SUMMARY
The success of immune checkpoint blockade in patients with a wide variety of malignancies has changed the treatment paradigm in oncology. However, combination therapies with immune checkpoint blockade will be needed to overcome resistance and broaden the clinical utility of immunotherapy. Here we discuss a framework for rationally designing combination therapy strategies based on enhancing major discriminatory functions of the immune system that are corrupted by cancer – namely, antigenicity, adjuvanticity, and homeostatic feedback inhibition. We review recent advances on how conventional genotoxic cancer therapies, molecularly targeted therapies, epigenetic agents, and immune checkpoint inhibitors can restore these discriminatory functions. Potential barriers that can impede response despite combination therapy are also discussed.
Introduction
Prior to the shift in the clinical research landscape brought about by the success of immune checkpoint blockade (ICB) in multiple tumor types, chemotherapy, radiation, surgery, and molecularly targeted agents were the predominant modalities employed across nearly all cancers. These therapies are often effective in early stage cancers but typically are not curative in advanced stage disease. Studies that examined response and resistance to radiation and systemic therapies focused on tumor cell intrinsic effects (Holohan et al., 2013). For instance, both chemotherapy and radiation can induce DNA damage, leading to cell cycle arrest or cell death. Thus, improving therapy often focused on enhancing these tumor cell intrinsic effects by incorporating agents with unique modes of action, or understanding how intrinsic resistance develops. For molecularly targeted agents against oncogenic drivers, resistance often develops through mutations in the kinase that prevent drug binding or through activation of alternate growth pathways, prompting design of next-generation drugs or blocking secondary pathways through combination therapy (Rotow and Bivona, 2017). Thus, efforts to improve conventional and molecularly targeted cancer therapies and how to combine therapies have focused on cell intrinsic modes of action such as mutations and/or other genetic influences with little attention to tumor extrinsic factors like the immune system.
The study of anti-tumor immune responses has led to the development of numerous therapeutic strategies. Among these approaches, antibodies that block “immune checkpoints,” negative regulators of T cell function (Topalian et al., 2015), and chimeric antigen receptors have now gained FDA approval (Lim and June, 2017). The therapeutic antibody ipilimumab, targeting cytotoxic T lymphocyte antigen 4 (CTLA-4), was the first checkpoint inhibitor to be approved for clinical use in cancer. CTLA-4 competes with the co-stimulatory receptor CD28 for binding to B7 ligands. A second immune checkpoint receptor, programmed cell death protein 1 (PD-1), is expressed by activated T cells, while its ligands PD-L1 and PD-L2, are expressed by tumor and immune cells. The PD-1 pathway is important for driving T cells into a dysfunctional state known as T cell exhaustion (Pauken and Wherry, 2015). Blocking either CTLA-4 or PD-1 has led to unprecedented durable responses with a generally favorable toxicity profile. However, it is clear from large clinical trials that only a fraction of patients respond and many will relapse. As with conventional cancer therapies, one way to improve clinical responses with immune checkpoint blockade (ICB) is through combination therapy strategies. In fact, extensive clinical efforts are currently underway to examine the safety and efficacy of combining ICB antibodies with each other, conventional cancer therapies, molecularly targeted agents, and novel immunomodulatory treatments. However, unlike with conventional therapies, the target of ICB is typically not the tumor cells directly but immune cells. Although the somatic mutation burden and cancer genetic mutations are undoubtedly important, as discussed by Wellenstein and de Visser in this issue and elsewhere (Schumacher and Schreiber, 2015), the nature of the immune infiltration in the tumor (Coussens et al., 2013), expression of PD-L1 on tumor and immune cells (Herbst et al., 2014; Tumeh et al., 2014), and developmental fate of T cells (Pauken and Wherry, 2015), are just some of the tumor cell extrinsic factors that influence response. Moreover, emerging evidence indicates that conventional genotoxic (e.g., chemotherapy, radiation) and molecularly targeted therapies can have immunomodulatory effects. Thus, designing rational combination strategies requires expanding our understanding of relevant determinants of response and resistance beyond cell intrinsic and genetic mechanisms.
In this review, we discuss tumor cell extrinsic factors, epigenetic properties, and feedback inhibition mechanisms important to consider for combination therapy strategies that target the anti-tumor immune response. We outline a framework based on the major discriminatory functions of the immune system. Within this context we discuss how cancers can corrupt these discriminatory functions, and how these functions might be restored through immunomodulatory effects of conventional therapies, targeted therapies, and other approaches.
Discriminatory Functions and Balancing Acts of the Immune System
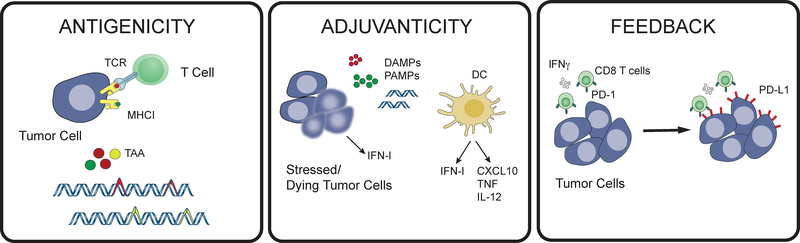
In the continuous struggle between pathogen and host, the selective pressures that have shaped the discriminatory functions of the immune system are manifold. Here, we put forth three discriminatory functions that are particularly relevant to cancer and cancer therapy by briefly reviewing their contributions to inflammation associated with host-pathogen interactions. These three discriminatory functions are: 1) antigenicity, 2) adjuvanticity, and 3) homeostatic feedback inhibition (Figure 1).
Figure 1: Three discriminatory functions of the immune system, antigenicity, adjuvanticity, and feedback regulation, are critical for promoting anti-tumor immunity.
T cells recognize tumor associated antigens (TAA), which can be generated by mutations in tumor cells, when presented in the context of class I MHC. The presence of non-self antigens must be accompanied by danger signaling to activate the innate immune system, promote dendritic cell (DC) maturation, and T cell activation. Normal homeostatic feedback mechanisms then curb the immune response to limit immunopathology after clearance of pathogens.
Antigenicity, or the first discriminatory function of the immune system, refers to the ability of antigens, typically peptides, to bind to and stimulate T and B cell receptors. Antigenicity contributes to self-nonself discrimination (G. Fu et al., 2014). Self-antigens are expressed by host tissues, while nonself-antigens are specifically expressed by pathogens and/or are not germline encoded. Since T cells bearing T cell receptors that recognize self-antigens are eliminated by thymic selection or are tolerized in the periphery, self-antigens generally have poor antigenicity. In contrast, nonself-antigens can exhibit strong antigenicity. Reliance upon the stronger antigenicity of nonself-antigens for immune activation allows effector cells to target pathogens while minimizing risk of autoimmunity. However, this method of self-nonself discrimination by the adaptive immune system is often not sufficient to generate a productive immune response against pathogens. Additional signals besides antigenicity are generally required to mount an effective adaptive immune response.
The generation of adaptive immunity often requires that nonself peptides are accompanied by adjuvants, which are either pathogen products or agents associated with cellular damage (Bonam et al., 2017). Thus, the second discriminatory function of the immune system is adjuvanticity: the ability to sense danger or damage and provide context for self-nonself recognition. These danger or damage signals are recognized by extracellular and intracellular pattern recognition receptors (PRRs) expressed by innate immune cells (Takeuchi and Akira, 2010). Major classes of PRRs include the membrane-bound toll-like receptors (TLRs), cytoplasmic NOD-like receptors (NLRs), as well as receptors primarily dedicated to recognizing nucleic acids such as RIG-like receptors (RLRs), the OAS-like receptors (OLRs) that includes cGAS, and AIM2-like receptors (ALRs). Ligands for PRRs can be pathogen-encoded and referred to as pathogen-associated molecular patterns (PAMPs), which are motifs such as bacterial LPS and viral nucleic acid features. Alternatively, endogenous molecules from stressed or damaged cells can bind to PRRs. These damage-associated molecular patterns (DAMPs) include extracellular ATP, cytoplasmic calreticulin, high mobility group box 1 (HMGB1) proteins, endogenous nucleic acids, and many intracellular proteins exposed by damaged or dying cells (Galluzzi et al., 2016). The cellular context provided by PRR signaling is important because nonself-antigens accompanied by PAMPs/DAMPs are much more likely to result from pathogen invasion rather than an accidental encounter due to failure of central or peripheral tolerance. Indeed, when PRRs expressed on macrophages and dendritic cells (DCs) are activated, this sets off a cascade of events to promote adaptive antigen-specific immunity (Banchereau et al., 2000). In the case of DCs, multiple proinflammatory cytokines (e.g., TNFα, IL-6, and IL-12) and anti-viral interferons (IFNs) are produced. PRR stimulation enables DCs to then migrate from tissue to regional draining lymph nodes, process antigen for presentation by class I and II MHC, and induce many co-stimulatory molecules needed by antigen-specific T cells.
Whereas triggering an inflammatory response is important for adaptive immunity, it is critical that this inflammatory response be temporally and spatially restricted. Homeostatic feedback inhibition, which is the third discriminatory function of the immune system, interprets environmental cues during inflammation and tunes immune effector function accordingly. This discriminatory function is evident during the resolution of inflammation that accompanies wound healing. The initial inflammatory phase of normal wound healing begins with the release of proinflammatory cytokines (Enyedi and Niethammer, 2015). Neutrophils are among the first innate immune cells that enter the wound to begin clearing tissue debris. Additional chemokines such as CCL2 and CCL3 then recruit monocytes to the inflamed area, which in turn differentiate into macrophages. These macrophages continue the process of phagocytosis and cytokine and chemokine production to recruit lymphocytes, but also produce VEGF, PDGF, and TGFβ to initiate angiogenesis and the proliferative phase of wound healing (Portou et al., 2015). During this phase, a transdifferentiation program called epithelial-to-mesenchymal transition (EMT) is thought to promote re-epithelialization, which is promoted by TGFβ and proinflammatory cytokines like TNFα (Thiery et al., 2009). In pathological situations such as diabetes that result in chronic and immune suppressed wounds, these early phases of wound healing fail to resolve. Interestingly, hyperactivation of TLRs such as TLR2 and TLR4, which contribute to early proinflammatory signals and neutrophil and macrophage infiltration, have been implicated in non-healing wounds (Lin Chen et al., 2013; Suga et al., 2014). In contrast, activation of TLR3 and TLR9, which recognize nucleic acids PAMPs, can accelerate wound healing (Lin et al., 2011; 2012; Sato et al., 2010). Moreover, TLR3 and TLR9 engagement increases production of type I IFNs (IFN-I), CXCL9, and CXCL10, all of which are cytokines that favor T cell recruitment and activation. Indeed, the recruitment of T cells proceeds innate immune cells and peaks during later stage of wound healing (Portou et al., 2015). Thus, productive wound healing requires proper timing and coordination of innate and adaptive immune populations, mesenchymal transdifferentiation programs, and disparate PRR signals. Dysregulation of these processes is associated with non-healing and immune suppressed wounds.
Feedback inhibition is also important when pathogens cannot be eliminated, requiring the establishment of a host-pathogen stalemate intended to limit immune-mediated pathology. This type of feedback inhibition can be controlled by the timing and duration of inflammatory signals. A prime example of this is how IFN pathways control host response to acute versus chronic viral infections (Snell and Brooks, 2015). Both interferon-gamma (IFNG) and IFN-I are critical in controlling acute viral infection (van den Broek et al., 1995a; 1995b). IFNs increase the expression of MHC-I, promote antigen processing, and coordinate both innate and adaptive immunity. Mice lacking IFN signaling rapidly succumb to disease or are unable to clear the virus. However, in several examples that include the LCMV model in mice, SIV model in primates, and chronic hepatitis in humans, chronic infection and immune suppression is paradoxically associated with high levels of multiple IFN-stimulated genes (ISGs). In this setting, persistent IFN-I signaling results in dominant suppressive effects attributed to IL-10, PD-L1, and dysfunctional lymphoid architecture. Blocking IFN-I signaling in the LCMV model at later times after chronic infection is established can decrease viral titers and improve chronic infection, indicating that chronic IFN signaling contributes to immune suppression and persistent infection (Sandler et al., 2014; Teijaro et al., 2013; Wilson et al., 2013). It is thought that these suppressive effects of IFNs may serve to minimize immunopathology that can occur with chronic infection.
Although feedback inhibition resulting from chronic infection impacts many types of immune cells, the tuning of effector function is particularly important for T cells. Antigen persistence or inflammation can result in T cell exhaustion, a dysfunctional state characterized by a progressive decline in effector function and cytokine secretion, altered metabolism, and eventual deletion (Pauken and Wherry, 2015). Although exhausted T cells (TEX) are hypofunctional compared to effector or memory T cells, TEX have important functions in controlling pathogens during chronic infection by maintaining a host-pathogen stalemate. The importance of PRR signaling for the development of TEX has been suggested in viral infection models whereby inadequate PRR signaling and insufficient CD4 T cell help promote exhaustion (Y. Wang et al., 2012). Moreover, persistent IFN signaling that can accompany chronic viral infection may predispose to “out-of-sequence” IFN signaling on T cells relative to T cell receptor engagement, resulting in inhibitory effects on T cell survival and proliferation (Crouse et al., 2015; Urban and Welsh, 2014). In fact, compared to other signal 3 cytokines such as IL-12, IFN-I is biased toward inducing higher levels of PD-1 on activated T cells and subsequent T cell exhaustion (Gerner et al., 2013). As discussed below, once these environmental cues favor development of TEX, the exhausted state may be difficult to reverse.
