Abstract
Background
Osteosarcoma is the most common primary aggressive bone tumor affecting children and young adolescents.
Metastases are often resistant to conventional chemotherapy and mean short-term survival.
Development of valuable diagnostic indicators and targeting agents will have important implications for clinical diagnosis by the identification and characterization of molecules that contribute to its aggressive behavior.
Methods
We examined differential expression levels of common stem cell markers in osteosarcoma parental and sphere cells. In addition, we further analyzed the changes of candidate common stem cell markers before and after in vitro chemotherapy of osteosarcoma cells. The biological functions of CD24+ subpopulation in osteosarcoma such as proliferation, migration, invasion, tumorigenesis and metastasis were systematically investigated, and the correlations of CD24 levels with prognosis in patients with osteosarcoma were analyzed.
Findings
CD24+ Cells presented characteristics of TICs and resist drug-induced apoptosis. The prevention of tumor formation and metastasis by CD24 knockdown highlights the potential of CD24 as a therapeutic target for osteosarcoma. Moreover, the levels of CD24 in osteosarcoma samples were significantly correlated with the prognosis of patients.
Interpretation
CD24+ cell subset played an important role in osteosarcoma invasion and metastasis.
Funding
National Natural Science Foundation of China (No.81772857); Shanghai Science and Technology Commission (18140902000); Shanghai Municipal Health Commission (2017ZZ01017; 17411950301)
Research in context.
Evidence before this study
CD24 is a mucin-like glycosyl phosphatidylinositol anchored cell surface protein that functions both in signal transduction and as an adhesion molecule. CD24 is well known as a negative marker for breast cancer stem cells. The pathophysiologic function of CD24 in osteosarcoma cells is not yet understood.
Added value of this study
In the present study, we performed a series of functional studies on the osteosarcoma CD24+ subpopulation and performed a prognostic analysis of clinical cases. The results of this study found that CD24 can be used as a positive marker for osteosarcoma tumour-initiating cells. While its pathophysiologic function largely remains unclear, CD24 has been suggested to play a key role in the invasive and metastatic stages of osteosarcoma cells. Our study shows in vitro and in vivo that CD24 is important in the oncogenesis of osteosarcoma.
Implications of all the available evidence
More importantly, we confirmed that CD24 is a functional osteosarcoma cell surface marker, which provides the basis for early detection, surveillance, and as a therapy target for osteosarcoma.
Alt-text: Unlabelled box
1. Introduction
As the most common primary bone tumour, osteosarcoma has a high degree of malignancy, shows early occurrence of metastasis and is the second most common cause of cancer-related death in the paediatric age group [1], [2], [3], [4], [5], [6]. Approximately 90% of cases show micrometastasis at the time of diagnosis; thus, systematic chemotherapy is the first treatment choice [7]. However, even when patients with high-grade osteosarcomas undergo intensive chemotherapy, the survival rate is only 50% to 80% [8]. Osteosarcoma relapse observed after chemotherapy was associated with < 20% survival, and metastasis indicates a poor prognosis [1,9]. Elucidation of the biological mechanisms of tumorigenesis and metastasis is important for the development of new treatment strategies and predictive markers of metastasis.
Tumour-initiating cells (TICs) are a subpopulation of chemo-resistant tumour cells that have been shown to cause tumour relapse following chemotherapy. In the past few years, a variety of TIC markers, such as CD133, CD117 and CD271, have been reported in osteosarcoma [10], [11], [12], [13]. Despite numerous efforts to identify osteosarcoma TIC markers, no reports have successfully shown the clinical significance of these markers, specifically functional markers that can be used as oncotargets of osteosarcoma metastasis. In the present study, we identified CD24 as a functional marker that affected osteosarcoma cell proliferation, invasion and migration and showed that CD24 was associated with osteosarcoma prognosis. These findings suggest that CD24 is a risk marker for metastasis and an attractive therapeutic target in osteosarcoma to achieve better clinical outcomes for osteosarcoma patients.
2. Material and methods
2.1. Flow cytometry
Table S1 showed the antibodies used for flow cytometry in this study. Corresponding fluorophore-labeled primary antibodies (20 µl each) were added in each test sample and incubate in dark at 4°C for 30 min. PBS was then used to wash the antibody-labeled cells twice, followed by spinning down the cell pellet. Cell pellet from each test sample was resuspended in 300 µl PBS and analyzed by MACS Quant(Miltenyi Biotech Inc, Bergisch Gladbach,Germany). Isotype control was also established.
2.2. Cell sorting
For magnetic cell sorting, cells were labeled with PE-conjugated CD24 anti-body (BD PharMingen, San Jose, CA) followed by anti-PE microbeads (Miltenyi Biotec, Germany). MACS was performed according to the manufacturer's instructions of Miltenyi Biotec MACS Cell Separation Kit (Miltenyi Biotech Inc, Bergisch Gladbach, Germany). Cells were repeatedly passing through the cell sorting column for at least three times (in order to reach >95% purity of the target cell population). For Flow cytometry cell sorting,was performed using MoFlo XDP Cell Sorter (Beckman Coulter, Fullerton, CA). Cells were incubated with 1 μg of each antibody for 30 min. Control experiments involved incubation with each antibody for 30 min and no apparent increase in the number of dead cells detected by propidium iodide (PI) staining. Flowjo v7.6.2 software (Treestar Inc, San Carlos, CA) was used for data analysis.
2.3. Cell culture and sarcosphere formation
The MG-63, MNNG/HOS, and U-2 OS cell lines were purchased from American Type Culture Collection (ATCC, Rockville, Maryland). The OSC228 cell line was established in our laboratory. Adherent cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum (FBS) according to conventional procedures. For sphere formation assays, 105 cells were placed on culture dishes coated with poly 2-hydroxyethyl methacrylate(poly-HEMA; 120 mg/ml in 95% ethanol; Sigma-Aldrich) containing DMEM/F12 (with 20 ng/ml epidermal growth factor [PeproTech, USA], 20 ng/ml fibroblast growth factor [PeproTech, USA], and 20 ng/ml insulin-like growth factor [PeproTech, USA]). Adherent cells and sarcospheres were incubated at 37 °C with 5% CO2. After 3 passages, the spheres were observed using an inverted phase contrast microscope. Sphere derived from one parent cell was trypsinized into single-cell suspension and performed cytometry analyses directly after dissociation.
2.4. Cell migration and invasion assays
Cell migration and invasion assays were performed in 24-well plates with an 8-μm pore size transwell chamber (BD Biosciences, NJ, USA). For the migration assay, 5 × 104 cells were seeded in the upper chamber of each transwell insert. For the invasion assay, the membrane was coated with Matrigel and 1 × 105 cells were placed in the upper chamber. In each lower chamber, 800 μl of DMEM with 10% FBS was added as the chemoattractant. After incubation at 37 °C, the membrane inserts were removed from the plate and the non-invading cells in the upper surface were removed with cotton swabs. Cells that moved to the bottom surface of the chamber were stained with 0.1% crystal violet in 20% methanol. The cells were imaged and counted using an IX71 inverted microscope (Olympus, Tokyo, Japan).
2.5. Apoptosis measurement
After cell collection, an Annexin V apoptosis detection kit (Roche Diagnostics) was used to detect apoptosis by flow cytometry in accordance with the manufacturer's instruction. The FlowJo v7.6.2 software (TreeStar Inc., San Carlos, CA) was used to analyse the test data.
2.6. In vivo tumorigenicity
For the tumorigenicity experiments in the tibial marrow cavity of nude mice (BALB/c-nu/nu, 4 weeks old), ELDA analysis method reported in previous literature was used [14]. A micro-injector was inserted into the tibial cavity and injected 10 µl of experimental cell suspension at different concentrations (cell numbers). The 10 µl cell suspension for the control group was injected into the contralateral tibial cavity as a self-control. SkyScan 1076 in vivo X-ray microtomography (SkyScan Inc., Belgium) and a Kodak DXS 4000 Pro-System (Carestream Health, Inc., CT, USA) for X-ray scanning in small animals were used to regularly observe the tumour growth in mouse tibia.
