Abstract
A substantial body of literature has provided evidence for the role of gut microbiota in metabolic diseases including type 2 diabetes. However, reports vary regarding the association of particular taxonomic groups with disease. In this systematic review, we focused on the potential role of different bacterial taxa affecting diabetes. We have summarized evidence from 42 human studies reporting microbial associations with disease, and have identified supporting preclinical studies or clinical trials using treatments with probiotics. Among the commonly reported findings, the genera of Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia and Roseburia were negatively associated with T2D, while the genera of Ruminococcus, Fusobacterium, and Blautia were positively associated with T2D. We also discussed potential molecular mechanisms of microbiota effects in the onset and progression of T2D.
Keywords: Type 2 diabetes, Microbiota, 16S rRNA, Metagenomics, Insulin resistance
1. Introduction
The microbiome has been associated with pathophysiology of most chronic diseases. Type 2 diabetes (T2D) is no exception to this rule. Indeed, there is evidence for the effects of microbiota on glucose metabolism in both preclinical animal models of T2D and in healthy animals. Therefore, there is considerable interest in potential use of microbiota in clinical applications for understanding and treating T2D. At first glance, however, the microbiome literature on T2D appears chaotic and concerns have been raised about variability of the results. Different taxa are reported to be associated with T2D in different studies. Furthermore, a recent large study observed that different microbes were found associated with the same metabolic outcomes in different geographical areas [1]. While this might appear somewhat discouraging it is important to remember that discrepancies between results and disagreements about interpretations are common features of any emerging field in science. As a research community, we should not shy away from these problems, rather understand which aspects of the current literature are robust and which ones are not. A key issue moving forward is to identify properties of the microbiome and T2D that contribute to this apparent lack of reproducibility. In this review, we researched recent literature regarding microbiome in type 2 diabetes patients and summarize the most reliable findings.
2. Bacteria involved in T2D
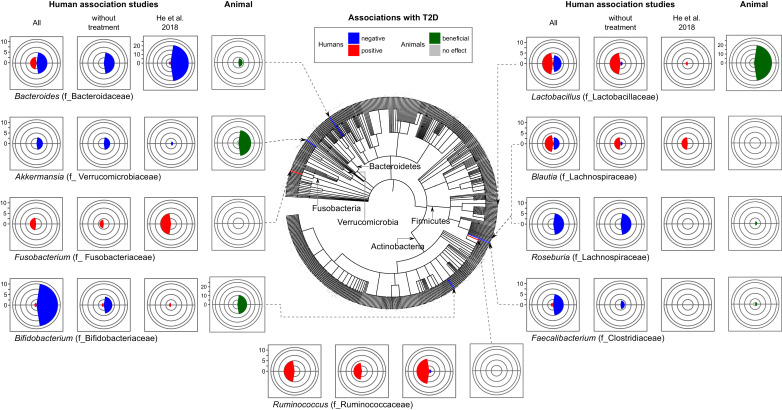
Out of 42 human observational studies that investigated T2D and the bacterial microbiome, the majority of studies reported associations between specific taxa and disease or its phenotypes (see Supporting Table 1 and “Search strategy and selection criteria” below). However, only a handful reported similar results. Among the commonly and consistently reported findings, the genera of Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia and Roseburia were negatively associated with T2D, while the genera of Ruminococcus, Fusobacterium, and Blautia were positively associated with T2D (Fig. 1). Lactobacillus genus, while frequently detected and reported, shows the most discrepant results among studies. Interestingly, different macro-metrics of microbial communities, such as several indexes of diversity and the Bacteroidetes/Firmicutes ratio that have been previously suggested as markers of metabolic disease did not show consistent associations with T2D (Table 1).
Table 1.
Number of reports examining association between T2D and diversity of microbiota or Bacteroides/Firmicutes ratio.
| Index | # Reports | No association | References (PMID) | Positive | References (PMID) | Negative | References (PMID) | |
|---|---|---|---|---|---|---|---|---|
| Alpha diversity | Shannon | 13 | 9 | 24013136, 29998997, 29280312, 29922272, 29596446, 27151248, 26756039 | 2 | 30397356, 26941724 | 2 | 27974055, 27151248 |
| Chao1 | 8 | 6 | 24013136, 29998997, 29280312, 29922272, 26756039, 27151248 | 2 | 26941724, 29789365 | 0 | ||
| Simpson | 3 | 1 | 29998997 | 1 | 26941724 | 1 | 29789365 | |
| Beta diversity | 8 | 7 | 24988476, 28530702, 24997786, 29280312, 29922272, 29596446, 27151248 | 0 | 1 | 27974055 | ||
| Bacteroides/Firmicutes ratio | 14 | 6 | 24013136, 26756039, 29789365, 29434314, 29657308, 29998997 | 3 | 20140211, 29434314, 23032991 | 4 | 23657005, 27974055, 26919743, 22293842 | |
Fig. 1.
Microbial genera most frequently found to be associated with T2D. Number of studies reporting one of the indicated genera in association with T2D (without treatment), and including anti-diabetic therapy (All) in addition to the largest human study by He et al., 2018 [1].
Bacteroides and bifidobacterium represent beneficial genera most frequently reported in studies of T2D.
Bifidobacterium appears to be the most consistently supported by the literature genus containing microbes potentially protective against T2D. Indeed, nearly all papers report a negative association between this genus and T2D [2], [3], [4], [5], [6], [7], [8], [9]; while only one paper reported opposite results [10]. Furthermore, some studies also found a negative association between specific species such as B. adolescentis, B. bifidum, B. pseudocatenulatum, B. longum, B. dentium and disease in patients treated with metformin or after undergoing gastric bypass surgery [6,11]. According to our literature search, Bifidobacterium has not been used alone as probiotics for T2D. However, almost all animal studies that tested several species from this genus (B. bifidum, B. longum, B. infantis, B. animalis, B. pseudocatenulatum, B. breve) showed improvement of glucose tolerance [12], [13], [14], [15], [16]. Thus, animal studies strengthen the idea that Bifidobacterium naturally habituating the human gut or introduced as probiotics play protective role in T2D.
The second most commonly reported genus was Bacteroides. Eight studies have reported associations between the abundance of this genus and T2D. Among these, five cross-sectional studies [3,[17], [18], [19], [20]] show negative associations with disease while three other studies [6,11,21] that involved some type of treatment reported positive associations. This apparent inconsistency can be explained by previously reported antibiotic effect of metformin [22] and/or potential feedback mechanisms on gut microbiota resulting from improved human physiology. Interestingly, in He et al. [1,23] 21 out of 23 OTUs of Bacteroides detected in their study were negatively associated with T2D. Accordingly, in investigations that analyzed this genus on the species level, Bacteroides intestinalis, Bacteroides 20–3 and Bacteroides vulgatus were decreased in T2D patients and Bacteroides stercoris were enriched after sleeve gastrectomy (SG) surgery in T2D patients with diabetes remission [5,11,17,24]. We also found only two experimental animal studies testing the ability of Bacteroides to treat diet induced metabolic disease. In these studies, administration of Bacteroides acidifaciens [25] and Bacteroides uniformis [26] improved glucose intolerance and insulin resistance in diabetic mice. Together, these studies indicate that Bacteroides plays a beneficial role on glucose metabolism in humans and experimental animals.
