Abstract
Ketamine is a dissociative anesthetic first developed in the 1960s but is increasingly used at subanesthetic doses for both clinical and non-clinical purposes. There is evidence from human recreational users of compulsive use and addiction. Sensitization is an increase in an effect of a drug with repeated use that is thought to be important in the development of addiction. Research on psychomotor stimulants has shown the development of sensitization in laboratory animals to be modified by factors that influence addiction. In the current paper we describe four experiments on the development of sensitization in laboratory rats aimed at determining if ketamine sensitization is also influenced by factors thought to be important in addiction. Adult, male Sprague-Dawley rats received ketamine (5, 10, 20 or 50 mg/kg i.p.) for five or more days and the development of locomotor sensitization was followed. Experiment 1 examined the ability of low doses of ketamine to produce sensitization and found sensitization at 5, 10 and 20 mg/kg. Experiment 2 examined the influence of environmental context and found that ketamine sensitization (20 mg/kg) was greater when administration occurred in a novel environment (the experimental apparatus) than in home cages. Experiment 3 found that ketamine sensitization (20 mg/kg) did not occur when animals were housed in social isolation but occurred readily in pair-housed animals. Finally, Experiment 4 found that ketamine sensitization (20 or 50 mg/kg) was similar whether drug was administered daily or at 3-day intervals. Together, the results demonstrate that ketamine sensitization is robust and reliable, occurring under a variety of circumstances. Moreover, ketamine sensitization is influenced by factors that influence the development of addiction in humans. The current results may lead to a better understanding of ketamine abuse and addiction and may help inform clinical use of the drug.
Introduction
Ketamine was first developed as an anesthetic in the 1960s, however interest in the use of this drug at subanesthetic doses has grown in recent years and continues to expand [1–5]. Among the therapeutic indications of subanesthetic ketamine are pathological pain (alone and in combination with opioids) [6–11] and major depression [12–16]. Most notably, ketamine as a treatment for depression has generated considerable excitement in recent years. Based on early successful studies, ketamine has been used off-label for treating individuals with depression for several years, and in 2019 esketamine (the S(+) isomer of ketamine), was approved by the Einited States Food and Drug Administration for the treatment of depression [17–19]. Importantly, ketamine has a different mechanism of action than other classes of antidepressants. Whereas most other antidepressants act via increasing monoaminergic neurotransmission, ketamine is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist. Moreover, ketamine’s antidepressant effects can be seen in minutes to hours (as opposed to traditional antidepressants, which require 3-6 weeks to act) and it has the ability to treat severe depression in individuals who are refractory to traditional antidepressants. The impact of this development is difficult to overstate, as ketamine represents the first new neurochemical class of antidepressants identified in many years [18].
Early after the approval of ketamine as an anesthetic it was “discovered” by recreational users who enjoy its rewarding and stimulating effects and its ability to evoke a unique subjective state at subanesthetic doses [2, 20–23]. Over the years, ketamine recreational use has increased, early-on through diversion of the drug from veterinary sources and more recently from clandestine laboratories [5, 20–23]. It has often been included in a class of drugs known as “club drugs” because of use at dance clubs and party settings [23–29]. But it is also used by spiritual seekers or “psychonauts” who enjoy the vivid visual imagery/hallucinations evoked by the drug, as well as out-of-body and transcendental experiences [28, 30–34]. Although ketamine is not used as widely as many other drugs of abuse, there is significant evidence that it can be addictive. Case reports and other studies have documented compulsive use of the drug, craving among users, and extreme difficulty in quitting after long-term use [5, 29, 31, 32, 34–37]. Preclinical studies utilizing self-administration and conditioned place preference approaches have demonstrated ketamine’s reinforcing effects in animal models [38–53].
Among the factors that are thought to contribute to addiction is a form of neurobehavioral plasticity known as behavioral sensitization. Sensitization is defined as an increase in an effect of drug with repeated use [54–57]. According to the “Incentive Sensitization Theory of Addiction” sensitization is responsible for an amplified desire (or wanting) for a drug that is ultimately expressed as craving in addicted individuals [55, 58–60]. In animal models sensitization is most often studied as an increase in drug-induced locomotor activity following repeated administration. Given the overlap between the circuits that mediate locomotor activity and incentive motivation, locomotor sensitization in laboratory animals can be evidence of increases in motivation for drug (for review see [58]). Sensitization has been most commonly investigated in response to psychomotor stimulants, such as amphetamine and cocaine, but is widely seen with known addictive drugs. Our laboratory and others have reported that repeated use of ketamine leads to progressive increases in locomotor stimulant effects of the drug, reflective of behavioral sensitization [61–69]. To the extent that ketamine sensitization is related to its addictive properties, a better understanding of this phenomenon may help in the prevention and treatment of ketamine addiction.
Extensive research with psychomotor stimulants has revealed that sensitization is modified by circumstances surrounding administration of the drug, factors that are also important to addiction in humans. Among the factors that have been found to modify psychostimulant sensitization include the physical environment in which drug is administered, the social environment surrounding administration, and the interval at which drug is administered [54, 70–76]. To date, very few published studies have studied these factors in ketamine sensitization. In the current experiments, we examined the development of sensitization at very low subanesthetic doses of ketamine to determine the threshold for the development of sensitization; as well as the influence of environment, social isolation and treatment interval.
Methods
Animals
Eighty-seven adult male Sprague Dawley rats (Harlan, now Envigo; Livermore, CA), approximately 300 grams at beginning of experiment, were used in four experiments. Animals were habituated to the vivarium for at least one week prior to the beginning of each study. The rats were provided water and food ad libitum and (with the exception of Experiment 4; see below) were housed in groups of 2-3 in clear plastic cages (10.5” x 19” x 8”). The vivarium was kept on a 12-hour light/dark cycle and all experiments were performed during the light phase. Prior to each experiment rats were handled and their weights were recorded for three consecutive days to familiarize them with the experimenter. All studies were reviewed and approved by the Institutional Animal Care and Use Committee at California State University San Marcos and are in compliance with the Guide for Care and Use of Laboratory Animals (National Academies Press).
Drugs
Ketamine hydrochloride (Sigma-Aldrich) was dissolved in 0.9% saline and injected intraperitoneally (i.p.) at a volume of 1 ml/kg of body weight. Saline was used as the vehicle control.
Apparatus
A Kinder Scientific Open Field Motor Monitor (16” x 16” x 15”) was used to assess locomotor activity (Kinder Scientific, Poway, CA). The system consists of eight clear Plexiglas chambers, each surrounded by two arrays of photocells (16x16) and interfaced with a personal computer. One array of photocells (5 cm above the floor) measures horizontal activity and the other (12.5 cm above the floor) measures vertical activity (rearing). Kinder Scientific software can provide multiple measures of activity, including ambulations (interruptions of successive photocell beams typically representing forward locomotion), fine movements (interruptions of a single photocell beam typically representing stereotyped behavior), time active, and rearing. In the experimental room sound was provided by a white noise generator (Lafayette Instruments, Lafayette, IN) and an incandescent light was set at low levels.
Procedures
Four experiments were performed, each examining a factor that might affect ketamine sensitization, including dose, treatment interval, environmental context and social environment. On each test day, animals were taken to the experimental room and habituated to the room for 30 minutes. They were then placed individually into the locomotor apparatus for an additional 30 minutes of habituation prior to injection and testing. At the end of habituation, animals received the assigned treatment and locomotor activity was assessed for 90 minutes.
