Abstract
Brown algae are important primary producers and ecosystem engineers in the ocean, and Ectocarpus has been established as a laboratory model for this lineage. Like most multicellular organisms, Ectocarpus is associated with a community of microorganisms, a partnership frequently referred to as holobiont due to the tight interconnections between the components. Although genomic resources for the algal host are well established, its associated microbiome is poorly characterized from a genomic point of view, limiting the possibilities of using these types of data to study host–microbe interactions. To address this gap in knowledge, we present the annotated draft genome sequences of seventy-two cultivable Ectocarpus-associated bacteria. A screening of gene clusters related to the production of secondary metabolites revealed terpene, bacteriocin, NRPS, PKS-t3, siderophore, PKS-t1, and homoserine lactone clusters to be abundant among the sequenced genomes. These compounds may be used by the bacteria to communicate with the host and other microbes. Moreover, detoxification and provision of vitamin B pathways have been observed in most sequenced genomes, highlighting potential contributions of the bacterial metabolism toward host fitness and survival. The genomes sequenced in this study form a valuable resource for comparative genomic analyses and evolutionary surveys of alga-associated bacteria. They help establish Ectocarpus as a model for brown algal holobionts and will enable the research community to produce testable hypotheses about the molecular interactions within this complex system.
Keywords: brown algae, holobiont, alga-associated bacteria, biosynthetic gene clusters, detoxification, metabolic networks
Introduction
Brown macroalgae are important primary producers and major ecosystem engineers on marine rocky shores, providing both shelter and nutrients for other forms of life (Brodie et al. 2017). They belong to the stramenopiles, an evolutionarily distinct lineage from the Achaeplastida, which comprise red and green algae as well as land plants (Charrier et al. 2008) and are of commercial importance in several regions of the world (Koru 2013; Raja et al. 2013; Venkatesan et al. 2015). Ectocarpus is a genus of brown algae that has been established as a laboratory model for this lineage (Peters et al. 2004) due to its small genome (Cock et al. 2010), the possibility of cultivation in the lab, and its short life cycle.
Like most if not all multicellular eukaryotes, brown algae, including Ectocarpus, are associated with bacteria (Paix et al. 2019). These interactions may be so intimate that the term holobiont has been suggested to describe the functional unit of a host and its associated microbiome (Zilber‐Rosenberg and Rosenberg 2008; Douglas and Werren 2016). For instance, it has been estimated that approximately half of all algae (including 49 out of 83 surveyed stramenopiles) rely on their bacteria associated to provide them with vitamin B12 (Croft et al. 2005; Tang et al. 2010). In Ectocarpus, associated bacteria are known to provide functions related to developmental transitions and growth of the algae (Pedersen 1968; Tapia et al. 2016). Furthermore, they may impact their capacity to tolerate environmental stressors (Dittami et al. 2016).
Collections of cultivable bacteria provide a valuable resource to study the mechanisms underlying these interactions, and in Ectocarpus three recent papers describe the generation of culture collections. In Ectocarpus siliculosusTapia et al. (2016) have reported the isolation of 9 bacterial strains, and in Ectocarpus subulatusKleinJan et al. (2017) cultivated 46 strains corresponding to 33 different bacterial genera from algal surfaces. An additional 95 strains corresponding to 27 different genera have also recently been isolated from field material of E. subulatus (Dittami et al. 2019).
In present study, we describe genomic resources for 72 of these cultivable Ectocarpus-associated bacteria. Sixty-two genomes were sequenced specifically for this study, plus ten previously sequenced genomes from the same culture collection (Burgunter-Delamare et al. 2019) were also included. These genomes constitute a valuable resource both to study the genomic adaptations of bacteria to life on the surface of brown algae, but also to generate hypotheses on potential beneficial interactions between the bacteria and their host, for example, via metabolic complementarity-based approaches (Frioux et al. 2018). They furthermore constitute a first step toward filling a big gap in our current knowledge: The fact that currently (September 2019), based on our research through Marine Metagenomics Portal (Robertsen et al. 2017; Klemetsen et al. 2018), only ∼100 draft and complete bacterial genomes isolated from algae/seaweed are publicly available in GenBank. Thus, the genomes from this study could add a great amount of information to algal microbiomes and will promote other studies aiming to decipher algal-microbial associations.
Materials and Methods
Bacterial Strains and DNA Extraction
Bacterial strains were isolated from a laboratory culture of E.subulatus (strain CCAP 1310/19; KleinJan et al. 2017) as well as from field samples of the same species (Dittami et al. 2019). Field samples were collected in March 2017 from two locations along the Hopkins River, Victoria, Australia, a few km upstream of Hopkins River falls, the original collection site of strain CCAP 1310/19 (West and Kraft 1996): Framlingham Forest reserve (–38.297064, 142.668291) and Kent's Ford (–38.191574, 142.698058). All bacterial strains were identified by Sanger-sequencing of the 16S rDNA gene using the 8F and the 1492R primer pair (Weisburg et al. 1991). Bacteria were grown on 90 mm Petri dishes with R2A medium (Reasoner and Geldreich 1985) Sigma–Aldrich at 19 °C for 4–7 days. Subsequently, a single colony was selected and grown at 25 °C in liquid R2A medium overnight. The bacterial genomic DNA was extracted using Promega Wizard Genomic DNA purification kit following the manufacturer’s instructions. The extracted DNA was quantified using a Qubit and its quality was determined using agarose gel electrophoresis.