While Roseburia, Faecalibacterium, and Akkermansia were not reported as frequently as the two genera above mentioned (Bifidobacterium, Bacteroides) in the 42 studies we reviewed, but those genera were also found to be consistently negatively associated with T2D in human studies.
In five case-control studies Roseburia was found in lower frequencies in T2D group than in healthy controls [3,17,[27], [28], [29]]. Accordingly, investigations that were able to assign Roseburia to a species level also reported a negative association with disease for Roseburia inulinivorans, Roseburia_272, and one unclassified OTU from this genus [11,17,24]. Only one paper reported an opposite result for Roseburia intestinalis [17].
Two case-control studies reported lower frequencies in the disease group for Faecalibacterium [2,28]. Nevertheless, this genus was also found to be decreased after different types of antidiabetic treatments ranging from metformin and herbal medicine [30] to bariatric surgery [11]; only one study reported an opposite effect [31]. Moreover, studies that were able to analyze this genus at species level usually detected F. prausnitzii. This species was found to be negatively associated with T2D in four out five human case control studies [17,24,[32], [33], [34]]. While it is a popular probiotic for colitis [35], there were few attempts to use F. prausnitzii as a probiotic for metabolic disease.
Interestingly, in one study the administration of F. prausnitzii resulted in improvement of hepatic function and decreased liver fat inflammation in mice with diet-induced metabolic disease without affecting blood glucose [36]. Finally, it was also shown that another species of this genus, Faecalibacterium cf, was associated with remission of diabetes after bariatric surgery [11].
Akkermansia muciniphila is a relatively recently discovered member of commensal microbiota [37]. Its beneficial effect on host glucose metabolism was first reported in animal models [38,39]. In agreement with animal studies, the negative association between the abundance of this bacterium and T2D has been reported in human studies [17,38].
In summary, a decrease in at least one of these five phylogenetically distant genera (Bacteroides, Bifidobacterium, Roseburia, Faecalibacterium, and Akkermansia) in patients was found in approximately half of T2D microbiome studies suggesting their potential role beyond serving as a biomarker. Supporting this notion, the majority of these bacteria have been tested as probiotics for metabolic disease in mice, but more rarely in humans [[12], [13], [14], [15], [16],25,26,[36], [37], [38], [39], [40], [41], [42]]. The potential mechanisms of interaction between these microbes and mammalian organisms are discussed later in this paper.
Lactobacillus genus presents a complex case of apparently discordant results when considering all association studies, i.e. including those that analyzed changes after treatments (Fig. 1). However, cross-sectional studies of patients versus controls reported positive association between abundances of this genus and T2D in five out of six papers [[3], [4], [5],29,43]. Furthermore, several associations of this genus tend to be species-specific. For example, while L. acidophilus [34], L. gasseri [24], L. salivarius [24] were increased, L. amylovorus [29] was decreased in T2D patients suggesting a high diversity in functional impact on host metabolism by bacteria from this genus. Moreover, several species from this genus have been also tested as probiotics. Experimental studies in mice show mostly beneficial effects in the models of T2D such as L. plantarum [44], [45], [46], [47], L. reuteri [48], L. casei [49], L. curvatus [50], L. gasseri [51], L. paracasei [52], L. rhamnosus [53], L. sakei [54]. More importantly, twenty-five human clinical trials [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79] employed twelve different species of Lactobacillus with ten of those studies [[55], [56], [57], [58], [59], [60], [61], [62],64,79] adding other probiotics. Out of eleven studies [[58], [59], [60], [61], [62], [63], [64],72,76,77,79] that showed some protective effect, the majority combined other genera, most frequently Bifidobacterium [[58], [59], [60], [61], [62],64,79], suggesting that Lactobacillus and Bifidobacterium may work in a synergistic manner. Species L. sporogenes [76,77], L. casei Shirota [63], L. reuteri [72] used as mono-probiotics have been reported to improve T2D related symptoms in humans.
L. plantarum, bacteria found in fermented food products, is intensively studied in animal systems, with many studies showing that L. plantarum improves glucose metabolism in diet-induced and genetic models of T2D [44], [45], [46], [47] mice; only one reported with no significant effect of this treatment [80]. However, this species had no significant effect on glucose metabolism in four clinical trials [68], [69], [70], [71]. Thus, it seems that Lactobacilli anti-diabetic effect is seen more frequently when they are a part of probiotic cocktail rather than administered individually [58,61,62,64].
Overall, Lactobacillusgenus is highly diverse and contains the highest number of OTUs in the human gut among potentially probiotic bacteria. Its effects on T2D seems to be species-specific or even strain-specific, which might explain why genus level analysis lacks consistency amongst studies using this bacteria (Fig. 1).
Fewer studies (11 out of 42) reported positive associations (increase in disease) of microbiota with T2D and/or hyperglycemia. Specifically, Ruminococcus, Fusobacterium, and Blautia have been reported in a positive association with T2D. On one hand, consistent findings have been reported in 5 studies on Ruminococcus genus [3,17,28,31,81] and 3 studies on Fusobacterium [2,4,6]. On the other hand, the studies reporting species levels of these bacteria reported conflicting results [6,11,34]. For example, while one study demonstrated that Ruminococcus sp. SR1/5 enriched by metformin treatment [6], another found Ruminococcus bromii enriched and Ruminococcus torques decreased after bariatric surgery and diabetes remission [11]. It is possible that different types of treatments might be a major reason for the inconsistences between results of these studies.
Blautia genus has been found increased in disease groups in three out of four cross-sectional studies for T2D [17,18,82,83] and reduced after bariatric surgery [31]. Disagreeing with these reports, Blautia spp. were reported to increase after treatment with metformin in another study [30]. Importantly, results by He et al. 2018 [1], are concordant with the genus level analyses demonstrating positive associations between T2D and several OTUs of all three of these genera. The question still remains whether these bacteria play a causal role in T2D since there are no studies investigating these potentially harmful bacteria in animal models of T2D.
In summary, our review of literature regarding overall diversity and other macro-metrics of microbial communities failed to show a relation to diabetes (Table 1). However, some taxa have been systematically implicated in T2D. Surprisingly, some taxa are consistently associated with protection from T2D at genus level (e.g. Bacteroides, Bifidobacterium, etc.) or even phylogenetically at higher levels (e.g. Actinobacteria [7,17]) whereas others (e.g. Lactobacilli) show only species- or strain-specific effects. This phenomenon might be to be associated with a diversity of a given genus habituating the human gut (i.e. the larger a number of strains of a given genus found in human gut, the more strain-specific effects are observed). Importantly, several of these microbes are currently tested as probiotics in mouse and human studies.
