taylor W, 2015. The Genotoxin Colibactin Is a Determinant of Virulence in Escherichia coli K1 Experimental Neonatal Systemic Infection 83, 3704–3711. doi: 10.1128/IAI.00716-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougayrede J-P, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E, 2006. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851. doi: 10.1126/science.1127059 [DOI] [PubMed] [Google Scholar]
- Österlund A, Kahlmeter G, Odén H, Kühn I, Landgren M, 2005. Diversity among 2481 Escherichia coli from women with community-acquired lower urinary tract infections in 17 countries. J. Antimicrob. Chemother 55, 928–937. doi: 10.1093/jac/dki122 [DOI] [PubMed] [Google Scholar]
- Padilla-Carlin DJ, McMurray DN, Hickey AJ, 2008. The guinea pig as a model of infectious diseases. Comp. Med 58, 324–340. [PMC free article] [PubMed] [Google Scholar]
- Pandey M, Khan A, Das SC, Sarkar B, Kahali S, Chakraborty S, Chattopadhyay S, Yamasaki S, Takeda Y, Nair GB, Ramamurthy T, 2003. Association of cytolethal distending toxin locus cdtB with enteropathogenic Escherichia coli isolated from patients with acute diarrhea in Calcutta, India. J. Clin. Microbiol 41, 5277–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricia E, Arnaud LM, Tony LG, Christine A, Stéphanie G, Bertrand P, David S, Erick D, 2006. Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ. Microbiol 8, 1975–1984. doi: 10.1111/j.1462-2920.2006.01077.x [DOI] [PubMed] [Google Scholar]
- Poolman JT, Wacker M, 2016. Extraintestinal Pathogenic Escherichia coli, a Common Human Pathogen: Challenges for Vaccine Development and Progress in the Field. J. Infect. Dis 213, 6–13. doi: 10.1093/infdis/jiv429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putze J, Hennequin C, Nougayrède J-P, Zhang W, Homburg S, Karch H, Bringer M-A, Fayolle C, Carniel E, Rabsch W, Oelschlaeger TA, Oswald E, Forestier C, Hacker J, Dobrindt U., 2009. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect. Immun 77, 4696–4703. doi: 10.1128/IAI.00522-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaser JK, Clark EE Jr, Meyers NM, 2008. All Creatures Great and Minute: A Public Policy Primer for Companion Animal Zoonoses. Zoonoses Public Health 55, 385–401. doi: 10.1111/j.1863-2378.2008.01123.x [DOI] [PubMed] [Google Scholar]
- Robertson S, Burakoff A, Stevenson L, Tompkins B, Patel K, Tolar B, Whitlock L, House J, Schlater L, Mackie T, Morningstar-Shaw B, Nichols M, Basler C, 2018. Notes from the Field: Recurrence of a Multistate Outbreak of Salmonella Enteritidis Infections Linked to Contact with Guinea Pigs - Eight States, 2015-2017. MMWR. Morb. Mortal. Wkly. Rep 67, 1195–1196. doi: 10.15585/mmwr.mm6742a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Siek KE, Giddings CW, Doetkott C, Johnson TJ, Fakhr MK, Nolan LK, 2005. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology 151, 2097–2110. doi: 10.1099/mic.0.27499-0 [DOI] [PubMed] [Google Scholar]
- Rosadi F, Fiorentini C, Fabbri A, 2016. Bacterial protein toxins in human cancers. Pathog. Dis 74, ftv105–ftv105. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Snider C, Braden CR, Boxrud D, Wunschmann A, Rudroff JA, Lockett J, Smith KE, 2007. Multidrug-resistant Salmonella enterica serotype Typhimurium associated with pet rodents. N. Engl. J. Med 356, 21–28. doi: 10.1056/NEJMoa060465 [DOI] [PubMed] [Google Scholar]
- Swennes AG, Buckley EM, Madden CM, Byrd CP, Donocoff RS, Rodriguez L, Parry NMA, Fox JG, 2013. Enteropathogenic Escherichia coli prevalence in laboratory rabbits. Vet. Microbiol 163, 395–398. doi: 10.1016/j.vetmic.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swennes AG, Buckley EM, Parry NMA, Madden CM, Garcia A, Morgan PB, Astrofsky KM, Fox JG, 2012. Enzootic enteropathogenic Escherichia coli infection in laboratory rabbits. J. Clin. Microbiol 50, 2353–2358. doi: 10.1128/JCM.00832-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautman EP, Healy AR, Shine EE, Herzon SB, Crawford JM, 2017. Domain-Targeted Metabolomics Delineates the Heterocycle Assembly Steps of Colibactin Biosynthesis. J. Am. Chem. Soc 139, 4195–4201. doi: 10.1021/jacs.7b00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL, Gerdes S, Henry CS, Kenyon RW, Machi D, Mao C, Nordberg EK, Olsen GJ, Murphy-Olson DE, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Vonstein V, Warren A, Xia F, Yoo H, Stevens RL, 2017. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 45, D535–D542. doi: 10.1093/nar/gkw1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carra A, Brennan CA, Chun E, Ngo L, Samson LD, Engelward BP, Garrett WS, Balbo S, Balskus EP, 2019. The human gut bacterial genotoxin colibactin alkylates DNA. Science 363. doi: 10.1126/science.aar7785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha L, Wilson MR, Brotherton CA, Balskus EP, 2016. Characterization of Polyketide Synthase Machinery from the pks Island Facilitates Isolation of a Candidate Precolibactin. ACS Chem. Biol 11, 1287–1295. doi: 10.1021/acschembio.6b00014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.