2.7. In vivo pulmonary metastasis
Luciferase-labelled osteosarcoma cells at different concentrations (cell numbers) were resuspended and intravenously injected via the tail vein of mice (BALB/c-nu/nu, 4 weeks old). An IVIS Lumina II (PerkinElmer Inc., MA, USA) was used to regularly detect the bioluminescence in immunodeficient mice. Animals were euthanised three months later and the lungs were rapidly removed and placed in a Petri dish for bioluminescence detection using an in vivo imaging system. The lung tissues were fixed, paraffin-embedded, and sectioned for histopathological examination.
2.8. Protein mass spectrometry
NanoAcquity UPLC(Waters Corp., MA,USA) combined with LTQ Orbitrap XL(Thermo Fisher Scientific Inc., MA, USA) was used to study CD24+ and CD24– subsets of cells isolated from the MNNG/HOS cells using a flow cytometry cell sorter. Collected data were searched in the BioWorksBrowser (version Rev. 3.3.1 SP1, Thermo Fisher Scientific Inc.) with the SEQUEST search engines and analyzed using an APEX Quantitative Proteomics Tool (Version1.0.0, Pathogen Functional Genomics Resource center; J. Craig Venter Institute)and other bioinformatics software. Through the Database for Annotation, Visualization, and Integrated Discovery (DAVID) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database platforms, differentially expressed proteins of the CD24+ and CD24− cell subsets were analyzed for KEGG pathways. Data was deposited to MendeleyData (Doi: 10.17632/gjpgg96zxx.1; https://data.mendeley.com/datasets/gjpgg96zxx/draft?a=65386055-0712-4b61-a911-49ef8569c12f).
2.9. Statistical analysis
SPSS17.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis of the experimental data. Student's t-test was used to compare the mRNA expression and protein levels in different samples. Chi-square (χ2) test and Fisher's exact test were used to evaluate the correlation between CD24 levels and the clinicopathological features of the patients with osteosarcoma. Survival analysis of the patients was conducted using the Kaplan-Meier method and log rank test. Cox regression analysis was performed to evaluate the prognostic impacts of univariate and multivariate factors. p < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of osteosarcoma spheres and screening of TICs surface markers
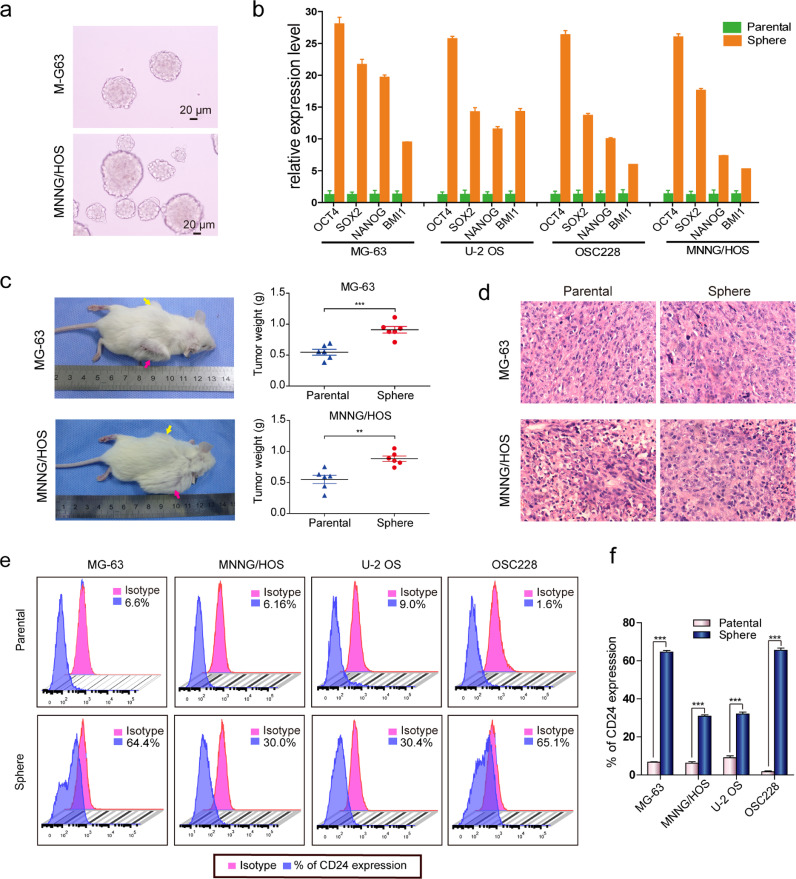
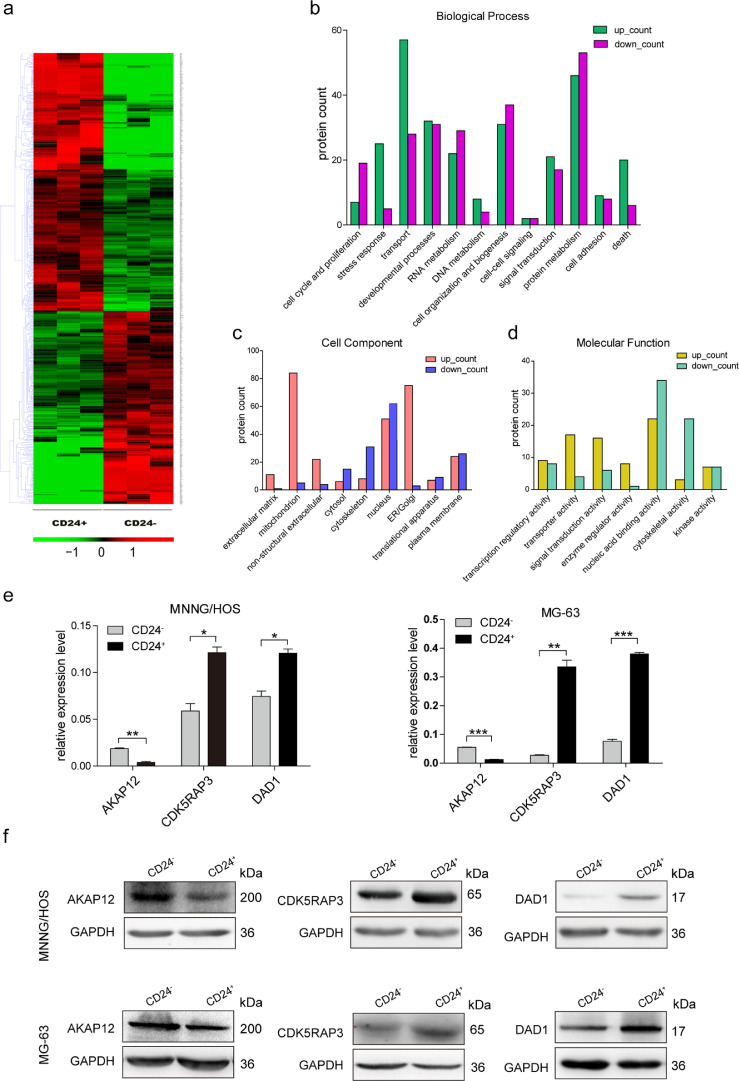
According to the cancer stem cell theory, serum deprivation culture is one strategy for enriching the sphere-forming ability of cancer stem cells. We observed sphere formation in MG-63 and MNNG/HOS osteosarcoma cells using the ultra-low adherent cell culture technique (Fig. 1a). Next, we examined the expression of four stemness-associated genes in the sphere and parental cells. We found that the mRNA expression levels of the OCT4, NANOG, SOX2, and BMI1 in sphere cells were higher than parental cells in MG-63, MNNG/HOS, U-2 OS, and OSC228 cell lines (Fig. 1b).We also detected the OCT4, NANOG, SOX2, and BMI1 expression by immunofluorescence analyses and the results showed that these four proteins are highly expressed in sphere cells (Figures S1a and S1b).To investigate the tumorigenic capacity of the sphere cells, we subcutaneously injected 1 × 106 MG-63 and MNNG/HOS sphere cells into the flanks of NOD/SCID mice. Corresponding parental cells were subcutaneously injected into the contralateral side as controls. Tumour tissues were collected from both sides one month after tumour cell inoculation. The results showed that sphere cells has stronger tumorigenicity and the tumor weight of sphere cells group was heavier than that of parental cells group(Fig. 1c). Hematoxylin and eosin-staining of the xenografts derived from sphere cells showed the similar pathological characteristics to those tumours derived from the corresponding parental cells (Fig. 1d).We screened 20 cell surface markers by flow cytometry in the sphere and corresponding parental cells of MG-63, MNNG/HOS, U-2 OS, and OSC228 (Table S3). Of the 20 cell surface markers, CD24 attracted our attention. The expression of CD24 all elevated in spheres formed by four osteosarcoma cell lines (MG-63, MNNG/HOS, U-2 OS, and OSC228), and the expression of other surface markers was inconsistent between these four spheres (Figs. 1e, f and S2a-S2c).
