Abstract
The purpose of study was to investigate the maturation of mesenchymal stem cells (MSC) laden in HA constructs with various combinations of chemically defined medium (CM) components and determine the impact of dexamethasone and serum on construct properties. Constructs were cultured in CM with the addition or withdrawal of media components or were transferred to serum containing media that partially represents and in vivo-like condition where pro-inflammatory signals are present. Constructs cultured in CM+ (CM with TGF-β3) and DEX- (CM+ without dexamethasone) conditions produced robust matrix, while those in ITS/BSA/LA- (CM+ without ITS/BSA/LA) and Serum+ (10% FBS with TGF-β3) produced little matrix. While construct properties in DEX- were greater than those in CM+ at 4 weeks, properties in CM+ and DEX- reversed by 8 weeks. While construct properties in DEX- were greater than those in CM+ at 4 weeks, the continued absence or removal of dexamethasone resulted in marked GAG loss by 8 weeks. Conversely, the continued presence or new addition of dexamethasone at 4 weeks further improved or maintained construct properties through 8 weeks. Finally, when constructs were converted to Serum (in the continued presence of TGF-β3 with or without dexamethasone) after pre-culture in CM+ for 4 weeks, GAG loss was attenuated with addition of dexamethasone. Interestingly, however, collagen content and type was not impacted. In conclusion, dexamethasone influences the functional maturation of MSC-laden HA constructs, and may help to maintain properties during long-term culture or with in vivo translation by repressing pro-inflammatory signals.
Keywords: Mesenchymal stem cells, Hyaluronic acid, Dexamethasone, Anti-inflammatory effect, Cartilage Tissue Engineering
Introduction
Strategies to engineering cartilaginous tissues using mesenchymal stem cells (MSCs) are promising given that these multipotent cells can undergo chondrogenesis in a variety of 3D contexts1–5. Particularly, MSCs in hyaluronic acid (HA) hydrogels1; 6–10 produce robust matrix equivalent to that produced by chondrocytes, when cultured in a chemically defined medium (CM) supplemented with TGF-β3 (CM+) 2; 11–13.
Building from the initial description of the CM formulation that supports chondrogenesis12; 14, a number of studies have explored which components are absolutely necessary, and over what time course. For instance, using both chondrocytes and MSCs, several studies have shown that transient inclusion of TGF-β3 with a very high dose (100 ng/mL) in CM over a relatively short period (< two weeks) results in greater properties than continual exposure to TGF-β3 with a low dose (10 ng/mL)2; 15–17. Translating this finding, others have shown that alginate-coated TGF-β3 microspheres co-encapsulated with human MSCs in HA hydrogels resulted in robust chondrogenesis18.
While these are compelling, the transition to the in vivo environment may be more complicated, especially when the in vivo environment is the synovial joint. Successful strategies for cartilage repair require not only fabrication of an engineered construct with native properties, but also stability of these properties within the native load-bearing synovial environment. Synovial fluid is a dialysate of the blood that is comparable to serum, and additionally contains HA, lubricin, proteinases, collagenases, and occasionally lymphocytes, monocytes, neutrophils and serum proteins19 including albumin, immunoglobulin, transferrin, fibrinogen, macroglobulin, antitrypsin, haptoglobin, glycoprotein and apolipoprotein. Recently, we showed that when MSC-laden constructs were transferred to serum containing media at the time of TGF withdrawal, construct properties rapidly decreased with marked GAG loss17. This suggests that while CM+ can foster maturation, conflict signals present in serum and synovial fluid may compromise long term functionality, and so may limit clinical translation.
One additional factor in CM, dexamethasone (DEX), is a synthetic glucocorticoid known to have pro-chondrogenic11; 14 and anti-inflammatory effects20; 21. Dexamethasone suppresses inflammatory signaling (e.g., IL-8 secretion regulated by p38MAPK, AP-1 and NF-kB activity)22. A recent study also showed that glucocorticoid combined with HA enhances glucocorticoid receptor activity to inhibit activation of the p38 MAPK signaling pathway23. Interestingly, several studies have shown that dexamethasone is an indispensable component in CM11; 14. One recent combinatorial screen coupled with RNA profiling showed that dexamethasone acted synergistically with TGF-β to promote MSC chondrogenesis24. In another recent study, however, it was shown that dexamethasone inhibits BMP-2 induced MSC chondrogenesis, and that removal of dexamethasone in CM improved GAG deposition in synovial explants25. In chick limb bud cultures, the dose of dexamethasone played a role, where lower doses improved chondrogenesis and higher doses promoted osteogenic conversion26. Similarly, others have reported a different impact of dexamethasone in young bovine and adult human MSCs in agarose and self-assembling peptide hydrogels20. Further, in short term cultures of equine MSCs in agarose hydrogels, withdrawal of dexamethasone had an adverse impact on matrix accumulation when this factor was absent for longer than two days27. Collectively, these data suggest there are species, age, cell source, material context, and time dependent effects of dexamethasone on MSCs and progenitor cell chondrogenesis.
Given these findings, the goal of this study was to investigate the effect of dexamethasone in long term cultures of MSCs in hydrogels, both in CM and under ‘conflict’ conditions wherein the constructs were transferred to a more in vivo-like serum containing setting. In a first set of studies, we investigated the maturation of MSC-laden constructs with various combinations of media components that make up CM, with a particular focus on dexamethasone and serum substitutes (e.g., ITS+ Premix) and the presence of serum. In a subsequent study, based on the differential effect of dexamethasone on functional properties during the culture period, we examined the short and long term effect of dexamethasone presence and absence in CM. Finally, given that previous studies indicated an anti-inflammatory role for dexamethasone, we assayed its role in attenuating matrix disruption when constructs were transferred to serum containing ‘conflict’ media for extended culture.
Methods
Preparation of cell/tissue culture media
Basal media (BM), CM, or media with addition/removal of CM components were prepared as outlined in Table 1. BM consisted of high glucose DMEM with 10% FBS (Gibco, Grand Island, NY) and 1% penicillin/streptomycin/fungizone (PSF)(Gibco, Grand Island, NY). This media was used to isolate and expand MSCs. CM contained high glucose DMEM with 1x PSF, 0.1 μM dexamethasone, 50 μg/mL ascorbate 2-phosphate, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenious acid (namely ITS), 1.25 mg/mL bovine serum albumin (BSA), and 5.36 μg/mL linoleic acid (LA). This CM was further supplemented with 10ng/mL TGF-β3 (R&D Systems, Minneapolis, MN) to produce a chondrogenic induction media. To identify the effect of the specific media components in CM+, additional formulations were developed including DEX- (removal of dexamethasone from CM+), ITS/BSA/LA- (removal of serum substitute from CM+) or Serum+ (addition of serum and removal of dexamethasone and ITS/BSA/LA) (Table 1).