In summary, antigenicity, adjuvanticity, and feedback inhibition are critical properties of normal immune responses. These discriminatory functions must be closely regulated to ensure proper recognition and clearance of pathogens, while maintaining normal tissue function. Antigenicity allows recognition of foreign proteins as nonself and is accompanied by PRR-driven danger signals, which provide the context for appropriately initiating adaptive immune responses. After these inflammatory pathways are initiated, feedback inhibition acts as a counterregulatory measure to curb immune function and restore normal tissue barriers once the pathogen is cleared. Conceptually, these discriminatory functions likely evolved to balance the “cost” associated with immune effector responses – specifically, direct detrimental effects of pathogens versus immunopathology from excessive immune stimulation (Iwasaki and Medzhitov, 2015). In this way, the immune system can maximize host fitness by either eliminating pathogens or drawing a host-pathogen stalemate. Below, we describe how cancers disrupt this balance to favor the tumor.
Cancers Corrupt the Discriminatory Functions of the Immune System
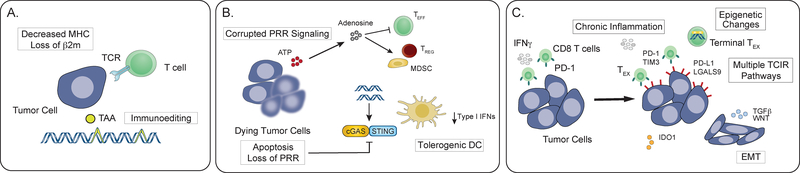
Since the discriminatory functions of the immune system have been largely shaped by host-pathogen interactions and balancing effector function with risk of immunopathology, there is an immense selective advantage for cancers to evolve low antigenicity, PRR signaling with poor adjuvanticity, and a tumor microenvironment (TME) that resembles chronically inflamed and non-healing tissue (Figure 2). A TME with these features is replete with signals that are unfavorable for immune effector function and that promote feedback inhibition mechanisms. As with host-pathogen interactions, some of the features of this immune suppression may be “locked-in” and difficult to reverse. There are numerous strategies tumors use to curtail anti-tumor immune responses by corrupting antigenicity and adjuvanticity, or by exploiting feedback inhibition.
Figure 2: Cancers corrupt the discriminatory functions of the immune response to evade elimination.
A. Decreased expression of class I MHC or genetic loss of β2-microglobulin prevent cell surface presentation of TAAs. Immunoediting leads to selection of tumor cells with decreased antigenicity, often by genetic loss or decreased expression of antigens. B. Tumors may inhibit cell intrinsic activation of pattern recognition receptor (PRR) signaling by genetic loss or silencing of pathways such as cGAS/STING. PRR signaling in the tumor microenvironment can also be corrupted to promote suppressive inflammatory signaling through activation of regulatory T cells, myeloid derived suppressor cells and macrophages, rather than dendritic cells. C. Feedback mechanisms that curb normal immune responses can be co-opted by tumor cells. Chronic inflammation (e.g., IFN signaling) mediated upregulation of immunosuppressive factors such as PD-L1 and IDO1 can impair T cell function. Persistent antigen and engagement of multiple T cell inhibitory receptors (TCIRs) can lead to epigenetic changes in effector T cells (Teff) that are only partially reversed by PD-1 blockade. TGFβ can have direct immunosuppressive effects as well as influence tumor cell fate.
Seminal experiments in mouse models and recent genomic evidence from cancer patients have firmly established the importance of immunoediting and neo-antigens in cancer progression and immunotherapy response. Since this topic is extensively reviewed elsewhere (Schumacher and Schreiber, 2015), we will only briefly discuss the critical role of neo-antigens here. Neo-antigens can be considered “non-self” or “altered-self” given that they are not germline encoded and subjected to central tolerance. Tumors arising in Rag2 knockout mice are more immunogenic than tumors from immunocompetent counterparts (DuPage et al., 2012), and the application of whole exome sequencing for neo-antigen identification has led to the discovery of relevant mutant proteins (Matsushita et al., 2012). Consistent with immunoediting, similar exome sequencing approaches from pre-therapy patient tumors show a correlation between ICB response and the number of non-synonymous somatic mutations or predicted neo-antigens (McGranahan et al., 2016; Rizvi et al., 2015; Snyder et al., 2014). After start of therapy, tumors responding to anti-PD-1 show loss of predicted neo-antigens and contraction of the subclones that express them (Riaz et al., 2017). Alternatively, patients that relapse after anti-PD-1 can have tumors that have lost MHC-I expression through mutation of either beta2-microglobulin or JAK signaling (Zaretsky et al., 2016). Tumors may also interfere with antigen presentation and priming of CD8 T cells by impairing recruitment of DCs (Salmon et al., 2016; Spranger et al., 2015). Thus, cancers pervasively cripple the self-nonself discriminatory function of the adaptive immune system through the survival of tumor clones lacking or unable to present adequate neo-antigens.
Tumors also avoid immune surveillance by favoring DAMP signals that promote cancer inflammation rather than priming adaptive immune responses. Due to cell intrinsic stress such as genomic instability and reactive oxygen species, or extrinsic stress such as metabolic limitations, hypoxia, and other harsh conditions present in the TME, cancer cells are constantly undergoing cell death (Galluzzi et al., 2016). This cell death may be accidental, such as necrosis, or programmed, such as apoptosis or necroptosis. Depending on the mode of cell death, various types of DAMPs are released, such as HMGB1, ATP, and adenosine. Even if cancer cells do not die, stressed cancer cells still expose DAMPs, such as cell surface calreticulin from ER stress or an unfolded protein response. However, despite this DAMP-rich environment, the TME may not promote anti-tumor immune responses. Rather, the cellular stress and/or the constellation of DAMPs liberated from dying cancer cells can favor the accumulation of dysfunctional innate immune cells such as dendritic cells (Cubillos-Ruiz et al., 2015), macrophages (Ruffell et al., 2014), and tumor-associated neutrophils (Coffelt et al., 2015) that facilitate tumor progression rather than generation of immunity.
Although it is often unclear which forms of cell death predominate in tumors, apoptosis and necrosis are likely pervasive. Apoptosis is generally considered immunologically silent (Green et al., 2009). Accordingly, apoptotic cells release immunomodulatory molecules and DAMPs, such as phosphatidylserine, that promote their clean elimination by acting as antiinflammatory and “eat me” signals for phagocytosis (Trahtemberg and Mevorach, 2017). Moreover, the immunologically silent nature of apoptosis is maintained by Caspase-mediated inactivation of Bax, preventing the release of mitochondrial DNA and subsequent activation of the endoplasmic reticulum associated signaling protein STING (Rongvaux et al., 2014; White et al., 2014). Analysis of cell lines and biopsies from melanoma or colon cancers has revealed frequent loss or epigenetic silencing of the cytosolic DNA sensor cGAS and/or STING, suggesting that cancers may suppress these pathways for immune evasion (Xia et al., 2016). Even necrosis of cancer cells can fail to prime a CD8 T cell response (Gamrekelashvili et al., 2014). The ATP that is released by necrosis and then subsequently metabolized to adenosine binds to purinergic receptors and interferes with the cytotoxic effects of T cells and NK cells, while enhancing regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) (Cekic and Linden, 2016). Interestingly, the failure of necrotic cells to prime T cells can be rescued by introduction of nucleic acid PAMP mimetics (Gamrekelashvili et al., 2014). Thus, despite the prevalence of cell death in tumors, the second discriminatory function of the immune system to reinforce recognition of non-self with DAMP or PAMP signaling is maladapted: DAMPs released by dying cancer cells favor cancer inflammation and tumor progression rather than adjuvanticity that supports an anti-tumor immune response. To achieve this, avoidance of strong nucleic acid DAMPs and the activation of their corresponding PRRs may be particularly important.
Cancer inflammation is associated with more than simply poorly immunogenic DAMPs unfavorable for adaptive immune responses. Tumor cells can also exploit feedback inhibition to avoid elimination. For instance, inherent cellular plasticity and chronic inflammatory signals associated with non-healing wounds can promote mesenchymal differentiation. In the TME, growth factors and morphogens, such as WNT and NOTCH, and numerous inflammation-associated cytokines, such as IL-6, IL-8, and TGFβ, can activate the EMT program in cancer cells (Sistigu et al., 2017; Thiery et al., 2009). In some cases, these growth factors and cytokines are produced by innate immune cells such as MDSCs and tumor-associated macrophages (TAMs). Moreover, inflammation associated with adaptive immunity may also promote EMT. In a breast cancer model, immunoediting by T cells was shown to promote EMT, generate cancer stem-like cells, and enhance therapy resistance (Reiman et al., 2010). Thus, inflammation resulting from both innate and adaptive immune responses are directly linked to EMT.
In addition to increased stemness, metastasis, and therapy resistance, cancers with mesenchymal properties can be highly immunosuppressive. For example, tumors formed from either MMTV-PyMT cancer cell lines or sorted subpopulations with epithelial traits express MHC-I, are infiltrated with CD8 T cells and M1 macrophages, and respond to ICB. In contrast, tumors from mesenchymal populations have lower MHC-I, higher PD-L1, and are infiltrated with CD8 T cells expressing multiple inhibitory receptors, Tregs, M2 macrophages, and MDSCs (Dongre et al., 2017). Accordingly, these mesenchymal tumors fail to respond to ICB. Interestingly, the immune suppressive features and ICB response associated with EMT is dominant, as suggested by mixing experiments. Furthermore, directly manipulating EMT with transcriptional regulators such as Brachyury (Hamilton et al., 2014) or the microRNA mir-200 (Limo Chen et al., 2014) enhances resistance to cytotoxic T cells and/or NK cells and increases tumor PD-L1 levels. Recent gene expression analysis of tumors from cancer patients show that an EMT signature is associated with higher expression of T cell inhibitory receptor pathways and Tregs (Lou et al., 2016; Mak et al., 2016). In contrast, there is no association with the somatic mutation burden. Melanoma patients treated with anti-PD-1 demonstrate enrichment of mesenchymal-related genes in tumors from non-responders, highlighting the potential clinical relevance of inflammation and cancer cell plasticity (Hugo et al., 2016). These findings support a potential dominant effect of EMT that can mask cancer cells from T cells irrespective of neoantigen repertoire. Indeed, tumor intrinsic mutations associated with EMT can actively orchestrate a TME that impedes dendritic cell function and T cell infiltration (see separate review in this issue).
Similar to their functions in pathogen infection, IFNG and IFN-I have critical roles in generating immunity against cancer. Mice with defects in IFN signaling, particularly in immune and stromal cells, exhibit compromised spontaneous and therapy-related anti-tumor immune responses (L. Deng et al., 2014; Katlinski et al., 2017; Woo et al., 2014). Disruption of IFN signaling in tumor cells can interfere with MHC-I expression1, alleviate IFN-mediated cytotoxicity, and likewise cause resistance to immunotherapies (Dighe et al., 1994; Manguso et al., 2017). In fact, tumors harboring mutations or copy number alterations in IFN signaling or MHC-I have been identified in patients that are either resistant or relapse after ICB (Gao et al., 2016; Zaretsky et al., 2016). However, activated CD8 T cells produce IFNG that increases PD-L1 and IDO1 in the tumor and TME (Spranger et al., 2013). This seemingly counter-productive response has been described as “adaptive resistance” (Taube et al., 2012) and is a consequence of the third discriminatory function of the immune system to provide feedback inhibition. In fact, blocking PD-L1-mediated adaptive resistance is thought to be a major mechanism of action for anti-PD-1/PDL therapy. Thus, in both chronic pathogen infections and in cancer, IFN drives initial immune stimulatory effects but then coordinates feedback inhibition through PD-L1.
Patients and mice with high levels of PD-L1 still often fail to respond to anti-PD-1/PD-L1 therapy, arguing that IFN may drive additional feedback inhibition pathways (Benci et al., 2016; Taube et al., 2014). Indeed, in mouse models, persistent IFNG signaling in tumor cells results in an altered epigenome/transcriptome and expression of multiple T cell inhibitory receptor ligands besides PD-L1 (Benci et al., 2016). This chronic IFNG signaling is sufficient to render tumors resistant to ICB. When IFNG and/or IFN-I signaling is blocked in ICB-resistant tumors, response is dramatically restored, suggesting that persistent IFN-I or IFNG is required to maintain adaptive resistance. Importantly, melanoma patients with high levels of ISGs such as IFIT1 and MX1, which are associated with the acquisition of IFN-driven adaptive resistance in mice, or high levels of serum IFNG prior to therapy are less likely to respond to anti-PD-1 (Benci et al., 2016; Huang et al., 2017). Thus, persistent IFN signaling in tumor cells that characterizes cancer inflammation can drive PD-L1-dependent and PD-L1-independent resistant states. When the latter develops, blockade of PD-1/PD-L1 may be insufficient to restore anti-tumor immunity.
While inflammation and plasticity of the TME allow tumors to passage into an immunosuppressive state, the epigenetic inflexibility of TEX that develops from persistent antigen and inflammation can galvanize immune suppression. Although PD-1 marks these TEX and blocking PD-1 can improve function, recent evidence reveals that exhaustion may not be easily reversed. In chronic viral infection or in cancer, PD-1 blockade rewires T cell transcriptional programs but does not remodel chromatin features after transitioning from an initial plastic state (Pauken et al., 2016; Philip et al., 2017; Sen et al., 2016). Consistent with the notion that lack of epigenetic plasticity limits the reversal of T cell exhaustion, TEX do not develop into memory T cells (TMEM) after PD-1 blockade but instead re-exhaust (Pauken et al., 2016). Furthermore, the TME may also adversely affect TMEM either by enriching for an exhaustion-related epigenetic state in pre-existing TMEM pools (Philip et al., 2017) or by interfering with their development through chronic inflammation and persistent IFN signaling (Stelekati et al., 2014). The potential clinical relevance of this epigenetic inflexibility and limited ability to reverse exhaustion, is highlighted by findings in melanoma and lung cancer patients treated with anti-PD-1. Not only are some of the exhaustion-associated chromatin features discernible in patient TILs (Philip et al., 2017), but most patients demonstrate only a single early proliferative burst in peripheral PD-1+ CD8 T cells after PD-1 blockade despite continued treatment (Huang et al., 2017; Kamphorst et al., 2017a).