Experiment 1: Ketamine Sensitization at Low Doses
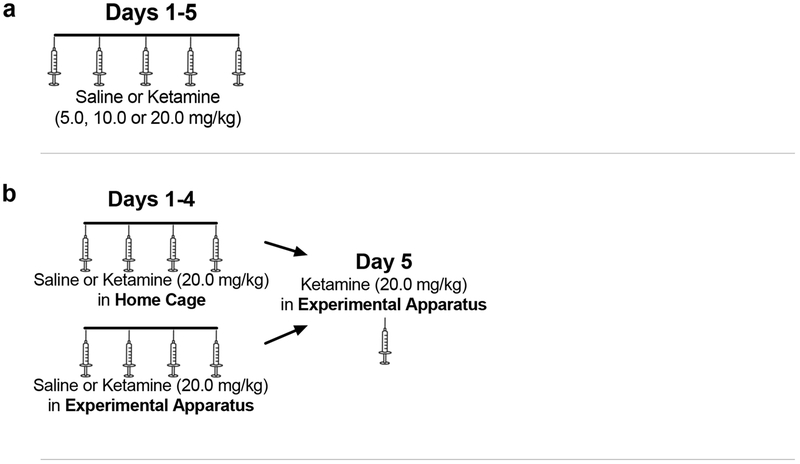
The purpose of this study was to examine the development of sensitization to very low doses of ketamine. Animals were assigned, in a counterbalanced manner, to receive saline or ketamine (5.0, 10.0, or 20.0 mg/kg i.p.) once daily for 5 days (n=7 in each of the three ketamine groups and n=3 in the saline reference group). Animals were tested as described above under Procedures. See Figure 1a.
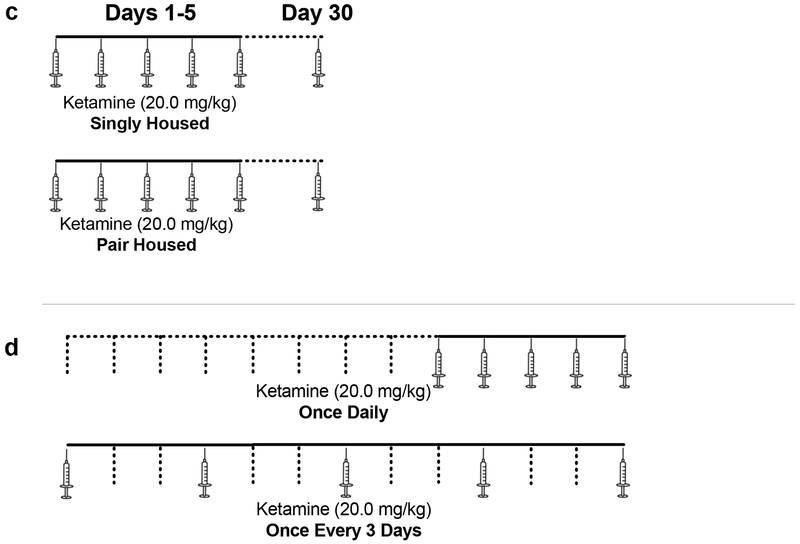
Figure 1.
Treatment and testing protocols for (a) Experiment 1: Ketamine Sensitization at Low Doses; (b) Experiment 2: Effect of Environmental Context on Ketamine Sensitization; (c) Experiment 3: Effect of Social Isolation on Ketamine Sensitization; and (d) Experiment 4: Effect of Treatment Interval on Ketamine Sensitization. Syringes show days with injections; dotted lines show days without injections.
Experiment 2: Effect of Environmental Context on Ketamine Sensitization
The aim of this study was to determine if the environment in which the animals received ketamine affected the expression of sensitization. Animals (n=6 per group) received saline or ketamine (20 mg/kg) once daily for four days in either the experimental testing apparatus or in the home cage. Thus, there were four groups: saline home cage (Sal Home), saline experimental apparatus (Sal Exp), ketamine home cage (Ket Home), ketamine experimental apparatus (Ket Exp). On each day, Exp animals were tested as described above under Procedures, however Home animals were injected and returned to their cages. On day 5 all animals were tested for the response to ketamine in the experimental apparatus (i.e., all four groups received ketamine) and were tested in the manner described under Procedures. Animals in the Home Cage groups had no exposure to the experimental apparatus prior to testing on day 5; the testing apparatus was therefore a novel environment for these animals. See Figure 1b.
Experiment 3: Effect of Social Isolation on Ketamine Sensitization
This study examined the influence of social isolation on the development of ketamine sensitization. Upon arrival from the vendor, animals were housed individually (n=7) or in pairs (n=8) for two weeks prior to the beginning of the experiment and remained housed in this manner for the entire duration of the experiment. Animals received ketamine (20 mg/kg) once daily for 5 days and then once again 25 days later (30 days from the first day of the experiment); animals remained unhandled and untreated during the 25 days between test days. This was done to explore the persistence of sensitization. On each test day animals were tested as described above under Procedures, except in this experiment activity was assessed for 60 minutes. See Figure 1c.
Experiment 4: Effect of Treatment Interval on Ketamine Sensitization
The purpose of this study was to determine if treatment interval affected the development of ketamine sensitization. Animals (n=6 per group) received ketamine (20 mg/kg or 50 mg/kg) either once daily or once every three days for five total injections. Test days were aligned so that the final testing day was the same for those treated daily and those treated at 3-day intervals. On each test day animals were tested as described above under Procedures. See Figure 1d.
Statistical Analyses
We utilized two measures used to assess activity: ambulations (reflecting horizontal locomotion) and fine movements (reflecting stereotypy). Horizontal activity and stereotypy are two prominent features of ketamine-induced stimulation of behavior [77–83]. For individual sessions, to illustrate the timecourse of drug effects and to compare groups we focused on ambulations. On these days, activity counts (ambulations) were analyzed by two-way repeated measures ANOVA (group x minutes post-injection) with minutes post-injection as the repeated measure. Post hoc analysis was performed using Bonferroni’s correction. To assess the development of sensitization across days ambulations or fine movements on each day was totaled and analyzed by two-way repeated measures ANOVA (group x test day), with test day as the repeated measure. Dunnett’s multiple comparisons test was used to determine changes from Day 1 of testing for each group. Due to differences in the duration of effect, at doses of 20 mg/kg or lower, 30 minutes was the timeframe totaled for analysis; at 50 mg/kg, 60 minutes was the timeframe totaled for analysis.
Results
Experiment 1: Ketamine Sensitization at Low Doses
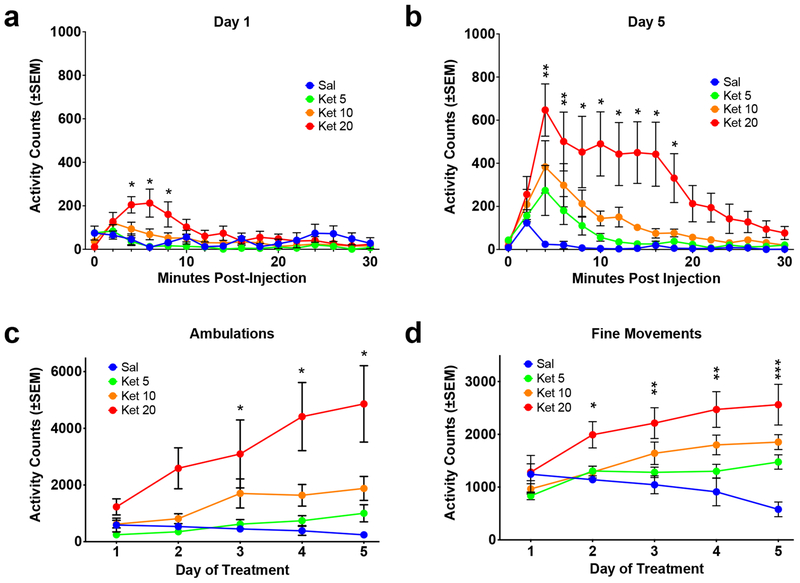
On day 1 of treatment, ketamine produced a very modest increase in activity (as assessed by ambulations) that was only evident at the highest dose, 20 mg/kg (Figure 2a). Two-way repeated measures ANOVA revealed a significant effect of treatment F(3, 20)=2.012, p=.0094; a significant effect of time F(14,280)=6.525, p<0.0001; and a significant interaction F(42,280)=2.554, p<0.0001. Post hoc analysis demonstrated that the 20 mg/kg dose had a significant increase relative to the saline group at 3 data points, but no other dose showed a significant difference from saline. By day 5 of treatment there were apparent increases in response relative to day 1 (compare Figures 2a, 2b). On day 5 (Figure 2b) there was a significant effect of treatment F(3, 20)=5.434, p=.0067; a significant effect of time F(14,280)=9.728, p<0.0001; and a significant interaction F(42,280)=2.263, p<0.0001. Post hoc analysis demonstrated that both the 10 mg/kg dose and the 20 mg/kg dose had significant increases relative to the saline group at multiple data points.