Table 1:
Combinational analysis of media components
| Media Component | Media Combinations | |||
|---|---|---|---|---|
| CM+ | DEX- | ITS/BSA/LA- | Serum+ | |
| DMEM (High glucose) | + | + | + | + |
| Penicillin/ Streptomycin/ Fungizone (PSF) | + | + | + | + |
| Ascorbate 2-Phosphate (AP) | + | + | + | + |
| Proline (PRO) | ||||
| Sodium Pyruvate (SP) | ||||
| Dexamethasone (DEX) | + | - | + | - |
| Insulin/Transferrin/Selenium Supplements (ITS) | + | + | - | - |
| Bovine Serum Albumin (BSA) | ||||
| Lenoleic Acid (LA) | ||||
| Fetal bovine serum (FBS) | - | - | - | + |
| TGF-ß3 | + | + | + | + |
Preparation of mesenchymal stem cells
MSCs were isolated from juvenile bovine knees from three different donors (Research 87, Bolyston, MA). To extract bone marrow, cubes of trabecular bone were segmented from the epi- and metaphyseal region of the femur and tibia and were washed with medium containing 0.2% heparin (Sigma-Aldrich, St. Louis, MO). The diluted bone marrow was pooled and washed in BM, resuspended and plated onto tissue culture plastic. Adherent cells were noted within 5 days, and were expanded to passage 3 to obtain sufficient cell numbers for hydrogel encapsulation.
MeHA synthesis
Synthesis of methacrylated hyaluronic acid (MeHA) was as previously described by Burdick and co-workers 7. Briefly, 1% w/v sodium hyaluronate (65 kDa MW; Lifecore, Chaska, MN) dissolved in deionized water was reacted with methacrylic anhydride (Sigma-Aldrich, St. Louis, MO) on ice at a pH of 8.0 with 5N NaOH (Sigma-Aldrich, St. Louis, MO) for 6 hours. This was followed by dialysis (No.132670, 6kDa MWCO, Spectrum Laboratories, Rancho Dominguez, CA) to remove unreacted byproducts for one week with repeated changes of distilled water. The MeHA solution was lyophilized and stored at −20°C.
MSC encapsulation and construct culture
To form gels, lyophilized MeHA was dissolved at 1% w/v in PBS with 0.05% w/v photoinitiator (Irgacure I2959, Ciba-Geigy, Tarrytown, NY). MSCs were resuspended at a concentration of 60 million cells/mL and poured into a gel casting apparatus (Hoefer, Inc., Hollison, MA) 28 and exposed to UV using a 365nm Blak Ray UV lamp (UVL-56, San Gabriel, CA) for 10 minutes. The range of the UV was 320–400 nm with a transmission maximum of 70% at 365 nm. To explore the effect of media components in chondrogenic maturation of MSCs laden in HA hydrogels, cylindrical constructs (Ø4 × 2.25 mm) were cored using a biopsy punch from the resulting hydrogel slabs and cultured in CM+, DEX-, ITS/BSA/LA- or Serum+ for 8 weeks (Table 1). Each construct received 2 mL of media, which was changed thrice weekly for the duration of the study.
Effect of dexamethasone on construct maturation
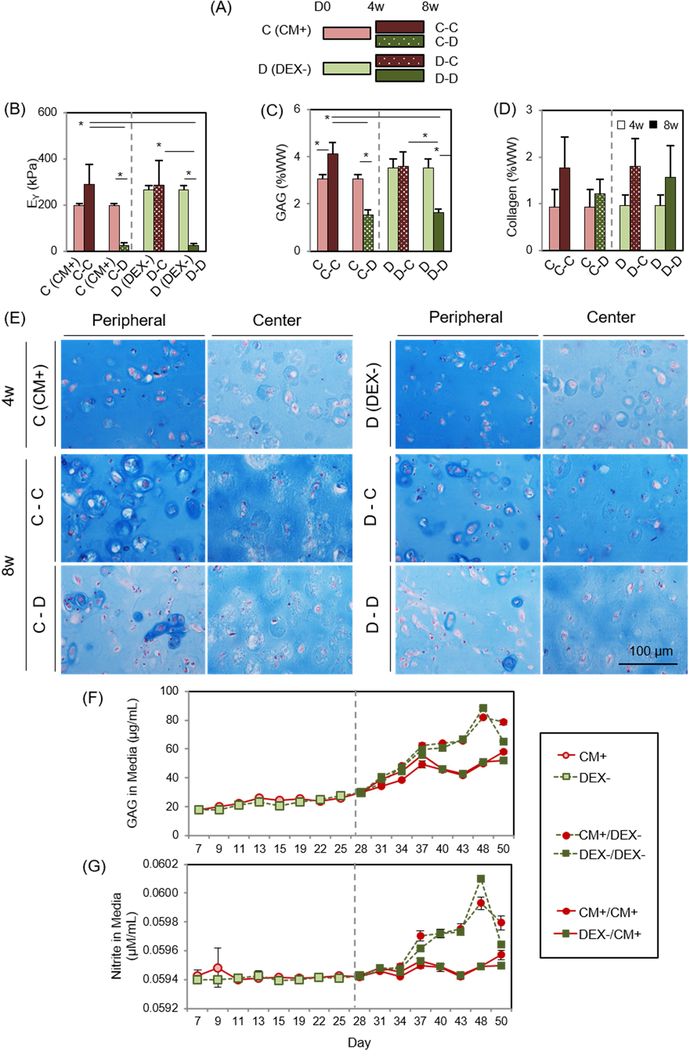
To explore the short and long-term effects of DEX inclusion or exclusion, MSC-laden HA constructs were cultured in CM+ (the C group) for 4 weeks, and then were maintained in CM+ (the C-C group) or were transferred to DEX- (the C-D group; removal of dexamethasone from CM+) for the next 4 weeks. Likewise, constructs cultured in DEX- (the D group) for the first 4 weeks were converted to CM+ (the D-C group) or were continuously cultured in DEX- (the D-D group) through 8 weeks (Figure 2A). The level and timing of GAG, collagen, and nitrite released from constructs during the culture period were assessed using supernatants collected from each media change.
Figure 2: Short- and long-term effect of dexamethasone on MSC-laden HA constructs.
(A) Study design to test for the effect of dexamethasone (DEX) on MSC chondrogenesis, (B) Equilibrium modulus (EY; kPa), (C) GAG (%WW), (D) Collagen (%WW), (E) Alcian blue staining at 4 weeks (the CM+ or DEX- group; top) and 8 weeks (the C-C, C-D, D-C or D-D group; middle and bottom), (F) GAG in media (μg/mL), (G) Nitrite in media (μM/mL). (the C-C group: constructs cultured in CM+ for the first 4 weeks followed by 4 weeks in CM+; the C-D groups: constructs cultured in CM+ for the first 4 weeks followed by 4 weeks in DEX-; the D-C group: DEX- for the first 4 weeks followed by CM+ for 4 weeks; the D-D group: DEX- for the first 4 weeks followed by DEX- for 4). (n = 4/group; lighter bars = 4w, darker bars = 8w; solid fill = CM+; pattern fill = DEX-; scale bar = 100 μm; 20X magnification; *p<0.05).