3. Potential mechanisms of microbiota effects on metabolism in the T2D patient
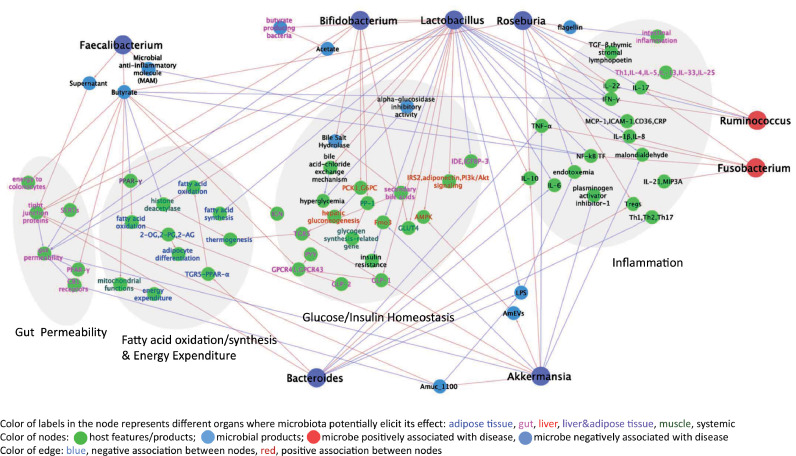
Multiple molecular mechanisms of gut microbiota contribution to metabolic disease and T2D have been recently reviewed elsewhere [84]. Microbiota modulates inflammation, interacts with dietary constituents, affects gut permeability, glucose and lipid metabolism, insulin sensitivity and overall energy homeostasis in the mammalian host (Fig. 2). Herein, we summarize the mechanisms whereby specific taxa highlighted earlier in this review can affect T2D.
Fig. 2.
Literature-based network analysis of potential effects on metabolism of bacterial taxa consistently found in association with human T2D (shown in Fig. 1). References corresponding to each edge can be found in the text.
3.1. Modulation of inflammation
Overall, T2D is associated with elevated levels of pro-inflammatory cytokines, chemokines and inflammatory proteins. While some gut microbes and microbial products especially lipopolysaccharides (LPS) promote metabolic endotoxemia and low-grade inflammation, others stimulate anti-inflammatory cytokines and chemokines. For example, induction of IL-10 by species of Roseburia intestinalis, Bacteroides fragilis, Akkermansia muciniphila, Lactobacillus plantarum, L. casei [37,[85], [86], [87], [88]] may contribute to improvement of glucose metabolism since overexpression of this cytokine in the muscle protects from ageing-related insulin resistance [89]. R. intestinalis can also increase IL-22 production, an anti-inflammatory cytokine [90,91] known to restore insulin sensitivity and alleviate diabetes [92]. It can also promote T regulatory cell differentiation, induce TGF-β and suppress intestinal inflammation [85,90,91]. Likewise, Bacteroides thetaiotaomicron induces expression of T regulatory cell gene expression [90].
Inhibition of pro-inflammatory cytokines and chemokines is another route used by beneficial microbes to prevent inflammation. Various species of Lactobacillus (L. plantarum, L. paracasei, L. casei) can decrease IL-1β, Monocyte Chemoattractant Protein-1, Intercellular adhesion molecule-1, IL-8, CD36 and C-reactive protein [93,94]. L. paracasei and B. fragilis inhibit expression of IL-6 [86,95]. Similarly, Lactobacillus, Bacteroides and Akkermansia have been found to suppress TNF-α [96,[86], [87], [88],95,97,98]. L. paracasei and microbial anti-inflammatory molecule from F. prausnitzii inhibit the activity of NF-kB [95,99]. Similarly, Roseburia and Faecalibacterium are butyrate producing bacteria and butyrate is also known to inhibit the activity of NF-kB [100,101]. Lactobacillus casei and Roseburia intestinalis decrease another pro-inflammatory cytokine IFN-γ [90,91,102] whereas Roseburia intestinalis can inhibit IL-17 production [90,91]. Bacteroides thetaiotaomicron reduces Th1, Th2 and Th17 cytokines in mono-associated mice [90].
Potentially detrimental microbes in T2D (pathobionts), like Fusobacterium nucleatum and Ruminococcus gnavus can increase several inflammatory cytokines, albeit in other inflammatory diseases [103,104].
3.2. Gut permeability
Increased intestinal permeability is a characteristic of human T2D. It results in translocation of gut microbial products into the blood and causes metabolic endotoxemia [105]. Two species (Bacteroides vulgatus and B. dorei) from the potentially beneficial for T2D genera have been found to upregulate the expression of tight junction genes in the colon leading to reduction in gut permeability, reduction of LPS production and amelioration of endotoxemia in a mouse model [106]. Another probiotic bacterium, Akkermansia muciniphila, decreased gut permeability using extracellular vesicles which improve intestinal tight junctions via AMPK activation in epithelium [42]. The outer membrane protein (Amuc_1100) of this bacterium enhances the expression of occludin and tight junction protein-1 (Tjp-1) and improves gut integrity [37]. Amuc_1100 also inhibits cannabinoid receptor type 1 (CB1) in the gut, which in turn, reduces gut permeability and systemic LPS levels [37]. While a specific bacterial component was not determined for Faecalibacterium prausnitzii, it was shown that the supernatant from the cultured bacterium enhances the expression of tight junction proteins improving intestinal barrier functions in colitis model [107]. Finally, butyrate, produced by Faecalibacterium, Roseburia, also have potential to reduce gut permeability through serotonin transporters and PPAR-γ pathways [101].
3.3. Glucose metabolism
Gut microbiota may also affect T2D by influencing glucose homeostasis and insulin resistance in major metabolic organs such as liver, muscle and fat, as well as by affecting digestion of sugars and production of gut hormones that control this process. For example, one of the potential probiotics discussed above (Bifidobacterium lactis) can increase glycogen synthesis and decrease expression of hepatic gluconeogenesis-related genes [108]. In the same report, B. lactis improved the translocation of glucose transporter-4 (GLUT4) and insulin-stimulated glucose uptake.