Figure 2. Experiment 1: Ketamine Sensitization at Low Doses.
(a) Timecourse of response to ketamine (5.0, 10.0 and 20.0 mg/kg) on Day 1 of treatment (ambulations). *=20 mg/kg different from saline. (b) Timecourse of response to ketamine on Day 5 of treatment (ambulations). Note the increases in response compared to Day 1. *=20 mg/kg different from saline. **=10 and 20 mg/kg different from saline. (c) Total activity across days of treatment (ambulations). *=20 mg/kg different from response on day 1. (d) Total activity across days of treatment (fine movements). *=20 mg/kg different from response on day 1. **=10 and 20 mg/kg different from response on day 1. ***=5, 10 and 20 mg/kg different from response on day 1. Although 20 mg/kg was the only dose that showed a significant increase in locomotion on Day 1, there was evidence of sensitization at all three doses.
To assess changes across days, the activity for the first 30 minutes post-injection was totaled for each day of treatment (this timeframe was used since ketamine’s effects were absent after 30 minutes). There was a clear escalation of activity across the 5 days of treatment, reflected in ambulations (horizontal locomotion), especially at the highest dose (Figure 2c). Two-way repeated measures ANOVA revealed a significant effect of treatment F(3, 20)=9.578, p=.0004; a significant effect of day F(4,80)=3.215, p=0.0168; but no interaction F(12,80)=1.370, p=0.1977. Post hoc analysis demonstrated significant increases at the 20 mg/kg dose on days 3, 4 and 5, relative to day 1. Changes in fine-movements were also examined across days, and showed a similar pattern to ambulations (Figure 2d). There was a significant effect of treatment F(3, 20)=7.439, p=.0016; a significant effect of day F(4,80)=8.150, p<0.0001; and a significant interaction F(12,80)=3.392, p<0.0005. Post hoc analysis demonstrated significant increases relative to day 1 for all three groups, which depended on the day of treatment (Figure 2c).
The results reveal that 20 mg/kg is near the threshold for a significant locomotor response to ketamine and ketamine sensitization; therefore, subsequent studies focused on this dose or higher.
Experiment 2: Effect of Environmental Context on Ketamine Sensitization
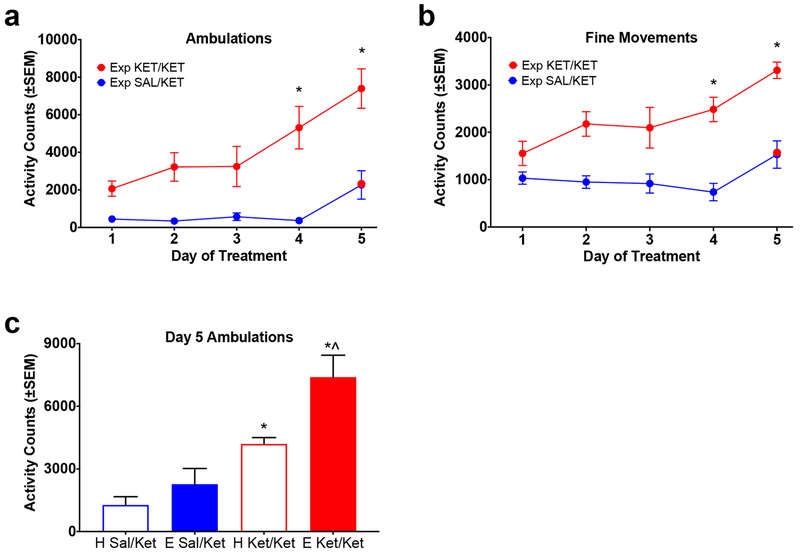
For this experiment, increases in ketamine response were evident across days in the Experimental Apparatus group for both ambulations and fine movements (Figure 3a, 3b). For ambulations (Figure 3a), two-way repeated measures ANOVA revealed a significant effect of treatment F(1,10)=38.0, p<0.0001; a significant effect of day F(4,40)=9.5, p<0.0001; and a significant interaction F(4,40)=2.8, p=0.0386. Post hoc analysis demonstrated significant increases in ketamine response on days 4 and 5 of treatment relative to day 1. For fine movements (Figure 3b) there was a significant effect of treatment F(1,10)=36.34, p=0.0001; a significant effect of day F(4,40)=7.890, p<0.0001; and a significant interaction F(4,40)=2.786, p=0.0393. As with ambulations, post hoc analysis revealed significant increases in ketamine response on days 4 and 5 of treatment relative to day 1. Repeated treatment with saline produced no significant changes in behavior across days.
Figure 3. Experiment 2: Effect of Environmental Context on Ketamine Sensitization.
SAL/KET = group received saline on days 1-4 and ketamine on day 5. KET/KET=group received ketamine all 5 days. (a) Total activity across days of treatment (ambulations). *=Significantly different from response on day 1. Red colored data point in SAL/KET group=ketamine treatment on day 5. (b) Total activity across days of treatment (fine movements). *=Significantly different from response on day 1. Red colored data point in SAL/KET group=ketamine treatment on day 5. (c) Total activity on Day 5 of treatment (ambulations). *=Significantly different from respective saline control group. ^=Significantly different from Home Cage KET/KET group. E=Treatment in Experimental Apparatus all 5 days; H=Treatment in Home Cage on days 1-4 and Experimental Apparatus on day 5. Although sensitization was evident in both the Home Cage and Experimental Apparatus groups, it was significantly greater in the Experimental Cage group.
The impact of environment on the development of sensitization was revealed on day 5 of treatment by comparing the response in animals that had received treatment repeatedly in the Home Cage and animals that had received treatment repeatedly in the Experimental Apparatus (Figure 3c). Animals that had received ketamine repeatedly in the Experimental Apparatus showed the greatest response to ketamine, while those that received ketamine in the Home Cage showed a smaller response. Two-way ANOVA revealed a significant effect of environment F(1,20)=15.43, p=0.0008; a significant effect of treatment F(1,20)=30.20; but no significant interaction F(1,20)=20.15, p=0.1712. Post hoc analysis revealed a significant difference between each group that received repeated administration of ketamine (Ket/Ket) and its respective saline control (Sal/Ket), and importantly, between the Experimental Apparatus Ket/Ket group and the Home Cage Ket/Ket group. There was no significant difference between Experimental Apparatus and Home Cage Sal/Ket groups.