Effect of dexamethasone in serum containing media
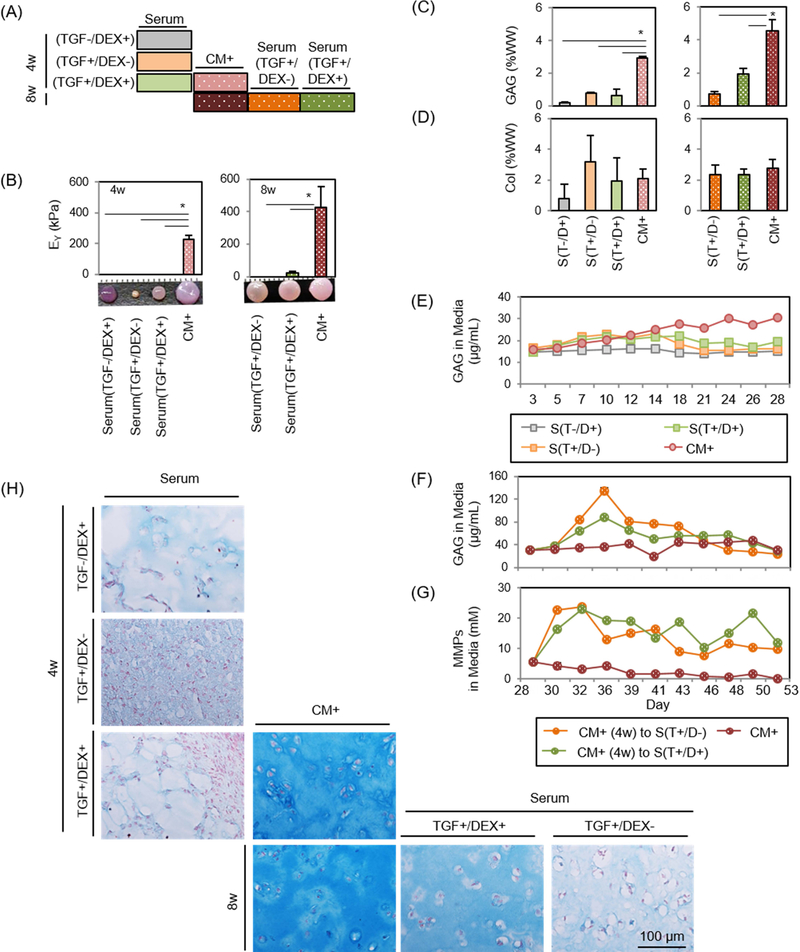
Early results showed that transfer of constructs from CM+ to serum containing media resulted in rapid loss of mechanical properties. To assess whether dexamethasone could inhibit GAG loss in the context of serum containing media, constructs were first cultured for 4 weeks in CM+ or Serum with or without dexamethasone and/or TGF (TGF-/DEX+, TGF+/DEX- or TGF+/DEX+) (Figure 4A). Constructs cultured in Serum were harvested at 4 weeks, while constructs maintained in CM+ for the first 4 weeks were either continuously cultured in CM+ (CM+_CM+) or were converted to BM with or without dexamethasone (TGF+/DEX- or TGF+/DEX+) for the next 4 weeks.
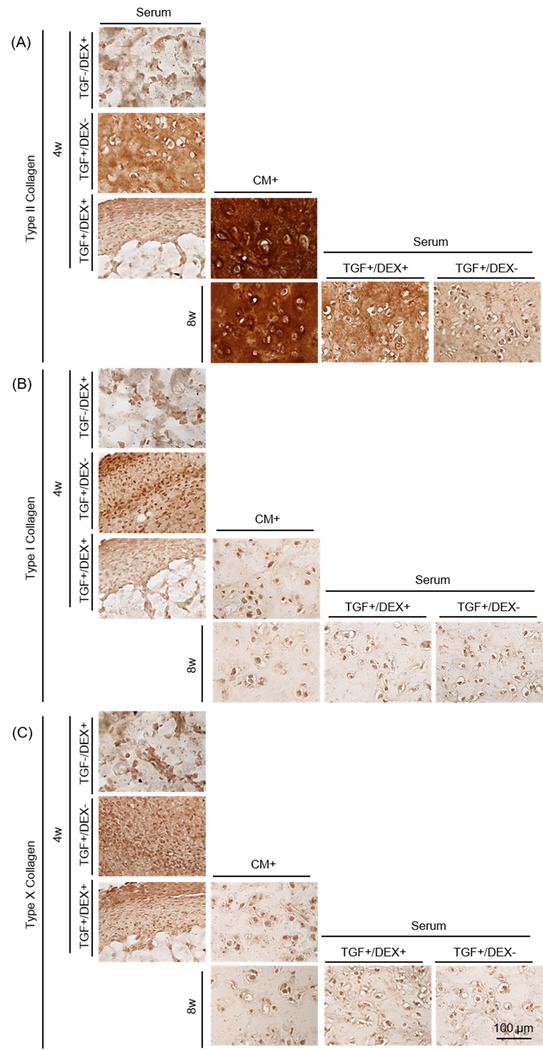
Figure 4: Functional properties of MSC-laden HA constructs in CM+ or with transfer to serum containing media.
(A) Schematic of impact of serum containing media and the effect of DEX. Constructs were cultured in CM+ or serum containing media with addition/removal of DEX or TGF for the first 4 weeks. Constructs were cultured in CM+ for the first 4 weeks and continued in CM+ or switched to Serum with the removal/addition of DEX/TGF. (B-D) (left). Afterwards, constructs cultured in CM+ were transferred to serum containing media with addition/removal of DEX or TGF for 4 weeks (right) (Inset: gross images of constructs cultured in CM+ or serum containing media at 4 or 8 weeks; marking = 1mm), (B) Equilibrium modulus (EY; kPa), (C) GAG (%WW), (D) Collagen (%WW) . (E and F) GAG in media (μg/mL) over first 4 weeks (E) and following 4 weeks (F). (G) MMPs in media (mM/mL) (n = 3/group; *p<0.05), (H) Alcian blue staining: constructs were cultured in CM+ or serum containing media for 4 weeks with the addition/removal of DEX or TGF (first three rows). Constructs cultured in CM+ for the first 4 weeks were continued in CM+ or switched to Serum with the removal/addition of DEX or TGF (bottom row).
Cell viability
Cell viability was assessed using the LIVE/DEAD staining kit (Molecular Probes, Invitrogen), and was determined by calcein AM and ethidium homodimer, respectively.
Analysis of mechanical properties
Unconfined compression testing was performed to determine bulk compressive equilibrium (EY) and dynamic (|G*|) moduli of constructs as in Mauck et al28; 29. Compressive modulus was determined via a stress-relaxation test including a step compression at 0.05%/sec to 10% strain after creep loading to 0.02 N for 5 min. The relaxation phase was for 1200 seconds. Subsequently, a 1% sinusoidal deformation was applied at 1.0 Hz to obtain the dynamic modulus. Prior to mechanical testing, the top and bottom surfaces of constructs were carefully planed using a freezing stage microtome to ensure an even contact surface.
Biochemical analysis
After mechanical testing, construct wet weight was measured followed by papain digestion. Sulfated glycosaminoglycan content was assessed using the 1,9-dimethylmethylene blue assay30. Orthohydroxyproline (OHP) content was measured via reaction with chloramine T and diaminobenzaldehyde. Collagen content was extrapolated from OHP using a 1:7.14 ratio of OHP31. In some studies, GAG, collagen, nitrite (Griess Reagent System; Promega, Madison, WI)32 and MMP activity (SensoLyte 520 Generic MMP kit; AnaSpec, Fremont, CA) was assessed using media supernatants collected at each media change over the culture period.
Histological analysis
Constructs were fixed in 4% paraformaldehyde for 24 hours and embedded in paraffin. Sections (8 μm) were deparaffinized in a graded series of ethanol and stained with Alcian Blue (pH 1.0) for proteoglycans (PG). Immunohistochemistry was carried out to visualize type I, II and X collagen using IHC Select HRP/DAB kit (Millipore, Billerica, MA). Primary antibodies to type I collagen (MAB3391; Millipore, Billerica, MA), type II collagen (II-II6B3; DSHB, Iowa City, IA) and type X collagen (X-AC9; DSHB, Iowa City, IA) were used for immunolabeling.