Genome Sequencing, Assembly, and Annotation
Paired-end DNA libraries with an average insert size of 500 bp were prepared using the Nextera XT DNA library kit (library average size ∼1,100 bp). Libraries were then sequenced using the Illumina MiSeq technology (V3, paired-end, 2 × 300 bp reads) at GENOMER platform (Station Biologique de Roscoff), multiplexing ∼20 bacterial genomes per run. Raw reads were first examined using FastQC (Andrews 2010). Low-quality sequences were trimmed or removed using Trimmomatic v.0.38 and a sliding window with a quality score of 15 as well as a minimal read length of 36 bp as filters. Trimmed read pairs were used for genome assembly with SPAdes v.3.12.0 (Bankevich et al. 2012) using default parameters. Genomic sequences encoding parts of the ribosome were identified using Barrnap v. 0.8 (https://github.com/tseemann/barrnap) and 16S rDNA sequences used to search for complete reference genomes in the GenBank. These reference genomes were used for scaffolding with Medusa version 1.6. Finally, gaps in the scaffolds were filled wherever possible using GapCloser 1.12 (Li et al. 2010) and the resulting draft genomes were annotated and prepared for submission to public databases using the MicroScope platform (Vallenet et al. 2017). The genomes were deposited at the European Nucleotide Archive.
Phylogenomic Analyses
Phylogenomic relationships among all studied strains were confirmed by running genome clustering based on pairwise distances and Average Nucleotide Identity (ANI) between all selected genomes using the Neighbor-Joining algorithm in MicroScope. Furthermore, the closest genome has been provided for all genomes, based on their resulting Tetra-nucleotide signature correlation index via the JSpeciesWS tool (Richter et al. 2016).
In Silico Analysis of Bacterial Metabolism
Models of primary metabolism for each sequenced bacterium were generated using the Pathway tools pipeline implemented in the MicroScope platform. The output of this pipeline is a pathway completion value, that is, the ratio between the number of reactions for a specific pathway in a bacterium and the total number of reactions for that pathway defined in the MetaCyc (Caspi et al. 2018) or KEGG (Kanehisa et al. 2008) databases. In addition, secondary metabolite-related gene clusters were predicted using antiSMASH (Blin et al. 2017).
Results and Discussion
Genome Characteristics
Here, we report the sequencing of 62 and the analysis of 72 genomes of Ectocarpus-associated bacterial strains corresponding to 43 different genera and 16 different orders. The individual strains as well as key attributes of their genome sequences are listed in table 1. The genome size of all strains ranged from 2.4 Mb to 6.8 Mb. The largest genome was that of Imperialibacter sp. strain SDR9 from the Bacteroidetes and the smallest was that of Micrococcus sp. strain 11B from the Actinobacteria. The analyzed genomes showed diverse GC contents with strains belonging to the Bacteroidetes and Firmicutes exhibiting GC contents <40% (e.g., 30% in Flavobacterium sp. 9AF) contrary to Actinobacteria, where most strains exhibit GC contents over 70%. Overall, the GC content was positively correlated with genome size (Pearson correlation r = 0.73, P = 0.042). CheckM analyses (Parks et al. 2015) suggest that the sequenced genomes are nearly complete (>98%, table 1) and free of or with very low levels of contamination (<2.5%; supplementary table S1, Supplementary Material online). The only exception was Arthrobacter sp. strain 9V with 4.8% contamination (22 marker genes). This indicates that, overall, the presented genomes are suitable for downstream analyses such as comparisons of metabolic capacities.
Table 1.