Lactobacillus gasseri BNR17 also increases GLUT-4 expression in the muscle with potential anti-diabetes effect [109]. Akkermansia muciniphila and Lactobacillus plantarum reduce the expression of hepatic flavin monooxygenase 3 (Fmo3) [37,93], a key enzyme of xenobiotic metabolism, whose knockdown has been found to prevent development of hyperglycemia and hyperlipidemia in insulin resistant mice [110]. Lactobacillus casei can ameliorate insulin resistance by increasing the mRNA level of phosphatidylinositol-3-kinase (PI3K), insulin receptor substrate 2 (IRS2), AMPK, Akt2 and glycogen synthesis in the liver [97,111]. The effect of this particular microbe is not limited to the effects on liver. Indeed, L. casei also reduces hyperglycemia via a bile acid-chloride exchange mechanism involving the up regulation of multiple genes, i.e., ClC1-7, GlyRα1, SLC26A3, SLC26A6, GABAAα1, Bestrophin-3 and CFTR [112]. It also decreases the insulin-degrading enzyme (IDE) in the caco-2 cells and insulin-like growth factor binding proteins-3 (IGFBP-3) in the white adipose tissue [97,111,113]. L. rhamnosus, another lactobacillus species, increases adiponectin level in the epididymal fat, thus, improving insulin sensitization [98].
Some species of Lactobacillii and Akkermansia muciniphila possess potent alpha-glucosidase inhibitory activity that prevents the breakdown of complex carbohydrates and reduces postprandial hyperglycemia [52]. Microbiota and their products can modulate gut hormones and enzymes and improve insulin resistance and glucose tolerance. Butyrate can act as ligand for G-protein coupled receptors (GPCR41 and GPCR43) in the gut and promotes the release of gut hormones GLP-1, PYY and GLP-2 from entero-endocrine l-cells (reviewed in [114,115]). Bifidobacterium and Lactobacillus produce bile salt hydrolases, which convert primary conjugated bile salts into deconjugated bile acids (BA) that are subsequently converted into secondary BA. Secondary BAs activate the membrane bile acid receptor (TGR5) to induce the production of GLP-1 (reviewed in [114]).
3.4. Fatty acid oxidation, synthesis and energy expenditure
Increasing fatty acid oxidation and energy expenditure and reducing synthesis of fatty acids ameliorates obesity and consequently T2D [116]. Akkermansia muciniphila, Bacteroides acidifaciens, Lactobacillus gasseri and short chain fatty acids have been reported to increase fatty acid oxidation in the adipose tissue.
For example, Akkermansia muciniphila has been found to increase the levels of 2-oleoyl glycerol (2-OG), 2-palmitoylglycerol (2-PG), 2-acylglycerol (2-AG) in the adipose tissue which increase the fatty acid oxidation and adipocyte differentiation [39]. Furthermore, Bacteroides acidifaciens also improves fatty acid oxidation in the adipose tissue via TGR5-PPAR-α pathway [25]. Likewise, butyrate can promote fatty acid oxidation and thermogenesis by inhibiting the histone deacetylation process in the muscle which increases energy expenditure partially by promoting mitochondrial functions in the muscle [117]. In liver and adipose tissue, butyrate and other two SCFAs, propionate and acetate, decrease the expression of PPAR-γ [118] which in turns increases fatty acid oxidation. Lactobacillus gasseri has been shown to reduce obesity by increasing the fatty acid oxidation genes and reducing fatty acid synthesis related genes [109]. Serum level of malonidialdehyde, a marker of oxidative damage of lipids, has been found to be reduced by Akkermansia muciniphila and Lactobacillus caseiin diabetic rodents [87,96]. Hence, members of microbiota with beneficial effect on T2D modulate fatty acid metabolism and associated energy expenditure in the host that results in alleviation of obesity and accompanying T2D.
3.5. Combined effects of bacteria
Besides the above-mentioned mechanisms, some microbes can also affect the host physiology by increasing other potential beneficial microbiota or by cross-feeding. Several species of Bifodobacterium were shown to have cross feeding interaction with other microbiota like Faecalibacterium and Roseburia [119,120]. Lactobacillus rhamnosus can increase Bifidobacterium abundance in the cecum of rats [98]. L. casei has been found to increase the butyrate producing bacteria [97,111].
4. Contribution of microbiota to the success of drug therapy for T2D
The interplay of drugs and gut microbiota is receiving much-deserved interest (reviewed in [121]). It is well known that antibiotics [122,123], non-antibiotic drugs [124] and anti-diabetic drugs (Table 2) can modulate microbiota and improve diabetes. Similarly, the baseline microbiota can positively and negatively affect the pharmacokinetics and pharmacodynamics of drugs and numerous chemicals via a variety of mechanisms (reviewed in [125]). Fewer studies, however, have examined how altering gut microbiota (via pre- and/or probiotics) changes the effects of anti-diabetic drugs.
Table 2.
Contribution of microbiota to the success of therapy of T2D.
| Anti-diabetic Drug | Effects on Microbiota | References (PMID) | References (PMID) | |
|---|---|---|---|---|
| Promotes | Reduces | |||
| Biguanides (Metformin) | Akkermansia muciniphila, Escherichia,Bifidobacterium adolescentis, Lactobacillus,Butyrivibrio, Bifidobacterium bifidum, Megasphaera, Prevetolla, Escherichi-Shigella, Erysipelotrichaceae incertate sedis, Fusobacterium, Flavonifractor, Lachnospiraceae, Lachnospiracea incertae sedis, and Clostridium XVIII and IV | 23804561, 28530702, 25038099, 27999002, 29056513, 30261008, 30815546, 29789365 | Intestinibacter, Romboutsia, Peptostreptococcaceae_unclassified, Clostridiaceae_1_unclassified, Asaccharospora, Alistipes, Oscillibacter, Bacteroides, Parabacteroides, un-Ruminococcaceae | 28530702, 30261008, 29789365 |
| Alpha-glucosidase Inhibitors (eg. Acarbose, voglibose, miglitol) | Lactobacillus, Faecalibacterium,Dialister,Subdoligranulum,Allisonella, Megasphaera, Bifidobacterium, Enterococcus, faecalis | 28130771, 29176714, 25327485 | Butyricicoccus, Phascolarctobacterium,Ruminococcus, Eggerthella, Bacteroides, Oribacterium, Erysipelotrichaceae,Coriobacteriaceae, Bacteroides | 28130771, 28349245, 29176714, 25327485 |
| GLP-1 Receptor agonist(eg. Liraglutide) | Akkermansia muciniphila, Bacteroides acidifaciens, Lachnoclostridium, Flavonifractor, Ruminococcus_gnavus,Allobaculum, Turicibacter, Anaerostipes, Lactobacillus, Butyricimonas, Desulfovibrio | 30815546, 30292107, 29171288, 27633081 | Helicobacter, Prevotella, Ruminococcaceae, Christensenellaceae, Roseburia, Candidatus Arthromitus, Marvinbryantia,Incertae Sedis | 30292,107,29171288, 27633081 |
| Thiazolidinediones (Pioglitazone) | Proteobacteria | 27751827 | ||
| DPP-4 Inhibitors (Vildagliptin,sitagliptin,saxagliptin) | Lactobacillus, Streptococcus, Bacteroides acidifaciens, Streptococcus hyointestinalis, Erysipelotrichaceae, Allobaculu, Turicibacter,Roseburia | 29797022, 29036231, 27633081, 27631013 | Oscillibacter, Ruminiclostridium_6, Anaerotruncus, Kurthia,Christensenellaceae, Prevotellaceae, Bacteroides,Prevotella,Blautia, | 29797022, 29036231, 27633081, 27631013 |
| SGLT2 Inhibitors (eg.Dapagliflozin) | Akkermansia, Enterococcus | 29703207 | Oscillospira | 29703207 |
One recent study examined effects of a probiotic Bifidobacterium animalis ssp. lactis 420, prebiotic polydextrose and their combination with sitagliptin in diabetic mice [126]. The combination of sitagliptin with pre- and probiotics was effective in reducing several T2D parameters. A similar study in Zucker diabetic rats observed that combining prebiotic polysaccharide with the antidiabetic drugs metformin and sitagliptin reduced hyperglycemia and adiposity compared to using only the drugs [127]. In another study, streptozotocin-induced diabetic mice were treated with a combination of a prebiotic and metformin. Improvements in fasting blood glucose, glucose tolerance and insulin resistance were observed with the combined therapy, as compared to metformin or MOS alone [128]. Thus, a new direction in the microbiome research has emerged focused on the interaction between anti-diabetic drugs and microbiota. These studies should answer important questions such as (1) how different anti-diabetic drugs affect microbiota; (2) which characteristics of gut microbiota are underlying different responses to anti-diabetic drugs; and (3) which co- pre- and probiotics are needed to improve response to medication.