Statistical Analysis
Statistical analysis was performed using the SYSTAT software (v10.2, SYSTAT software Inc., San Jose, CA). Significance was determined by two-way ANOVA with Tukey’s post hoc test (p<0.05).
Results
Influence of media components on the functional maturation of MSC-laden HA hydrogel constructs
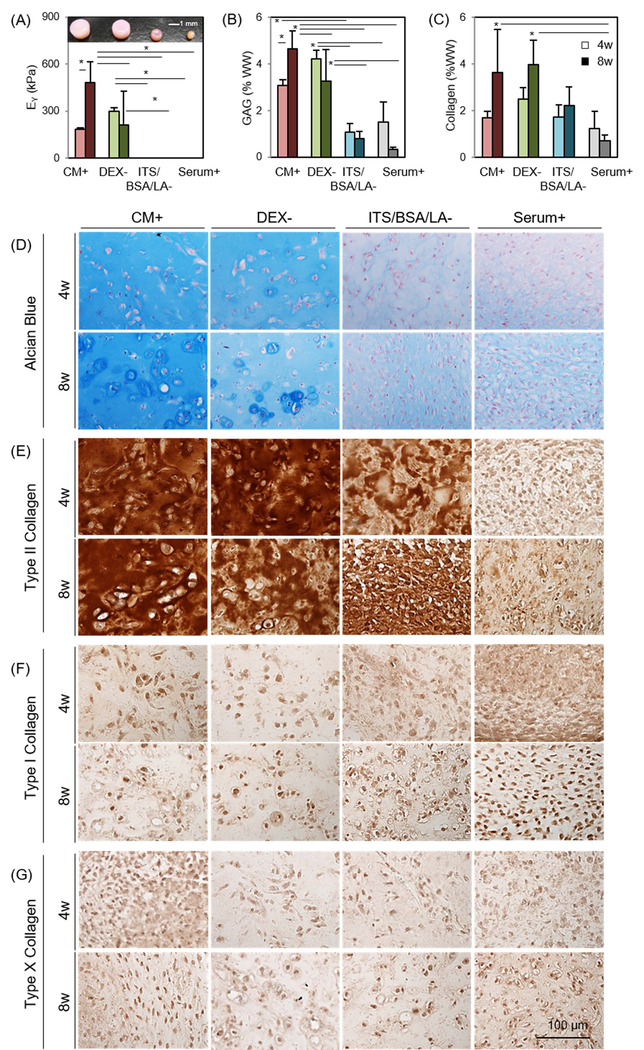
Four media conditions were initially screened (Table 1). MSCs were viable only in CM+ and DEX- (Supplementary 1); constructs in these viable conditions maintained their initial shape and produced robust matrix (Figure 1A). Conversely, cells in ITS/BSA/LA- and Serum+ did not remain viable, and these constructs contracted significantly over the culture period. Constructs cultured in Serum+ contracted very quickly, MSCs appeared to aggregate centrally, and this culminated in the formation of a central pellet-like structure that separated from the HA hydrogel. Equilibrium modulus (EY) and GAG content of constructs in CM+ and DEX- reached to 181 kPa (3.1 %WW) and 296 kPa (4.2 %WW) at 4 weeks, respectively, while the constructs in ITS/BSA/LA- and Serum+ had no measurable mechanical properties and lower GAG content (1.1 and 1.5 %WW, respectively) (Figure 1A and B and Table 2). At 4 weeks, the mechanical properties and GAG content of constructs in the DEX- were 63% (p=0.838) and 37% (p=0.461) greater than those cultured in CM+, respectively. Interestingly, however, properties in CM+ and DEX- reversed at 8 weeks, reaching 480 kPa/4.7 %WW and 211 kPa/3.3 %WW, respectively. Mechanical properties and GAG content of constructs in CM+ were 2.2 (p=0.002) and 1.42 (p=0.032) times greater than those cultured in DEX- at this 8 week time point. Collagen content increased with time for all media conditions, except for Serum+, regardless of the presence or absence of DEX (Figure 1C). Similarly, Alcian blue staining showed marked PG production in constructs cultured in CM+ and DEX- at 4 weeks compared to ITS/BSA/LA- and Serum+. However, PG accumulation of constructs in DEX- decreased after 8 weeks. (Figure 1D). Conversely, immunohistochemistry showed positive staining of type II collagen for all groups except for BM+. The production of type II collagen was independent of addition and/or removal of dexamethasone and/or ITS/BSA/LA. Moreover, in the presence of TGF-β3, all groups showed little staining for type I or X collagen, regardless of the variations in PG and type II collagen (Figure 1E–G).
Figure 1: Influence of media components on functional properties of MSC-laden HA constructs.
(A) Equilibrium modulus (EY; kPa) (Inset: gross images of constructs cultured in different media combinations at 8 weeks), (B) GAG (%WW), (C) Collagen (%WW), (D) Alcian blue staining at 4 weeks (top) and 8 weeks (bottom), (E-G) Immunohistochemistry for type II, I and X collagen (n = 4/group; lighter bars = 4w, darker bars = 8w; scale bar = 100μm; *p<0.05).
Table 2:
Mechanical and biochemical properties of MSC-laden constructs with various media formulations at 4 and 8 weeks (mean ± SD; n = 4/group).