Fig. 1.
Features of osteosarcoma spheres. (a) Floating sarcospheres derived from MG-63 and MNNG/HOS cells after 3 passages. Scale bar = 20 µm. (b) Comparison of OCT4, SOX2, NANOG, and BMI1 mRNA expression between parental cells and corresponding sarcosphere cells (t-test, ***p < 0.001; **p < 0.01; and *p < 0.05). Error bars represent the standard deviation (SD) from at least three independent experiments. (c) 1 × 106 parental and corresponding sarcosphere cells from MG-63 and MNNG/HOS cells were subcutaneously injected into the flank of the NOD/SCID mice, respectively. (6 mice in MG-63 group and MNNG/HOS group respectively) After 1 month, the mice were sacrificed and the tumor tissues from parental and sphere cells were weighed and analyzed. Yellow arrows: parental cells inoculation. Pink arrows: sphere cells inoculation. (d) H&E staining of tumours formed subcutaneously in the NOD/SCID mice after inoculation of parental and sarcosphere cells from MG-63 and MNNG/HOS cells (scale bar=20 µm). (e) CD24 levels in the parental cells and corresponding sarcospheres cells analyzed by flow cytometry; independent experiments were repeated three times. Data are presented as the mean ± standard deviation. (t-test, ***p < 0.001; **p < 0.01; and *p < 0.05) (see Table S3 for relevant details).
3.2. CD24+ cells resist drug-induced apoptosis
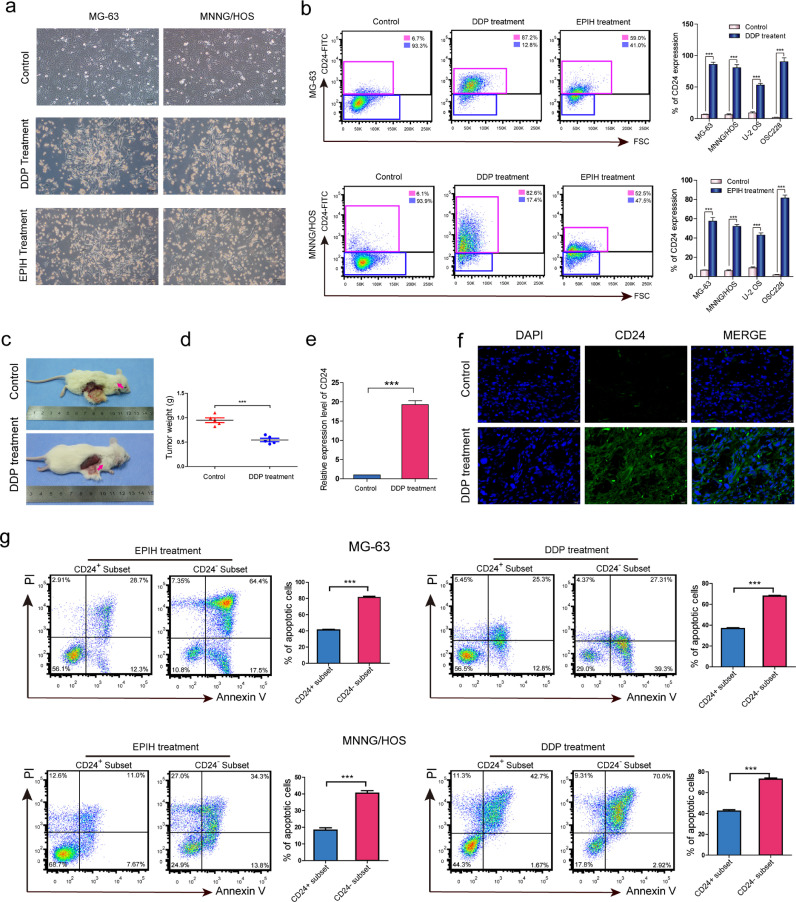
As we known, cancer stem cells can also be enriched by chemotherapy. In the present study, cis-platinum (DDP) and epirubicin hydrochloride (EPIH) were used for chemotherapy in vitro (Fig. 2a, S3a-S3d).After the administration of DDP and EPIH, the cell surface markers protein expression were detected using flow cytometry in MG-63, MNNG/HOS, U-2 OS, and OSC228 osteosarcoma cells (Table S4).among these cell surface markers, we found that CD24+ osteosarcoma cells increased in proportion after chemotherapy (Figs. 2b).However, the levels of other cell surface markers, including CD14, CD133 and CD117, varied among the different osteosarcoma cell lines after chemotherapy (Figures S4a-S4c, S5a-S5c and Table S4). In animal models with in vivo chemotherapy, the tumour sizes in the mice that underwent DDP chemotherapy were smaller than that observed in mice from the control group after one month (Fig. 2c and 2d). Dissected xenografts from both groups were examined using Qrt-PCR, and the results showed that CD24 expression in the DDP-treated group was higher than in the control group (Fig. 2e), and similar results were also observed by immunofluorescence staining of the xenograft tissues (Fig. 2f). Next, we isolated CD24+ and CD24- subset from MG-63 and MNNG/HOS sphere cells and investigate apoptosis induced by chemotherapy. Compared with CD24− cells, CD24+ cells have stronger anti-apoptotic ability in both MG-63 and MNNG/HOS cells (Fig. 2g).
Fig. 2.
Chemoresistance of CD24+osteosarcoma cells. (a) Micrographs of MG63 and MNNG/HOS cells after 7 days of chemotherapy with DDP and EPIH. The cells in the control group grew well. In the experimental group, a large number of cells floated and some surviving cells adhered to the bottom of the culture dish. (b) Flow cytometry shows the levels of CD24 in osteosarcoma cells 7 days after DDP and EPIH chemotherapy. Independent experiments were repeated three times. Data are presented as the mean ± standard deviation (t-test, ***p < 0.001; **p < 0.01; and *p < 0.05) (see Table S4 for relevant details). (c-f) (c, d) Tumorigenic NOD/SCID mouse model established by the subcutaneous inoculation of MNNG/HOS cells used for in vivo chemotherapy (n = 5 per group). Arrows indicate subcutaneous osteosarcoma xenografts in immunodeficient mice (30 days). (e) CD24 expression of the two groups was assessed by real-time PCR. The CD24 mRNA expression in the xenografts treated with DDP was higher than that in the untreated xenografts (t-test, ***p < 0.001; **p < 0.01; and *p < 0.05). (f) Immunofluorescence staining (green/FITC) showed that the CD24 levels in DDP-treated xenografts were higher than that in that in the untreated xenografts (scale bar = 20 µm). (g) Flow cytometry for the detection of apoptosis in the CD24+ and CD24− subsets from MG-63 and MNNG/HOS cells after DDP and EPIH chemotherapy. Independent experiments were repeated three times. Data are presented as the mean ± standard deviation (t-test, ***p < 0.001; **p < 0.01; and *p < 0.05).