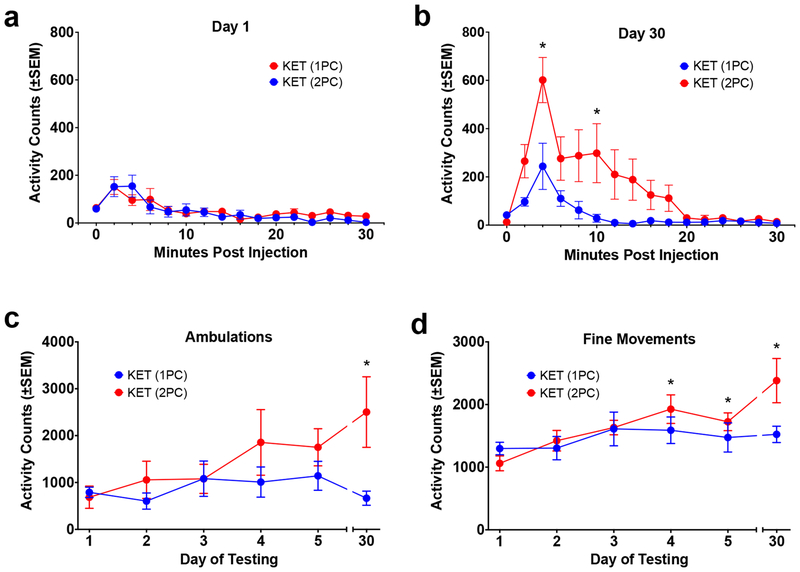
Experiment 3: Effect of Social Isolation on Ketamine Sensitization
In this experiment, animals were housed 1 per cage or 2 per cage and the development of ketamine sensitization was determined. There was no apparent difference between the two groups in the response to ketamine on day 1 of treatment (Figure 4a). Two-way repeated measures ANOVA of ambulations on day 1 revealed no significant effect of housing F(1,13)=0.1435, p=0.7109; a significant effect of time F(14,182)=11.86, p<0.0001; and no interaction F(14,182)=0.97, p=0.4849. However, on the last day of treatment and testing (day 30), there were differences between the groups (Figure 4b). Two-way repeated measures ANOVA revealed a significant effect of housing F(1,13)=4.999, p=0.0435; a significant effect of time F(14,182)=13.51, p<0.0001; and a significant interaction F(14,182)=3.503, p= p<0.0001. Post hoc analysis showed that the response to ketamine in the 2 per cage group was significantly greater than the 1 per cage group for a portion of the timecourse.
Figure 4. Experiment 3: Effect of Social Isolation on Ketamine Sensitization.
(a) Timecourse of response to ketamine (20.0 mg/kg) on Day 1 of treatment (ambulations). There were no differences between animals housed one per cage (1PC) and those housed two per cage (2PC). (b) Timecourse of response to ketamine on Day 30 (ambulations). *=2 per cage group significantly greater than 1 per cage group. (c) Total activity across days of treatment (ambulations). *=2 per cage group significantly different from response on day 1 (the 1 per cage group showed no significant increases). (d) Total activity across days of treatment (fine movements). *=2 per cage group significantly different from response on day 1 (the 1 per cage group showed no significant increases). There was evidence of sensitization in animals housed 2 per cage, but not in animals housed 1 per cage.
Considering the development of sensitization across days of treatment, the animals housed 2 per cage showed an apparent escalation in response across days, while the animals housed 1 per cage did not. For ambulations (Figure 4c), two-way ANOVA revealed no significant effect of housing F(1,13)=2.972, p=0.1084; no significant effect of day F(5,65)=1.827, p=0.1198; and no significant interaction F(5,65)=1.806, p=0.1240. Post hoc analysis revealed a significant increase for the 2 per cage group on day 30 relative to day 1, but no significant increases for the 1 per cage group. For fine movements (Figure 4d), two-way ANOVA revealed no significant effect of housing F(1,13)=1.816, p=0.2008; a significant effect of day F(5,65)=4.665, p=0.0011; and an interaction that approached significance F(5,65)=2.083, p=0.0787. Post hoc analysis revealed a significant increase for the 2 per cage group on days 4, 5 and 30 relative to day 1, but no significant increases for the 1 per cage group.
Experiment 4: Effect of Treatment Interval on Ketamine Sensitization
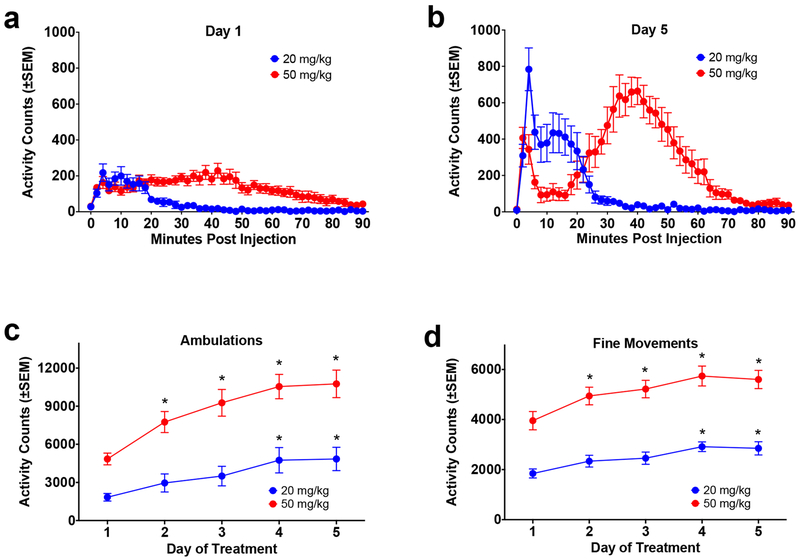
For this experiment, animals received ketamine treatment either daily or once every three days. Two doses of ketamine were examined, and because the response was so different, the results of each dose were initially analyzed separately. On day 1 at 20 mg/kg there was a modest short-lived increase in activity in response to ketamine (Figure 5a). Two-way repeated measures ANOVA of ambulations revealed no significant effect of treatment interval F(1,10)=0.0052, p=0.9439; a significant effect of time F(14,140)=5.596, p<0.0001, and no significant interaction F(14,140)=0.4413, p=0.9583 (data not shown). Results on day 5 of treatment revealed similar effects (albeit greater in magnitude; compare Figures 5a and 5b). There was no significant effect of treatment interval F(1,10)=0.0173, p=0.8979; a significant effect of time F(14,140)=8.904, p<0.0001, and no significant interaction F(14,140)=0.5052, p=0.9271 (data not shown).
Figure 5. Experiment 4: Effect of Treatment Interval on Ketamine Sensitization (20 mg/kg).
Because there were no differences between groups treated at 1-day and 3-day intervals, the data from these groups were collapsed and the development of sensitization at the two doses was assessed (see text for details). (a) Timecourse of response to ketamine (20.0 mg/kg and 50 mg/kg) on Day 1 of treatment (ambulations). (b) Timecourse of response to ketamine (20.0 mg/kg and 50 mg/kg) on Day 5 of treatment (ambulations). (c) Total activity across days of treatment (ambulations). *=significantly different from response on Day 1. (d) Total activity across days of treatment (fine movements). *=significantly different from response on Day 1.
At 50 mg/kg, on day 1 there was an increase in activity in response to ketamine that lasted approximately 80-90 minutes (Figure 5a). Two-way repeated measures ANOVA of ambulations revealed no significant effect of treatment interval F(1,10)=0.0130, p=0.9114; a significant effect of time F(44,440)=4.897, p<0.0001, and no significant interaction F(44,440)=1.138, p=0.2589 (data not shown). On day 5 the data from one animal was lost due to a technical problem. The response to ketamine on day 5 was strongly increased from day 1 (compare Figures 5a and 5b). Two-way repeated measures ANOVA of ambulations on day 5 revealed no significant effect of treatment interval F(1,9)=1.039, p=0.3348; a significant effect of time F(44,396)=10.03, p<0.0001, and no significant interaction F(44,396)=0.4397, p=0.9994 (data not shown).
Because there were no significant differences between the groups treated daily and those treated at 3-day intervals, the groups were combined and the results were analyzed for sensitization across days. Both doses showed an escalation in response across days (Figure 5). For ambulations (Figure 5c) two-way repeated measures ANOVA revealed a significant effect dose F(1,22)=35.51, p<0.0001; a significant effect of day of treatment F(4,87)=15.29, p<0.0001; but no significant interaction F(4,87)=1.683, p=0.1611. Post hoc analysis showed a significant increase in response relative to day 1 on days 4 and 5 at the 20 mg/kg dose; for the 50 mg/kg dose, there was a significant increase relative to day 1 on days 2, 3, 4 and 5 (Figure 5c). For fine movements (Figure 5d) two-way repeated measures ANOVA revealed a significant effect of dose F(1,22)=103.8, p<0.0001; a significant effect of day of treatment F(4,87)=8.641, p<0.0001; but no significant interaction F(4,87)=0.5619, p=0.6909. As with ambulations, post hoc analysis showed a significant increase in response relative to day 1 for the 20 mg/kg dose on days 4 and 5. For the 50 mg/kg dose, there was a significant increase relative to day 1 on days 2, 3, 4 and 5 (Figure 5d).