| CM+ | DEX- | ITS/BSA/LA- | Serum+ | |||||
|---|---|---|---|---|---|---|---|---|
| 4w | 8w | 4w | 8w | 4w | 8w | 4w | 8w | |
| EY(kPa) | 182 ± 12 | 480 ± 132 | 297 ± 23 | 211 ± 215 | 0 | 0 | 0 | 0 |
| |G*| (kPa) | 2181 ± 181 | 3835 ± 597 | 3009 ± 193 | 2527 ± 1330 | 151 ± 80 | 674 ± 241 | 0 | 0 |
| Diameter (mm) | 3.8 ± 0.1 | 4.1 ± 0.2 | 3.8 ± 0.2 | 3.9 ± 0.2 | 2.7 ± 0.2 | 2.4 ± 0.2 | 1 | 1 |
| GAG (%WW) | 3.1 ± 0.3 | 4.7 ± 0.8 | 4.2 ± 0.4 | 3.3 ± 1.4 | 1.1 ± 0.4 | 0.8 ± 0.3 | 1.5 ± 0.8 | 0.4 ± 0.1 |
| GAG (μg/const) | 756 ± 67 | 1543 ± 275 | 951 ± 156 | 804 ± 490 | 62 ± 11 | 47 ± 9 | 14 ± 2 | 15 ± 8 |
| Collagen (%WW) | 1.7 ± 0.3 | 3.6 ± 1.9 | 2.5 ± 0.5 | 4.0 ± 1.1 | 1.7 ± 0.5 | 2.2 ± 0.8 | 1.3 ± 0.7 | 0.7 ± 0.2 |
| Collagen (μg/const) | 414 ± 89 | 1243 ± 795 | 559 ± 69 | 926 ± 292 | 102 ± 11 | 135 ± 22 | 11 ± 4 | 27 ± 5 |
Short and long term effects of dexamethasone on construct maturation
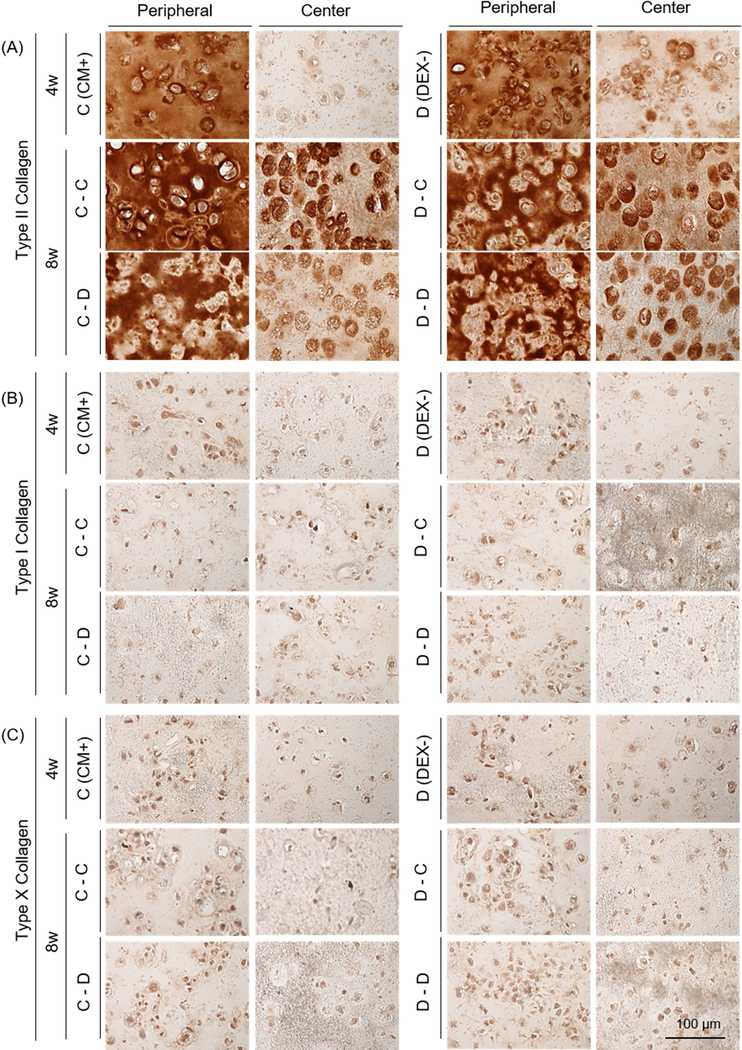
Given that dexamethasone seemed dispensable in the first period of culture, we next carried out a study exploring the timing of exposure.). Withdrawal (the C-D group) or continued absence (the D-D group) of dexamethasone reduced viability near the peripheral region (Supplementary 2). The mechanical properties and GAG content of constructs in the DEX- (267 kPa and 3.5 %WW) were greater than these in CM+ (198 kPa and 3.1 %WW) at 4 weeks (Figure 2B and C, and Table 3). However, when continuously cultured in DEX-, construct properties decreased by 8 weeks (25 kPa; p<0.0001 and 1.6 %WW; p<0.0001, respectively). Likewise, when constructs cultured in CM+ were switched to DEX-, construct properties markedly decreased (25 kPa; p=0.008 and 1.5 %WW; p<0.0001, respectively) by 8 weeks. However, constructs that were continuously cultured in CM+ or were switched to CM+ after the first 4 weeks of culture in DEX- slightly increased or maintained their properties (290 kPa and 4.1 %WW (p=0.009) for the C-C group and 285 kPa and 3.6 %WW for the D-C group). Collagen content was also independent of the addition or removal of dexamethasone, generally increasing with culture duration (Figure 2D). Alcian blue staining showed robust PG deposition in CM+ and DEX- after 4 weeks, with greater intensity in the peripheral region compared to the core. Constructs continuously cultured or switched to CM+ accumulated intense ECM and PCM in the periphery and core regions, whereas those cultured or switched to DEX- decreased in PG content, particularly in the peripheral region (Fig 2E). GAG and nitrite release from constructs increased with the switch to DEX- (at week 4) and continued to increase over the next 3 weeks whereas constructs maintained in CM+ showed a lower release of both (Figure 2F and G). The amount of collagen released from constructs was similar and steady for all groups (data not shown). Immunostaining showed a peripheral region with greater type II collagen staining compared to the center, but the matrix intensity was similar for all groups regardless of presence or partial/continuous absence of dexamethasone (Figure 3A). No staining for type I or X collagen matrix was observed (Figure 3B and C).
Table 3:
Effect of DEX on the functional maturation of MSC-laden HA constructs at 4 and 8 weeks (mean ± SD; n = 4/group).
| CM+ | C-C | C-D | DEX- | D-C | D-D | |
|---|---|---|---|---|---|---|
| 4w | 8w | 4w | 8w | |||
| EY(kPa) | 198 ± 8 | 291 ± 83 | 26 ± 83 | 267 ± 19 | 286 ± 109 | 26 ± 9 |
| |G*| (MPa) | 1518 ± 96 | 2279 ± 382 | 921 ± 229 | 2125 ± 100 | 2507 ± 671 | 812 ± 66 |
| Diameter (mm) | 4.1 ± 0.1 | 4.2 ± 0.1 | 4.1 ± 0.1 | 4.1 ± 0.1 | 4.1 ± 0.1 | 4.1 ± 0.1 |
| GAG (%WW) | 3.1 ± 0.2 | 4.1 ± 0.5 | 1.5 ± 0.2 | 3.5 ± 0.4 | 3.6 ± 0.6 | 1.6 ± 0.1 |
| GAG (μg/const) | 1087 ± 49 | 1501 ± 310 | 484 ± 82 | 1120 ± 153 | 1027 ± 272 | 436 ± 43 |
| Collagen (%WW) | 0.9 ± 0.4 | 1.8 ± 0. 7 | 1.2 ± 0.3 | 1.0 ± 0.2 | 1.8 ± 0.6 | 1.6 ± 0.7 |
| Collagen (μg/const) | 325 ± 119 | 645 ± 269 | 381 ± 96 | 309 ± 104 | 524 ± 239 | 494 ± 180 |
Figure 3: Immunohistochemistry of MSC-laden HA constructs with short- and long-term addition or removal of dexamethasone.
(A) Type II collagen, (B) Type I collagen and (C) Type X collagen (the CM+ or DEX- group at 4 weeks (top); the C-C, C-D, D-C or D-D group at 8 weeks (middle and bottom); scale bar = 100 μm).