Genome Features of Algal-Associated Bacteria Analyzed in This Study
| Strain | Complete-ness (%)a | Genome Size (Mb) | Coverage (X) | N50 (Mb) | %GC | Scaffold Nb. | CDS Nb. | Mean CDS Length | tRNA Nb. | rRNA Nb. | Closest Relative | Accession Numbers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | ||||||||||||
| Aeromicrobium sp. 9AM | 99.7 | 4.2 | 144 | 2.98 | 68 | 9 | 4,422 | 897 | 46 | 3 | Aeromicrobium sp. Root236 | LR733303–LR733311 |
| Arthrobacter sp. 8AJ | 99.7 | 4.3 | 88 | 4.22 | 66 | 4 | 4,228 | 944 | 51 | 5 | Moraxella osloensis NCTC10465 | LR733289–LR733292 |
| Arthrobacter sp. 9AX | 99.7 | 4.4 | 230 | 4.41 | 66 | 7 | 4,453 | 918 | 50 | 6 | Pseudarthrobacter siccitolerans 4J27 | LR733289–LR733292 |
| Arthrobacter sp. 9V | 99.7 | 5.1 | 221 | 4.82 | 62 | 158 | 5,091 | 925 | 62 | 9 | Arthrobacter sp. EpRS71 | LR732912–LR733069 |
| Citricoccus sp. K5 | 99.2 | 3.9 | 324 | 3.74 | 69 | 9 | 3,708 | 974 | 47 | 5 | Citricoccus muralis DSM 14442 | LR732817–LR732825 |
| Curtobacterium sp. 8I–2 | 99 | 3.6 | 109 | 2.80 | 71 | 5 | 3,767 | 911 | 47 | 6 | Curtobacterium flaccumfaciens UCD-AKU | LR732826–LR732830 |
| Frigoribacterium sp. 9N | 98.5 | 3.3 | 151 | 2.53 | 71 | 16 | 3,339 | 926 | 45 | 5 | Frigoribacterium sp. Leaf8 | LR733390–LR733405 |
| Microbacterium sp. 8M | 99.5 | 3.7 | 185 | 3.68 | 71 | 2 | 3,659 | 961 | 44 | 4 | Microbacterium azadirachtae DSM 23848 | LR733284–LR733285 |
| Micrococcus sp. 116 | 98.6 | 2.6 | 215 | 2.49 | 73 | 19 | 2,526 | 943 | 48 | 5 | Micrococcus luteus 2385 | LR732370–LR732388 |
| Micrococcus sp. 11B | 98.1 | 2.4 | 450 | 1.89 | 73 | 52 | 2,398 | 952 | 48 | 5 | Micrococcus luteus 2385 | LR733070–LR733121 |
| Micrococcus sp. 80W | 98.1 | 2.5 | 224 | 1.78 | 73 | 80 | 2,521 | 942 | 48 | 4 | Micrococcus luteus 2385 | LR732389–LR732468 |
| Nocardioides sp. AX2bis | 98.7 | 4.2 | 221 | 3.96 | 73 | 37 | 4,397 | 915 | 45 | 4 | Marmoricola aurantiacus DSM 12652* | LR733215–LR733251 |
| Plantibacter sp. T3 | 99.5 | 4 | 287 | 3.98 | 69 | 3 | 4,131 | 924 | 48 | 4 | Plantibacter flavus VKM Ac-2504 | LR733286–LR733288 |
| Pseudoclavibacter sp. 8L | 98.2 | 4.1 | 98 | 1.43 | 68 | 30 | 4,137 | 921 | 45 | 4 | Microbacterium sp. TS-1* | LR733185–LR733214 |
| Bacteroidetes | ||||||||||||
| Imperialibacter sp. SDR9 | 100 | 6.8 | 111 | 0.96 | 47 | 65 | 5,767 | 1069 | 38 | 4 | Arcticibacter pallidi-corallinus CGMCC 1.9313* | LR701573–LR701637 |
| Marinoscillum sp. 108 | 99.1 | 5.2 | 83 | 3.73 | 46 | 12 | 4,489 | 1086 | 37 | 4 | Marinoscillum furvescens DSM 4134* | LR734808–LR734819 |
| Chryseobacterium sp. 8AT | 100 | 4.7 | 114 | 4.43 | 34 | 31 | 4,483 | 931 | 70 | 7 | Chryseobacterium scophthalmum DSM 16779 | LR733314–LR733344 |
| Flavobacterium sp. 9AF | 98.9 | 4.2 | 101 | 2.95 | 30 | 74 | 3,871 | 992 | 51 | 5 | Flavobacterium sp. 316* | LR733556–LR733629 |
| Flavobacterium sp. 9R | 99.6 | 3.6 | 184 | 3.42 | 35 | 16 | 3,175 | 1006 | 42 | 6 | Flavobacterium succinicans DD5b* | LR733413–LR733428 |
| Maribacter sp. 151 | 99.7 | 4.4 | 59 | 4.35 | 36 | 4 | 3,857 | 1044 | 36 | 6 | Maribacter litoralis SDRB-Phe2 | LR733271–LR733274 |
| Sphingobacterium sp. 8BC | 100 | 5.8 | 129 | 5.73 | 40 | 14 | 5,379 | 960 | 70 | 9 | Sphingobacterium multivorum NCTC11343 | LR733857–LR733870 |
| Firmicutes | ||||||||||||
| Bacillus sp. 348 | 99.6 | 3.8 | 246 | 3.58 | 41 | 5 | 4,070 | 846 | 79 | 9 | Bacillus stratosphericus LK33 | LR732831–LR732835 |