In two cases of osteosarcoma that were treated with preoperative chemotherapy, the levels of CD24, CD117, CD14 and CD133 were 87.1%, 14.4%, 7.88%, and 3.12%, respectively, in case #88 and 59.8%, 13.1%, 10.9%, and 16.1%, respectively, in case #89 (Figure S6a). Two cases without preoperative chemotherapy (cases #22 and #26) were randomly selected to analyse the levels of CD24, CD117, CD14 and CD133 and compared to cases that received preoperative chemotherapy. The levels of CD24, CD117, CD14 and CD133 in case #22 were 8.44%, 10.2%, 6.04%, and 3.23%, respectively, and 1.59%, 12.7%, 8.75%, and 9.2%, respectively, in case #26 (Figure S6b). Immunohistochemical staining of CD24 showed similar results to the flow cytometry analysis (Figures S6c and S6d).
3.3. CD24+cells present characteristics of TICs
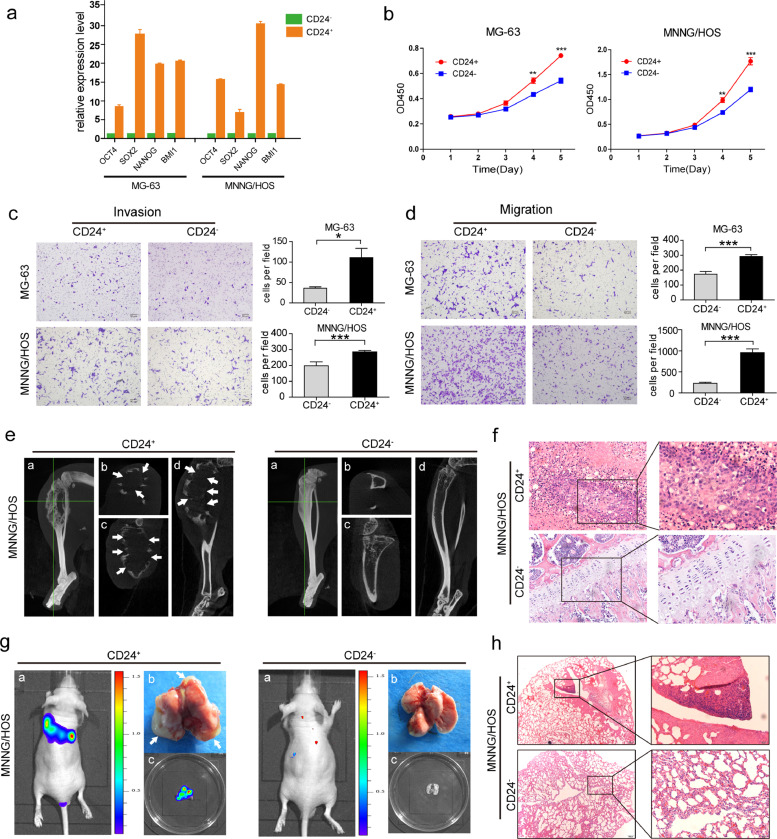
We sorted CD24+ and CD24− subset cells from osteosarcoma cells using flow cytometry. In MG-63 and MNNG/HOS cells, the OCT4, NANOG, SOX2, and BMI1 Mrna levels in the CD24+subset were higher than those in the CD24−subset (Fig. 3a). The CD24 levels in purified CD24+ cells were substantially decreased in serum-containing medium after 6 days of culture (Figures S7a, S7c, S7e and S6f). However, the purified CD24− cells maintained a stable and low level of CD24 at four different time points under the same culture conditions (Figures S7b, S7d-S7f). In the present study, we found that the proliferative capacity of CD24+ cells was higher than that of CD24−cells (Figs. 3b and S8a). Furthermore, transwell assays showed that the invasive and migratory capacities of the CD24+ subset cells was higher than that of the CD24−cells (Figs. 3c,3d, S8b and S8c).
Fig. 3.
Characteristics of CD24+cells isolated from osteosarcoma cells. (a)The mRNA expression of OCT4, SOX2, NANOG, and BMI1 in CD24+ cells sorted by FACS from MG-63 and MNNG/HOS cells was higher than in CD24−cells (***p < 0.001; **p < 0.01; and *p < 0.05). Error bars represent the standard deviation (SD) from at least three independent experiments. (b) The proliferative rate of CD24+ cells isolated from MG-63 and MNNG/HOS cells was higher than CD24−cells. Independent experiments were repeated three times. Data are presented as the mean ± standard deviation (***p < 0.001; **p < 0.01; and *p < 0.05). (c and d) Compared with CD24− osteosarcoma cells, CD24+ cells exhibited stronger (c) invasive and (d) migratory capacities. Ten high power fields were selected for comparison. Data are presented as the mean ± standard deviation (***p < 0.001; **p < 0.01; and *p < 0.05). (e and f) (e) CD24+ osteosarcoma cells from MNNG/HOS cells showed a stronger tumorigenic capacity than CD24− osteosarcoma cells. Representative images of CD24+and CD24− cell-induced tumour formation at 8 weeks after 1 × 104 cell injection. a: The whole image of the three-dimensional CT reconstruction. b: Cross section. c: Coronal section. d: Sagittal section. White arrow showing cortical destruction. (f) Corresponding paraffin-embedded tissues were processed for H&E staining (scale bar = 50 µm; magnified: scale bar = 20 µm) (see Table S5 for relevant details). (g and h) (g) Representative images of CD24+and CD24− MNNG/HOS cell-induced lung metastasis at 8 weeks after 1 × 104 cell injection. a: In vivo bioluminescence imaging of the mice. b: Lung tissues isolated from the mice (arrows indicate the pulmonary metastases). c: In vitro bioluminescence imaging of isolated lung tissues. (h) H&E staining of the corresponding paraffin-embedded lung tissues from mice (scale bar = 200 µm; magnified: scale bar = 50 µm) (see Table S7 for relevant details).
3.4. Tumorigenic and metastatic capacities of CD24+ cells
To test the tumorigenic capacity of CD24+ cells, we performed limiting dilution analysis in MNNG/HOS cells and revealed that CD24+ subset was more tumorigenic than CD24– subset (Figs. 3e, 3f and Table S5). For example, the CD24+ subset formed tumours from 500 cells (2/5), while the CD24− subset formed no tumours from 2500 cells (0/5) in mice after 3 months. Furthermore, we combined CD24 and other reported osteosarcoma TIC surface markers, including CD133 and CD117, to conduct gradient tumorigenic experiments in mouse tibias (Table S6). On the other hand, to investigate the metastatic capacity of CD24+ cells, CD24+ subset of MNNG/HOS cells (1 × 104 were injected into mice. After two months, pulmonary metastases were observed in all mice (n = 5/5).However, injection of the same amount of CD24- cells resulted in pulmonary metastasis in one mouse (n = 1/5) (Figs. 3g, 3h and Table S7).