Dexamethasone attenuates GAG loss with transfer to serum-containing media
Constructs cultured in Serum in the absence or presence of TGF and/or dexamethasone (Serum [TGF-/DEX+], Serum [TGF+/DEX-] and Serum [TGF+/DEX+]) failed to mature from the outset, contracted and/or produced little matrix after 4 weeks (0 kPa and 0.2–0.8 %WW). Conversely, those in CM+ produced robust matrix (230 kPa; p=0.001 vs. all Serum and 2.9 %WW; p=0.001, 0.009 and 0.005 vs. Serum, respectively) (Figure 4B–D and Table 4). Likewise, GAG release from Serum was low, similar to day 0 levels (Figure 4E). When constructs were switched to Serum (Serum[TGF+/DEX-] or Serum[TGF+/DEX+]) after pre-culture in CM+ for 4 weeks, constructs lost mechanical properties entirely (0 kPa for Serum[TGF+/DEX-]; p<0.0001) or significantly (21 kPa for Serum[TGF+/DEX+]; p<0.0001) compared to those that were in CM+ for the entire 8 weeks. Interestingly, their gross appearance did not change (Figure 4B). However, GAG loss in the group cultured in the presence of dexamethasone (Serum[TGF+/DEX+]) was 34% of the level at 4 weeks while that in the absence of dexamethasone (Serum[TGF+/DEX-]), GAG loss reached 80% over the ensuing 4 weeks (Figure 4C). Consistent with the results from the first and second studies, collagen content did not change on a per wet weight basis (Figure 4D). GAG release from constructs occurred almost immediately when converted to Serum, and lasted through the culture duration. GAG loss in Serum supplemented with dexamethasone was slightly lower (Figure 4F). MMPs released to the media were similar to GAG, with no pronounced effect of dexamethasone (MMP level in BM: 5 mM) (Figure 4G). Nitrite and collagen release (data not shown) showed no changes, and cells remained viable for all groups even after conversion to Serum (Supplementary 3). Alcian blue staining showed that constructs cultured in Serum produced little to no matrix and showed marked contraction (left column). Constructs in CM+ grew well and produced robust matrix with time in culture (middle). However, when the constructs were switched to Serum, GAG loss was partially inhibited for constructs in the presence of dexamethasone, whereas constructs switched to Serum in the absence of dexamethasone showed marked GAG depletion (Figure 4H). Similarly, constructs cultured in Serum in the presence of TGF (T+/D- or T+/D+; left column) produced some positive staining for type II collagen, but also showed some positive staining for type I and X collagen (Figure 5). Constructs in CM+ showed dense type II collagen staining with no type I and X collagen staining (middle). When transferred to Serum (T+/D+ or T+/D-) after 4 weeks, a marked decrease of type II collagen was observed, but the presence of dexamethasone (T+/D+) reduced the collagen loss (right). There was no positive staining for type I and X collagen for constructs initially cultured in CM+.
Table 4:
Impact of serum containing media and the effect of DEX on the functional properties of MSC-laden constructs (mean ± SD; n = 4/group).
| 4w | 8w | ||||||
|---|---|---|---|---|---|---|---|
| Serum | CM+ | Serum | CM+ | ||||
| TGF-/DEX+ | TGF+/DEX- | TGF+/DEX+ | TGF+/DEX- | TGF+/DEX+ | |||
| EY (kPa) | 0 | 0 | 0 | 231 ± 26 | 0 | 21 ± 10 | 426 ± 128 |
| |G*| (kPa) | 95 ± 21 | 0 | 123 ± 33 | 2208 ± 162 | 284 ± 63 | 916 ± 247 | 3330 ± 603 |
| Diameter (mm) | 3.0 ± 0.0 | 1 ± 0.0 | 2.4 ± 0.1 | 4 ± 0.0 | 3.8 ± 0.0 | 3.9 ± 0.1 | 4.0 ± 0.0 |
| GAG (%WW) | 0.2 ± 0.0 | 0.8 ± 0.0 | 0.6 ± 0.4 | 2.9 ± 0.1 | 0.7 ± 0.1 | 1.9 ± 0.4 | 4.6 ± 0.7 |
| GAG (μg/const) | 15.4 ±3.7 | 11.6 ± 2.5 | 26.1 ± 1.6 | 795 ± 16.3 | 193 ± 35 | 524 ± 92 | 1247 ± 209 |
| COL (%WW) | 0.8 ± 0.9 | 3.2 ± 1.7 | 2.0 ± 1.5 | 2.1 ± 0.6 | 2.4 ± 0.6 | 2.4 ± 0.4 | 2.8 ± 0.6 |
| COL (μg/const) | 48 ± 37 | 45 ± 20 | 86 ± 38 | 581 ± 193 | 654 ± 184 | 647 ± 95 | 757 ± 154 |
Figure 5: Immunohistochemistry of MSC-laden HA constructs in CM+ or with transfer to serum containing media.
(A) Type II collagen, (B) Type I collagen and (C) Type X collagen (Constructs cultured in Serum for 4 weeks (left colum), CM+ for 4 and 8 weeks (middle) and transferred to Serum at 8 weeks (right); scale bar = 100 μm).
Discussion
In this study, we determined the roles of various CM components on the chondrogenesis and functional maturation of MSCs-laden constructs. Further, we defined the role of dexamethasone during culture in these CM conditions and after transition of constructs to a conflicting (in vivo-like) environment that contained serum. Our findings show that MSC-laden constructs produce robust matrix in CM+, and that mechanical properties and GAG content were greater in DEX- after 4 weeks. However, we also noted that with the prolonged absence of dexamethasone, the mechanical properties and GAG content of these constructs markedly decreased by 8 weeks, whereas those constructs cultured in CM+ for entire period showed persistent increases throughout this time period. This indicates that dexamethasone plays a key role in regulating pro-chondrogenic and anti-inflammatory activities, consistent with previous studies11; 20, and supports long term tissue formation and maturation.
Our data also showed that no matrix was produced when constructs were cultured from the outset in ITS/BSA/LA- or in Serum+. ITS/BSA/LA include essential components in CM, namely insulin, transferrin, selenium, bovine serum albumin (BSA) and linoleic acid 33–35, and is commonly used as a serum replacement (i.e., ITS Premix+) to improve consistency of in vitro studies. Insulin is crucial for glucose transport into cultured cells, and transferrin is an ion carrier. Selenium, as sodium selenite, is a cofactor for selenium-dependent enzyme activities (e.g., glutathione reductase, glutathione peroxidase), and inhibits oxidation. Linoleic acid is a precursor of prostaglandins, glycolipids, and vitamins, while albumin serves as a plasma substitute for the growth of cultured cells. The poor growth of constructs in ITS/BSA/LA- indicate that these molecules are imperative for growth in CM. This indicates that some serum elements (i.e., transferrin and insulin) are essential for construct growth, but that other elements in serum can limit matrix formation by MSCs.
Based on our observation of a differential effect of dexamethasone on functional maturation during the culture period, we further investigated how dexamethasone regulated functional properties with time in culture. Consistent with previous results, constructs produced greater mechanical properties and GAG content in the absence of dexamethasone through 4 weeks, compared to constructs cultured in the presence of dexamethasone. However, constructs cultured with the continuous absence of dexamethasone showed a marked decrease in properties by 8 weeks compared to those with the continuous addition of dexamethasone. Interestingly, when dexamethasone was added later in culture, construct properties were maintained through the 8 weeks. Conversely, when dexamethasone was withdrawn at 4 weeks, construct properties decreased to the level of the continuous absence of dexamethasone. This suggests that the presence of dexamethasone is essential during the latter phases of construct maintenance, but may not be necessary during the rapid growth phase of construct maturation.