| Bacillus sp. 349Y | 99.3 | 4.5 | 114 | 0.12 | 48 | 85 | 4,616 | 839 | 97 | 9 | Bacillus sp. Leaf406 | LR733732–LR733816 |
| Bacillus sp. 71 | 99.3 | 5.7 | 116 | 5.69 | 35 | 14 | 6,092 | 796 | 98 | 18 | Bacillus cereus HuA2-4 | LR733376–LR733389 |
| Bacillus sp. 9J | 99.6 | 3.8 | 179 | 3.74 | 42 | 76 | 4,109 | 834 | 86 | 9 | Bacillus sp. Leaf49 | LR732836–LR732911 |
| Exiguobacterium sp. 8A | 99.3 | 3.1 | 184 | 2.87 | 48 | 77 | 3,234 | 868 | 63 | 13 | Exiguobacterium sp. AT1b | LR733630–LR733706 |
| Exiguobacterium sp. 8H | 99.3 | 3 | 296 | 0.87 | 48 | 40 | 3,154 | 868 | 63 | 14 | Exiguobacterium sp. AT1b | LR733429–LR733468 |
| Exiguobacterium sp. 9Y | 99.3 | 3 | 88 | 1.61 | 47 | 20 | 3,070 | 876 | 65 | 11 | Exiguobacterium oxidotolerans JCM 12280 | LR732308–LR732327 |
| Staphylococcus sp. 8AQ | 99.2 | 2.5 | 269 | 2.49 | 31 | 4 | 2,501 | 886 | 62 | 9 | Staphylococcus pasteuri BAB3 | LR733871–LR733874 |
| Proteobacteria | ||||||||||||
| Aeromonas sp. 8C | 100 | 4.6 | 345 | 4.57 | 59 | 3 | 4,769 | 899 | 114 | 11 | Aeromonas veronii TTU2014-115ASC | LR732797–LR732799 |
| Aeromonas sp. 9A | 100 | 4.8 | 105 | 4.70 | 59 | 11 | 4,590 | 925 | 114 | 16 | Aeromonas salmonicida Y577 | LR732779–LR732789 |
| Alteromonas sp. 38 | 100 | 4.7 | 209 | 4.70 | 44 | 3 | 4,324 | 975 | 62 | 6 | Alteromonas stellipolaris LMG 21856 | LR733300–LR733302 |
| Marinobacter sp. HK377 | 100 | 4.4 | 172 | 4.34 | 57 | 7 | 4,176 | 976 | 45 | 6 | Marinobacter salarius R9SW1 | LR701480–LR701486 |
| Marinobacter sp. N1 | 100 | 4.4 | 152 | 4.35 | 57 | 2 | 4,125 | 978 | 45 | 6 | Marinobacter salarius R9SW1 | LR733269–LR733270 |
| Burkholderia sp. 8Y | 100 | 6.3 | 61 | 2.36 | 63 | 37 | 6,403 | 874 | 52 | 8 | Burkholderia sp. MR1 | LR733519–LR733555 |
| Limnobacter sp. 130 | 99 | 3.3 | 74 | 1.82 | 52 | 6 | 3,034 | 1007 | 37 | 3 | Limnobacter sp. MED105* | LR732328–LR732333 |
| Massilia sp. 9I | 100 | 5.5 | 195 | 5.51 | 66 | 9 | 5,242 | 984 | 70 | 7 | Massilia alkalitolerans DSM 17462 | LR733275–LR733283 |
| Burkholderiales bacterium 8X | 99.8 | 4.8 | 141 | 4.78 | 67 | 3 | 4,776 | 973 | 44 | 5 | Variovorax sp. WDL1* | LR732703–LR732705 |
| Brevundimonas sp. G8 | 99.7 | 3.3 | 375 | 3.32 | 66 | 1 | 3,308 | 927 | 47 | 3 | Brevundimonas sp. Leaf280 | LR732816–LR732816 |
| Oceanicaulis sp. 350 | 99.8 | 3.1 | 185 | 2.98 | 62 | 4 | 3,035 | 939 | 47 | 6 | Oceanicaulis alexandrii DSM 11625 | CABWMW010000001–CABWMW010000008 |
| Pantoea sp. 111 | 100 | 4.9 | 62 | 4.09 | 56 | 35 | 4,807 | 890 | 73 | 9 | Pantoea brenneri LMG 5343 | LR733469–LR733503 |
| Enterobacterales bacterium 8AC | 100 | 5.3 | 134 | 4.81 | 53 | 63 | 4,858 | 936 | 74 | 10 | Serratia oryzae J11-6 | LR733916–LR733978 |
| Halomonas sp. 153 | 100 | 5.5 | 35 | 5.44 | 55 | 11 | 5,045 | 972 | 59 | 5 | Halomonas titanicae BH1 | LR733721–LR733731 |
| Halomonas sp. 98 | 100 | 5.5 | 109 | 5.43 | 55 | 14 | 5,029 | 975 | 59 | 6 | Halomonas titanicae BH1 | LR733707–LR733720 |
| Acinetobacter sp. 8BE | 100 | 4.4 | 144 | 3.94 | 41 | 35 | 4,368 | 891 | 61 | 7 | Acinetobacter sp. NIPH 809 | LR732744–LR732778 |
| Acinetobacter sp. 8I-beige | 100 | 3.5 | 138 | 2.08 | 41 | 7 | 3,452 | 895 | 73 | 7 | Acinetobacter johnsonii DSM 6963 | LR732790–LR732796 |
| Moraxellaceae bacterium 17A | 100 | 3 | 194 | 2.75 | 43 | 37 | 2,973 | 897 | 41 | 6 | Moraxella osloensis CCUG 57516 | LR732269–LR732305 |