3.5. Characteristics of the CD24+ subset isolated from clinical osteosarcoma samples
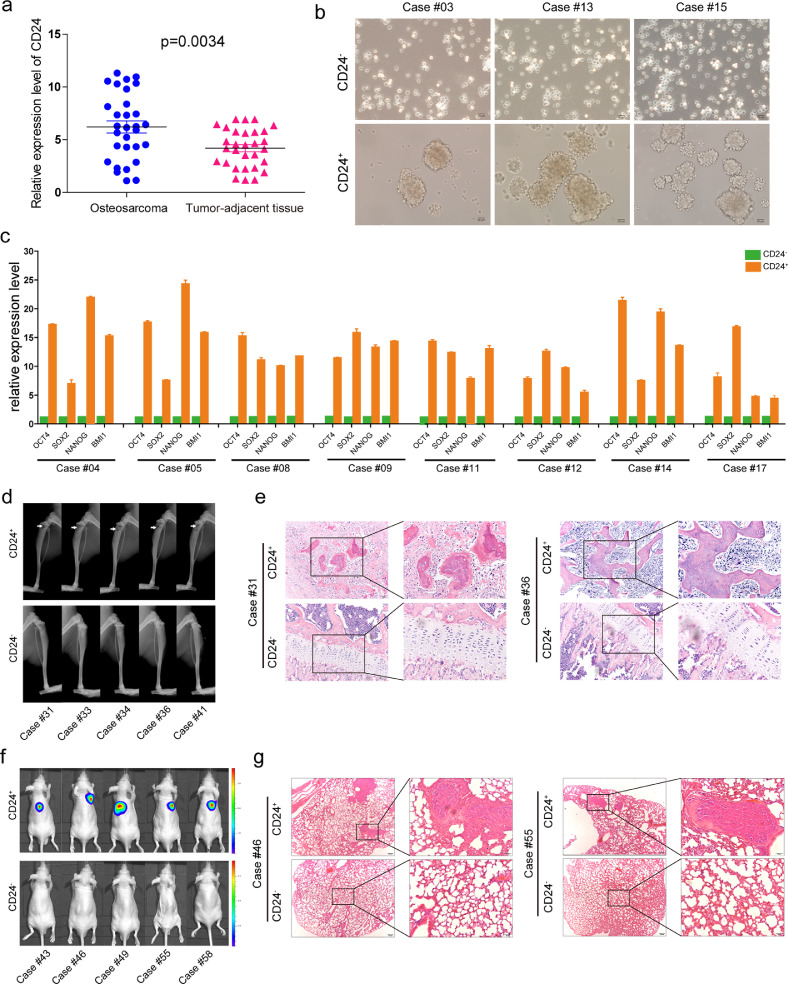
We tested the expression of CD24 in 30 paired osteosarcoma tissues and adjacent noncancerous tissues and found that the CD24 mRNA expression was higher in osteosarcoma tissues than in adjacent noncancerous tissues (Fig. 4a). We performed primary culture of human osteosarcoma and then CD24+and CD24−subset cells were isolated using flow cytometry. Sphere formation assays showed that, compared with CD24− cells, CD24+ cells have a stronger ability to form spheres (Fig. 4b). The results of qRT-PCR showed that OCT4, NANOG, SOX2, and BMI1 expression in the CD24+subset was higher than that observed in the CD24−subset (Fig. 4c). We validated the tumorigenic and metastatic potential of CD24+ cells from clinical samples of osteosarcoma in vivo. We performed limiting dilution analysis in 5 osteosarcoma samples and revealed that the CD24+ subset formed tumours even only 500 cells, while the CD24− subset formed no tumours from 2500 cells (0/5) in mice after 3 months (Figs. 4d, 4e and Table S8).In addition, compared with CD24− cells, CD24+ cells isolated from osteosarcoma clinical samples have stronger metastatic (Figs. 4f, 4g and Table S9) potential. According to the Kaplan-Meier analysis and log rank test results, among another cohort of 87 cases of osteosarcoma, cases with high levels of CD24 had a shorter three-year overall survival (OS) and three-year disease-free survival (DFS) than cases with low levels of CD24 (Figure S9).We then analyzed the correlation of CD24 expression with clinico-pathologic features in osteosarcoma patients. We found that CD24 expression was significantly correlated with tumour size (p = 0.029), tumor metastasis (p = 0.003) and Enneking surgical stage of the tumour (p = 0.034) (Table S10). The results from the univariate analysis suggested that CD24 levels, Enneking tumour stage, and tumour size were associated with the three-year OS and three-year DFS (Table S11). To evaluate the impact of multiple parameters on patient survival, we used Cox regression models to conduct multivariate analyses of the seven factors. Our results showed that age, gender, histological type, tumour location, and tumour size had no significant correlation with patients’ three-year OS and three-year DFS. Stepwise regression analyses showed that CD24 levels and Enneking staging were the only risk factors that affected the three-year OS and the three-year DFS. High CD24 levels and Enneking staining were associated with decreased survival in osteosarcoma patients (Table S12).
Fig. 4.
Characteristics of the CD24+ subset isolated from clinical osteosarcoma samples. (a) Compared to corresponding adjacent tissues, the mRNA expression of CD24 was higher in the 30 primary osteosarcoma samples. Independent experiments were repeated three times. Data are presented as the mean ± standard deviation (t-test, ***p < 0.001; **p < 0.01; and *p < 0.05). (b))1 × 105 CD24+ cells and CD24− cells were sorted by Flow cytometry.CD24− cells isolated from osteosarcoma samples (cases #03, #13, and #15) generated fewer and smaller sarcospheres than CD24+ cells after 7 days of serum deprivation and ultra-low adherent cell culture. (c)1 × 106 CD24+ cells and CD24− cells were sorted by Flow cytometry.The mRNA levels of OCT4, SOX2, NANOG, and BMI1 in CD24+ cells from clinical osteosarcoma samples were higher than those in CD24−cells (cases #04, #05, #08, #09, #11, #12, #14, and #17). Independent experiments were repeated three times. Data are presented as the mean ± standard deviation. Error bars represent the standard deviation (SD) from at least three independent experiments (t-test, ***p < 0.001; **p < 0.01; and *p < 0.05). (d and e) (d) CD24+ cells and CD24− cells were sorted by Flow cytometry. Osteosarcoma cells with different gradients were injected into the medullary cavity of the mouse tibia (No. of mouse = 3 per group). CD24+ osteosarcoma cells from samples (cases #31, #33, #34, #36, and #41) showed a higher tumour-forming incidence than CD24− osteosarcoma cells. (e) Corresponding paraffin-embedded tissues (cases #31 and #36) were processed for H&E staining (scale bar = 100 µm; magnified: scale bar = 50 µm) (see Table S8 for relevant details). (f and g) CD24+ cells and CD24− cells were sorted by Flow cytometry. Osteosarcoma cells with different gradients were injected into the tail vein of the mice(No. of mouse = 3 per group). (f) CD24+ osteosarcoma cells from samples (cases #43, #46, #49, #55, and #58) showed a stronger metastatic capacity than CD24− osteosarcoma cells. (g) H&E staining of corresponding paraffin-embedded lung tissues (#46 and #55) from mice (scale bar = 200 µm; magnified: scale bar = 50 µm) (see Table S9 for relevant details).
3.6. Differentially expressed proteins between CD24+ and CD24− subset cells
Mass spectrometry analysis showed that the CD24+ and CD24− subsets of MNNG/HOS cells had 510 differentially expressed proteins, with a fold change > 1.5 used as the standard (p < 0.05). among these proteins, 218 were downregulated and 292 were upregulated (Fig. 5a and Table S13). We performed gene ontology (GO) analysis on the differentially expressed proteins according to the biological process, cell component, and molecular function categories. We found that a relatively large number of proteins are related to protein transport and metabolism (Fig. 5b–d). Next, we validated three differentially expressed proteins, AKAP12, CDK5RAP3, and DAD1, in CD24+ and CD24– cells sorted from MG-63 and MNNG/HOS cells. Real-time PCR and western blot assays showed that AKAP12 was downregulated and that CDK5RAP3 and DAD1 were upregulated in the CD24+ subset (Fig. 6e and f). The functions of these three proteins in CD24+ cells still need further study.
Fig. 5.
Analysis of differential expressed proteins between CD24+ subset and CD24−subset in osteosarcoma cells. (a) Hierarchical clustering analysis of differential expressed proteins in CD24+ and CD24−subsets of MNNG/HOS cells were performed. (b-d)(B)Biological process analysis,(c)cell component analysis and (d) molecular function analysis of differential expressed proteins in CD24+ and CD24− subsets of MNNG/HOS cells were performed. (e) Comparative analysis of AKAP12, CDK5RAP3, and DAD1 mRNA expression in CD24+ and CD24−subsets from MG-63 and MNNG/HOS cells were performed by using RT-PCR. Independent experiments were repeated three times. Data were presented using mean ± standard deviation (t-test, ***p < 0.001; **p < 0.01; *p < 0.05). (f) The levels of AKAP12, CDK5RAP3, and DAD1 in CD24+ and CD24−subsets from MG-63 and MNNG/HOS cells were detected by using Western Blot analysis.