In summary, under the selective pressure to avoid immune recognition, cancers have developed numerous strategies to corrupt the discriminatory functions of the immune system. Decreased antigen presentation or expression of low affinity antigens can limit antigenicity. Even when antigens are expressed, tumors can limit the generation of adaptive immune responses by interfering with adjuvanticity. Here, tumors can silence PRR signaling or subvert PRR signals to favor suppressive inflammatory pathways. Cancers also broadly corrupt the discriminatory function of the immune system that interprets environmental cues to properly tune immune effector function. In response to inflammatory signals, the epigenetic plasticity of cancer cells allows them to adapt an immune suppressive state. While for immune cells, cancer inflammation instructs T cells to adopt a dysfunctional and epigenetically “locked-in” state. For both cancer cells and immune cells, the timing, duration, and magnitude of inflammatory signals such as IFN can potently reinforce an overall immune suppressive TME.
Restoring Immunogenic PRR Signaling with Genotoxic Therapies
Since tumors exhibit PRR signaling that favors cancer inflammation and tumor progression rather than an anti-tumor immune response, an important consideration in improving ICB is to enhance the adjuvanticity of PRR signals in the TME. One approach is to use genotoxic agents that are already widely employed in cancer treatment such as chemotherapy and radiation. Indeed, this strategy of combining ICB with either chemotherapy or radiation is being extensively studied in clinical trials (Garg et al., 2017; Shabason and Minn, 2017). Notably, the significance of restoring adjuvanticity is dependent on adequate tumor antigenicity. Although genotoxic agents can be mutagenic and potentially alter the neo-antigen repertoire, the nature of the subclonal mutations generated may have little impact on antigenicity (McGranahan et al., 2016). Other reviews discuss tumor antigenicity in detail and cover the challenges and promising approaches to therapeutically enhance it (Lim and June, 2017; Schumacher and Schreiber, 2015). Here, we discuss recent developments that have shed important insight into how genotoxic agents, targeted therapies, and other approaches can enhance adjuvanticity by activating nucleic acid sensing.
In multiple syngeneic transplantation models, tumor response to genotoxic cancer agents can be strongly influenced by the adaptive immune system. For example, depletion of CD8 T cells or transplanting tumors into immunocompromised mice can result in markedly diminished tumor response to certain chemotherapeutic agents or to radiation (Galluzzi et al., 2015; Lee et al., 2009). Moreover, vaccination experiments with cancer cells treated in vitro show that these agents can generate anti-tumor immunity. Such observations strongly argue that determinants of effective tumor response extend beyond cancer cell intrinsic effects. Seminal studies have defined that this immunogenic cell death (ICD) can involve the release of now familiar DAMPs such as calreticulin, extracellular ATP, HMGB1, and ANXA1, followed by the activation of their cognate PRRs (Galluzzi et al., 2015), including newly identified PRRs such as formyl peptide receptor 1 (FPR1) (Vacchelli et al., 2015). These PRRs promote the uptake of antigens, activate APCs, and facilitate the interaction between APCs and damaged cells. Thus, conventional genotoxic cancer therapies can have immunogenic effects by enhancing PRR signaling.
The ability of radiation to improve response to ICB was also demonstrated in early studies in mice and later suggested in patients based on several case reports (Demaria et al., 2015). In mice, combining radiation with anti-CTLA-4 resulted in regression not only of the irradiated tumor but improved response of unirradiated (abscopal) tumors as well, confirming a systemic anti-tumor effect from radiation (Demaria et al., 2005; Twyman-Saint Victor et al., 2015). A similar abscopal effect was also observed in a patient undergoing radiation after initial anti-CTLA-4 (Postow et al., 2012). In experimental models, radiation has effects consistent with improved adjuvanticity, including diversification of the intratumoral T cell repertoire (Twyman-Saint Victor et al., 2015), improved antigen processing, and increased MHC-I expression (Reits, 2006). Improvements in T cell immune parameters are also observed in cancer patients undergoing radiation (Muraro et al., 2017; T. Zhang et al., 2017). Moreover, as with spontaneous T cell priming against tumors, the immunogenic effects of radiation are dependent on STING activation in dendritic cells and subsequent IFN-I production (L. Deng et al., 2014). Similarly, the combination of chemotherapies with favorable immunomodulatory properties can also improve tumor control when combined with ICB (Pfirschke et al., 2016), and preliminary evidence suggests improvement in response rates with certain combinations in patients (Langer et al., 2016). Thus, these observations suggest that combining conventional genotoxic therapies with ICB may improve the discriminatory function of the immune system to sense damage and evoke immunogenic PRR signaling.
For radiation and many chemotherapies, the principle insult is on DNA and disruption of genomic integrity. These insults include DNA double-stranded breaks, DNA cross-links, chromosome bridges, and other chromosomal abnormalities during mitosis. Surprisingly, DNA damage has also been intimately linked to pathogen responses and IFN signaling. For example, the serine/threonine kinase ATM, which is the key kinase activated after DNA damage, regulates PRR activation and early IFN-I signaling that can influence viral and bacterial defense (Härtlova et al., 2015; Purbey et al., 2017). Recently, several studies have described how the DNA damage response and perturbations in genomic integrity are sensed to activate nucleic acid PRRs such as cGAS/STING and modulate the immune system.
The OLR cGAS is a cytosolic DNA sensor and nucleotidyltransferase that produces cyclic GMP-AMP (cGAMP) to subsequently activate STING (Qi Chen et al., 2016). STING coordinates TBK1-mediated phosphorylation of IRF3/7, IFN-I production, and ISG induction. Recent structural studies reveal that cGAS forms dimers that can either cooperatively bind to shorter DNA to form ladder-like networks or can recognize longer DNA with the assistance of DNA-bending proteins like HMGB1 and TFAM (Andreeva et al., 2017). These requirements bias cGAS to recognize structured DNA or DNA that is bound to nucleoid proteins. After DNA damage, cGAS/STING is activated in multiple cancer cell lines over the course of several days. This delay is attributed to the time needed for cells with DNA damage to progress through mitosis and form micronuclei (Harding et al., 2017; Mackenzie et al., 2017). These micronuclei contain damaged chromosomal fragments with a compromised nuclear envelope. cGAS prominently localizes to micronuclei, resulting in STING activation and ISG induction. In mice, this signaling in cancer cells is at least partially responsible for the immunogenic effects of radiation when combined with anti-CTLA-4 or PD-1/PD-L1 blockade (Harding et al., 2017; Vanpouille-Box et al., 2017; H. Wang et al., 2017). Indeed, cGAS has a more general role in inflammation after tissue damage and in immunosurveillance against cancer (Dou et al., 2017; Glück et al., 2017). Interestingly, activation of cGAS/STING exhibits a threshold effect and can be negatively regulated by the exonuclease TREX1, which presumably degrades the DNA that stimulates cGAS (Vanpouille-Box et al., 2017). This threshold effect may have therapeutic consequences when trying to exploit cGAS for ICB therapy, since such properties may impact optimal radiation or chemotherapy dose and schedule.
The RLR founding member RIG-I is a cytosolic RNA sensor that recognizes short double-stranded or highly structured RNA typically with a blunt and triphosphorylated 5’ end (Schlee and Hartmann, 2016). Like cGAS/STING, the anti-viral response after RLR stimulation results from IRF3/7 phosphorylation, IFN-I production, and ISG induction. Recent structural studies highlight that a 2-O-methyl cap, which is an RNA modification coupled to RNA polymerase II transcription, actively interferes with efficient binding of RNA to RIG-I (Devarkar et al., 2016; Schuberth-Wagner et al., 2015). This modification biases RIG-I against recognition of typical cellular mRNAs, which are RNA polymerase II products, and toward highly structured non-coding RNAs (ncRNAs). Direct introduction of RNA ligands to activate RIG-I in cancer models promotes an IFN-I response, DC activation, and stimulates innate and adaptive immune cells (Nabet et al., 2017; Poeck et al., 2008). In fact, the adjuvanticity of RIG-I ligands for CD8 T cell priming can be particularly strong compared to other nucleic acid PRR ligands (Hochheiser et al., 2016). Likewise, radiation of cancer cells also activates RIG-I through ncRNAs that accumulate in the cytoplasm. These ncRNAs may include U1 snRNA, which is also recognized by TLR3 after UV damage (Bernard et al., 2012; Ranoa et al., 2016). Disruption of either RIG-I or TLR3 in tumor cells interferes with IFN-I production and response to DNA damage in vitro and in vivo, suggesting that RNA sensing in tumor cells can have a cell autonomous role. For all the RNA sensors, how ligand-receptor recognition is regulated after DNA damage is an important issue. Although expression of normally silenced transcripts or export of strictly nuclear RNA are implicated (Ranoa et al., 2016), recent studies in viral infection and cancer indicate additional layers of regulation through RNA binding proteins that mask ncRNAs from RIG-I recognition (Chiang et al., 2018; Nabet et al., 2017).
In summary, chemotherapy and radiation are standard cancer therapies that are widely used and readily incorporated into combination ICB strategies (Table 1). One of the mechanisms by which they improve ICB efficacy in syngeneic mouse models is by stimulating PRR signaling upon recognizing endogenous DNA or RNA. Multiple DAMPs and PRRs in cancer cells are engaged after chemotherapy or radiation. As with the complexity of PRRs in pathogen infection, the integration of these multiple PRR signals and associated regulatory mechanisms likely will impact net adjuvanticity and immunomodulatory effects.
Table 1.
Strategies to restore PRR signaling
| Target | Examples under clinical investigation ± ICB | References |
|---|---|---|
| PRR activation | Chemotherapy | Galluzzi et al, 2015 |
| Radiation | Shabason and Minn, 2017 | |
| HER2-directed antibody | Park et al, 2010, Stagg et al, 2011 | |
| PRR agonists | Fu et al, 2015, Corrales et al, 2015, Charlebois et al, 2017, Kranz et al 2016, Shekarian et al, 2017 | |
| Oncolytic viruses | Ribas et al, 2017 | |
| Endogenous retroviruses | DNMT inhibitors | Chiappinelli et al, 2015; Roulois et al, 2015 Topper et al, 2017 |
| HDAC inhibitors | ||
| CDK4/6 inhibitors | Goel et al, 2017, Zhang et al, 2018 | |
| Costimulatory agonist | CD40 agonist | Byrne and Vonderheide, 2016 |
Restoring Immunogenic PRR Signaling with Targeted Therapies
In addition to chemotherapy and radiation, other major classes of cancer therapies also appear to have immunogenic effects through nucleic acid DAMPs and PRR signaling. In mouse models, monoclonal antibodies against HER2 can elicit cytotoxicity that activates MYD88-dependent PRR signaling and requires CD8 T cells (Park et al., 2010; Stagg et al., 2011). More recently, CDK4/6 inhibitors, which are approved for use in hormone-receptor positive breast cancer patients and being tested clinically in multiple other tumor types, were also shown to have immunomodulatory functions (J. Deng et al., 2017; Goel et al., 2017; J. Zhang et al., 2018). These include effects on PD-L1 expression, induction of IFN signaling in tumor cells, suppression of Treg proliferation, and enhancement of T cell activation. Mechanistically in tumor cells, CDK4/6 inhibition decreases the DNA methyltransferase DNMT1, resulting in derepression of endogenous retroviruses (ERV), which are repetitive elements that can form dsRNA (Goel et al., 2017). These ERVs then trigger a type III interferon response. As expected, direct epigenetic therapy with the DNA methyltransferase inhibitor (DNMTi) azacytidine also derepresses ERVs, resulting in TLR- and RLR-mediated IFN signaling (Chiappinelli et al., 2015; Roulois et al., 2015). Combining azacytidine with anti-CTLA-4 improves responses in mice, suggesting that like with radiation, ncRNAs can enhance tumor adjuvanticity and ICB response through nucleic acid DAMPs and PRR signaling. These epigenetic therapies may also modulate anti-tumor immune responses by decreasing the expression of oncogenes such as MYC (Topper et al., 2017). Interestingly, emerging evidence indicates that tumor suppressor and oncogene pathways in the TME regulate production of RNA DAMPs (Moroishi et al., 2016; Nabet et al., 2017), providing insight into why blocking oncogenic drivers can cause PRR activation and immune-mediated regression. Overall, these findings suggest that multiple classes of targeted and epigenetic therapies can unexpectedly initiate nucleic acid DAMPs and PRR signaling with favorable immune consequences.