| Enhydrobacter sp. 8BJ | 100 | 2.8 | 301 | 2.62 | 43 | 31 | 2,628 | 919 | 45 | 7 | Moraxella osloensis NCTC10465 | LR733345–LR733375 |
| Enhydrobacter sp. AX1 | 99.7 | 2.7 | 350 | 2.65 | 44 | 16 | 2,517 | 943 | 49 | 6 | Enhydrobacter aerosaccus SK60 | LR732800–LR732815 |
| Pseudomonas sp. 8AS | 98.1 | 4.3 | 199 | 4.26 | 66 | 7 | 4,113 | 945 | 57 | 4 | Pseudomonas alcaligenes NBRC 14159 | LR733406–LR733412 |
| Pseudomonas sp. 8BK | 100 | 4.5 | 145 | 4.38 | 60 | 11 | 4,205 | 960 | 63 | 9 | Pseudomonas peli DSM 17833 | LR733252–LR733262 |
| Pseudomonas sp. 8O | 99.8 | 5.2 | 78 | 1.61 | 62 | 6 | 4,949 | 949 | 60 | 5 | Pseudomonas pseudoalcaligenes AD6 | LR733263–LR733268 |
| Pseudomonas sp. 8Z | 99.4 | 4.8 | 144 | 1.12 | 61 | 12 | 4,625 | 935 | 61 | 8 | Pseudomonas composti CCUG 59231* | LR733824–LR733835 |
| Pseudomonas sp. 9Ag | 100 | 4.7 | 136 | 4.62 | 60 | 4 | 4,465 | 946 | 52 | 4 | Pseudomonas sp. 10B238 | LR733836–LR733839 |
| Pseudomonas sp. 9AZ | 99.7 | 4.5 | 235 | 4.46 | 60 | 4 | 4,260 | 961 | 60 | 8 | Pseudomonas peli DSM 17833 | LR733840–LR733843 |
| Bosea sp. 125 | 99.1 | 6.3 | 46 | 6.12 | 67 | 63 | 6,435 | 899 | 46 | 3 | Bosea sp. Root483D1 | LR733122–LR733184 |
| Bosea sp. 127 | 99.1 | 6.3 | 78 | 6.28 | 67 | 8 | 6,705 | 876 | 46 | 3 | Bosea sp. Root483D1 | LR733511–LR733518 |
| Bosea sp. 29B | 99.1 | 6.3 | 137 | 6.32 | 67 | 7 | 6,422 | 904 | 46 | 3 | Bosea sp. Root483D1 | LR733817–LR733823 |
| Bosea sp. 62 | 99.1 | 6.3 | 154 | 6.28 | 67 | 7 | 6,411 | 905 | 46 | 3 | Bosea sp. Root483D1 | LR733504–LR733510 |
| Bosea sp. HK365B | 99.1 | 6.3 | 133 | 1.03 | 67 | 18 | 6,738 | 876 | 46 | 3 | Bosea sp. Root483D1 | LR701663–LR701680 |
| Hoeflea sp. HK425 | 99.9 | 5.2 | 326 | 4.68 | 61 | 28 | 5,266 | 898 | 43 | 3 | Hoeflea halophila KCTC 23107 | LR701545–LR701572 |
| Rhizobium sp. SD404 | 100 | 4.2 | 148 | 4.22 | 62 | 18 | 4,192 | 920 | 42 | 3 | Pararhizobium haloflavum XC0140* | LR701442–LR701459 |
| Roseovarius sp. SD190 | 99.3 | 4.7 | 80 | 3.89 | 61 | 17 | 4,794 | 902 | 44 | 3 | Roseovarius sp. TM1035 | LR701460–LR701476 |
| Erythrobacter sp. HK427 | 99.1 | 3.1 | 157 | 3.12 | 63 | 3 | 3,097 | 947 | 45 | 3 | Porphyrobacter sp. AAP60* | LR701477–LR701479 |
| Novosphingobium sp. 9U | 99.6 | 4.6 | 221 | 2.82 | 65 | 75 | 4,843 | 867 | 49 | 5 | Novosphingobium resinovorum SA1* | LR732469–LR732543 |
| Sphingomonas sp. 8AM | 99.7 | 3.8 | 119 | 3.66 | 67 | 13 | 3,739 | 929 | 48 | 4 | Sphingomonas phyllosphaerae FA2 | LR733844–LR733856 |
| Sphingomonas sp. AX6 | 99.4 | 3 | 228 | 3.01 | 64 | 1 | 3,161 | 892 | 44 | 3 | Sphingomonas echinoides ATCC 14820* | LR733857–LR733870 |
| Sphingomonas sp. HK361 | 99.7 | 3.3 | 150 | 1.78 | 66 | 8 | 3,274 | 935 | 45 | 3 | Hephaestia caeni DSM 25527* | LR701487–LR701494 |
| Sphingomonas sp. SD391 | 99.5 | 4.6 | 114 | 4.15 | 66 | 34 | 4,682 | 903 | 49 | 5 | Sphingomonas sp. Leaf28 | LR701495–LR701528 |
| Sphingomonas sp. T1 | 99.3 | 4.5 | 243 | 3.83 | 66 | 41 | 4,647 | 900 | 50 | 3 | Sphingomonas sp. Leaf30 | LR733875–LR733915 |
| Sphingorhabdus sp. 109 | 99.2 | 3.6 | 97 | 3.56 | 58 | 5 | 3,585 | 928 | 45 | 6 | Sphingorhabdus sp. M41* | LR732707–LR732711 |
| Luteimonas sp. 9C | 100 | 3.3 | 77 | 2.83 | 69 | 2 | 3,207 | 957 | 48 | 3 | Xanthomonas sp. Mitacek01 | LR733312–LR733313 |
Note.—The closest relative with the similarity below Cut-off [z-score (<0.98)] is marked with asterisk. Nb, number; CDS, coding sequence.