Discussion
The major conclusion from these studies is that ketamine sensitization is a robust phenomenon that occurs with repeated administration under a variety of circumstances and that sensitization can be influenced by different conditions, including dose, environmental context and social isolation, but apparently not treatment interval. As described in more detail below, this research complements and extends previous research demonstrating ketamine sensitization in laboratory animals [61–69]. In addition, the results show that ketamine sensitization is modified by factors that affect the development of sensitization to other drugs and that influence the development of addiction. Although the number of injections needed to induce sensitization varied across the current experiments, significant increases in activity were evident by the second injection in some cases and by the fourth injection in all experiments. Thus, sensitization to ketamine has the ability to develop rapidly in laboratory rats. To the extent that locomotor sensitization is a relevant animal model of addiction, the results are consistent with the idea that ketamine is an addictive drug.
In Experiment 1 we found that the threshold dose for a locomotor stimulant response to ketamine on the first day of treatment was 20 mg/kg i.p. This is a conservative estimate, given the low number of animals in the saline reference group; it is possible that significant stimulation would’ve been evident at lower doses with a larger number of animals. Nonetheless, this dose range is consistent with unpublished work from our laboratory and with the findings of others on locomotor stimulant effects of ketamine in laboratory rats (e.g., [84–86], but see also [63]). Importantly, however, increases in response following repeated administration, reflective of ketamine sensitization, occurred not only at 20 mg/kg, but also at 5 and 10 mg/kg, despite the lack of a significant stimulant response at the lower doses on day 1. Therefore, significant neurobehavioral plasticity is evident at doses that are near or below the threshold for locomotor stimulation. This is consistent with previous research demonstrating ketamine sensitization at very low doses [63]. Although sensitization occurred at all three doses, the onset of sensitization was dose dependent, appearing by day 2 at the highest dose, day 3 at the middle dose and day 5 at the lowest dose. Considering the human relevance of the doses used in this experiment, it’s difficult to make a direct translation; however, there is good reason to believe that these doses are in the range that parallel human antidepressant effects and drug abuse. The dose most often cited for antidepressant effects in the forced swim test in laboratory rats is 10 mg/kg (for review see [87, 88]), which is the middle dose used in this study; this is also the threshold dose for producing reward as assessed by conditioned place preference in laboratory rats [49, 50, 69]. Sensitization therefore occurs at doses that are analogous to clinical and recreational doses in standard animal models.
In Experiment 2, the environment in which the animals received treatment had a potent effect on sensitization. Animals that had received treatment in the experimental apparatus showed sensitization that was much stronger than animals that had received treatment in their home cages. These results are similar to findings with psychostimulants, which show greater sensitization in animals treated in the experimental apparatus than those that received treatment in home cages [for review see 54, 70–72]. Moreover, these findings replicate and extend our earlier findings demonstrating an influence of the environment on ketamine sensitization when the drug was administered at weekly intervals [64]; to our knowledge, no other published research has examined the impact of environment on ketamine sensitization. The traditional interpretation of these differences, based largely on studies with cocaine and amphetamine, is that environmental cues and conditioned drug-environment interactions are important to the development of sensitization. In fact, sensitization has been found in some cases to be “context-dependent,” requiring environmental cues to develop [for review see 54, 70, 71, 89, 90]. However, an alternative explanation is that ketamine sensitization developed in the home cage group, but the expression of sensitization was suppressed in the novel environment of the experimental cage [70]. Since we did not follow the development of sensitization across days in the home cage group, it is possible that sensitization developed, but that the expression of sensitization was suppressed in the experimental cage environment. According to this explanation, mild stress induced by the novel environment may have suppressed locomotor behavior, accounting for the difference between the groups. It should be noted, however, that evidence from the respective control groups suggests that this may not have been the case, as ketamine-induced locomotor behavior was not significantly different in the home cage group that received ketamine for the first time on day 5 (the Home Sal-Ket group) and the experimental apparatus group that received ketamine for the first time on day 5 (the Exp Sal-Ket group). One additional point is that ketamine sensitization did indeed develop in the home cage group in the current experiment, but it was reduced in magnitude when compared to the experimental cage group. Thus, ketamine sensitization was lower in the home cage group, but it was not entirely context-dependent. It is noteworthy that ketamine self-administration, in both animal models and human users is dependent on the environment in which the drug is administered [34, 39], supporting an important role of context in ketamine abuse and addiction. Further research is necessary to clarify the role of environmental context in the development and the expression of ketamine sensitization.
Experiment 3 examined the influence of social isolation on sensitization to ketamine. In this experiment, animals that were housed 2 per cage developed sensitization, while those that were housed singly did not. The effect was evident by the fourth day of treatment but was most pronounced when animals were retested for sensitization 25 days following the five days of treatment. We are unaware of any previous research that has examined the impact of social isolation on ketamine sensitization. There is considerable interest in the social influence on response to drugs, since social factors are considered to be important in the development of addiction, in relapse, and in addiction treatment [73–75, 91]. One important theme of this work is that social isolation increases the likelihood of addiction and decreases the effectiveness of treatment. The current results are in contrast to this idea, in that socially isolated animals did not develop sensitization, but socially housed animals did. The stressful effects of isolation in rats have been well-documented and may have contributed to the lack of sensitization seen in the singly housed animals [for review see 92]. Research on sensitization to psychomotor stimulants has been inconclusive regarding the influence of social factors. In some studies, sensitization was increased by isolation, in other cases it was decreased, while in others no effect was seen [for review see 75]. The differences in findings are likely due to differences in experimental approaches, including age of isolation, duration of isolation, number of conspecifics in the social housed comparison group, specific drug and drug doses examined, and others. The current study utilized adult animals, pair-housed or singly-housed for 14 days prior to the beginning of and throughout the study. The results demonstrate that isolation housing interferes with the development of ketamine sensitization.
Experiment 4 examined the effect of treatment interval on the development of ketamine sensitization at two doses: 20 mg/kg and 50 mg/kg. In this study, sensitization developed similarly in animals treated once daily and those treated at 3-day intervals regardless of dose. There appeared to be a trend toward more rapid sensitization in the daily treatment group but this did not achieve statistical significance. Since there was no difference in response at the two treatment intervals we combined the groups and assessed the development of sensitization at the two doses. Sensitization developed by the day 2 at 50 mg/kg and by day 4 at 20 mg/kg. This reinforces the finding in Experiment 1 that sensitization develops more quickly at higher subanesthetic doses relative to lower doses. Considering the impact of treatment intervals, the dogma arising from research on cocaine and amphetamine is that sensitization develops best following treatment at long intervals, and that tolerance is more likely following treatment at shorter intervals [for review see 54, 76]. The current results suggest that this is not the case for sensitization to ketamine – sensitization developed similarly at both treatment intervals. It should be noted that the dogma from research on cocaine and amphetamine may not generalize to other drugs of abuse, since sensitization to the locomotor stimulating effects of opioids occurs following continuous administration [64], whereas tolerance develops to amphetamine under similar circumstances [76, 93–98]. Additionally, ketamine has a very short duration of action, which likely influences the interdose interval necessary for the development of tolerance or sensitization. For example, in the current studies, the response to 20 mg/kg of ketamine on the first day of treatment was only 10-20 minutes in duration (e.g., Experiment 1 and current experiment). It should be added that ketamine sensitization can develop following treatment at longer intervals. In previous research we demonstrated strong ketamine sensitization with an interdose interval as long as 7 days [64]. With regard to the human relevance of these findings, potent sensitization would be expected whether the drug is used daily or at more prolonged intervals, such as weekend parties [64].