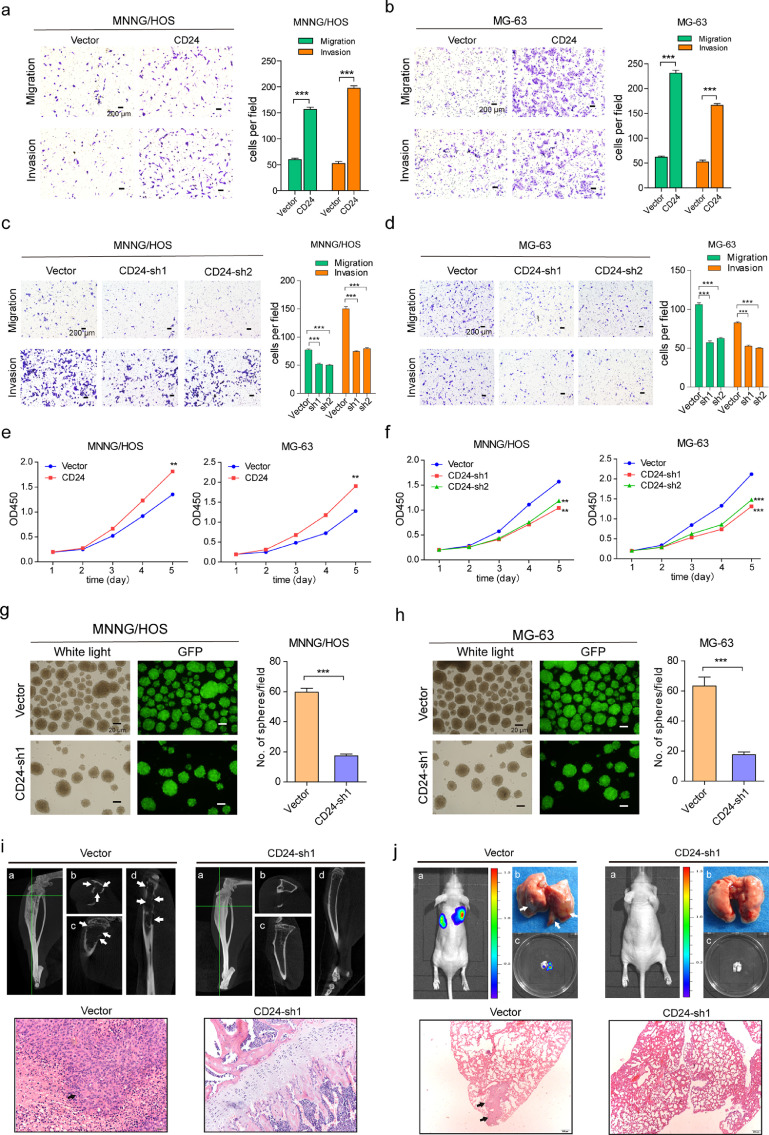
Fig. 6.
Functional effects of CD24 on osteosarcoma cells. (a and b) CD24 overexpression promotes migration and invasion of MNNG/HOS(a) and MG-63(b) cells. (c and d) Knockdown of CD24 in MNNG/HOS(c) and MG-63(d) cells resulted in a significant decrease in the invasive and migratory capacities. Ten high power fields were selected for comparison. Data are presented as the mean ± standard deviation (***p < 0.001; **p < 0.01; and *p < 0.05). (e) CD24 overexpression promoted proliferation of MNNG/HOS cells and MG-63 cells (t-test, ***p < 0.001; **p < 0.01; *p < 0.05) . (f) Down-regulation of CD24 expression decreased proliferation of MNNG/HOS cells and MG-63 cells(t-test, ***p < 0.001; **p < 0.01; *p < 0.05) (g and h) Knockdown of CD24 reduced the number of sarcospheres formed in MNNG/HOS(g) and MG-63(h) cells. Left panels: light microscopy. right panels: fluorescence microscopy (green fluorescent protein, GFP); ten high power fields were selected for comparison. Data are presented as the mean ± standard deviation (***p < 0.001; **p < 0.01; and *p < 0.05). (i) Representative images of shCD24 and control cell-induced tumour formation at 8 weeks after 1 × 105 cell injection. Top panels: a: The whole image of three-dimensional CT reconstruction. b: Cross section; c: Coronal section; d: Sagittal section. Bottom panels: The corresponding paraffin-embedded tissues were processed for H&E staining (scale bar = 50 µm; magnified: scale bar = 20 µm) (see Table S14 for relevant details). (j) Representative images of shCD24 and control cell-induced lung metastasis at 12 weeks after 1 × 105 cells injection. Top panels: a: In vivo bioluminescence imaging of the mice; b: Lung tissues isolated from the mice (arrows indicate the pulmonary metastases from osteosarcoma); c: In vitro bioluminescence imaging of isolated lung tissues. Bottom panels: H&E staining of corresponding paraffin-embedded lung tissue from mice (scale bar = 200 µm; magnified: scale bar = 50 µm) (see Table S15 for relevant details).
3.7. CD24 is a functional tic marker in human osteosarcoma
We further tested the biological function of CD24 in osteosarcoma. The invasive and migration ability of MG-63 and MNNG/HOS cells were significantly enhanced after up-regulating CD24 expression (Fig. 6a and b). We designed two shRNA sequences for knocking down the expression of CD24 in osteosarcoma cells and found that both sequences can achieve the purpose of knocking down CD24 in osteosarcoma cells(Figure S10a). The invasive and migratory capacities of MG-63 and MNNG/HOS cells were weakened after CD24 knockdown (Fig. 6c and d). Up-regulation of CD24 expression can enhanced the proliferative capacity of osteosarcoma cells (Fig. 6e), while down-regulation of CD24 expression reduced the proliferation of osteosarcoma cells (Fig. 6f).The number of spheres formed by MG-63 and MNNG/HOS cells was reduced after CD24 was knocked out, and there was a substantial difference in number of spheres between the CD24 knockdown and control groups (Fig. 6g and h). No significant changes in the growth cycle characteristics of osteosarcoma cells after CD24 knockdown(Figure S11a and S11b). To assess the tumorigenicity of osteosarcoma after CD24 knockdown, we injected CD24 knockdown cells and control cells (1 × 105) into the tibial marrow cavity of the mice.
To assess the tumorigenicity of osteosarcoma after CD24 knockdown, we injected CD24 knockdown cells and control cells (1 × 105) into the tibial marrow cavity of the mice. The incidence of tumour formation was 100% (5/5) in the control group, whereas it was 0–20% (MG-63 group: 0/5; MNNG/HOS group: 1/5) in the CD24 knockdown group after two months (Fig. 6i and Table S14).
The metastatic capacity of CD24 knockdown cells was also examined in immunodeficient mice via tail vein injection, followed by monitoring pulmonary metastases for three months. In the control group injected with 1 × 105 osteosarcoma cells without CD24 knockdown, all mice (MNNG/HOS group, 5/5) or most of the mice (MG-63 group, 4/5) showing tumour formation exhibited lung metastasis; however, only 1 animal in the MNNG/HOS group (1/5) and none in the MG-63 group (0/5) showed lung metastasis in the CD24 knockdown set (Fig. 6j and Table S15).