Determined using the CheckM tool.
Phylogenomic Tree
Several of the sequenced bacteria in this study correspond to bacteria with no or only few closely related sequences in the databases. Notably, Enterobacterales bacterium 8AC, and Moraxellaceae bacterium 17A could be confidently identified only to the family level through RDP classifier (supplementary table S1, Supplementary Material online), making these strains candidates for new species or genera. Besides, fifteen strains including Imperialibacter sp. EC-SDR9, Marinoscillum sp. 108, Sphingomonas sp., AX6, and Novosphingobium sp., and Burkholderiales bacterium 8X have low similarity (z-score below cutoff < 0.989) with their closest genome-sequenced relatives (based on the tetra-nucleotide signature correlation index, table 1 and supplementary fig. S1, Supplementary Material online). This phylogenomic analysis yielded a tree generally grouping together bacteria from the same taxon (supplementary fig. S1, Supplementary Material online). However, Imperialibacter sp. EC-SDR9 and Sphingobacterium sp. 8BC from Bacteroidetes clustered with Firmicutes.
Secondary Metabolic Activities and Potentially Symbiosis-Related Metabolites
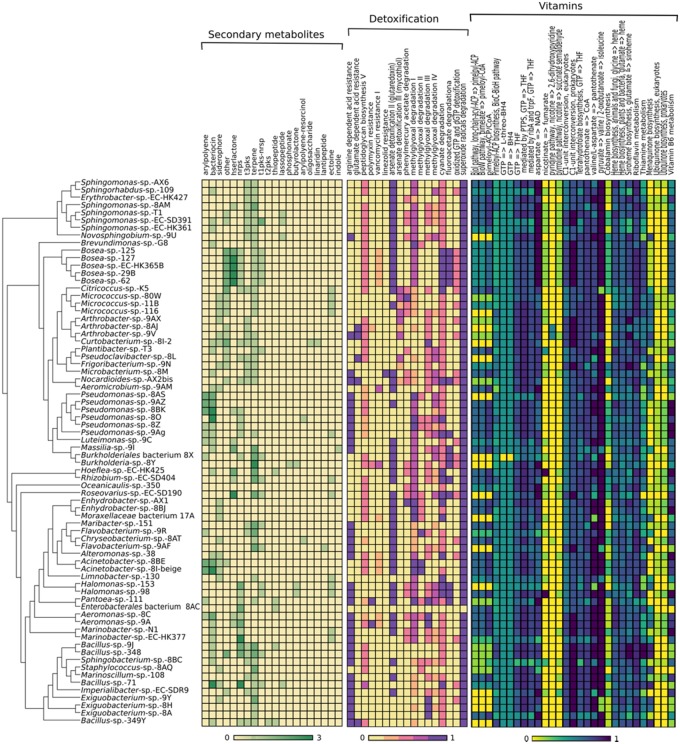
Algal-associated microbes are likely to interact with both the host and other microbes within the community. Secondary metabolites are metabolites not essential for normal growth of microorganisms, but they play a major role as chemical signals for interaction with other microorganisms (Netzker et al. 2015), restriction of pathogens (antimicrobial activities), and biofouling (Wiese et al. 2009; Nasrolahi et al. 2012; Susilowati et al. 2015). For instance, terpenes as the largest class of natural compounds have protective roles against competitors and are involved in interspecies signaling (Gershenzon and Dudareva 2007; Yamada et al. 2015). Similarly, bacteriocins, peptidic toxins produced by bacteria, have been suggested to play a role in pathogenesis by induction of cell lysis (Li and Tian 2012). The annotation of the 72 bacterial genomes with respect to genes involved in secondary metabolism obtained from AntiSMASH via the MicroScope platform showed that all analyzed strains except Oceanicaulis sp. strain 350, had at least one secondary biosynthetic gene cluster. Furthermore, 68% of genomes have at least one predicted terpene cluster gene, followed by bacteriocin (40.2%), nonribosomal Peptide Synthetases (NRPS, 36%), Type 3 polyketide synthases (PKS-t3, 33.33%), siderophores (23.6%), Type 1 polyketide synthases (PKS-t1, 20.8%), and homoserine lactone synthesis genes (16.6%; fig. 1 and supplementary table S1, Supplementary Material online). These genes are likely to be at least partially involved in the communication with the host and between microbes.
Fig. 1.
—Heatmap of representative secondary metabolite clusters, detoxification-, and vitamin biosynthetic genes in the studied bacterial genomes. The dendrogram represents a whole-genome phylogeny, secondary metabolite gene clusters were predicted via AntiSMASH, detoxification genes were identified based on the MicroCyc database, and vitamin biosynthesis capacities were assess based on KEGG entries. The color code represents the number of genes per cluster (secondary metabolites) or the proportion of genes found in a particular organism and pathway.
Detoxification Role of Symbionts and Provision of Vitamins
In terms of detoxification mechanisms, one pathway that was complete in all studied genomes was the capacity to degrade superoxide radicals. Moreover, 46 strains of 72 possessed the complete pathway for glutaredoxin synthesis (fig. 1). This mechanism is important for the degradation reactive oxygen species (ROS), which are formed by the algae through metabolic processes and in response to different stressors (Cosse et al. 2007). ROS can cause significant damage to the cell; thus, microorganisms have developed defense systems to detoxify ROS in order to survive.