5. Outstanding questions
T2D is a multi-organ, heterogeneous, multi-factorial disease making the dissection of causative microbes from the gut microbiome challenging. In human studies, confounding factors like geographic location, race, culture, health status and drug-use lead to inconsistency in identifying microbiota associated with T2D [1]. Moreover, due to challenges in sampling from the intestine of humans, most studies use stool samples for microbiota analysis. However, the stool microbiota profile does not fully reflect the gut microbiome. Furthermore, most studies focused on genomics, rarely studying the transcriptome, proteome or metabolome. Even at the genomic level, deep shotgun sequencing is expensive, making marker-based amplicon sequencing such as 16S rRNA gene prevailing. Further, the existing sequencing and analysis technologies rarely identify (annotate) microbes at species or strain levels. Considering that the functional capacity varies between strains from the same species, identification of microbes and microbial genes associated with disease is challenging.
A significant problem in the field is that the majority of human association studies do not attempt to infer microbes that may have contributing and/or causal role in T2D. Although inference of causality is a complex statistical problem, it is possible for host-microbiome interactions. Indeed, new approaches, such as Transkingdom Network Analysis [122] and novel application of Mendelian Randomization methods [129], have been recently developed and validated to answer which microbes and microbial genes/pathways are in control of host physiological processes.
Finally, challenges related to animal studies testing effects of microbiota on diabetes hamper progress. First, discrepancies between results caused by differences between microbiomes of otherwise genetically identical animals is one problem. Second, current advanced technologies in gnotobiotics such as studies of germfree and mono/oligo-colonized animals are currently incompatible with functional metabolic studies employing metabolic cages and hyperinsulinemic-euglycemic clamp techniques. Our research community should overcome these technical challenges and develop robust experimental systems to validate predictions coming from human studies and investigate mechanisms of host-microbiota interactions in metabolic diseases.
Future research is needed to develop new diagnostic, preventive and therapeutic microbiota tools for personalized/precision medicine of T2D. First, design of microbiome studies will need to account for clinical, molecular, and genetic as well as drug response diversity of T2D patients stratifying patient populations for analyses. Second, non-invasive approaches to collect microbiota samples from different sites of intestinal tract are needed as fecal material is limited in representation of gut microbiota. Third, while it is easier to focus on individual causal microbes, identifying combination of microbes is required to truly capture the community-level dynamics of the gut microbiota. In addition to taxon-based analysis, grouping microbes by function regardless of taxonomic similarity and function-based analysis should be pursued. Accordingly, we anticipate development of a new generation of analytical methods that will model cause-effect relationships and infer targets of therapeutic interventions. Finally, in order to test new drugs and probiotics as well as drug-microbiota interactions, well-defined gnotobiotic models, specifically humanized microbiota, will become a main tool in animal studies.
6. Conclusion
Despite multiple studies supporting the importance of gut microbiota in pathophysiology of T2D, the field is in early stage. Currently, we have reached a point in our understanding that some microbial taxa and related molecular mechanisms may be involved in glucose metabolism related to T2D. However, the heterogeneity of T2D and redundancy of gut microbiota do not promise simple interpretations (e.g. low diversity) and easy solutions (such as fecal transplant from non-diabetic/non-obese donor). In contrast, we should work towards precision/personalized medicine selecting anti-diabetics and probiotics for a given patient based on the combination of her/his mammalian and microbial genomes.
7. Search strategy and selection criteria
PubMed and Google Scholar literature searches were performed. To identify gut microbiome composition of T2D patients, articles between 2006 and 2018 were included with combinations of the terms “T2D”, “Glucose”, “gut” “Microbiome” “16S rRNA”, “metagenomics”, and “sequencing”. Additional papers relevant to our research were manually sought through bibliography search. Inclusion criteria in our review were (1) Human case-controlled studies; (2) articles focused on T2D (3) gut microbiota quantified from stool samples; (4) Glucose testing performed during the study (5) Either 16S rRNA gene sequencing or metagenomic sequencing performed in stool samples.
Google scholar and PubMed found 42 papers relevant to our focus. Articles were rejected if it was determined from the title and the abstract that the study failed to meet the inclusion criteria. Any ambiguities regarding the application of the selection criteria were resolved through discussions between at least 3 researchers involved. Each publication was an academic and peer-reviewed study.
Majority (79%) of studies utilized 16S rRNA gene sequencing with V3 and V4 regions most frequently (33% and 42%, respectively) targeted for sequencing (Supporting Table 1). Human subjects across all studies had mean age of 53 years (standard deviation 10 years) and were equivalently distributed between sexes. On average, patients had body mass index 28.3 ± 3 whereas controls 25.8 ± 4.
We searched for mouse colonization studies for the top 8 microbes found in the human-case studies. Articles between 1997 and 2018 were included with combinations of the terms “Mouse”, “Glucose”, “[selected microbe]”. Selected microbes included: Bacteroides, Bifidobacterium, Lactobacillus, Blautia, Faecalibacterium, Ruminococcus, Roseburia, and Fusobacterium. Inclusion criteria were (1) Mouse colonization studies; (2) Articles focused on T2D; (3) Glucose testing performed during the study. We also analyzed the literature on Akkermansia muciniphila, though it is in the species level, because of its recent emergence as an important potential probiotic microbe.
Similar to the mouse colonization study literature search, we searched for results from clinical trials with microbes/probiotic supplementation. Inclusion criteria were: (1) Human Clinical study w/ microbes/probiotic supplementation; (2) Glucose testing performed during the study; and (3) Microbes or Probiotics from genera identified in our papers as frequently found in human association studies.