4. Discussion
TICs can grow and form spheres under specific culture conditions [11, 15], and this characteristic was used to enrich osteosarcoma TICs in the present study. In canine and human osteosarcomas, the expression of OCT4 in sarcosphere cells is higher than in the parental cells [16]. OCT4+-expressing osteosarcoma cells showed TIC-like characteristics [17]. When the tumour-suppressing STF cDNA 3 was overexpressed in osteosarcoma TICs, OCT4, NANOG, and SOX2 expression were reduced, resulting in the loss of TIC characteristics and the presence of apoptosis [18]. Bone morphogenetic protein 2 (BMP2) weakened the tumorigenic properties of osteosarcoma cells by downregulating OCT4, NANOG, and SOX2 expression [19]. In the present study, compared to osteosarcoma parental cells, osteosarcoma sarcosphere cells highly expressed TIC-related genes, which is consistent with previous reports.
CD117 expression in 318–1, p932, and mouse K7M2 osteosarcoma sarcosphere cells was significantly higher than that observed in the corresponding parental cells [12]. However, increased levels of CD117 were not observed in the sarcospheres of MG-63, U-2 OS and OSC228 cells in the present study. Previous reports have shown that the in vitro application of doxorubicin significantly increased CD117 expression in 318–1, P932, and K7M2 osteosarcoma cells [12]. In our study, similar results were observed in the EPIH treatment groups; In the DDP chemotherapy group, CD117 expression was significantly increased in MNNG/HOS, U-2 OS, and OSC228 cells after DDP treatment but decreased in MG-63 cells. Tirino et al. showed that CD133 expression was < 5% in SAOS2, MG-63, and U-2 OS osteosarcoma cells, and that the CD133+subset showed higher proliferation and sarcosphere formation than the CD133− subset [10]. Interestingly, a subsequent study from Tirino et al. group demonstrated that 21 clinical osteosarcoma samples all showed negative CD117 expression (<0.1%) [11], suggesting that CD133 is more likely to be a marker of osteosarcoma TIC than CD117. In our study, we obtained the similar results that CD133 levels were < 5% in the parental MG-63, MNNG/HOS, U-2 OS, and OSC228 cells. But it is worth noting that we found the CD133 levels in the corresponding sarcosphere cells derived from parental cells did not significantly increase and were still < 5%.
CD24 is a negative TIC marker in breast cancer and prostate cancer. The CD44+CD24− population in breast and prostate cancer cells shares with normal stem cells the ability to proliferate extensively, and to give rise to diverse cell types with reduced developmental or proliferative potential. The extensive proliferative potential of the tumorigenic population was demonstrated by the ability of as few as 200(breast cancer cells) or 100 (prostate cancer cells) CD44+CD24− cells to give rise to tumors. This extensive proliferative potential contrasts with the bulk of CD44− and/or CD24+ cancer cells that lacked the ability to form detectable tumors [20, 21]. CD24 expression impedes myeloid-derived suppressor cell expansion and function and therefore slows oral cancer oncogenesis [22]. CD24 suppresses the malignant phenotype by downregulating Sonic Hedgehog transcription in breast cancer cells [23]. The proliferation of TICs is controversial. Compared with non-TIC, CD133+ TICpopulation isolated from the hepatoma carcinoma cells possess higher proliferative and clonogenic potential in vitro. However, colony formation assay revealed that CD24+ TICpopulation derived from hepatoma carcinoma cells proliferated at a significantly lower rate than CD24−cells [24, 25]. it is possible that different TIC markers may display different tumorigenic and metastatic potentials.In this study,we found that the CD24+ subpopulation had stronger migration, invasion and tumorigenicity capacities than the CD24− subpopulation, and tumours formed in mice had similar histological features to the parental cell xenografts. CD24+ can differentiate into CD24− cells under conventional in vitro culture conditions and exhibited a stronger anti-apoptotic ability. Previous reports showed that TICs were associated with tumorigenesis and played an important role in tumour metastasis [26, 27]. However, Pang et al. demonstrated that tumorigenicity and tumour metastasis might be induced by different TIC subsets [28]. In the present study, we confirmed that the osteosarcoma CD24+subset not only had a strong tumorigenic capacity but that it is was also correlated with metastasis.
CD24 overexpression in fresh osteosarcoma tissue was significantly associated with a reduction in tumour-free survival in patients with osteosarcoma. Additionally, CD24 overexpression is also associated with early recurrence in patients with osteosarcoma, suggesting that CD24+ tumour cells can re-accelerate tumour recurrence. Existing studies have shown that the promotion of tumour proliferation and metastasis may be due to different subsets of cancer stem cells. However, in this study, CD24 overexpression was associated with early metastasis of tumours, all of which indicate that the CD24+ subpopulation can maintain tumour growth and eventually lead to tumour metastasis. Patients with high CD24 expression in primary osteosarcoma had an increased risk of metastasis after surgical resection. Osteosarcoma metastasis is considered the major mechanism responsible for poor prognosis in patients with osteosarcoma. These results also support previous studies that demonstrated that CD24 expression correlates with the prognosis of patients with osteosarcoma. Although CD133, CD117, and CD271 have been reported as markers of osteosarcoma stem cells, in these reports, the metastasis of osteosarcoma subpopulations has not been further studied.
We used protein mass spectrometry to analyse the differentially expressed proteins in osteosarcoma CD24+and CD24− cells. AKAP12 expression is deficient or downregulated in a variety of tumours. The level of AKAP12 expression was determined to correlate with signal transduction and protein localization [29, 30]. Upregulation of AKAP12 expression suppresses cancer cell proliferation and metastasis [31, 32]. In the present study, AKAP12 expression was downregulated in CD24+osteosarcoma cells, suggesting that AKAP12 might be associated with strong cell proliferation and metastatic capacity in CD24+cells. CDK5RAP3 is overexpressed in hepatocarcinoma and promotes liver cancer metastasis [33, 34]. DAD1 is a oligosaccharyltransferase subunit in mammals [35]. The apoptosis-related protein DAD1 is necessary for protein N-glycosylation [36]. DAD1 has certain anti-apoptotic effects on tumorigenesis [37, 38]. In the present study, CDK5RAP3 and DAD1 were significantly upregulated in CD24+ cells, suggesting that these proteins might be associated with the high proliferation rate and metastatic capacity of osteosarcoma cells.
After CD24 knockdown in osteosarcoma cells, the stemness genes OCT4, Nanog, and Sox2 were all downregulated, suggesting that CD24 expression is associated with these genes. Compared with osteosarcoma cells without CD24 knockdown, the shCD24 group cells displayed reduced sphere formation abilities and significantly reduced migration and invasion. In the in vivo mouse experiments, CD24 expression not only decreased tumour growth but also suppressed lung metastasis in mice. Our findings also suggest that by inhibiting the expression of CD24 in osteosarcoma cells, the biological function of the osteosarcoma can be affected, which means that CD24 can not only be used as a prognostic factor, but is also expected to become a target of osteosarcoma treatment in the future.
Declaration of Competing Interests
The authors declare no competing financial interests.
Acknowledgments
Funding sources
This work was supported by the grants from the National Natural Science Foundation of China (No. 81772857), Shanghai Science and Technology Commission (18140902000) and Shanghai Municipal Health Commission (2017ZZ01017; 17411950301). The funders had no role in the study design, data collection, data analysis, data interpretation, and writing of the report.
Acknowledgements
We are thankful to Pro. Zailong Cai for laboratory help.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2019.102598.
Contributor Information
Zhiwei Wang, Email: shwangzhiwei@vip.sina.com.
Jianru Xiao, Email: jianruxiao63@126.com.