Furthermore, the cyanate degradation pathway was complete or semicomplete in all bacteria except in strains 8BE, 8AC, and 8AQ. Cyanate is a common compound in marine environments and may serve as both an energy source for marine microbes (Palatinszky et al. 2015) as well as a potential source of nitrogen (Kamennaya et al. 2008; Sáez et al. 2019). Whether this pathway also plays a role during the interactions of microbes with their algal host, for example, by enabling the microbes to provide nitrogen to their host, remains to be tested.
Finally, most genomes analyzed encoded nearly complete or complete pathways for production of B vitamins like biotin (B7), folate (B9), riboflavin (B2), thiamine (B1), and pyridoxine (B6) (fig. 1). They may thus be contributors of vitamin B for the algal host, as has previously been suggested for diatom-bacteria associations (Behringer et al. 2018). All in all, these studied metabolic features highlight the possible contributions of the alga-associated bacteria to maintain host fitness and survival.
The genomic resources provided here constitute a valuable resource for comparative genomic analyses and evolutionary surveys of alga-associated bacteria and will allow us to produce testable hypotheses about the molecular interactions between the microbes and their host. They may, among other uses, facilitate metabolic complementarity centered approach as proposed by Dittami et al. (2014), to identify potential beneficial interactions between the partners. They will also form the bases for more targeted molecular approaches, for example, gene knockouts or gene expression analyses once specific interactions are being targeted in coculture experiments.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
We appreciate the LABGeM (CEA/Genoscope & CNRS UMR8030), the France Génomique and French Bioinformatics Institute national infrastructures (funded as part of Investissement d'Avenir program managed by Agence Nationale pour la Recherche, contracts ANR-10-INBS-09 and ANR-11-INBS-0013) for their technical support within the MicroScope annotation platform and thank to Sylvie Rousvoal for help with DNA extractions.
This study was supported partially by the CNRS Momentum call, the ANR project IDEALG [ANR-10-BTBR-04] “Investissements d’Avenir, Biotechnologies-Bioressources,” and the European Union’s Horizon 2020 research and innovation Programme under the Marie Sklodowska-Curie grant agreement [624575 (ALFF)]. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author Contributions
E.K. participated in the conception and design of the study, sample processing, genome sequencing and assembly, data analysis, writing the manuscript. E.G. participated in the genome assembling, submission of genomes to MicroScope, and helped with the preparation of the figures. H.K. participated in the isolation of bacteria and genome sequencing. G.T. and E.L. both contributed to the sequencing of the genomes. E.C. participated in the assembling protocol and revision. S.M.D. participated in the conception and design of the study, isolation of bacteria, genome assembly and writing the manuscript. All authors approved the final draft.
Data deposition: The projects PRJEB31339 and PRJEB34356 have been deposited at European Nucleotide Archive - European Molecular Biology Laboratory- EBI under the accession numbers given in Table 1 (http://www.ebi.ac.uk/ena/data/view/ <ACCESSION NUMBER>).
Literature Cited
- Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. In: Babraham Bioinformatics. Cambridge: Babraham Institute.
- Bankevich A, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5): 455–477. doi:10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer G, et al. 2018. Bacterial communities of diatoms display strong conservation across strains and time. Front Microbiol. 9:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, et al. 2017. antiSMASH 4.0-improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 45(W1):W36–W41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie J, et al. 2017. The algal revolution. Trends Plant Sci. 22(8):726–738. [DOI] [PubMed] [Google Scholar]
- Burgunter-Delamare B, et al. 2019. Metabolic complementarity between a brown alga and associated cultivable bacteria provide indications of beneficial interactions. bioRxiv 813683. doi:10.1101/813683. [Google Scholar]
- Caspi R, et al. 2018. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 46(D1):D633–D639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier B, et al. 2007. Development and physiology of the brown alga Ectocarpus siliculosus: two centuries of research. New Phytol. 177(2):319–332. [DOI] [PubMed] [Google Scholar]
- Cock JM, et al. 2010. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465(7298):617–621. [DOI] [PubMed] [Google Scholar]
- Cosse A, Leblanc C, Potin P.. 2007. Dynamic defense of marine macroalgae against pathogens: from early activated to gene‐regulated responses In: Adv. Bot. Res. Academic Press; p. 221–266. [Google Scholar]
- Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG.. 2005. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438(7064):90–93. [DOI] [PubMed] [Google Scholar]
- Dittami SM, et al. 2016. Host–microbe interactions as a driver of acclimation to salinity gradients in brown algal cultures. ISME J. 10(1):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittami SM, Eveillard D, Tonon T.. 2014. A metabolic approach to study algal–bacterial interactions in changing environments. Mol Ecol. 23(7):1656–1660. [DOI] [PubMed] [Google Scholar]
- Dittami SM, et al. 2019. Revisiting Australian Ectocarpus subulatus (Phaeophyceae) from the Hopkins River: distribution, abiotic environment, and associated microbiota. bioRxiv 821579. doi:10.1101/821579. [DOI] [PubMed]