Acknowledgements
This work was partially supported by NIH R01 DK103761 (NS) and DK112360 (DBJ). The funding source had no involvement in study design, analysis, writing of the report, and in the decision to submit the paper for publication. The corresponding authors confirm to have full access to all the data and have final responsibility for the decision to submit for publication. The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2019.11.051.
Contributor Information
Andrey Morgun, Email: andriy.morgun@oregonstate.edu.
Natalia Shulzhenko, Email: natalia.shulzhenko@oregonstate.edu.
Appendix. Supplementary materials
References
- 1.He Y. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 2018;24(10):1532–1535. doi: 10.1038/s41591-018-0164-x. [DOI] [PubMed] [Google Scholar]
- 2.Gao R. Dysbiosis signatures of gut microbiota along the sequence from healthy, young patients to those with overweight and obesity. Obesity (Silver Spring) 2018;26(2):351–361. doi: 10.1002/oby.22088. [DOI] [PubMed] [Google Scholar]
- 3.Candela M. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80–93. doi: 10.1017/S0007114516001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedighi M. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb Pathog. 2017;111:362–369. doi: 10.1016/j.micpath.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 5.Wu X. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010;61(1):69–78. doi: 10.1007/s00284-010-9582-9. [DOI] [PubMed] [Google Scholar]
- 6.Wu H. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 7.Barengolts E. Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS ONE. 2018;13(3) doi: 10.1371/journal.pone.0194171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015;9(3):552–562. doi: 10.1038/ismej.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen C. Host-microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br J Nutr. 2016;116(11):1869–1877. doi: 10.1017/S0007114516004086. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki M. Transglucosidase improves the gut microbiota profile of type 2 diabetes mellitus patients: a randomized double-blind, placebo-controlled study. BMC Gastroenterol. 2013;13:81. doi: 10.1186/1471-230X-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy R. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27(4):917–925. doi: 10.1007/s11695-016-2399-2. [DOI] [PubMed] [Google Scholar]
- 12.Le T.K. Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed Res. 2015;36(1):63–70. doi: 10.2220/biomedres.36.63. [DOI] [PubMed] [Google Scholar]
- 13.Moya-Perez A, Neef A, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0126976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi K., Ben Othman M., Sakamoto K. Sterilized bifidobacteria suppressed fat accumulation and blood glucose level. Biochem Biophys Res Commun. 2018;501(4):1041–1047. doi: 10.1016/j.bbrc.2018.05.105. [DOI] [PubMed] [Google Scholar]
- 15.Aoki R. A proliferative probiotic bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep. 2017;7:43522. doi: 10.1038/srep43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9(1):1–15. doi: 10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. 2013;8(8):e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippert K. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes. 2017;8(4):545–556. doi: 10.3920/BM2016.0184. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi Y. Association of intestinal microbiota with metabolic markers and dietary habits in patients with type 2 diabetes. Digestion. 2016;94(2):66–72. doi: 10.1159/000447690. [DOI] [PubMed] [Google Scholar]
- 20.Munukka E. Women with and without metabolic disorder differ in their gut microbiota composition. Obesity. 2012;20(5):1082–1087. doi: 10.1038/oby.2012.8. [DOI] [PubMed] [Google Scholar]
- 21.Sun L. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24(12):1919–1929. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik F. Is metformin poised for a second career as an antimicrobial? Diabetes Metab Res Rev. 2018;34(4):e2975. doi: 10.1002/dmrr.2975. [DOI] [PubMed] [Google Scholar]
- 23.He Y. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome. 2018;6(1):172. doi: 10.1186/s40168-018-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson FH. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 25.Yang J.Y. Gut commensal bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017;10(1):104–116. doi: 10.1038/mi.2016.42. [DOI] [PubMed] [Google Scholar]
- 26.Cano G. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS ONE. 2012;7(7):e41079. doi: 10.1371/journal.pone.0041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen N. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salamon D. Characteristics of gut microbiota in adult patients with type 1 and type 2 diabetes based on nextgeneration sequencing of the 16S rRNA gene fragment. Pol Arch Intern Med. 2018;128(6):336–343. doi: 10.20452/pamw.4246. [DOI] [PubMed] [Google Scholar]
- 29.Forslund K. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong X. Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: a multicenter, randomized, open label clinical trial. MBio. 2018;9(3) doi: 10.1128/mBio.02392-17. pii: e02392-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrone V. Postoperative changes in fecal bacterial communities and fermentation products in obese patients undergoing bilio-intestinal bypass. Front Microbiol. 2016;7:200. doi: 10.3389/fmicb.2016.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remely M. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2014;537(1):85–92. doi: 10.1016/j.gene.2013.11.081. [DOI] [PubMed] [Google Scholar]
- 33.Furet JP. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graessler J. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514–522. doi: 10.1038/tpj.2012.43. [DOI] [PubMed] [Google Scholar]
- 35.Rossi O. Faecalibacterium prausnitzii strain HTF-F and its extracellular polymeric matrix attenuate clinical parameters in DSS-Induced colitis. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0123013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munukka E. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017;11(7):1667–1679. doi: 10.1038/ismej.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plovier H. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 38.Greer R.L. Akkermansia muciniphila mediates negative effects of IFNgamma on glucose metabolism. Nat Commun. 2016;7:13329. doi: 10.1038/ncomms13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everard A. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao S. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol. 2017;58(1):1–14. doi: 10.1530/JME-16-0054. [DOI] [PubMed] [Google Scholar]
- 41.Hanninen A. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in nod mice. Gut. 2018;67(8):1445–1453. doi: 10.1136/gutjnl-2017-314508. [DOI] [PubMed] [Google Scholar]
- 42.Chelakkot C. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50(2):e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni Y. Characteristics of gut microbiota and its response to a Chinese herbal formula in elder patients with metabolic syndrome. Drug Discov Ther. 2018;12(3):161–169. doi: 10.5582/ddt.2018.01036. [DOI] [PubMed] [Google Scholar]
- 44.Martinic A. Supplementation of lactobacillus plantarum improves markers of metabolic dysfunction induced by a high fat diet. J Proteome Res. 2018;17(8):2790–2802. doi: 10.1021/acs.jproteome.8b00282. [DOI] [PubMed] [Google Scholar]
- 45.Lee E. Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients. 2018;10(5) doi: 10.3390/nu10050643. pii: E643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balakumar M. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur J Nutr. 2018;57(1):279–295. doi: 10.1007/s00394-016-1317-7. [DOI] [PubMed] [Google Scholar]