Another approach to stimulate PRR signaling is to directly introduce attenuated viral pathogens. A potential advantage of this approach is that multiple viral PAMPs and cellular DAMPs are likely sensed, resulting in PRR signal integration and more robust adjuvanticity (Kohlhapp and Kaufman, 2016). Indeed, a recent phase I clinical trial for melanoma combining anti-PD-1 with an attenuated oncolytic virus talimogene laherparepvec demonstrated impressive clinical responses of 62%, compared to approximately 30% response expected for anti-PD-1 alone (Ribas et al., 2017). Importantly, responses were observed in patients with low baseline CD8 T cell infiltration, which has previously been correlated with response to anti-PD-1. A more direct, albeit more restricted, method to activate PRRs is utilizing direct PRR agonists. In preclinical studies, use of STING agonists is effective alone or when combined with cell-based vaccines and PD-1/PD-L1 blockade, especially with large established tumors that are difficult to treat with ICB alone (J. Fu et al., 2015). Similar to how radiation mediates abscopal tumor responses through cGAS/STING, STING agonists also elicited tumor responses at distant sites (Corrales et al., 2015). Other PRR agonists, particularly RNA-based and a variety of TLR agonists represent a similar strategy that is being combined with ICB or immunomodulatory agents (Charlebois et al., 2017; Kranz et al., 2016; Shekarian et al., 2017). Alternatively, rather than directly stimulating PRRs to enhance adjuvanticity, agonists of costimulatory pathways on APCs may bypass the need for PRR signaling. Using a mouse pancreatic cancer model, an agonist CD40 antibody was shown to bypass the typical requirement for innate sensors, including cGAS/STING and TLRs, and mount immune-mediated rejection when combined with chemotherapy (Byrne and Vonderheide, 2016).
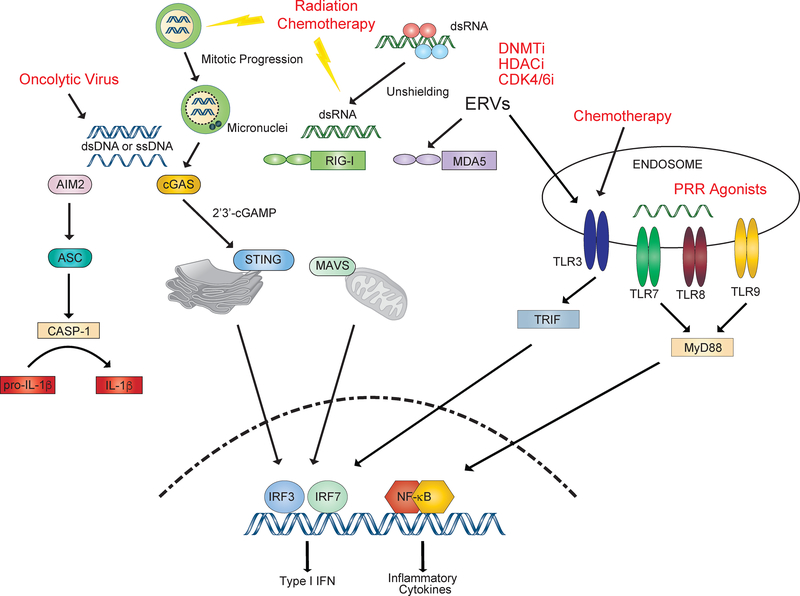
In summary, in addition to genotoxic cancer therapies, molecularly targeted and epigenetic therapies can stimulate nucleic acid PRRs and mediate immunogenic effects (Figure 3). This broadens the possible types of targeted cancer therapies that can be effectively combined with ICB to improve PRR signaling (Table 1). Oncolytic viruses that intrinsically generate multiple PAMP and DAMP signals, direct agonists of nucleic acid PRR pathways, and co-stimulatory pathways on APCs that bypass PRR requirements all provide novel and directed strategies to restore adjuvanticity.
Figure 3: Activation of pattern recognition receptors by conventional and targeted therapies can promote innate immune signaling.
Radiation and genotoxic agents can lead to activation of the cGAS/STING pathway intrinsically in tumor cells as well as in dendritic cells. Altered cell cycle machinery in tumor cells allows progression through mitosis despite DNA damage, leading to the accumulation of cGAS positive nuclei. Epigenetic therapy with DNA methyltransferase inhibitors (DNMTi) and histone deacetylase inhibitors (HDACi) can derepress endogenous retroviruses (ERVs) in a mechanism termed virus mimicry. Targeted therapies such as CDK4/6 inhibitors have also been shown to promote interferon signaling through this mechanism. Changes in RNA binding proteins can lead to unshielding of dsRNA, allowing RIG-I activation. Chemotherapy, such as adriamycin, can activate TLR3 signaling to promote anti-tumor immune responses. Oncolytic viruses have multi-faceted effects on innate immune signaling through activation of PRR and promotion of immunogenic cell death (ICD). Direct STING and TLR agonists are also being developed.
Interfering with Feedback Inhibition Imposed by Immune Cells
Proper tuning of immune effector function to maximize fitness of the host is an important regulatory function of the immune system, which is dysregulated in cancer by skewing feedback inhibition to maximize fitness of the tumor. Thus, despite the importance of therapeutically enhancing PRR signaling to augment the adjuvanticity of cancer, effectively addressing feedback inhibition mechanisms is a key step for successful combination strategies. The importance of blocking feedback inhibition is highlighted by the success of anti-PD-1/PD-L1 agents in the clinic. In fact, adaptive resistance through PD-L1 may be one of the first tactics employed by cancer to regain an advantage once the immune system begins gaining ground (Minn and Wherry, 2016). In mice and patient tumors treated with radiation combined with anti-CTLA-4, increased PD-L1 expression occurs and either promotes or associates with relapse or resistance (Twyman-Saint Victor et al., 2015). PD-L1 is similarly increased in mouse models after radiation plus anti-TGFβ or after combination therapy employing STING agonists (J. Fu et al., 2015; Vanpouille-Box et al., 2015). Consistent with anti-CTLA-4 failure enriching for PD-1/PD-L1-driven exhaustion, TEX-related genes expressed in the tumor may be preferentially predictive in melanoma patients previously treated with anti-CTLA-4 compared to naïve patients (Riaz et al., 2017). Even molecularly targeted therapies such as CDK4/6 inhibitors, which promote PRR activation, T cell function, and immune-mediated cytotoxicity, can increase tumor PD-L1 expression due to the coupling of cell cycle progression to PD-L1 expression. Here, CDK4/6 inhibitors increase PD-L1 by antagonizing the ability of Cyclin D-CKD4 to regulate PD-L1 stability through the Cullin-3 E3 ligase and its adaptor protein SPOP (J. Zhang et al., 2018). Thus, early adaptive resistance mediated by PD-L1 is an argument for using PD-1/PD-L1 blockade as a cornerstone for combination strategies. Nonetheless, even combinations that block PD-1/PD-L1 and augment PRR signals will often be insufficient. Emerging evidence provides potential strategies to more broadly combat feedback inhibition affecting immune cells.
In chronic viral infection models, the severity of exhaustion is progressive and leads to co-expression of multiple T cell inhibitory receptors in addition to PD-1 (Pauken and Wherry, 2015). In this setting, multi-agent ICB blockade is superior to anti-PD-1 alone at improving TEX function (Blackburn et al., 2008). Given that anti-tumor T cells in patients are also enriched for T cells co-expressing multiple T cell inhibitory receptors (Gros et al., 2014), this motivates the notion that multi-agent ICB may also be superior to monotherapy in cancer. Lending support to this strategy, recent evidence suggests that combination CTLA-4 and PD-1 blockade is more clinically effective than monotherapy (Larkin et al., 2015; Postow et al., 2015). These two immune checkpoints are thought to be largely non-redundant both spatiotemporally and in their immune effects (Topalian et al., 2015; Wei et al., 2017); however, recent interactions through CD28 have been uncovered that implicate potentially more mechanistic overlap than previously appreciated (Kamphorst et al., 2017b; “T cell costimulatory receptor CD28 is a primary target for PD-1,” 2017).
Lack of deep mechanistic insight and the sheer number of permutations makes it a challenge to find ICB combinations with potent rather than marginal benefit (Baumeister et al., 2016), a criterion that is important given that combination ICB can markedly increase immune-related toxicities (Larkin et al., 2015; Postow et al., 2015). One approach is to use combination ICB to target distinct TEX populations that are either most critical for the reactivation effects of ICB or are the most dysfunctional (Table 2). For example, a PD-1+ TIM3− CXCR5+ population of T cells are responsible for the proliferative burst observed after PD-1 blockade (He et al., 2016; Im et al., 2016; Utzschneider et al., 2016), while a PD-1+ TIM3+ subset enriches for particularly dysfunctional TEX (Singer et al., 2017). Indeed, co-blockade of TIM3 and PD-1 is more effective than individual therapy in mouse tumor models (Sakuishi et al., 2010), and higher levels of TIM3 are observed on PD-1+ T cells in mice and patients exhibiting adaptive resistance after anti-PD-1 therapy (Koyama et al., 2016). A second approach is to integrate additional ICBs with distinct mechanisms of action. Again, using TIM3 as an example, structural studies reveal that TIM3 is a receptor for phosphatidylserine, a DAMP found on apoptotic cells and associated with immune tolerance (DeKruyff et al., 2010). Interfering with TIM3 may antagonize both the T cell inhibitory function of TIM3 and a toleragenic DAMP signal. A third approach to designing effective combination ICB strategies is to broaden the relevant immune cell types targeted. For example, the Ig superfamily member VISTA is expressed not only on T cells but on macrophages, dendritic cells, Tregs, and NK cells. In mouse models, VISTA synergizes with PD-1 to promote T cell activation, and anti-VISTA plus anti-PD-1/PD-L1 improves multiple immune parameter and tumor response (Liu et al., 2015; L. Wang et al., 2011). Combinations can also be designed to broaden coverage by using one agent that selectively targets an important population like Tregs. For example, modified antibodies against CD25 can potently deplete Tregs and improve PD-1 blockade especially against large established tumors (Arce Vargas et al., 2017). Finally, rather than blockade of immune checkpoints, co-stimulatory agonists that can decrease multiple inhibitory receptors may offer functionally similar benefits. Lack of CD4 T cell help promotes CD8 T cell exhaustion and either improving CD4 help or recapitulating the help using CD27 agonists can decrease multiple CD8 T cell inhibitory receptors (Ahrends et al., 2017; Sanmamed et al., 2015).
Table 2.
Approaches for targeting feedback inhibition in the tumor-immune microenvironment
| Target | Examples under clinical investigation ± ICB | References |
|---|---|---|
| Dual checkpoint blockade | Anti-CTLA-4 Anti-TIM3 Anti-LAG3 Anti-TIGIT Anti-VISTA |
Larkin et al., 2015; Postow et al., 2015
Sakuishi et al., 2010; Koyama et al., 2016 Liu et al., 2015; L. Wang et al., 2011 |
| Costimulatory agonist | Anti-GITR Anti-ICOS Anti-OX40 Anti-CD27 |
Sanmamed et al, 2015 |
| Regulatory T cells | Anti-CD25 | Arce Vargas et al., 2017 |
| IDO1 inhibitors | Prendergast et al, 2017 | |
| Epigenetic changes | DNMT inhibitors HDAC inhibitors |
Topper et al, 2017 Ghoneim et al., 2017 |
| Myeloid suppressor cells | CSF1R inhibitor/antibodies PI3Kγ inhibitor Class IIa HDAC inhibitors |
Zhu et al, 2014 |
| De Henau et al., 2016; Kaneda et al., 2016 | ||
| Guerriero et al., 2017 | ||
| Cytokines | JAK inhibitors | Benci et al, 2017 |
| TGFβ inhibitors/antibodies | Tauriello et al, 2018; Mariathasan et al, 2018 | |
| MET inhibitors | Glodde et al, 2017 | |
| Immunometabolism | IDO1 inhibitors A2AR antagonists Arginase inhibitors Glutaminase inhibitors |
Prendergast et al, 2017 Cekic and Linden, 2016 |
Even with the blockade of multiple T cell inhibitory receptors, a critical unresolved issue is the extent to which this strategy can overcome “locked-in” epigenetic features of TEX. As previously discussed, TEX adopt an inflexible epigenetic state that is distinct from effector or memory T cells (Figure 4), and this dysfunction may occur early during tumorigenesis (Pauken et al., 2016; Philip et al., 2017; Schietinger et al., 2016; Sen et al., 2016). Although anti-PD-1 improves TEX function, the inability to convert TEX into effector T cells places a limit on the ability of reinvigorated TEX to handle antigen load, while the failure to convert into memory T cells may compromise long-term durable response. The notion that TEX have limited function despite ICB is evident in both mice and patients. For example, despite improving TEX function, the effectiveness of even combination therapy utilizing radiation and blockade of PD-L1 and/or CTLA-4 rapidly falls off if the TEX burden is high – a finding observed in mouse models and suggested by patient data (Twyman-Saint Victor et al., 2015). In comprehensive studies of melanoma and lung cancer patients, most patients treated with anti-PD-1 demonstrate only a single early proliferative burst in PD-1+ CD8 peripheral T cells (Huang et al., 2017; Kamphorst et al., 2017a). Thus, the degree to which combination ICB will significantly improve TEX function given epigenetic constraints is an outstanding question. However, recent proof-of-principle studies in mice demonstrate that interfering with the DNA methyltransferase DNMT3A, which controls some of the epigenetic changes that occur in TEX, antagonizes TEX development and dysfunction (Ghoneim et al., 2017). Furthermore, combining decitabine, a DNMTi, with PD-1/PD-L1 blockade in a mouse prostate cancer model increases proliferation of tumor antigen-specific T cells and improves ICB response. Similarly, DNMTi combined with a HDACi decreases exhaustion-associated genes expressed by TILs in a lung adenocarcinoma model (Topper et al., 2017). Notably, durable responses have been observed in non-small cell lung cancer patients initially treated with DNMTi and HDACi prior to ICB (Juergens et al., 2011). Thus, epigenetic therapies may hold promise to improve TEX function by interfering with loci that maintain exhaustion.