Together, the current results demonstrate that ketamine sensitization develops under a variety of experimental conditions. Moreover, it is influenced by factors that modify sensitization to other drugs, albeit not always in the same direction. Therefore, ketamine sensitization is reliable, reproducible and robust. Compulsive ketamine use and addiction have been widely reported [5, 29, 31, 32, 35–37], however it is currently unclear if sensitization contributes to the development of ketamine addiction. As discussed above, sensitization is thought to be responsible for drug wanting and craving that are key elements of addiction. It is therefore possible that the current results point toward a valid animal model of the development of ketamine addiction. Further research will help to clarify the role of sensitization in addiction to ketamine. In addition to the factors examined in the current experiments, ketamine sensitization has been reported to be affected by age and sex of the animals [62, 63, 65, 67]. It will be of interest to determine how age and/or sex intersect with these other variables.
In addition to being a prominent drug of abuse, ketamine is also used clinically at subanesthetic doses for a variety of conditions, including pathological pain and major depression. An early retrospective look at responses in humans that had received repeated doses of ketamine in clinical studies found no evidence of sensitization [99]. However, with more clinical experience, evidence of sensitization may emerge. It will be of interest and importance to more closely evaluate the potential for sensitization in individuals treated for depression. It will be of particular interest to determine if sensitization contributes to the antidepressant effects of the drug and/or if it leads to increases in side-effects with repeated treatment. A better understanding of ketamine sensitization may lead to a better prevention and treatment of ketamine abuse and addiction and will inform future therapeutic use of the drug.
Highlights.
Ketamine produces locomotor stimulation at low subanesthetic doses.
Repeated administration of ketamine produces robust and reliable sensitization.
Dose, environment, and social isolation affect sensitization.
Ketamine sensitization is influenced by factors that influence addiction.
Acknowledgements
The authors would like to thank Dr. Shawn Bates for helpful comments on the manuscript. This research was supported by the National Institute of General Medical Sciences (GM 64783, GM 08807 and GM 81069) and the Office for Training, Research, and Education in the Sciences. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Trujillo KA, Smith ML, Sullivan B, Heller CY, Garcia C, Bates M, The neurobehavioral pharmacology of ketamine: implications for drug abuse, addiction, and psychiatric disorders, ILAR J 52(3) (2011) 366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Domino EF, Taming the ketamine tiger. 1965, Anesthesiology 113(3) (2010) 678–84. [DOI] [PubMed] [Google Scholar]
- [3].Wolff K, Winstock AR, Ketamine : from medicine to misuse, CNS Drugs 20(3) (2006) 199–218. [DOI] [PubMed] [Google Scholar]
- [4].Tyler MW, Yourish HB, Ionescu DF, Haggarty SJ, Classics in Chemical Neuroscience: Ketamine, ACS Chem Neurosci 8(6) (2017) 1122–1134. [DOI] [PubMed] [Google Scholar]
- [5].Morgan CJ, Curran HV, Independent D Scientific Committee on, Ketamine use: a review, Addiction 107(1) (2012) 27–38. [DOI] [PubMed] [Google Scholar]
- [6].Cohen SP, Bhatia A, Buvanendran A, Schwenk ES, Wasan AD, Hurley RW, Viscusi ER, Narouze S, Davis FN, Ritchie EC, Lubenow TR, Hooten WM, Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists, Reg Anesth Pain Med 43(5) (2018) 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Subramaniam K, Subramaniam B, Steinbrook RA, Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review, Anesth Analg 99(2) (2004) 482–95, table of contents. [DOI] [PubMed] [Google Scholar]
- [8].Bell RF, Eccleston C, Kalso E, Ketamine as adjuvant to opioids for cancer pain. A qualitative systematic review, J Pain Symptom Manage 26(3) (2003) 867–75. [DOI] [PubMed] [Google Scholar]
- [9].Hocking G, Cousins MJ, Ketamine in chronic pain management: an evidence-based review, Anesth Analg 97(6) (2003) 1730–9. [DOI] [PubMed] [Google Scholar]
- [10].Pourmand A, Mazer-Amirshahi M, Royall C, Alhawas R, Shesser R, Low dose ketamine use in the emergency department, a new direction in pain management, Am J Emerg Med 35(6) (2017) 918–921. [DOI] [PubMed] [Google Scholar]
- [11].Bell RF, Dahl JB, Moore RA, Kalso E, Perioperative ketamine for acute postoperative pain, Cochrane Database Syst Rev (1) (2006) CD004603. [DOI] [PubMed] [Google Scholar]
- [12].Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH, Antidepressant effects of ketamine in depressed patients, Biol Psychiatry 47(4) (2000) 351–4. [DOI] [PubMed] [Google Scholar]
- [13].Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA Jr., Charney DS, Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds, Annu Rev Pharmacol Toxicol 54 (2014) 119–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zanos P, Gould TD, Mechanisms of ketamine action as an antidepressant, Mol Psychiatry 23(4) (2018) 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Abdallah CG, Sanacora G, Duman RS, Krystal JH, The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation?, Pharmacol Ther 190 (2018) 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression, Arch Gen Psychiatry 63(8) (2006) 856–64. [DOI] [PubMed] [Google Scholar]
- [17].Hashimoto K, Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective, Psychiatry and Clinical Neurosciences Epub ahead of print (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim J, Farchione T, Potter A, Chen Q, Temple R, Esketamine for Treatment-Resistant Depression - First FDA-Approved Antidepressant in a New Class, N Engl J Med 381(1) (2019) 1–4. [DOI] [PubMed] [Google Scholar]
- [19].Swainson J, Thomas RK, Archer S, Chrenek C, Baker G, Dursun S, MacKay MA, Klassen LJ, Chokka P, Demas ML, Esketamine for treatment resistant depression, Expert Rev Neurother (2019). [DOI] [PubMed] [Google Scholar]
- [20].Liu Y, Lin D, Wu B, Zhou W, Ketamine abuse potential and use disorder, Brain Res Bull 126(Pt 1) (2016) 68–73. [DOI] [PubMed] [Google Scholar]
- [21].Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M, A Review of Ketamine Abuse and Diversion, Depress Anxiety 33(8) (2016) 718–27. [DOI] [PubMed] [Google Scholar]
- [22].Stewart CE, Ketamine as a street drug, Emerg Med Serv 30(11) (2001) 30, 32, 34 passim. [PubMed] [Google Scholar]
- [23].Kalsi SS, Wood DM, Dargan PI, The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use, Emerg Health Threats J 4 (2011) 7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Freese TE, Miotto K, Reback CJ, The effects and consequences of selected club drugs, J Subst Abuse Treat 23(2) (2002) 151–6. [DOI] [PubMed] [Google Scholar]
- [25].Hopfer C, Mendelson B, Van Leeuwen JM, Kelly S, Hooks S, Club drug use among youths in treatment for substance abuse, Am J Addict 15(1) (2006) 94–9. [DOI] [PubMed] [Google Scholar]
- [26].Klein M, Kramer F, Rave drugs: pharmacological considerations, AANA J 72(1) (2004) 61–7. [PubMed] [Google Scholar]
- [27].Maxwell JC, Spence RT, Profiles of club drug users in treatment, Subst Use Misuse 40(9-10) (2005) 1409–26. [DOI] [PubMed] [Google Scholar]