- 47.Okubo T. KK/Ta mice administered lactobacillus plantarum strain no. 14 have lower adiposity and higher insulin sensitivity. Biosci Microbiota Food Health. 2013;32(3):93–100. doi: 10.12938/bmfh.32.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fak F, Backhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe-/- mice. PLoS ONE. 2012;7(10):e46837. doi: 10.1371/journal.pone.0046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naito E. Beneficial effect of oral administration of lactobacillus casei strain shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110(3):650–657. doi: 10.1111/j.1365-2672.2010.04922.x. [DOI] [PubMed] [Google Scholar]
- 50.Park DY. Supplementation of lactobacillus curvatus HY7601 and lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS ONE. 2013;8(3):e59470. doi: 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yun S.I., Park H.O., Kang J.H. Effect of lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107(5):1681–1686. doi: 10.1111/j.1365-2672.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- 52.Dang F. Administration of lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. 2018;9(7):3630–3639. doi: 10.1039/c8fo00081f. [DOI] [PubMed] [Google Scholar]
- 53.Park K.Y., Kim B., Hyun C.K. Lactobacillus rhamnosus GG improves glucose tolerance through alleviating er stress and suppressing macrophage activation in db/db mice. J Clin Biochem Nutr. 2015;56(3):240–246. doi: 10.3164/jcbn.14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim S.M. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr Res. 2016;36(4):337–348. doi: 10.1016/j.nutres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Sabico S. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naive T2DM patients: a randomized clinical trial. J Transl Med. 2017;15(1):249. doi: 10.1186/s12967-017-1354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazloom Z., Yousefinejad A., Dabbaghmanesh M.H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci. 2013;38(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 57.Ivey K.L. The effects of probiotic bacteria on glycaemic control in overweight men and women: a randomised controlled trial. Eur J Clin Nutr. 2014;68(4):447–452. doi: 10.1038/ejcn.2013.294. [DOI] [PubMed] [Google Scholar]
- 58.Ejtahed H.S. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539–543. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 59.Moroti C. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11:29. doi: 10.1186/1476-511X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kijmanawat A. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: a double-blind randomized controlled trial. J Diabetes Investig. 2019;10(1):163–170. doi: 10.1111/jdi.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asemi Z. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63(1–2):1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- 62.Tajabadi-Ebrahimi M. A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes. 2017;125(1):21–27. doi: 10.1055/s-0042-105441. [DOI] [PubMed] [Google Scholar]
- 63.Hulston C.J., Churnside A.A., Venables M.C. Probiotic supplementation prevents high-fat, overfeeding-induced insulin resistance in human subjects. Br J Nutr. 2015;113(4):596–602. doi: 10.1017/S0007114514004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohamadshahi M. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts. 2014;4(2):83–88. doi: 10.5681/bi.2014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung SP. Effect of lactobacillus gasseri BNR17 on overweight and obese adults: a randomized, double-blind clinical trial. Korean J Fam Med. 2013;34(2):80–89. doi: 10.4082/kjfm.2013.34.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brahe L.K. Dietary modulation of the gut microbiota–a randomised controlled trial in obese postmenopausal women. Br J Nutr. 2015;114(3):406–417. doi: 10.1017/S0007114515001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karlsson Videhult F. Probiotics during weaning: a follow-up study on effects on body composition and metabolic markers at school age. Eur J Nutr. 2015;54(3):355–363. doi: 10.1007/s00394-014-0715-y. [DOI] [PubMed] [Google Scholar]
- 68.Feizollahzadeh S. Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type II diabetes mellitus. Probiotics Antimicrob Proteins. 2017;9(1):41–47. doi: 10.1007/s12602-016-9233-y. [DOI] [PubMed] [Google Scholar]
- 69.Sharafedtinov K.K. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients–a randomized double-blind placebo-controlled pilot study. Nutr J. 2013;12:138. doi: 10.1186/1475-2891-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naruszewicz M. Effect of lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. 2002;76(6):1249–1255. doi: 10.1093/ajcn/76.6.1249. [DOI] [PubMed] [Google Scholar]
- 71.Hutt P. Impact of probiotic lactobacillus plantarum TENSIA in different dairy products on anthropometric and blood biochemical indices of healthy adults. Benef Microbes. 2015;6(3):233–243. doi: 10.3920/BM2014.0035. [DOI] [PubMed] [Google Scholar]
- 72.Simon MC. Intake of lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: a proof of concept. Diabetes Care. 2015;38(10):1827–1834. doi: 10.2337/dc14-2690. [DOI] [PubMed] [Google Scholar]
- 73.Jones M.L. Evaluation of clinical safety and tolerance of a lactobacillus reuteri NCIMB 30242 supplement capsule: a randomized control trial. Regul Toxicol Pharmacol. 2012;63(2):313–320. doi: 10.1016/j.yrtph.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Hsieh M.C. The beneficial effects of lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. 2018;8(1):16791. doi: 10.1038/s41598-018-35014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vajro P. Effects of lactobacillus rhamnosus strain gg in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52(6):740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 76.Asemi Z. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2014;33(2):198–203. doi: 10.1016/j.clnu.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Asemi Z. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2016;35(4):819–825. doi: 10.1016/j.clnu.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 78.Shakeri H. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49(7):695–701. doi: 10.1007/s11745-014-3901-z. [DOI] [PubMed] [Google Scholar]
- 79.Kobyliak N. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr. 2018;12(5):617–624. doi: 10.1016/j.dsx.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Axling U. Green tea powder and lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 2012;9(1):105. doi: 10.1186/1743-7075-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allin KH. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia. 2018;61(4):810–820. doi: 10.1007/s00125-018-4550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Egshatyan L. Gut microbiota and diet in patients with different glucose tolerance. Endocr Connect. 2016;5(1):1–9. doi: 10.1530/EC-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inoue R. Prediction of functional profiles of gut microbiota from 16S rRNA metagenomic data provides a more robust evaluation of gut dysbiosis occurring in Japanese type 2 diabetic patients. J Clin Biochem Nutr. 2017;61(3):217–221. doi: 10.3164/jcbn.17-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aw W., Fukuda S. Understanding the role of the gut ecosystem in diabetes mellitus. J Diabetes Investig. 2018;9(1):5–12. doi: 10.1111/jdi.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen Z. Insights into roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J Gastroenterol Hepatol. 2018;33(10):1751–1760. doi: 10.1111/jgh.14144. [DOI] [PubMed] [Google Scholar]
- 86.Chang YC. TLR2 and interleukin-10 are involved in bacteroides fragilis-mediated prevention of DSS-induced colitis in gnotobiotic mice. PLoS ONE. 2017;12(7) doi: 10.1371/journal.pone.0180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li X. Effects of lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J Appl Microbiol. 2016;121(6):1727–1736. doi: 10.1111/jam.13276. [DOI] [PubMed] [Google Scholar]