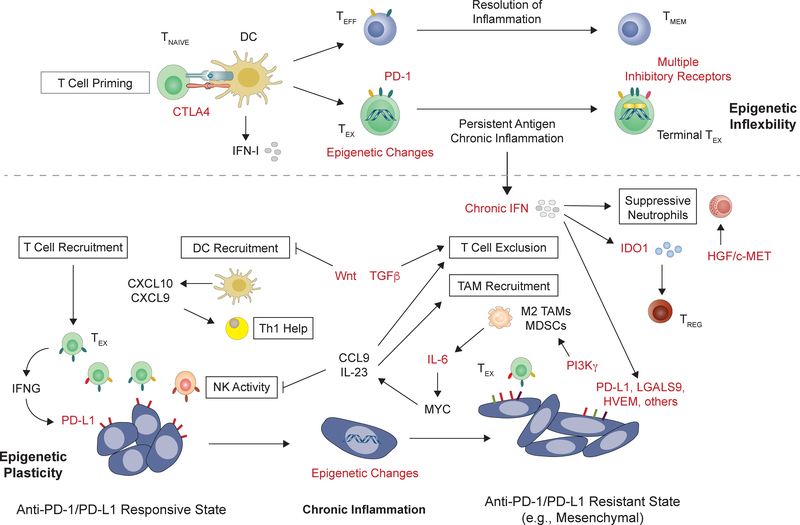
Figure 4: Targets to interfere with feedback inhibition mechanisms from immune cells and the tumor microenvironment.
Naïve T cells (TNAIVE) undergo different cell fates depending on the nature of the antigen and the course of inflammation (above dashed line). After T cell priming, persistent antigen and chronic inflammation, which typifies cancer, results in T cell exhaustion as a mechanism to limit immune-mediated pathology. In contrast to effector T cells (TEFF) that differentiate into memory T cells (TMEM), these exhausted T cells (TEX) have poor effector function that may be epigenetically “locked-in”. Targeting multiple co-expressed T cell inhibitory receptors can improve TEX function. In contrast to TEX, the tumor microenvironment (TME) generally demonstrates plasticity (below dashed line). Instructed by aberrant environmental cues and feedback signals associated with chronic inflammation and non-healing wounds, cancers can acquire various immune-TME phenotypes (boxed in black). DC and T cell recruitment favor response to PD1–1/PD-L1 blockade. However, tumors that transition into mesenchymal states by inflammatory cytokines or oncogenic signals exhibit an immune-TME that excludes T cells and supports suppressive TAMs and MDSCs. Chronic cytokine signals such as IFN can exacerbate immune suppression through various mechanisms, which can contrast with their typical stimulatory roles during productive immune responses that resolve. When multiple immune suppressive mechanisms dominate, resistance or relapse to anti-PD-1/PD-L1 typically occurs. Potential targets for combination ICB therapy is indicated in red and include the epigenetic state of tumor and immune cells, intracellular and extracellular signaling pathways, suppressive cytokines, and non-redundant T cell inhibitory receptor pathways. See Table 2 for examples of agents against these targets.
Interfering with Feedback Inhibition Co-opted by Cancer Cells
Even if TEX can be reversed or avoided to allow for anti-tumor effector T cell development, the insidious ability of cancers to remodel tumor cells and stroma may enforce a dominant immune suppressive microenvironment. Indeed, adoptively transferred anti-tumor effector T cells can remain functionally inert due to a TME that excludes T cells (Spranger et al., 2017). In addition, aforementioned mixing experiments between epithelial and mesenchymal cancer cells also suggest that immunosuppression by the TME exhibits a dominant effect (Dongre et al., 2017). Promising options for disabling these dominant suppressive effects span from interfering with chromatin-level changes, blocking extracellular and intracellular signals, to aggressive cytoreduction (Figure 4).
The ability of chronic inflammatory signaling such as various proinflammatory cytokines, TGFβ, mitogens, or prolonged IFN signaling to drive cancer cells into mesenchymal states or states associated with adaptive resistance, suggests epigenetic regulation. Recent studies have examined the notion that mesenchymal or stem cell-like properties gained by tumor cells may suppress their expression of TH1-type cytokines (Peng et al., 2015). Indeed, epigenetic therapy using an inhibitor to EZH2, which trimethylates histone H3 at lysine 27, combined with DNMTi restores expression of CXCL9 and CXCL10 in mouse models. This results in enhanced T cell infiltration and response to either anti-PD-L1 or to adoptive T cell transfer. In models of non-small cell lung cancer, DNMT and HDAC inhibition depletes MYC, a known transcriptional regulator of EMT, and reverses multiple immunosuppressive features of the TME (Topper et al., 2017). This includes a decrease in TAMs mirrored by an increase in tumor-infiltrating CD8 T cells. Direct manipulation of MYC results in similar immune consequences to the TME (Kortlever et al., 2017). Here, MYC activation in mouse lung adenomas conversely leads to expulsion of T, B, and NK cells and an influx in macrophages – effects attributed to IL-23 and CCL9. Tumor regression induced by decreasing MYC expression in this model was NK cell dependent. These experimental findings elucidated by genetic manipulation of MYC have relevance to EMT associated with cancer inflammation. MYC is a potent driver of EMT and is a downstream transcription factor of multiple inflammatory cytokine pathways such as IL-6 and STAT3 (Hirano et al., 2000). Apart from altering the epigenetic landscape of tumor cells, epigenetic agents such as class IIa HDAC inhibitors can reprogram suppressive stromal immune cells by recruiting or favoring the development of anti-tumor TAMs (Guerriero et al., 2017). Thus, targeting chromatin-level regulators of the epigenetic state of tumor cells or stromal cells, like with TEX, may antagonize how cancers co-opt chronic inflammation to expel favorable adaptive and innate immune cells from the TME (Table 2).
Direct targeting of inflammatory cytokines and the associated signaling pathways that cancers use to enforce immunosuppression offers another strategy for combination therapy. Although there are many therapeutic opportunities, several intriguing combinations are notable. Emerging evidence that mesenchymal features are associated with anti-PD-1 resistance make pathways such as TGFβ, AXL, HGF/c-MET, JAK/STAT, and WNT attractive targets (Dongre et al., 2017; Hugo et al., 2016). Some of these pathways such as TGFβ and AXL can be inhibited by selective agents (e.g., galunisertinib) to either block extracellular EMT stimuli (i.e., TGFβ, WNT) or intracellular EMT signals (i.e. AXL) (Marcucci et al., 2016). In addition to its role in EMT, recent evidence from mouse models and analysis of patient samples suggests that TGFβ may be particularly relevant in immune excluded tumors. In a genetically engineered mouse model of metastatic colon cancer, TGFβ inhibition cooperates with PD-L1 blockade to promote T cell-mediated clearance of liver metastases (Tauriello et al., 2018). Similarly, in metatastatic urothelial carcinoma, an increased TGFβ signature is associated with resistance to PD-L1 blockade specifically in patients with immune excluded tumors (Mariathasan et al., 2018). In T cell inflamed tumors, JAK/STAT signaling can contribute to immunotherapy resistance. For example, the production of IFNγ by intratumoral T cells drives PD-L1-dependent and PD-L1-independent resistance pathways on tumor and immune cells (Benci et al., 2016; Spranger et al., 2013), including promoting immunosuppressive properties of recruited neutrophils (Glodde et al., 2017). In mouse models, pharmacological JAK1/2 inhibition can interfere with resistance mechanisms associated with chronic IFN signaling and restore response of ICB-resistant tumors (Benci et al., 2016). However, like in the setting of chronic viral infection (Ng et al., 2015; Sandler et al., 2014; Wilson et al., 2013), improving ICB requires delayed JAK inhibitor administration to preserve favorable attributes of IFNs (e.g., antigen processing and early immune cell activation), while selectively blocking the suppressive effects of persistent signaling. Alternatively, a potentially less complex approach is to target the pathways that the suppressive properties of JAK/STAT signaling impact. For example, reactive tumor-infiltrating neutrophils that acquire suppressive features upon IFNγ stimulation rely upon HGF/c-MET signaling for recruitment. Thus, the selective c-MET inhibitor capmatinib can improve ICB in mouse models (Glodde et al., 2017). Similarly, inhibitors of PI3Kγ or CSF/CSF1R can interfere with suppressive myeloid and macrophage features of the TME to improve response to checkpoint blockade (De Henau et al., 2016; Kaneda et al., 2016; Y. Zhu et al., 2014). Finally, small molecular inhibitors targeting IDO1, an immunosuppressive metabolic factor regulated by IFN signaling, are being combined with ICB (Prendergast et al., 2017). Thus, targeting extracellular and intracellular signaling pathways associated with cancer inflammation may improve immunotherapy by antagonizing EMT or suppressive immune features of the TME (Table 2).
As cancer outcomes improve, aggressive surgery or radiation is increasingly utilized in patients with limited metastatic disease. Since the TME exerts a dominant immunosuppressive effect and/or tumor antigen load can overwhelm the limited function of reactivated TEX, incorporation of strategies that involve significant tumor cytoreduction may have merit as an approach to combat feedback inhibition imposed by tumor and immune cells. In mouse models of viral infection, higher antigen load facilitates T cell exhaustion (Zuniga and Harker, 2012), and in cancer models, the effectiveness of ICB rapidly diminishes when tumor burden increases (Benci et al., 2016). Indeed, the restricted proliferative burst of TEX in metastatic melanoma patients after anti-PD-1 is associated with clinical response when overall tumor burden is low (Huang et al., 2017). In contrast, patients with an unfavorable ratio of reinvigorated TEX to tumor burden are not likely to respond. These observations suggest that therapeutically decreasing tumor burden in the metastatic setting may address a barrier that can limit ICB efficacy. Alternatively, moving combination therapies to earlier stage and non-metastatic disease settings is another strategy. Under these circumstances, not only is tumor burden generally lower but all gross tumor is typically treated deliberately for cytoreduction. In the non-metastatic setting, the overall tumor-immune microenvironment may also be more favorable. Recent examination of multiple metastases that evolved in different organs in an ovarian cancer patient revealed the co-existence of heterogeneous tumor-immune microenvironments (Jiménez-Sánchez et al., 2017). These metastases differed in T cell infiltration, PD-L1 status, and genomic features. Such observations suggest obtaining complete responses in patients with widespread metastases can be a challenge due to increased likelihood that some metastatic lesions harbor an immunosuppressive TME. Indeed, evidence from autochthonous mouse models support how diverse TMEs can influence immunotherapy response and the importance of having model systems to capture this diversity (J. Zhu et al., 2017). Consistent with a potential benefit of using ICB in non-metastatic and lower tumor burden settings, an unusually impressive improvement from anti-PD-L1 therapy has been observed in stage III (non-metastatic) lung cancer patients first treated with concurrent chemotherapy and radiation to all gross disease (Antonia et al., 2017). Thus, incorporation of aggressive cytoreduction into combination therapy can be a readily employed strategy to address feedback inhibition mechanisms imposed by tumor and immune cells.
Concluding Remarks
The clinical efficacy and durability of responses seen with ICB has spurred a dramatic change in the approach to the treatment of cancer, driving interest in understanding how to extend the impact of ICB to more patients across additional cancer types. This goal requires an understanding of how inherent properties of cancer cells influence therapy response, how cancer cells program the TME to alter the immune system, and how immune cells reciprocally react to these tumor intrinsic and extrinsic effects. The overall impact on antigenicity, adjuvanticity, and feedback inhibition is of particular importance in reactivating an anti-tumor immune response. Therefore, maximizing each of these three discriminatory functions of the immune system should be considered when rationally designing combination strategies. However, given the potential for added toxicity from combination therapies, biomarkers that assess the sufficiency of each of these discriminatory functions for a given patient are needed. Some methods such as predicted neo-antigen load and PD-L1 status already provide some information about these functions, while other stratification schemes such as “immune cell exclusion”, “immune desert”, and “inflamed tumors” represent important phenotypes that need more granular molecular explanation. Development of multivariate classifiers using multidimensional tumor and immune features will help to match rational combinations that improve immune discriminatory functions with properly selected patients.
Antigenicity is a critical and largely inherent property of cancer cells. Addressing poor antigenicity with vaccines, adoptive cell therapies, and CAR T cells can pose challenges in clinical deployment; however, when successful these approaches result in clear responses. In contrast, addressing adjuvanticity can be accomplished with conventional genotoxic therapies that are in widespread clinical use; however, since adjuvants rarely cause immune effects on their own, the effectiveness of these interventions when combined with ICB is often unclear. Mechanistically, chemotherapy and radiation can impact adjuvanticity by activation of PRRs that sense endogenous DNA and RNA. Surprisingly, multiple classes of cancer therapies also appear to engage these nucleic acid PRRs with favorably immune effects. However, how and to what extent therapy-induced activation of nucleic acid PRRs and numerous other PRRs enhance adjuvanticity to improve ICB response requires further investigation. Since genotoxic therapies and many other diverse therapies that potentially impact adjuvanticity are readily available in the clinic, a solid understanding of mechanism (and limitations) could quickly result in rational adaptation and meaningful clinical impact.