- [28].Jansen KL, A review of the nonmedical use of ketamine: use, users and consequences, J Psychoactive Drugs 32(4) (2000) 419–33. [DOI] [PubMed] [Google Scholar]
- [29].Dillon P, Copeland J, Jansen K, Patterns of use and harms associated with non-medical ketamine use, Drug Alcohol Depend 69(1) (2003) 23–8. [DOI] [PubMed] [Google Scholar]
- [30].Collier BB, Ketamine and the conscious mind, Anaesthesia 27(2) (1972) 120–34. [DOI] [PubMed] [Google Scholar]
- [31].Siegel RK, Phencyclidine and ketamine intoxication: a study of four populations of recreational users, NIDA Res Monogr (21) (1978) 119–47. [PubMed] [Google Scholar]
- [32].Muetzelfeldt L, Kamboj SK, Rees H, Taylor J, Morgan CJ, Curran HV, Journey through the K-hole: phenomenological aspects of ketamine use, Drug Alcohol Depend 95(3) (2008) 219–29. [DOI] [PubMed] [Google Scholar]
- [33].Stirling J, McCoy L, Quantifying the psychological effects of ketamine: from euphoria to the k-Hole, Subst Use Misuse 45(14) (2010) 2428–43. [DOI] [PubMed] [Google Scholar]
- [34].De Luca MT, Meringolo M, Spagnolo PA, Badiani A, The role of setting for ketamine abuse: clinical and preclinical evidence, Rev Neurosci 23(5-6) (2012) 769–80. [DOI] [PubMed] [Google Scholar]
- [35].Jansen KL, Darracot-Cankovic R, The nonmedical use of ketamine, part two: A review of problem use and dependence, J Psychoactive Drugs 33(2) (2001) 151–8. [DOI] [PubMed] [Google Scholar]
- [36].Copeland J, Dillon P, The health and psycho-social consequences of ketamine use, International Journal of Drug Policy 16 (2005) 122–131. [Google Scholar]
- [37].Morgan CJ, Rees H, Curran HV, Attentional bias to incentive stimuli in frequent ketamine users, Psychol Med 38(9) (2008) 1331–40. [DOI] [PubMed] [Google Scholar]
- [38].Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR, Prediction of abuse liability of drugs using IV self-administration by rats, Psychopharmacology (Berl) 82(1-2) (1984) 6–13. [DOI] [PubMed] [Google Scholar]
- [39].De Luca MT, Badiani A, Ketamine self-administration in the rat: evidence for a critical role of setting, Psychopharmacology (Berl) 214(2) (2011) 549–56. [DOI] [PubMed] [Google Scholar]
- [40].Marquis KL, Moreton JE, Animal models of intravenous phencyclinoid self-administration, Pharmacol Biochem Behav 27(2) (1987) 385–9. [DOI] [PubMed] [Google Scholar]
- [41].Moreton JE, Meisch RA, Stark L, Thompson T, Ketamine self-administration by the rhesus monkey, J Pharmacol Exp Ther 203(2) (1977) 303–9. [PubMed] [Google Scholar]
- [42].Risner ME, Intravenous self-administration of phencyclidine and related compounds in the dog, J Pharmacol Exp Ther 221(3) (1982) 637–44. [PubMed] [Google Scholar]
- [43].Wright KN, Hagarty DP, Strong CE, Schoepfer KJ, Kabbaj M, Sex-Dependent Ketamine Addiction-Like Behavior Profile Following Exposure to Chronic Mild Stress, Chronic Stress (Thousand Oaks) 3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Young AM, Woods JH, Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories, J Pharmacol Exp Ther 218(3) (1981) 720–7. [PubMed] [Google Scholar]
- [45].Botanas CJ, de la Pena JB, Dela Pena IJ, Tampus R, Yoon R, Kim HJ, Lee YS, Jang CG, Cheong JH, Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: Evidence of its abuse potential, Pharmacol Biochem Behav 133 (2015) 31–6. [DOI] [PubMed] [Google Scholar]
- [46].Suzuki T, Aoki T, Kato H, Yamazaki M, Misawa M, Effects of the 5-HT(3) receptor antagonist ondansetron on the ketamine- and dizocilpine-induced place preferences in mice, Eur J Pharmacol 385(2-3) (1999) 99–102. [DOI] [PubMed] [Google Scholar]
- [47].Suzuki T, Kato H, Aoki T, Tsuda M, Narita M, Misawa M, Effects of the non-competitive NMDA receptor antagonist ketamine on morphine-induced place preference in mice, Life Sci 67(4) (2000) 383–9. [DOI] [PubMed] [Google Scholar]
- [48].van der Kam EL, De Vry J, Tzschentke TM, The mGlu5 receptor antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP) supports intravenous self-administration and induces conditioned place preference in the rat, Eur J Pharmacol 607(1-3) (2009) 114–20. [DOI] [PubMed] [Google Scholar]
- [49].van der Kam EL, De Vry J, Tzschentke TM, 2-Methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates ketamine and heroin reward as assessed by acquisition, extinction, and reinstatement of conditioned place preference in the rat, Eur J Pharmacol 606(1-3) (2009) 94–101. [DOI] [PubMed] [Google Scholar]
- [50].Xu DD, Mo ZX, Yung KK, Yang Y, Leung AW, Individual and combined effects of methamphetamine and ketamine on conditioned place preference and NR1 receptor phosphorylation in rats, Neurosignals 15(6) (2006) 322–31. [DOI] [PubMed] [Google Scholar]
- [51].Caffino L, Piva A, Giannotti G, Di Chio M, Mottarlini F, Venniro M, Yew DT, Chiamulera C, Fumagalli F, Ketamine Self-Administration Reduces the Homeostasis of the Glutamate Synapse in the Rat Brain, Mol Neurobiol 54(9) (2017) 7186–7193. [DOI] [PubMed] [Google Scholar]
- [52].Caffino L, Piva A, Mottarlini F, Di Chio M, Giannotti G, Chiamulera C, Fumagalli F, Ketamine Self-Administration Elevates alphaCaMKII Autophosphorylation in Mood and Reward-Related Brain Regions in Rats, Mol Neurobiol 55(7) (2018) 5453–5461. [DOI] [PubMed] [Google Scholar]
- [53].Venniro M, Mutti A, Chiamulera C, Pharmacological and non-pharmacological factors that regulate the acquisition of ketamine self-administration in rats, Psychopharmacology (Berl) 232(24) (2015) 4505–14. [DOI] [PubMed] [Google Scholar]
- [54].Robinson TE, Becker JB, Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis, Brain Res 396(2) (1986) 157–98. [DOI] [PubMed] [Google Scholar]
- [55].Robinson TE, Berridge KC, The neural basis of drug craving: an incentive-sensitization theory of addiction, Brain Res Brain Res Rev 18(3) (1993) 247–91. [DOI] [PubMed] [Google Scholar]
- [56].Steketee JD, Kalivas PW, Drug wanting: behavioral sensitization and relapse to drug-seeking behavior, Pharmacol Rev 63(2) (2011) 348–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vezina P, Leyton M, Conditioned cues and the expression of stimulant sensitization in animals and humans, Neuropharmacology 56 Suppl 1 (2009) 160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Robinson TE, Berridge KC, Review. The incentive sensitization theory of addiction: some current issues, Philos Trans R Soc Lond B Biol Sci 363(1507) (2008) 3137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Berridge KC, Robinson TE, Liking, wanting, and the incentive-sensitization theory of addiction, Am Psychol 71(8) (2016) 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Robinson TE, Berridge KC, Addiction, Annu Rev Psychol 54 (2003) 25–53. [DOI] [PubMed] [Google Scholar]
- [61].Uchihashi Y, Kuribara H, Morita T, Fujita T, The repeated administration of ketamine induces an enhancement of its stimulant action in mice, Jpn J Pharmacol 61(2) (1993) 149–51. [DOI] [PubMed] [Google Scholar]
- [62].Rocha A, Hart N, Trujillo KA, Differences between adolescents and adults in the acute effects of PCP and ketamine and in sensitization following intermittent administration, Pharmacol Biochem Behav 157 (2017) 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Strong CE, Schoepfer KJ, Dossat AM, Saland SK, Wright KN, Kabbaj M, Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats, Neuropharmacology 121 (2017) 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Trujillo KA, Zamora JJ, Warmoth KP, Increased response to ketamine following treatment at long intervals: implications for intermittent use, Biol Psychiatry 63(2) (2008) 178–83. [DOI] [PubMed] [Google Scholar]