- 88.Chen P. Antidiabetic effect of lactobacillus casei CCFM0412 on mice with type 2 diabetes induced by a high-fat diet and streptozotocin. Nutrition. 2014;30(9):1061–1068. doi: 10.1016/j.nut.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 89.Dagdeviren S. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J. 2017;31(2):701–710. doi: 10.1096/fj.201600832R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffmann T.W. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. ISME J. 2016;10(2):460–477. doi: 10.1038/ismej.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu C. Roseburia intestinalis inhibits interleukin17 excretion and promotes regulatory T cells differentiation in colitis. Mol Med Rep. 2018;17(6):7567–7574. doi: 10.3892/mmr.2018.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514(7521):237–241. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- 93.Liu WC. Lactobacillus plantarum reverse diabetes-induced Fmo3 and ICAM expression in mice through enteric dysbiosis-related c-Jun NH2-terminal kinase pathways. PLoS ONE. 2018;13(5) doi: 10.1371/journal.pone.0196511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian P. Antidiabetic (type 2) effects of lactobacillus G15 and Q14 in rats through regulation of intestinal permeability and microbiota. Food Funct. 2016;7(9):3789–3797. doi: 10.1039/c6fo00831c. [DOI] [PubMed] [Google Scholar]
- 95.Sun KY. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine. 2017;92:1–11. doi: 10.1016/j.cyto.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Zhang L. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog Dis. 2018;76(4) doi: 10.1093/femspd/fty028. fty028. [DOI] [PubMed] [Google Scholar]
- 97.Wang G. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. 2017;8(9):3155–3164. doi: 10.1039/c7fo00593h. [DOI] [PubMed] [Google Scholar]
- 98.Singh S. Lactobacillus rhamnosus NCDC17 ameliorates type-2 diabetes by improving gut function, oxidative stress and inflammation in high-fat-diet fed and streptozotocintreated rats. Benef Microbes. 2017;8(2):243–255. doi: 10.3920/BM2016.0090. [DOI] [PubMed] [Google Scholar]
- 99.Breyner N.M. Microbial anti-inflammatory molecule (MAM) from faecalibacterium prausnitzii shows a protective effect on DNBS and DSS-Induced colitis model in mice through inhibition of NF-kappaB pathway. Front Microbiol. 2017;8:114. doi: 10.3389/fmicb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inan M.S. The luminal short-chain fatty acid butyrate modulates nf-kappab activity in a human colonic epithelial cell line. Gastroenterology. 2000;118(4):724–734. doi: 10.1016/s0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- 101.Kinoshita M., Suzuki Y., Saito Y. Butyrate reduces colonic paracellular permeability by enhancing PPARgamma activation. Biochem Biophys Res Commun. 2002;293(2):827–831. doi: 10.1016/S0006-291X(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 102.Matsuzaki T. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of lactobacillus casei. APMIS. 1997;105(8):643–649. doi: 10.1111/j.1699-0463.1997.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 103.Yang Y. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear Factor-kappaB, and up-regulating expression of microRNA-21. Gastroenterology. 2017;152(4):851–866. doi: 10.1053/j.gastro.2016.11.018. e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hall A.B. A novel ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017;9(1):103. doi: 10.1186/s13073-017-0490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cani PD. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 106.Yoshida N. Bacteroides vulgatus and bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486–2498. doi: 10.1161/CIRCULATIONAHA.118.033714. [DOI] [PubMed] [Google Scholar]
- 107.Carlsson A.H. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol. 2013;48(10):1136–1144. doi: 10.3109/00365521.2013.828773. [DOI] [PubMed] [Google Scholar]
- 108.Kim S.H. The anti-diabetic activity of bifidobacterium lactis HY8101 in vitro and in vivo. J Appl Microbiol. 2014;117(3):834–845. doi: 10.1111/jam.12573. [DOI] [PubMed] [Google Scholar]
- 109.Kang JH. Anti-obesity effect of lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE. 2013;8(1):e54617. doi: 10.1371/journal.pone.0054617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miao J. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X. Effects of lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef Microbes. 2017;8(3):421–432. doi: 10.3920/BM2016.0167. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y. Lactobacillus casei reduces susceptibility to type 2 diabetes via microbiota-mediated body chloride ion influx. Sci Rep. 2014;4:5654. doi: 10.1038/srep05654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Neyazi N. Potential efficacy of lactobacillus casei IBRC_M10711 on expression and activity of insulin degrading enzyme but not insulin degradation. In Vitro Cell Dev Biol Anim. 2017;53(1):12–19. doi: 10.1007/s11626-016-0083-4. [DOI] [PubMed] [Google Scholar]
- 114.Allin KH, Nielsen T, Pedersen O. Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2015;172(4):R167–R177. doi: 10.1530/EJE-14-0874. [DOI] [PubMed] [Google Scholar]
- 115.Arora T., Backhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. J Intern Med. 2016;280(4):339–349. doi: 10.1111/joim.12508. [DOI] [PubMed] [Google Scholar]
- 116.Houmard J.A. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1111–R1116. doi: 10.1152/ajpregu.00396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gao Z. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.den Besten G. Short-Chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-Dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 119.Moens F., Weckx S., De Vuyst L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and faecalibacterium prausnitzii. Int J Food Microbiol. 2016;231:76–85. doi: 10.1016/j.ijfoodmicro.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 120.Rios-Covian D. Enhanced butyrate formation by cross-feeding between faecalibacterium prausnitzii and bifidobacterium adolescentis. FEMS Microbiol Lett. 2015;362(21) doi: 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- 121.Whang A, Nagpal R, Yadav H. Bi-directional drug-microbiome interactions of anti-diabetics. EBioMedicine. 2019;39:591–602. doi: 10.1016/j.ebiom.2018.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morgun A. Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut. 2015;64(11):1732–1743. doi: 10.1136/gutjnl-2014-308820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rodrigues R.R. Antibiotic-Induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol. 2017;8:2306. doi: 10.3389/fmicb.2017.02306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maier L. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klaassen CD, Cui JY. Review: mechanisms of how the intestinal microbiota alters the effects of drugs and bile acids. Drug Metab Dispos. 2015;43(10):1505–1521. doi: 10.1124/dmd.115.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stenman L.K. Probiotic B420 and prebiotic polydextrose improve efficacy of antidiabetic drugs in mice. Diabetol Metab Syndr. 2015;7:75. doi: 10.1186/s13098-015-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reimer R.A. Combining sitagliptin/metformin with a functional fiber delays diabetes progression in zucker rats. J Endocrinol. 2014;220(3):361–373. doi: 10.1530/JOE-13-0484. [DOI] [PubMed] [Google Scholar]
- 128.Zheng J. Prebiotic mannan-oligosaccharides augment the hypoglycemic effects of metformin in correlation with modulating gut microbiota. J Agric Food Chem. 2018;66(23):5821–5831. doi: 10.1021/acs.jafc.8b00829. [DOI] [PubMed] [Google Scholar]
- 129.Sanna S. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51(4):600–605. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.