Perhaps the most daunting discriminatory function to therapeutically skew toward an anti-tumor immune response is feedback inhibition. The immune system is wired to balance potent effector function with the risk for immune-mediated pathology or poor restoration of barrier integrity. Cancers exploit a variety of these homeostatic feedback mechanisms to tip the balance toward immune suppression. This includes programming the TME to resemble the immune contexture of non-healing wounds, activation of T cell inhibitory receptor pathways, and transitioning into immune suppressive mesenchymal states. Thus, combination strategies to combat feedback inhibition consist of blocking signals that enforce suppressive innate immune cells, antagonizing exhaustion-related T cell inhibitory receptors, and interfering with epigenetic changes and chronic signaling needed to maintain immunosuppressive tumor states. On the immune side, T cells have evolved to “lock-in” the consequences of feedback inhibition pathways. A lack of epigenetic plasticity once T cells become exhausted may be a principle mechanism for this inflexibility. Currently, it is unclear the extent to which these tumor and immune feedback mechanisms can and need to be targeted in order to perpetuate durable anti-tumor immunity. Nonetheless, incorporating epigenetic strategies to force TEX plasticity, ways to compensate for the limited function of reactivated TEX, or methods to prevent TEX altogether may hold promise for successful combination therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahrends T, Spanjaard A, Pilzecker B, Bąbała N, Bovens A, Xiao Y, Jacobs H, Borst J, 2017. CD4+T Cell Help Confers a Cytotoxic T Cell Effector Program Including Coinhibitory Receptor Downregulation and Increased Tissue Invasiveness. Immunity 47, 848–861.e5. doi: 10.1016/j.immuni.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Andreeva L, Hiller B, Kostrewa D, Lässig C, de Oliveira Mann CC, Jan Drexler D, Maiser A, Gaidt M, Leonhardt H, Hornung V, Hopfner K-P, 2017. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein–DNA ladders. Nature 549, 394–398. doi: 10.1038/nature23890 [DOI] [PubMed] [Google Scholar]
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M, PACIFIC Investigators, 2017. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 377, 1919–1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A, Ben Aissa A, Franz D, Werner Sunderland M, Wong YNS, Henry JY, O’Brien T, Nicol D, Challacombe B, Beers SA, Gore M, Larkin J, Swanton C, Chester KA, Pule M, Ravetch JV, Marafioti T, Peggs KS, Quezada SA, Wotherspoon A, Francis N, Smith M, Strauss D, Hayes A, Soultati A, Stares M, Spain L, Lynch J, Fotiadis N, Fernando A, Hazell S, Chandra A, Pickering L, Rudman S, Chowdhury S, Jamal-Hanjani M, Veeriah S, Shafi S, Czyzewska-Khan J, Johnson D, Laycock J, Bosshard-Carter L, Goh G, Rosenthal R, Gorman P, Murugaesu N, Hynds RE, Wilson G, Birkbak NJ, Watkins TBK, McGranahan N, Horswell S, Mitter R, Escudero M, Stewart A, Van Loo P, Rowan A, Xu H, Turajlic S, Hiley C, Abbosh C, Goldman J, Stone RK, Denner T, Matthews N, Elgar G, Ward S, Biggs J, Costa M, Begum S, Phillimore B, Chambers T, Nye E, Graca S, Bakir Al, M., Hartley JA, Lowe HL, Herrero J, Lawrence D, Hayward M, Panagiotopoulos N, Kolvekar S, Falzon M, Borg E, Simeon C, Hector G, Smith A, Aranda M, Novelli M, Oukrif D, Janes SM, Thakrar R, Forster M, Ahmad T, Lee SM, Papadatos-Pastos D, Carnell D, Mendes R, George J, Navani N, Ahmed A, Taylor M, Choudhary J, Summers Y, Califano R, Taylor P, Shah R, Krysiak P, Rammohan K, Fontaine E, Booton R, Evison M, Crosbie P, Moss S, Idries F, Joseph L, Bishop P, Chaturved A, Quinn AM, Doran H, Leek A, Harrison P, Moore K, Waddington R, Novasio J, Blackhall F, Rogan J, Smith E, Dive C, Tugwood J, Brady G, Rothwell DG, Chemi F, Pierce J, Gulati S, Naidu B, Langman G, Trotter S, Bellamy M, Bancroft H, Kerr A, Kadiri S, Webb J, Middleton G, Djearaman M, Fennell D, Shaw JA, Le Quesne J, Moore D, Nakas A, Rathinam S, Monteiro W, Marshall H, Nelson L, Bennett J, Riley J, Primrose L, Martinson L, Anand G, Khan S, Amadi A, Nicolson M, Kerr K, Palmer S, Remmen H, Miller J, Buchan K, Chetty M, Gomersall L, Lester J, Edwards A, Morgan F, Adams H, Davies H, Kornaszewska M, Attanoos R, Lock S, Verjee A, MacKenzie M, Wilcox M, Bell H, Iles N, Hackshaw A, Ngai Y, Smith S, Gower N, Ottensmeier C, Chee S, Johnson B, Alzetani A, Shaw E, Lim E, De Sousa P, Barbosa MT, Bowman A, Jorda S, Rice A, Raubenheimer H, Proli C, Cufari ME, Ronquillo JC, Kwayie A, Bhayani H, Hamilton M, Bakar Y, Mensah N, Ambrose L, Devaraj A, Buderi S, Finch J, Azcarate L, Chavan H, Green S, Mashinga H, Nicholson AG, Lau K, Sheaff M, Schmid P, Conibear J, Ezhil V, Ismail B, Irvin-sellers M, Prakash V, Russell P, Light T, Horey T, Danson S, Bury J, Edwards J, Hill J, Matthews S, Kitsanta Y, Suvarna K, Fisher P, Keerio AD, Shackcloth M, Gosney J, Postmus P, Feeney S, Asante-Siaw J, 2017. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity 46, 577–586. doi: 10.1016/j.immuni.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K, 2000. Immunobiology of dendritic cells. Annu Rev Immunol 18, 767–811. doi: 10.1146/annurev.immunol.18.1.767 [DOI] [PubMed] [Google Scholar]
- Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH, 2016. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol 34, 539–573. doi: 10.1146/annurevimmunol-032414-112049 [DOI] [PubMed] [Google Scholar]
- Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ, 2016. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 167, 1–28. doi: 10.1016/j.cell.2016.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu BD, Gallo RL, 2012. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med. 18, 1286–1290. doi: 10.1038/nm.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ, 2008. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10, 29–37. doi: 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonam SR, Partidos CD, Halmuthur SKM, Muller S, 2017. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 38, 771–793. doi: 10.1016/j.tips.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Byrne KT, Vonderheide RH, 2016. CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer. Cell Reports 15, 2719–2732. doi: 10.1016/j.celrep.2016.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic C, Linden J, 2016. Purinergic regulation of the immune system. Nat Rev Immunol 16, 177–192. doi: 10.1038/nri.2016.4 [DOI] [PubMed] [Google Scholar]
- Charlebois R, Allard B, Allard D, Buisseret L, Turcotte M, Pommey S, Chrobak P, Stagg J, 2017. PolyI:C and CpG Synergize with Anti-ErbB2 mAb for Treatment of Breast Tumors Resistant to Immune Checkpoint Inhibitors. Cancer Research 77, 312–319. doi: 10.1158/0008-5472.CAN-16-1873 [DOI] [PubMed] [Google Scholar]
- Chen Limo, Gibbons DL, Goswami S, Cortez MA, Ahn Y-H, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal J, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Peng D, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FX-F, 2014. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Comms 5, 5241. doi: 10.1038/ncomms6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Lin, Guo S, Ranzer MJ, DiPietro LA, 2013. Toll-like receptor 4 has an essential role in early skin wound healing. J. Invest. Dermatol. 133, 258–267. doi: 10.1038/jid.2012.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Qi, Sun L, Chen ZJ, 2016. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat Immunol 17, 1142–1149. doi: 10.1038/ni.3558 [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Sparrer KMJ, van Gent M, Lässig C, Huang T, Osterrieder N, Hopfner K-P, Gack MU, 2018. Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity. Nat Immunol 19, 53–62. doi: 10.1038/s41590-017-0005-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, Hein A, Rote NS, Cope LM, Snyder A, Makarov V, Buhu S, Slamon DJ, Wolchok JD, Pardoll DM, Beckmann MW, Zahnow CA, Mergoub T, Chan TA, Baylin SB, Strick R, 2015. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 162, 974–986. doi: 10.1016/j.cell.2015.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau C-S, Verstegen NJM, Ciampricotti M, Hawinkels LJAC, Jonkers J, de Visser KE, 2015. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348. doi: 10.1038/nature14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo S-R, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW Jr., Gajewski TF, 2015. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Reports 11, 1018–1030. doi: 10.1016/j.celrep.2015.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, Palucka AK, 2013. Neutralizing Tumor-Promoting Chronic Inflammation: A Magic Bullet? Science 339, 286–291. doi: 10.1126/science.1232227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse J, Kalinke U, Oxenius A, 2015. Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol 15, 231–242. doi: 10.1038/nri3806 [DOI] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, Ellenson LH, Caputo T, Lee A-H, Conejo-Garcia JR, Glimcher LH, 2015. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 161, 1527–1538. doi: 10.1016/j.cell.2015.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, Douglas M, Tibbitts T, Sharma S, Proctor J, Kosmider N, White K, Stern H, Soglia J, Adams J, Palombella VJ, McGovern K, Kutok JL, Wolchok JD, Merghoub T, 2016. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 539, 443–447. doi: 10.1038/nature20554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim Y-LE, Lee H-H, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM, 2010. T Cell/Transmembrane, Ig, and Mucin-3 Allelic Variants Differentially Recognize Phosphatidylserine and Mediate Phagocytosis of Apoptotic Cells. The Journal of Immunology 184, 1918–1930. doi: 10.4049/jimmunol.0903059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Golden EB, Formenti SC, 2015. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol 1, 1325–1332. doi: 10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC, 2005. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734. [PubMed] [Google Scholar]
- Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, Ivanova E, Paweletz CP, Bowden M, Zhou CW, Herter-Sprie GS, Sorrentino JA, Bisi JE, Lizotte PH, Merlino AA, Quinn MM, Bufe LE, Yang A, Zhang Y, Zhang H, Gao P, Chen T, Cavanaugh ME, Rode AJ, Haines E, Roberts PJ, Strum JC, Richards WG, Lorch JH, Parangi S, Gunda V, Boland GM, Bueno R, Palakurthi S, Freeman GJ, Ritz J, Haining WN, Sharpless NE, Arthanari H, Shapiro GI, Barbie DA, Gray NS, Wong K-K, 2017. CDK4/6 Inhibition Augments Anti-Tumor Immunity by Enhancing T Cell Activation. Cancer Discov CD–17–0915. doi: 10.1158/2159-8290.CD-17-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li X-D, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu Y-X, Weichselbaum RR, 2014. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 41, 843–852. doi: 10.1016/j.immuni.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarkar SC, Wang C, Miller MT, Ramanathan A, Jiang F, Khan AG, Patel SS, Marcotrigiano J, 2016. Structural basis for m7G recognition and 2’-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc Natl Acad Sci USA 113, 596–601. doi: 10.1073/pnas.1515152113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighe AS, Richards E, Old LJ, Schreiber RD, 1994. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFNγ receptors. Immunity 1, 447–456. doi: 10.1016/1074-7613(94)90087-6 [DOI] [PubMed] [Google Scholar]
- Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, Weinberg RA, 2017. Epithelial-to-Mesenchymal Transition Contributes to Immunosuppression in Breast Carcinomas. Cancer Research 77, 3982–3989. doi: 10.1158/0008-5472.CAN-16-3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, Simithy J, Lan Y, Lin Y, Zhou Z, Capell BC, Xu C, Xu M, Kieckhaefer JE, Jiang T, Shoshkes-Carmel M, Tanim KMAA, Barber GN, Seykora JT, Millar SE, Kaestner KH, Garcia BA, Adams PD, Berger SL, 2017. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550, 402–406. doi: 10.1038/nature24050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T, 2012. Expression of tumour-specific antigens underlies cancer immunoediting. Nature 482, 405–409. doi: 10.1038/nature10803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi B, Niethammer P, 2015. Mechanisms of epithelial wound detection. Trends in Cell Biology 25, 398–407. doi: 10.1016/j.tcb.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Rybakin V, Brzostek J, Paster W, Acuto O, Gascoigne NRJ, 2014. Fine-tuning T cell receptor signaling to control T cell development. Trends Immunol 35, 311–318. doi: 10.1016/j.it.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, Sivick KE, Zeng Q, Soares KC, Zheng L, Portnoy DA, Woodward JJ, Pardoll DM, Dubensky TW, Kim Y, 2015. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 7, 283ra52–283ra52. doi: 10.1126/scitranslmed.aaa4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G, 2016. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17, 97–111. doi: 10.1038/nri.2016.107 [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G, 2015. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 28, 690–714. doi: 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Gamrekelashvili J, Ormandy LA, Heimesaat MM, Kirschning CJ, Manns MP, Korangy F, Greten TF, 2014. Primary sterile necrotic cells fail to cross-prime CD8 +T cells. Oncoimmunology 1, 1017–1026. doi: 10.4161/onci.21098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen P-L, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P, 2016. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 1–18. doi: 10.1016/j.cell.2016.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AD, More S, Rufo N, Mece O, Sassano ML, Agostinis P, Zitvogel L, Kroemer G, Galluzzi L, 2017. Trial watch: Immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology 6, e1386829. doi: 10.1080/2162402X.2017.1386829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner MY, Heltemes-Harris LM, Fife BT, Mescher MF, 2013. Cutting edge: IL-12 and type I IFN differentially program CD8 T cells for programmed death 1 re-expression levels and tumor control. J. Immunol. 191, 1011–1015. doi: 10.4049/jimmunol.1300652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, Carter R, Awad W, Neale G, Thomas PG, Youngblood B, 2017. De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell 170, 142–157.e19. doi: 10.1016/j.cell.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, Brandes M, Eickhoff S, Das I, Shridhar N, Hinze D, Rogava M, van der Sluis TC, Ruotsalainen JJ, Gaffal E, Landsberg J, Ludwig KU, Wilhelm C, Riek-Burchardt M, Müller AJ, Gebhardt C, Scolyer RA, Long GV, Janzen V, Teng MWL, Kastenmüller W, Mazzone M, Smyth MJ, Tüting T, Hölzel M, 2017. Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy. Immunity 47, 789–802.e9. doi: 10.1016/j.immuni.2017.09.012 [DOI] [PubMed] [Google Scholar]