- Douglas AE, Werren JH.. 2016. Holes in the hologenome: why host–microbe symbioses are not holobionts. mBio 7(2):e02099–02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frioux C, Fremy E, Trottier C, Siegel A.. 2018. Scalable and exhaustive screening of metabolic functions carried out by microbial consortia. Bioinformatics 34(17):i934–i943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, Dudareva N.. 2007. The function of terpene natural products in the natural world. Nat Chem Biol. 3(7):408–414. [DOI] [PubMed] [Google Scholar]
- Kamennaya NA, Chernihovsky M, Post AF.. 2008. The cyanate utilization capacity of marine unicellular Cyanobacteria. Limnol Oceanogr. 53(6):2485–2494. [Google Scholar]
- Kanehisa M, et al. 2008. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36 (suppl_1):D480–D484. doi:10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KleinJan H, Jeanthon C, Boyen C, Dittami SM.. 2017. Exploring the cultivable Ectocarpus microbiome. Front Microbiol. 8:2456. doi:10.3389/fmicb.2017.02456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemetsen T, et al. 2018. The MAR databases: development and implementation of databases specific for marine metagenomics. Nucleic Acids Res. 46(D1):D692–D699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koru E. 2013. Seaweeds for food and industrial applications. In: Muzzalupo I, editor. IntechOpen; p. 735–748. [Google Scholar]
- Li R, et al. 2010. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 20(2):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-H, Tian X.. 2012. Quorum sensing and bacterial social interactions in biofilms. Sensors (Basel) 12(3):2519–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrolahi A, Stratil SB, Jacob KJ, Wahl M.. 2012. A protective coat of microorganisms on macroalgae: inhibitory effects of bacterial biofilms and epibiotic microbial assemblages on barnacle attachment. FEMS Microbiol Ecol. 81(3):583–595. [DOI] [PubMed] [Google Scholar]
- Netzker T, et al. 2015. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front Microbiol. 6:299. doi:10.3389/fmicb.2015.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix B, Othmani A, Debroas D, Culioli G, Briand J-F.. 2019. Temporal covariation of epibacterial community and surface metabolome in the Mediterranean seaweed holobiont Taonia atomaria. Environ Microbiol. 21(9):3346–3363. [DOI] [PubMed] [Google Scholar]
- Palatinszky M, et al. 2015. Cyanate as an energy source for nitrifiers. Nature 524(7563):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW.. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25(7):1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M. 1968. Ectocarpus fasciculatus: marine brownalga requiring kinetin. Nature 218:776.5690088 [Google Scholar]
- Peters AF, Marie D, Scornet D, Kloareg B, Mark Cock J.. 2004. Proposal of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) as a model organism for brown algal genetics and genomics. J Phycol. 40(6):1079–1088. [Google Scholar]
- Raja A, Vipin C, Aiyappan A.. 2013. Biological importance of marine algae—an overview. Int J Curr Microbiol Appl Sci. 2:222–227. [Google Scholar]
- Reasoner DJ, Geldreich EE.. 1985. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 49(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Rosselló-Móra R, Oliver Glöckner F, Peplies J.. 2016. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 32(6):929–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsen E, et al. 2017. ELIXIR pilot action: marine metagenomics? Towards a domain specific set of sustainable services [version 1; peer review: 1 approved, 2 approved with reservations]. F1000Research 6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez LP, et al. 2019. Cyanate assimilation by the alkaliphilic cyanide-degrading bacterium pseudomonas pseudoalcaligenes CECT5344: mutational analysis of the cyn gene cluster. IJMS 20(12):3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susilowati R, Sabdono A, Widowati I.. 2015. Isolation and characterization of bacteria associated with brown algae Sargassum spp. from Panjang island and their antibacterial activities. Proc Environ Sci. 23:240–246. [Google Scholar]
- Tang YZ, Koch F, Gobler CJ.. 2010. Most harmful algal bloom species are vitamin B1and B12 auxotrophs. Proc Natl Acad Sci USA. 107(48):20756–20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia JE, González B, Goulitquer S, Potin P, Correa JA.. 2016. Microbiota influences morphology and reproduction of the brown alga Ectocarpus sp. Front Microbiol. 7:197. doi:10.3389/fmicb.2016.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallenet D, et al. 2017. MicroScope in 2017: an expanding and evolving integrated resource for community expertise of microbial genomes. Nucleic Acids Res. 45(D1):D517–D528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan J, Manivasagan P, Kim S-K.. 2015. Chapter 1—Marine microalgae biotechnology: present trends and future advances In: Kim S-K, editor. Handbook of marine microalgae. Boston: Academic Press; p. 1–9. [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ.. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173(2):697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J, Kraft G.. 1996. Ectocarpus siliculosus (Dillwyn) Lyngbye from the Hopkins River Falls, Victoria. The first record of a freshwater brown alga in Australia. Muelleria 9:29–33. [Google Scholar]
- Wiese J, Thiel V, Nagel K, Staufenberger T, Imhoff JF.. 2009. Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic sea. Mar Biotechnol. 11(2):287–300. [DOI] [PubMed] [Google Scholar]
- Yamada Y, et al. 2015. Terpene synthases are widely distributed in bacteria. Proc Natl Acad Sci USA. 112(3):857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber‐Rosenberg I, Rosenberg E.. 2008. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 32(5):723–735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.