These mechanical and biochemical analyses were largely confirmed by histological assessment, which also showed that constructs with continuous addition of dexamethasone or with dexamethasone added later retained a dense matrix in both peripheral and central regions. Conversely, the removal or absence of dexamethasone resulted in marked depletion of GAG, with greater loss in the peripheral region. Analysis of loss of GAG and nitrite content (indicative of inflammation) in media showed that all groups had low levels, regardless of the presence of dexamethasone, at 4 weeks. Later, however, levels of both indicators of a catabolic state gradually increased for constructs cultured without dexamethasone, while release of GAG and nitrite production by constructs with dexamethasone remained lower. These data suggest that constructs produce greater properties in the absence of dexamethasone over the short term (through week 4), but require dexamethasone to retain these properties over longer durations (through week 8), as shown in the first study. Given the finding of increased nitrite in media over this period, the role of dexamethasone during this period may arise from the state of cells after maturation is complete. It has been reported that IGF-1 signaling, which maintains cartilage homeostasis in healthy cartilage, is sensitive to nitric oxide (NO). The presence of NO decreases IGF receptor expression and makes cartilage insensitive to the anabolic effects of IGF-136; 37. This effect of NO was enhanced when glutathione (an anti-oxidant) was depleted from the media36. This implies that reactive oxygen species that arise during long term culture (due to an increase of oxidative stress) may be mitigated by the action of dexamethasone, making this factor essential for the stability of MSC-based engineered cartilage cultured in vitro for long periods.
Based on the finding that dexamethasone is essential for the long term stability of chondrogenic MSC-based constructs, we next queried the potential protective role of this factor when constructs were transferred to in vivo like conditions including serum, a ‘conflict’ signal. Constructs cultured from the outset in serum containing media, with or without dexamethasone, failed to mature and produced no matrix. However, when constructs were pre-cultured in CM+ for 4 weeks, and then converted to serum containing media, dexamethasone had a positive effect on long term outcomes. That is, while all constructs lost mechanical properties compared to their week 4 values in serum containing media, GAG loss was attenuated with dexamethasone (34% loss compared to 80% loss without dexamethasone at 8 weeks). Interestingly, GAG release after transfer to serum containing media occurred very rapidly, reaching a maximum in one week. Collagen content was largely unaffected in the absence of dexamethasone (DEX-), but constructs cultured or transferred to Serum showed inferior staining and/or some depletion of type II collagen, suggesting that collagen synthesis and maturation may be influenced by catabolic elements in serum. Since synovial fluid contains serum elements and other factors, the response of MSC-laden constructs in serum may not fully reflect those to synovial fluid. Despite this, our results clearly showed an effect of serum components in the production and maturation of matrix. When constructs were transferred to Serum, released GAG and MMPs in the media increased. Since measured MMP activity in Serum (10% FBS) is low (5mM), this implies that MMPs secretion by cells was induced by the addition of serum.
While the literature on the effect of dexamethasone offer diverse findings20; 24; 25; 27, results from our study and many others suggest that dexamethasone supports matrix accumulation, especially combined with growth factors (e.g., TGF-β3), and suppresses matrix catabolism. This suggests that delivery of dexamethasone could be optimized to sustain construct properties by systemic or local delivery post-construct implantation, and that the timing and concentration of this delivery should be considered25; 27. Since local delivery of dexamethasone may be required, it is auspicious that numerous methods to deliver dexamethasone and other growth factors have been introduced for cartilage repair. For example, early work by Nuttelman et al., generated dexamethasone-releasing poly(ethylene glycol) (PEG)-based hydrogels, where dexamethasone was covalently linked to a photo-reactive PEG molecule via a degradable lactide bond. This allowed for sustained release of dexamethasone with in vivo hydrolysis of the ester bond38. Other work, by Na et al., fabricated a thermo-reversible hydrogel using poly(NiPAAm-co-AAc) as an injectable drug delivery vehicle with co-encapsulated dexamethasone and TGF-β339; 40. Similarly, local dual delivery of dexamethasone/TGF-β3 via with heparin in or on PLGA microspheres has been demonstrated to promote rabbit MSC chondrogenesis41. Most recently, Roach et al., demonstrated delivery of dexamethasone via PLGA microspheres in a chondrocyte-laden agarose hydrogel42. Kopesky et al., utilized a self-assembled peptide hydrogel (i.e., AcN-(KLDL)3-CNH2) encapsulated with TGF-β1 for sustained delivery of TGF-β143. Jung et al., introduced monoCB[6]/DAH-HA (monofunctionalized cucurbit[6]uril-HA (CB[6]-HA), diamino-hexane conjugated HA (DAH-HA), and drug conjugated CB[6] (drug-CB[6])) for controlled chondrogenesis of human MSCs44. Finally, Bajpayee et al., introduced avidin for charge based intraarticular delivery of a single dose of dexamethasone, and demonstrated that this single dose was both quickly and slowly released45; 46.
Collectively, our findings support the importance of dexamethasone in improving and maintaining functional MSC chondrogenesis in HA hydrogels. Targeted delivery and controlled release of dexamethasone (and TGF-β3) and recent advances in drug delivery systems to enable their release, may enable successful cartilage repair with MSC-based constructs by controlling tissue homoeostasis and promoting long term function after implantation.
Supplementary Material
Supplementary 1: Viability of MSC-laden constructs cultured in various media components at 8 weeks. Live (green; left column) and dead (red; right) cells in various media conditions; scale bar = 100 μm.
Supplementary 2: Viability of MSC-laden constructs with and without dexamethasone in defined media. Live cells near the edge (left column) and at the center (middle) of constructs; Dead cells (right column) at 8 weeks; Constructs with continuous (C-C or D-D) or addition/removal (C-D or D-C) of dexamethasone in defined media; scale bar = 100 μm.
Supplementary 3: Viability of MSC-laden constructs in CM+ or with transfer to serum containing media. Constructs cultured in CM+ for 8 weeks (top row); constructs transferred to serum containing media with TGF and/or dexamethasone (T+/D+ (middle) and T+/D- (bottom)) after culture for 4 weeks in CM+; scale bar = 100 μm.
Acknowledgments
This work was supported by the National Institutes of Health (R01 EB008722) and the Department of Veterans’ Affairs (I01 RX000700).