- [65].Wiley JL, Evans RL, Grainger DB, Nicholson KL, Age-dependent differences in sensitivity and sensitization to cannabinoids and ‘club drugs’ in male adolescent and adult rats, Addict Biol 13(3-4) (2008) 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yamamoto T, Nakayama T, Yamaguchi J, Matsuzawa M, Mishina M, Ikeda K, Yamamoto H, Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase, Neurosci Lett 610 (2016) 48–53. [DOI] [PubMed] [Google Scholar]
- [67].Bates MLS, Trujillo KA, Long-lasting effects of repeated ketamine administration in adult and adolescent rats, Behav Brain Res 369 (2019) 111928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Popik P, Kos T, Sowa-Kucma M, Nowak G, Lack of persistent effects of ketamine in rodent models of depression, Psychopharmacology (Berl) 198(3) (2008) 421–30. [DOI] [PubMed] [Google Scholar]
- [69].Schoepfer KJ, Strong CE, Saland SK, Wright KN, Kabbaj M, Sex- and dose-dependent abuse liability of repeated subanesthetic ketamine in rats, Physiol Behav 203 (2019) 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Robinson TE, Browman KE, Crombag HS, Badiani A, Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration, Neurosci Biobehav Rev 22(2) (1998) 347–54. [DOI] [PubMed] [Google Scholar]
- [71].Stewart J, Badiani A, Tolerance and sensitization to the behavioral effects of drugs, Behav Pharmacol 4(4) (1993) 289–312. [PubMed] [Google Scholar]
- [72].Caprioli D, Celentano M, Paolone G, Badiani A, Modeling the role of environment in addiction, Prog Neuropsychopharmacol Biol Psychiatry 31(8) (2007) 1639–53. [DOI] [PubMed] [Google Scholar]
- [73].Neisewander JL, Peartree NA, Pentkowski NS, Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction, Psychopharmacology (Berl) 224(1) (2012) 33–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bardo MT, Neisewander JL, Kelly TH, Individual differences and social influences on the neurobehavioral pharmacology of abused drugs, Pharmacol Rev 65(1) (2013) 255–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vannan A, Powell GL, Scott SN, Pagni BA, Neisewander JL, Animal Models of the Impact of Social Stress on Cocaine Use Disorders, Int Rev Neurobiol 140 (2018) 131–169. [DOI] [PubMed] [Google Scholar]
- [76].Post RM, Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance, Life Sci 26(16) (1980) 1275–82. [DOI] [PubMed] [Google Scholar]
- [77].Danysz W, Essmann U, Bresink I, Wilke R, Glutamate antagonists have different effects on spontaneous locomotor activity in rats, Pharmacology, biochemistry, and behavior 48(1) (1994) 111–8. [DOI] [PubMed] [Google Scholar]
- [78].Dematteis M, Lallement G, Mallaret M, Dextromethorphan and dextrorphan in rats: common antitussives--different behavioural profiles, Fundamental & clinical pharmacology 12(5) (1998) 526–37. [DOI] [PubMed] [Google Scholar]
- [79].Irifune M, Shimizu T, Nomoto M, Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice, Pharmacol Biochem Behav 40(2) (1991) 399–407. [DOI] [PubMed] [Google Scholar]
- [80].Irifune M, Shimizu T, Nomoto M, Fukuda T, Involvement of N-methyl-D-aspartate (NMDA) receptors in noncompetitive NMDA receptor antagonist-induced hyperlocomotion in mice, Pharmacology, biochemistry, and behavior 51(2-3) (1995) 291–6. [DOI] [PubMed] [Google Scholar]
- [81].Kelland MD, Soltis RP, Boldry RC, Walters JR, Behavioral and electrophysiological comparison of ketamine with dizocilpine in the rat, Physiology & behavior 54(3) (1993) 547–54. [DOI] [PubMed] [Google Scholar]
- [82].Ogren SO, Goldstein M, Phencyclidine- and dizocilpine-induced hyperlocomotion are differentially mediated, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 11(3) (1994) 167–77. [DOI] [PubMed] [Google Scholar]
- [83].Tricklebank MD, Singh L, Oles RJ, Preston C, Iversen SD, The behavioural effects of MK-801: a comparison with antagonists acting non-competitively and competitively at the NMDA receptor, European journal of pharmacology 167(1) (1989) 127–35. [DOI] [PubMed] [Google Scholar]
- [84].Garcia LS, Comim CM, Valvassori SS, Reus GZ, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J, Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels, Basic Clin Pharmacol Toxicol 103(6) (2008) 502–6. [DOI] [PubMed] [Google Scholar]
- [85].Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J, Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus, Prog Neuropsychopharmacol Biol Psychiatry 32(1) (2008) 140–4. [DOI] [PubMed] [Google Scholar]
- [86].Yang C, Li WY, Yu HY, Gao ZQ, Liu XL, Zhou ZQ, Yang JJ, Tramadol pretreatment enhances ketamine-induced antidepressant effects and increases mammalian target of rapamycin in rat hippocampus and prefrontal cortex, J Biomed Biotechnol 2012 (2012) 175619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Browne CA, Lucki I, Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants, Front Pharmacol 4 (2013) 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Pesic V, Petrovic J, M.J. M, Molecular Mechanism and Clinical Relevance of Ketamine as Rapid-Acting Antidepressant, Drug Dev Res 77(7) (2016) 414–422. [DOI] [PubMed] [Google Scholar]
- [89].Pert A, Post R, Weiss SR, Conditioning as a critical determinant of sensitization induced by psychomotor stimulants, NIDA Res Monogr 97 (1990) 208–41. [PubMed] [Google Scholar]
- [90].Post RM, Weiss SR, Fontana D, Pert A, Conditioned sensitization to the psychomotor stimulant cocaine, Ann N Y Acad Sci 654 (1992) 386–99. [DOI] [PubMed] [Google Scholar]
- [91].Pelloux Y, Giorla E, Montanari C, Baunez C, Social modulation of drug use and drug addiction, Neuropharmacology (2019). [DOI] [PubMed] [Google Scholar]
- [92].Hall FS, Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences, Crit Rev Neurobiol 12(1-2) (1998) 129–62. [DOI] [PubMed] [Google Scholar]
- [93].Izenwasser S, French D, Tolerance and sensitization to the locomotor-activating effects of cocaine are mediated via independent mechanisms, Pharmacol Biochem Behav 73(4) (2002) 877–82. [DOI] [PubMed] [Google Scholar]
- [94].Kunko PM, French D, Izenwasser S, Alterations in locomotor activity during chronic cocaine administration: effect on dopamine receptors and interaction with opioids, J Pharmacol Exp Ther 285(1) (1998) 277–84. [PubMed] [Google Scholar]
- [95].King GR, Joyner C, Ellinwood EH Jr., Continuous or intermittent cocaine administration: effects of flupenthixol treatment during withdrawal, Pharmacol Biochem Behav 49(4) (1994) 883–9. [DOI] [PubMed] [Google Scholar]
- [96].King GR, Joyner C, Ellinwood EH Jr., Continuous or intermittent cocaine administration: effects of amantadine treatment during withdrawal, Pharmacol Biochem Behav 47(3) (1994) 451–7. [DOI] [PubMed] [Google Scholar]
- [97].Nelson LR, Ellison G, Enhanced stereotypies after repeated injections but not continuous amphetamines, Neuropharmacology 17(12) (1978) 1081–4. [DOI] [PubMed] [Google Scholar]
- [98].Reith ME, Benuck M, Lajtha A, Cocaine disposition in the brain after continuous or intermittent treatment and locomotor stimulation in mice, J Pharmacol Exp Ther 243(1) (1987) 281–7. [PubMed] [Google Scholar]
- [99].Cho HS, D’Souza DC, Gueorguieva R, Perry EB, Madonick S, Karper LP, Abi-Dargham A, Belger A, Abi-Saab W, Lipschitz D, Bennet A, Seibyl JP, Krystal JH, Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine, Psychopharmacology (Berl) 179(1) (2005) 136–43. [DOI] [PubMed] [Google Scholar]