- Glück S, Guey B, Gulen MF, Wolter K, Kang T-W, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L, Ablasser A, 2017. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 19, 1061–1070. doi: 10.1038/ncb3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, Hoog J, Ellis MJ, Ma CX, Ramm S, Krop IE, Winer EP, Roberts TM, Kim H-J, McAllister SS, Zhao JJ, 2017. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475. doi: 10.1038/nature23465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Ferguson T, Zitvogel L, Kroemer G, 2009. Immunogenic and tolerogenic cell death. Nat Rev Immunol 9, 353–363. doi: 10.1038/nri2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K-I, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA, 2014. PD-1 identifies the patient-specific CD8⁺ tumor-reactive repertoire infiltrating human tumors. J Clin Invest 124, 2246–2259. doi: 10.1172/JCI73639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, Johnson SF, Carrasco RD, Lazo S, Bronson RT, Davis SP, Lobera M, Nolan MA, Letai A, 2017. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 543, 428–432. doi: 10.1038/nature21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DH, Huang B, Fernando RI, Tsang K-Y, Palena C, 2014. WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Research 74, 2510–2519. doi: 10.1158/0008-5472.CAN-13-1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA, 2017. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature Publishing Group 1, e00047. doi: 10.1038/nature23470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtlova A, Erttmann SF, Raffi FA, Schmalz AM, Resch U, Anugula S, Lienenklaus S, Nilsson LM, Kröger A, Nilsson JA, Ek T, Weiss S, Gekara NO, 2015. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity 42, 332–343. doi: 10.1016/j.immuni.2015.01.012 [DOI] [PubMed] [Google Scholar]
- He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, Xu L, Chen X, Hao Y, Wang P, Zhu C, Ou J, Liang H, Ni T, Zhang X, Zhou X, Deng K, Chen Y, Luo Y, Xu J, Qi H, Wu Y, Ye L, 2016. Follicular CXCR5-expressing CD8(+) T cells curtail chronic viral infection. Nature 537, 412–428. doi: 10.1038/nature19317 [DOI] [PubMed] [Google Scholar]
- Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HEK, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS, 2014. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M, 2000. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 19, 2548–2556. doi: 10.1038/sj.onc.1203551 [DOI] [PubMed] [Google Scholar]
- Hochheiser K, Klein M, Gottschalk C, Hoss F, Scheu S, Coch C, Hartmann G, Kurts C, 2016. Cutting Edge: The RIG-I Ligand 3pRNA Potently Improves CTL Cross-Priming and Facilitates Antiviral Vaccination. J. Immunol. 196, 2439–2443. doi: 10.4049/jimmunol.1501958 [DOI] [PubMed] [Google Scholar]
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG, 2013. Cancer drug resistance: an evolving paradigm. Nature Reviews Cancer 13, 714–726. doi: 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D’Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ, 2017. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65. doi: 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, Lo RS, 2016. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165, 35–44. doi: 10.1016/j.cell.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue H-H, Ahmed R, 2016. Defining CD8(+) T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421. doi: 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R, 2015. Control of adaptive immunity by the innate immune system. Nat Immunol 16, 343–353. doi: 10.1038/ni.3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Sánchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O, Konner J, Ricca J, Zamarin D, Walther T, Aghajanian C, Wolchok JD, Sala E, Merghoub T, Snyder A, Miller ML, 2017. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell 170, 927–938.e20. doi: 10.1016/j.cell.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens RA, Wrangle J, Vendetti FP, Murphy SC, Zhao M, Coleman B, Sebree R, Rodgers K, Hooker CM, Franco N, Lee B, Tsai S, Delgado IE, Rudek MA, Belinsky SA, Herman JG, Baylin SB, Brock MV, Rudin CM, 2011. Combination Epigenetic Therapy Has Efficacy in Patients with Refractory Advanced Non-Small Cell Lung Cancer. Cancer Discovery 1, 598–607. doi: 10.1158/2159-8290.CD-11-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, Behera M, Wu H, McCausland M, Chen Z, Zhang C, Khuri FR, Owonikoko TK, Ahmed R, Ramalingam SS, 2017a. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA 114, 4993–4998. doi: 10.1073/pnas.1705327114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, Sica GL, Sharpe AH, Freeman GJ, Blazar BR, Turka LA, Owonikoko TK, Pillai RN, Ramalingam SS, Araki K, Ahmed R, 2017b. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355, 1423–1427. doi: 10.1126/science.aaf0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, Schmid MC, Pink M, Winkler DG, Rausch M, Palombella VJ, Kutok J, McGovern K, Frazer KA, Wu X, Karin M, Sasik R, Cohen EEW, Varner JA, 2016. PI3Kγ is a molecular switch that controls immune suppression. Nature 539, 437–442. doi: 10.1038/nature19834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katlinski KV, Gui J, Katlinskaya YV, Ortiz A, Chakraborty R, Bhattacharya S, Carbone CJ, Beiting DP, Girondo MA, Peck AR, Puré E, Chatterji P, Rustgi AK, Diehl JA, Koumenis C, Rui H, Fuchs SY, 2017. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer Cell 31, 194–207. doi: 10.1016/j.ccell.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhapp FJ, Kaufman HL, 2016. Molecular Pathways: Mechanism of Action for Talimogene Laherparepvec, a New Oncolytic Virus Immunotherapy. Clin. Cancer Res. 22, 1048–1054. doi: 10.1158/1078-0432.CCR-15-2667 [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Sodir NM, Wilson CH, Burkhart DL, Pellegrinet L, Brown Swigart L, Littlewood TD, Evan GI, 2017. Myc Cooperates with Ras by Programming Inflammation and Immune Suppression. Cell 171, 1301–1315.e14. doi: 10.1016/j.cell.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Jänne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong K-K, Hammerman PS, 2016. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Comms 7, 10501. doi: 10.1038/ncomms10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, Vormehr M, Hüsemann Y, Selmi A, Kuhn AN, Buck J, Derhovanessian E, Rae R, Attig S, Diekmann J, Jabulowsky RA, Heesch S, Hassel J, Langguth P, Grabbe S, Huber C, Türeci O, Sahin U, 2016. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401. doi: 10.1038/nature18300 [DOI] [PubMed] [Google Scholar]
- Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JC-H, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H, Gandhi L, KEYNOTE-021 investigators, 2016. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. The Lancet Oncology 17, 1497–1508. doi: 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD, 2015. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 373, 23–34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Auh SL, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX, 2009. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595. doi: 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WA, June CH, 2017. The Principles of Engineering Immune Cells to Treat Cancer. Cell 168, 724–740. doi: 10.1016/j.cell.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Fang D, Fang J, Ren X, Yang X, Wen F, Su SB, 2011. Impaired wound healing with defective expression of chemokines and recruitment of myeloid cells in TLR3-deficient mice. J. Immunol. 186, 3710–3717. doi: 10.4049/jimmunol.1003007 [DOI] [PubMed] [Google Scholar]
- Lin Q, Wang L, Lin Y, Liu X, Ren X, Wen S, Du X, Lu T, Su SY, Yang X, Huang W, Zhou S, Wen F, Su SB, 2012. Toll-like receptor 3 ligand polyinosinic:polycytidylic acid promotes wound healing in human and murine skin. J. Invest. Dermatol. 132, 2085–2092. doi: 10.1038/jid.2012.120 [DOI] [PubMed] [Google Scholar]
- Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, Miller HE, Guleria I, Barth RJ, Huang YH, Wang L, 2015. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc. Natl. Acad. Sci. U.S.A. 112, 6682–6687. doi: 10.1073/pnas.1420370112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Diao L, Cuentas ERP, Denning WL, Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens C, Rodriguez JC, Hwu P, Wistuba II, Heymach JV, Gibbons DL, 2016. Epithelial-Mesenchymal Transition Is Associated with a Distinct Tumor Microenvironment Including Elevation of Inflammatory Signals and Multiple Immune Checkpoints in Lung Adenocarcinoma. Clin. Cancer Res. 22, 3630–3642. doi: 10.1158/1078-0432.CCR-15-1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie KJ, Carroll P, Martin C-A, Murina O, Fluteau A, Simpson DJ, Olova N, Sutcliffe H, Rainger JK, Leitch A, Osborn RT, Wheeler AP, Nowotny M, Gilbert N, Chandra T, Reijns MAM, Jackson AP, 2017. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465. doi: 10.1038/nature23449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak MP, Tong P, Diao L, Cardnell RJ, Gibbons DL, William WN, Skoulidis F, Parra ER, Rodriguez-Canales J, Wistuba II, Heymach JV, Weinstein JN, Coombes KR, Wang J, Byers LA, 2016. A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin. Cancer Res. 22, 609–620. doi: 10.1158/1078-0432.CCR-15-0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, Weiss SA, Lo J, Fisher DE, Miao D, Van Allen E, Root DE, Sharpe AH, Doench JG, Haining WN, 2017. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature Publishing Group 547, 1–17. doi: 10.1038/nature23270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci F, Stassi G, De Maria R, 2016. Epithelial–mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov 15, 311–325. doi: 10.1038/nrd.2015.13 [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel Iii EE, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T, 2018. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548. doi: 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen Y-S, Shea LK, Hundal J, Wendl MC, Demeter R, Wylie T, Allison JP, Smyth MJ, Old LJ, Mardis ER, Schreiber RD, 2012. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404. doi: 10.1038/nature10755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TBK, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C, 2016. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469. doi: 10.1126/science.aaf1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Wherry EJ, 2016. Combination Cancer Therapies with Immune Checkpoint Blockade: Convergence on Interferon Signaling. Cell 165, 272–275. doi: 10.1016/j.cell.2016.03.031 [DOI] [PubMed] [Google Scholar]
- Moroishi T, Hayashi T, Pan W-W, Fujita Y, Holt MV, Qin J, Carson DA, Guan K-L, 2016. The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 167, 1525–1539.e17. doi: 10.1016/j.cell.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro E, Furlan C, Avanzo M, Martorelli D, Comaro E, Rizzo A, Fae DA, Berretta M, Militello L, Del Conte A, Spazzapan S, Dolcetti R, Trovo M, 2017. Local High-Dose Radiotherapy Induces Systemic Immunomodulating Effects of Potential Therapeutic Relevance in Oligometastatic Breast Cancer. Front. Immunol. 8, 1476. doi: 10.3389/fimmu.2017.01476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, Benci JL, DeMichele AM, Tchou J, Marcotrigiano J, Minn AJ, 2017. Exosome RNA Unshielding Couples Stromal Activation to Pattern Recognition Receptor Signaling in Cancer. Cell 170, 352–366.e13. doi: 10.1016/j.cell.2017.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CT, Sullivan BM, Teijaro JR, Lee AM, Welch M, Rice S, Sheehan KCF, Schreiber RD, Oldstone MBA, 2015. Blockade of interferon Beta, but not interferon alpha, signaling controls persistent viral infection. Cell Host Microbe 17, 653–661. doi: 10.1016/j.chom.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, Liu Y, Tang J, Wang S, Fu Y-X, 2010. The Therapeutic Effect of Anti-HER2/neu Antibody Depends on Both Innate and Adaptive Immunity. Cancer Cell 18, 160–170. doi: 10.1016/j.ccr.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen D, Kurachi M, Barnitz RA, Bartman C, Bengsch B, Huang AC, Schenkel JM, Vahedi G, Haining WN, Berger SL, Wherry EJ, 2016. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. doi: 10.1126/science.aaf2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Wherry EJ, 2015. Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36, 265–276. doi: 10.1016/j.it.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, Kotarski J, Tarkowski R, Dou Y, Cho K, Hensley-Alford S, Munkarah A, Liu R, Zou W, 2015. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253. doi: 10.1038/nature15520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, Lin Y-J, Wojtkiewicz G, Iwamoto Y, Mino-Kenudson M, Huynh TG, Hynes RO, Freeman GJ, Kroemer G, Zitvogel L, Weissleder R, Pittet MJ, 2016. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 44, 343–354. doi: 10.1016/j.immuni.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, Hellmann MD, Wolchok JD, Leslie CS, Schietinger A, 2017. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456. doi: 10.1038/nature22367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, Kirschnek S, Gaffal E, Landsberg J, Hellmuth J, Schmidt A, Anz D, Bscheider M, Schwerd T, Berking C, Bourquin C, Kalinke U, Kremmer E, Kato H, Akira S, Meyers R, Häcker G, Neuenhahn M, Busch D, Ruland J, Rothenfusser S, Prinz M, Hornung V, Endres S, Tüting T, Hartmann G, 2008. 5′-triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat. Med. 14, 1256–1263. doi: 10.1038/nm.1887 [DOI] [PubMed] [Google Scholar]