References
- 1.Chung C, Burdick JA. 2009. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A 15:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byers BA, Mauck RL, Chiang IE, et al. 2008. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A 14:1821–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams CG, Kim TK, Taboas A, et al. 2003. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 9:679–688. [DOI] [PubMed] [Google Scholar]
- 4.Bosnakovski D, Mizuno M, Kim G, et al. 2006. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 93:1152–1163. [DOI] [PubMed] [Google Scholar]
- 5.Dickhut A, Gottwald E, Steck E, et al. 2008. Chondrogenesis of mesenchymal stem cells in gel-like biomaterials in vitro and in vivo. Front Biosci 13:4517–4528. [DOI] [PubMed] [Google Scholar]
- 6.Bian L, Guvendiren M, Mauck RL, et al. 2013. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A 110:10117–10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdick JA, Chung C, Jia X, et al. 2005. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 6:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung C, Beecham M, Mauck RL, et al. 2009. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials 30:4287–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson IE, Huang AH, Sengupta S, et al. 2009. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage 17:1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khetan S, Chung C, Burdick JA. 2009. Tuning hydrogel properties for applications in tissue engineering. Conf Proc IEEE Eng Med Biol Soc 2009:2094–2096. [DOI] [PubMed] [Google Scholar]
- 11.Mackay AM, Beck SC, Murphy JM, et al. 1998. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4:415–428. [DOI] [PubMed] [Google Scholar]
- 12.Pittenger MF, Mackay AM, Beck SC, et al. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JU, Barthel TS, Nishimura K, et al. 1998. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 80:1745–1757. [DOI] [PubMed] [Google Scholar]
- 14.Johnstone B, Hering TM, Caplan AI, et al. 1998. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238:265–272. [DOI] [PubMed] [Google Scholar]
- 15.Lima EG, Bian L, Mauck RL, et al. 2006. The effect of applied compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Conf Proc IEEE Eng Med Biol Soc 1:779–782. [DOI] [PubMed] [Google Scholar]
- 16.Huang AH, Stein A, Tuan RS, et al. 2009. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A 15:3461–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M, Erickson IE, Choudhury M, et al. 2012. Transient exposure to TGF-beta3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater 11:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian L, Zhai DY, Tous E, et al. 2011. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32:6425–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt TA, Gastelum NS, Nguyen QT, et al. 2007. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum 56:882–891. [DOI] [PubMed] [Google Scholar]
- 20.Florine EM, Miller RE, Porter RM, et al. 2013. Effects of Dexamethasone on Mesenchymal Stromal Cell Chondrogenesis and Aggrecanase Activity: Comparison of Agarose and Self-Assembling Peptide Scaffolds. Cartilage 4:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grodzinsky AJ, Wang Y, Kakar S, et al. 2017. Intra-articular dexamethasone to inhibit the development of post-traumatic osteoarthritis. J Orthop Res 35:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebeyrol C, Saint-Criq V, Guillot L, et al. 2012. Glucocorticoids reduce inflammation in cystic fibrosis bronchial epithelial cells. Cell Signal 24:1093–1099. [DOI] [PubMed] [Google Scholar]
- 23.Lv Q 2016. Glucocorticoid combined with hyaluronic acid enhance glucocorticoid receptor activity through inhibiting p-38MAPK signal pathway activation in treating acute lung injury in rats. Eur Rev Med Pharmacol Sci 20:3920–3929. [PubMed] [Google Scholar]
- 24.Jakobsen RB, Ostrup E, Zhang X, et al. 2014. Analysis of the effects of five factors relevant to in vitro chondrogenesis of human mesenchymal stem cells using factorial design and high throughput mRNA-profiling. PLoS One 9:e96615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shintani N, Hunziker EB. 2011. Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. Eur Cell Mater 22:302–319; discussion 319–320. [DOI] [PubMed] [Google Scholar]
- 26.Cheng X, Chen JL, Ma ZL, et al. 2014. Biphasic influence of dexamethasone exposure on embryonic vertebrate skeleton development. Toxicol Appl Pharmacol 281:19–29. [DOI] [PubMed] [Google Scholar]
- 27.Tangtrongsup S, Kisiday JD. 2016. Effects of Dexamethasone Concentration and Timing of Exposure on Chondrogenesis of Equine Bone Marrow-Derived Mesenchymal Stem Cells. Cartilage 7:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauck RL, Soltz MA, Wang CC, et al. 2000. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122:252–260. [DOI] [PubMed] [Google Scholar]
- 29.Mauck RL, Wang CC, Oswald ES, et al. 2003. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage 11:879–890. [DOI] [PubMed] [Google Scholar]
- 30.Farndale RW, Buttle DJ, Barrett AJ. 1986. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883:173–177. [DOI] [PubMed] [Google Scholar]
- 31.Neuman RE, Logan MA. 1950. The determination of hydroxyproline. J Biol Chem 184:299–306. [PubMed] [Google Scholar]
- 32.Bredt DS, Snyder SH. 1994. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63:175–195. [DOI] [PubMed] [Google Scholar]
- 33.Gstraunthaler G 2003. Alternatives to the use of fetal bovine serum: serum-free cell culture. ALTEX 20:275–281. [PubMed] [Google Scholar]
- 34.Morris JG, Cripe WS, Chapman HL Jr., et al. 1984. Selenium deficiency in cattle associated with Heinz bodies and anemia. Science 223:491–493. [DOI] [PubMed] [Google Scholar]
- 35.Yamane I, Murakami O, Kato M. 1975. Role of bovine albumin in a serum-free suspension cell culture medium. Proc Soc Exp Biol Med 149:439–442. [DOI] [PubMed] [Google Scholar]
- 36.Studer RK. 2004. Nitric oxide decreases IGF-1 receptor function in vitro; glutathione depletion enhances this effect in vivo. Osteoarthritis Cartilage 12:863–869. [DOI] [PubMed] [Google Scholar]
- 37.Studer RK, Levicoff E, Georgescu H, et al. 2000. Nitric oxide inhibits chondrocyte response to IGF-I: inhibition of IGF-IRbeta tyrosine phosphorylation. Am J Physiol Cell Physiol 279:C961–969. [DOI] [PubMed] [Google Scholar]
- 38.Nuttelman CR, Tripodi MC, Anseth KS. 2006. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. J Biomed Mater Res A 76:183–195. [DOI] [PubMed] [Google Scholar]
- 39.Na K, Park JH, Kim SW, et al. 2006. Delivery of dexamethasone, ascorbate, and growth factor (TGF beta-3) in thermo-reversible hydrogel constructs embedded with rabbit chondrocytes. Biomaterials 27:5951–5957. [DOI] [PubMed] [Google Scholar]
- 40.Na K, Kim S, Woo DG, et al. 2007. Combination material delivery of dexamethasone and growth factor in hydrogel blended with hyaluronic acid constructs for neocartilage formation. J Biomed Mater Res A 83:779–786. [DOI] [PubMed] [Google Scholar]
- 41.Park JS, Na K, Woo DG, et al. 2009. Determination of dual delivery for stem cell differentiation using dexamethasone and TGF-beta3 in/on polymeric microspheres. Biomaterials 30:4796–4805. [DOI] [PubMed] [Google Scholar]
- 42.Roach BL, Kelmendi-Doko A, Balutis EC, et al. 2016. Dexamethasone Release from Within Engineered Cartilage as a Chondroprotective Strategy Against Interleukin-1alpha. Tissue Eng Part A 22:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopesky PW, Byun S, Vanderploeg EJ, et al. 2014. Sustained delivery of bioactive TGF-beta1 from self-assembling peptide hydrogels induces chondrogenesis of encapsulated bone marrow stromal cells. J Biomed Mater Res A 102:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung H, Park JS, Yeom J, et al. 2014. 3D tissue engineered supramolecular hydrogels for controlled chondrogenesis of human mesenchymal stem cells. Biomacromolecules 15:707–714. [DOI] [PubMed] [Google Scholar]
- 45.Bajpayee AG, Quadir MA, Hammond PT, et al. 2016. Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthritis Cartilage 24:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bajpayee AG, Wong CR, Bawendi MG, et al. 2014. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials 35:538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1: Viability of MSC-laden constructs cultured in various media components at 8 weeks. Live (green; left column) and dead (red; right) cells in various media conditions; scale bar = 100 μm.
Supplementary 2: Viability of MSC-laden constructs with and without dexamethasone in defined media. Live cells near the edge (left column) and at the center (middle) of constructs; Dead cells (right column) at 8 weeks; Constructs with continuous (C-C or D-D) or addition/removal (C-D or D-C) of dexamethasone in defined media; scale bar = 100 μm.
Supplementary 3: Viability of MSC-laden constructs in CM+ or with transfer to serum containing media. Constructs cultured in CM+ for 8 weeks (top row); constructs transferred to serum containing media with TGF and/or dexamethasone (T+/D+ (middle) and T+/D- (bottom)) after culture for 4 weeks in CM+; scale bar = 100 μm.