Appendix. Supplementary materials
References
- 1.Ek E.T., Dass C.R., Choong P.F. Commonly used mouse models of osteosarcoma. Crit Rev Oncol Hematol. 2006;60(1):1–8. doi: 10.1016/j.critrevonc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Kager L., Zoubek A., Potschger U., Kastner U., Flege S., Kempf-Bielack B. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2003;21(10):2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 3.Kaste S.C., Pratt C.B., Cain A.M., Jones-Wallace D.J., Rao B.N. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer. 1999;86(8):1602–1608. doi: 10.1002/(sici)1097-0142(19991015)86:8<1602::aid-cncr31>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Mialou V., Philip T., Kalifa C., Perol D., Gentet J.C., Marec-Berard P. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome–the French pediatric experience. Cancer. 2005;104(5):1100–1109. doi: 10.1002/cncr.21263. [DOI] [PubMed] [Google Scholar]
- 5.Dahlin D.C., Coventry M.B. Osteogenic sarcoma. A study of six hundred cases. J Bone Joint Surg Am Vol. 1967;49(1):101–110. [PubMed] [Google Scholar]
- 6.Taylor W.F., Ivins J.C., Pritchard D.J., Dahlin D.C., Gilchrist G.S., Edmonson J.H. Trends and variability in survival among patients with osteosarcoma: a 7-year update. Mayo Clin Proc. 1985;60(2):91–104. doi: 10.1016/s0025-6196(12)60293-6. [DOI] [PubMed] [Google Scholar]
- 7.Himelstein B.P., Asada N., Carlton M.R., Collins M.H. Matrix metalloproteinase-9 (MMP-9) expression in childhood osseous osteosarcoma. Med Pediatr Oncol. 1998;31(6):471–474. doi: 10.1002/(sici)1096-911x(199812)31:6<471::aid-mpo2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Meyers P.A. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. 2009;9(8):1035–1049. doi: 10.1586/era.09.69. [DOI] [PubMed] [Google Scholar]
- 9.Fan T.M. Animal models of osteosarcoma. Expert Rev Anticancer Ther. 2010;10(8):1327–1338. doi: 10.1586/era.10.107. [DOI] [PubMed] [Google Scholar]
- 10.Tirino V., Desiderio V., d'Aquino R., De Francesco F., Pirozzi G., Graziano A. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PLoS ONE. 2008;3(10):e3469. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tirino V., Desiderio V., Paino F., De Rosa A., Papaccio F., Fazioli F. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB J. 2011;25(6):2022–2030. doi: 10.1096/fj.10-179036. [DOI] [PubMed] [Google Scholar]
- 12.Adhikari A.S., Agarwal N., Wood B.M., Porretta C., Ruiz B., Pochampally R.R. CD117 and stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010;70(11):4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian J., Li X., Si M., Liu T., Li J. CD271+ osteosarcoma cells display stem-like properties. PLoS ONE. 2014;9(6):e98549. doi: 10.1371/journal.pone.0098549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y., Smyth GK.ELDA. extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1–2):70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Fujii H., Honoki K., Tsujiuchi T., Kido A., Yoshitani K., Takakura Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol. 2009;34(5):1381–1386. [PubMed] [Google Scholar]
- 16.Wilson H., Huelsmeyer M., Chun R., Young K.M., Friedrichs K., Argyle D.J. Isolation and characterisation of cancer stem cells from canine osteosarcoma. Veterin J (London, England: 1997) 2008;175(1):69–75. doi: 10.1016/j.tvjl.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Levings P.P., McGarry S.V., Currie T.P., Nickerson D.M., McClellan S., Ghivizzani S.C. Expression of an exogenous human OCT-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res. 2009;69(14):5648–5655. doi: 10.1158/0008-5472.CAN-08-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y., Dai H., Guo Q.N. TSSC3 overexpression reduces stemness and induces apoptosis of osteosarcoma tumor-initiating cells. Apoptosis. 2012;17(8):749–761. doi: 10.1007/s10495-012-0734-1. [DOI] [PubMed] [Google Scholar]
- 19.Wang L., Park P., Zhang H., La Marca F., Claeson A., Valdivia J. BMP-2 inhibits the tumorigenicity of cancer stem cells in human osteosarcoma OS99-1 cell line. Cancer Biol Ther. 2011;11(5):457–463. doi: 10.4161/cbt.11.5.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurt E.M., Kawasaki B.T., Klarmann G.J., Thomas S.B., Farrar W.L. CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98(4):756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fugle C.W., Zhang Y., Hong F., Sun S., Westwater C., Rachidi S. CD24 blunts oral squamous cancer development and dampens the functional expansion of myeloid-derived suppressor cells. Oncoimmunology. 2016;5(10) doi: 10.1080/2162402X.2016.1226719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suyama K., Onishi H., Imaizumi A., Shinkai K., Umebayashi M., Kubo M. CD24 suppresses malignant phenotype by downregulation of SHH transcription through STAT1 inhibition in breast cancer cells. Cancer Lett. 2016;374(1):44–53. doi: 10.1016/j.canlet.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Lee T.K., Castilho A., Cheung V.C., Tang K.H., Ma S., Ng I.O. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated Nanog regulation. Cell Stem Cell. 2011;9(1):50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Ma S., Chan K.W., Hu L., Lee T.K., Wo J.Y., Ng I.O. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Hermann P.C., Huber S.L., Herrler T., Aicher A., Ellwart J.W., Guba M. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Marcato P., Dean C.A., Pan D., Araslanova R., Gillis M., Joshi M. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;29(1):32–45. doi: 10.1002/stem.563. [DOI] [PubMed] [Google Scholar]
- 28.Pang R., Law W.L., Chu A.C., Poon J.T., Lam C.S., Chow A.K. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6(6):603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Nauert J.B., Klauck T.M., Langeberg L.K., Scott J.D. Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Curr Biol. 1997;7(1):52–62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- 30.Shih M., Lin F., Scott J.D., Wang H.Y., Malbon C.C. Dynamic complexes of beta2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J Biol Chem. 1999;274(3):1588–1595. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi M., Nomoto S., Kanda M., Okamura Y., Nishikawa Y., Yamada S. Identification of the A kinase anchor protein 12 (AKAP12) gene as a candidate tumor suppressor of hepatocellular carcinoma. J Surg Oncol. 2012;105(4):381–386. doi: 10.1002/jso.22135. [DOI] [PubMed] [Google Scholar]
- 32.Liu W., Guan M., Hu T., Gu X., Lu Y. Re-expression of AKAP12 inhibits progression and metastasis potential of colorectal carcinoma in vivo and in vitro. PLoS ONE. 2011;6(8):e24015. doi: 10.1371/journal.pone.0024015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mak G.W., Chan M.M., Leong V.Y., Lee J.M., Yau T.O., Ng I.O. Overexpression of a novel activator of PAK4, the CDK5 kinase-associated protein CDK5RAP3, promotes hepatocellular carcinoma metastasis. Cancer Res. 2011;71(8):2949–2958. doi: 10.1158/0008-5472.CAN-10-4046. [DOI] [PubMed] [Google Scholar]
- 34.Mak G.W., Lai W.L., Zhou Y., Li M., Ng I.O., Ching Y.P. CDK5RAP3 is a novel repressor of p14ARF in hepatocellular carcinoma cells. PLoS ONE. 2012;7(7):e42210. doi: 10.1371/journal.pone.0042210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelleher D.J., DAD1 Gilmore R. the defender against apoptotic cell death, is a subunit of the mammalian oligosaccharyltransferase. Proc Natl Acad Sci USA. 1997;94(10):4994–4999. doi: 10.1073/pnas.94.10.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makishima T., Nakashima T., Nagata-Kuno K., Fukushima K., Iida H., Sakaguchi M. The highly conserved DAD1 protein involved in apoptosis is required for N-linked glycosylation. Genes Cells. 1997;2(2):129–141. doi: 10.1046/j.1365-2443.1997.1070303.x. [DOI] [PubMed] [Google Scholar]
- 37.Chopra M., Dharmarajan A.M., Meiss G., Schrenk D. Inhibition of UV-C light-induced apoptosis in liver cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2009;111(1):49–63. doi: 10.1093/toxsci/kfp128. [DOI] [PubMed] [Google Scholar]
- 38.True L., Coleman I., Hawley S., Huang C.Y., Gifford D., Coleman R. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci USA. 2006;103(29):10991–10996. doi: 10.1073/pnas.0603678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.