- Portou MJ, Baker D, Abraham D, Tsui J, 2015. The innate immune system, toll-like receptors and dermal wound healing: A review. Vascular Pharmacology 71, 31–36. doi: 10.1016/j.vph.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman R-A, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD, 2012. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N Engl J Med 366, 925–931. doi: 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS, 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372, 2006–2017. doi: 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ, 2017. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Research 77, 6795–6811. doi: 10.1158/00085472.CAN-17-2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purbey PK, Scumpia PO, Kim PJ, Tong A-J, Iwamoto KS, McBride WH, Smale ST, 2017. Defined Sensing Mechanisms and Signaling Pathways Contribute to the Global Inflammatory Gene Expression Output Elicited by Ionizing Radiation. Immunity 47, 421–434.e3. doi: 10.1016/j.immuni.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranoa DRE, Parekh AD, Pitroda SP, Huang X, Darga T, Wong AC, Huang L, Andrade J, Staley JP, Satoh T, Akira S, Weichselbaum RR, Khodarev NN, 2016. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs. Oncotarget 7, 26496–26515. doi: 10.18632/oncotarget.8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman JM, Knutson KL, Radisky DC, 2010. Immune Promotion of Epithelial-mesenchymal Transition and Generation of Breast Cancer Stem Cells. Cancer Research 70, 3005–3008. doi: 10.1158/0008-5472.CAN-09-4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reits EA, 2006. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. Journal of Experimental Medicine 203, 1259–1271. doi: 10.1084/jem.20052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martín-Algarra S, Mandal R, Sharfman WH, Bhatia S, Hwu W-J, Gajewski TF, Slingluff CL, Chowell D, Kendall SM, Chang H, Shah R, Kuo F, Morris LGT, Sidhom J-W, Schneck JP, Horak CE, Weinhold N, Chan TA, 2017. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 171, 934–949.e15. doi: 10.1016/j.cell.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, Kirkwood JM, Gajewski TF, Chen L, Gorski KS, Anderson AA, Diede SJ, Lassman ME, Gansert J, Hodi FS, Long GV, 2017. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 170, 1109–1119.e10. doi: 10.1016/j.cell.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA, 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Jackson R, Harman CCD, Li T, West AP, de Zoete MR, Wu Y, Yordy B, Lakhani SA, Kuan C-Y, Taniguchi T, Shadel GS, Chen ZJ, Iwasaki A, Flavell RA, 2014. Apoptotic Caspases Prevent the Induction of Type I Interferons by Mitochondrial DNA. Cell 159, 1563–1577. doi: 10.1016/j.cell.2014.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotow J, Bivona TG, 2017. Understanding and targeting resistance mechanisms in NSCLC. Nature Reviews Cancer 17, 637–658. doi: 10.1038/nrc.2017.84 [DOI] [PubMed] [Google Scholar]
- Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, Han H, Liang G, Jones PA, Pugh TJ, O’Brien C, De Carvalho DD, 2015. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell 162, 961–973. doi: 10.1016/j.cell.2015.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CMT, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM, 2014. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26, 623–637. doi: 10.1016/j.ccell.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC, 2010. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194. doi: 10.1084/jem.20100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, Chakarov S, Rivera C, Hogstad B, Bosenberg M, Hashimoto D, Gnjatic S, Bhardwaj N, Palucka AK, Brown BD, Brody J, Ginhoux F, Merad M, 2016. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 44, 924–938. doi: 10.1016/j.immuni.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Bosinger SE, Estes JD, Zhu RTR, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, Hill BJ, Timmer JK, Reiss E, Yarden G, Darko S, Contijoch E, Todd JP, Silvestri G, Nason M, Norgren RB, Keele BF, Rao S, Langer JA, Lifson JD, Schreiber G, Douek DC, 2014. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511, 601–605. doi: 10.1038/nature13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmamed MF, Pastor F, Rodriguez A, Perez-Gracia JL, Rodriguez-Ruiz ME, Jure-Kunkel M, Melero I, 2015. Agonists of Co-stimulation in Cancer Immunotherapy Directed Against CD137, OX40, GITR, CD27, CD28, and ICOS. Seminars in Oncology 42, 640–655. doi: 10.1053/j.seminoncol.2015.05.014 [DOI] [PubMed] [Google Scholar]
- Sato T, Yamamoto M, Shimosato T, Klinman DM, 2010. Accelerated wound healing mediated by activation of Toll-like receptor 9. Wound Repair Regen 18, 586–593. doi: 10.1111/j.1524-475X.2010.00632.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, Lauer P, Brockstedt DG, Knoblaugh SE, Hämmerling GJ, Schell TD, Garbi N, Greenberg PD, 2016. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity 45, 389–401. doi: 10.1016/j.immuni.2016.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M, Hartmann G, 2016. Discriminating self from non-self in nucleic acid sensing. Nat Rev Immunol 16, 566–580. doi: 10.1038/nri.2016.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuberth-Wagner C, Ludwig J, Bruder AK, Herzner A-M, Zillinger T, Goldeck M, Schmidt T, Schmid-Burgk JL, Kerber R, Wolter S, Stümpel J-P, Roth A, Bartok E, Drosten C, Coch C, Hornung V, Barchet W, Kümmerer BM, Hartmann G, Schlee M, 2015. A Conserved Histidine in the RNA Sensor RIG-I Controls Immune Tolerance to N1–2’O-Methylated Self RNA. Immunity 43, 41–51. doi: 10.1016/j.immuni.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD, 2015. Neoantigens in cancer immunotherapy. Science 348, 69–74. doi: 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao H-W, Godec J, LaFleur MW, Brown FD, Tonnerre P, Chung RT, Tully DC, Allen TM, Frahm N, Lauer GM, Wherry EJ, Yosef N, Haining WN, 2016. The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169. doi: 10.1126/science.aae0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabason JE, Minn AJ, 2017. Radiation and Immune Checkpoint Blockade: From Bench to Clinic. Semin Radiat Oncol 27, 289–298. doi: 10.1016/j.semradonc.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Shekarian T, Valsesia-Wittmann S, Brody J, Michallet MC, Depil S, Caux C, Marabelle A, 2017. Pattern recognition receptors: immune targets to enhance cancer immunotherapy. Ann. Oncol. 28, 1756–1766. doi: 10.1093/annonc/mdx179 [DOI] [PubMed] [Google Scholar]
- Singer M, Wang C, Cong L, Marjanovic ND, Kowalczyk MS, Zhang H, Nyman J, Sakuishi K, Kurtulus S, Gennert D, Xia J, Kwon JYH, Nevin J, Herbst RH, Yanai I, Rozenblatt-Rosen O, Kuchroo VK, Regev A, Anderson AC, 2017. A Distinct Gene Module for Dysfunction Uncoupled from Activation in Tumor-Infiltrating T Cells. Cell 171, 1221–1223. doi: 10.1016/j.cell.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistigu A, Di Modugno F, Manic G, Nisticò P, 2017. Deciphering the loop of epithelial-mesenchymal transition, inflammatory cytokines and cancer immunoediting. Cytokine & Growth Factor Reviews 36, 67–77. doi: 10.1016/j.cytogfr.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Snell LM, Brooks DG, 2015. New insights into type I interferon and the immunopathogenesis of persistent viral infections. Current Opinion in Immunology 34, 91–98. doi: 10.1016/j.coi.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA, 2014. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371, 2189–2199. doi: 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, Gajewski TF, 2015. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235. doi: 10.1038/nature14404 [DOI] [PubMed] [Google Scholar]
- Spranger S, Dai D, Horton B, Gajewski TF, 2017. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 31, 711–723.e4. doi: 10.1016/j.ccell.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF, 2013. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci Transl Med 5, 200ra116–200ra116. doi: 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ, 2011. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl. Acad. Sci. U.S.A. 108, 7142–7147. doi: 10.1073/pnas.1016569108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelekati E, Shin H, Doering TA, Dolfi DV, Ziegler CG, Beiting DP, Dawson L, Liboon J, Wolski D, Ali M-AA, Katsikis PD, Shen H, Roos DS, Haining WN, Lauer GM, Wherry EJ, 2014. Bystander Chronic Infection Negatively Impacts Development of CD8+ T Cell Memory. Immunity 40, 801–813. doi: 10.1016/j.immuni.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Sugaya M, Fujita H, Asano Y, Tada Y, Kadono T, Sato S, 2014. TLR4, rather than TLR2, regulates wound healing through TGF-β and CCL5 expression. J. Dermatol. Sci. 73, 117–124. doi: 10.1016/j.jdermsci.2013.10.009 [DOI] [PubMed] [Google Scholar]
- T cell costimulatory receptor CD28 is a primary target for PD-1, 2017. T cell costimulatory receptor CD28 is a primary target for PD-1 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S, 2010. Pattern Recognition Receptors and Inflammation. Cell 140, 805–820. doi: 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L, 2012. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4, 127ra37–127ra37. doi: 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA, 2014. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin. Cancer Res. 20, 5064–5074. doi: 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS-O, Batlle E, 2018. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543. doi: 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KCF, Welch M, Schreiber RD, Carlos de la Torre J, Oldstone MBA, 2013. Persistent LCMV Infection Is Controlled by Blockade of Type I Interferon Signaling. Science 340, 207–211. doi: 10.1126/science.1235214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RYJ, Nieto MA, 2009. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 139, 871–890. doi: 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM, 2015. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 27, 1–12. doi: 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper MJ, Vaz M, Chiappinelli KB, DeStefano Shields CE, Niknafs N, Yen R-WC, Wenzel A, Hicks J, Ballew M, Stone M, Tran PT, Zahnow CA, Hellmann MD, Anagnostou V, Strissel PL, Strick R, Velculescu VE, Baylin SB, 2017. Epigenetic Therapy Ties MYC Depletion to Reversing Immune Evasion and Treating Lung Cancer. Cell 171, 1284–1300.e21. doi: 10.1016/j.cell.2017.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahtemberg U, Mevorach D, 2017. Apoptotic Cells Induced Signaling for Immune Homeostasis in Macrophages and Dendritic Cells. Front. Immunol. 8, 1356. doi: 10.3389/fimmu.2017.01356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A, 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ, 2015. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377. doi: 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban SL, Welsh RM, 2014. Out-of-sequence signal 3 as a mechanism for virus-induced immune suppression of CD8 T cell responses. PLoS Pathog. 10, e1004357. doi: 10.1371/journal.ppat.1004357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, Pradervand S, Thimme R, Zehn D, Held W, 2016. T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity 45, 415–427. doi: 10.1016/j.immuni.2016.07.021 [DOI] [PubMed] [Google Scholar]
- Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, Signore M, De Ninno A, Lucarini V, Peschiaroli F, Businaro L, Gerardino A, Manic G, Ulas T, Günther P, Schultze JL, Kepp O, Stoll G, Lefebvre C, Mulot C, Castoldi F, Rusakiewicz S, Ladoire S, Apetoh L, Bravo-San Pedro JM, Lucattelli M, Delarasse C, Boige V, Ducreux M, Delaloge S, Borg C, André F, Schiavoni G, Vitale I, Laurent-Puig P, Mattei F, Zitvogel L, Kroemer G, 2015. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 350, 972–978. doi: 10.1126/science.aad0779 [DOI] [PubMed] [Google Scholar]
- van den Broek MF, Müller U, Huang S, Aguet M, Zinkernagel RM, 1995a. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol 69, 4792–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek MF, Müller U, Huang S, Zinkernagel RM, Aguet M, 1995b. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 148, 5–18. [DOI] [PubMed] [Google Scholar]
- Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S, 2017. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Comms 8, 15618. doi: 10.1038/ncomms15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S, 2015. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Research 75, 2232–2242. doi: 10.1158/0008-5472.CAN-14-3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, Chen ZJ, 2017. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl. Acad. Sci. U.S.A. 114, 1637–1642. doi: 10.1073/pnas.1621363114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, Gondek D, Wang Y, Fava RA, Fiser A, Almo S, Noelle RJ, 2011. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. Journal of Experimental Medicine 208, 577–592. doi: 10.1084/jem.20100619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Swiecki M, Cella M, Alber G, Schreiber RD, Gilfillan S, Colonna M, 2012. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe 11, 631–642. doi: 10.1016/j.chom.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang N-AAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe’er D, Allison JP, 2017. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 170, 1120–1133.e17. doi: 10.1016/j.cell.2017.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, Huang DCS, Kile BT, 2014. Apoptotic Caspases Suppress mtDNA-Induced STING-Mediated Type I IFN Production. Cell 159, 1549–1562. doi: 10.1016/j.cell.2014.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG, 2013. Blockade of Chronic Type I Interferon Signaling to Control Persistent LCMV Infection. Science 340, 202–207. doi: 10.1126/science.1235208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-R, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MYK, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre M-L, Gajewski TF, 2014. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842. doi: 10.1016/j.immuni.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Konno H, Ahn J, Barber GN, 2016. Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates With Tumorigenesis. Cell Reports 14, 282–297. doi: 10.1016/j.celrep.2015.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TNM, Lo RS, Ribas A, 2016. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 375, 819–829. doi: 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, Tan Y, Ci Y, Wu F, Dai X, Guo J, Huang Y-H, Fan C, Ren S, Sun Y, Freeman GJ, Sicinski P, Wei W, 2018. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 553, 91–95. doi: 10.1038/nature25015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Yu H, Ni C, Zhang T, Liu L, Lv Q, Zhang Z, Wang Z, Wu D, Wu P, Chen G, Wang L, Wei Q, Huang J, Wang X, 2017. Hypofractionated stereotactic radiation therapy activates the peripheral immune response in operable stage I non-small-cell lung cancer. Scientific Reports 7, 65. doi: 10.1038/s41598-017-04978-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Powis de Tenbossche CG, Cané S, Colau D, van Baren N, Lurquin C, Schmitt-Verhulst A-M, Liljeström P, Uyttenhove C, Van den Eynde BJ, 2017. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat Comms 8, 1404. doi: 10.1038/s41467-017-00784-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, Denardo DG, 2014. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Research 74, 5057–5069. doi: 10.1158/0008-5472.CAN-13-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga EI, Harker JA, 2012. T-cell exhaustion due to persistent antigen: Quantity not quality? Eur. J. Immunol. 42, 2285–2289. doi: 10.1002/eji.201242852 [DOI] [PMC free article] [PubMed] [Google Scholar]