Abstract
Background and Aims
Roots take up phosphorus (P) as inorganic phosphate (Pi). Enhanced root proliferation in Pi-rich patches enables plants to capture the unevenly distributed Pi, but the underlying control of root proliferation remains largely unknown. Here, the role of auxin in this response was investigated in maize (Zea mays).
Methods
A split-root, hydroponics system was employed to investigate root responses to Pi supply, with one (heterogeneous) or both (homogeneous) sides receiving 0 or 500 μm Pi.
Key results
Maize roots proliferated in Pi-rich media, particularly with heterogeneous Pi supply. The second-order lateral root number was 3-fold greater in roots of plants receiving a heterogeneous Pi supply than in roots of plants with a homogeneous Pi supply. Root proliferation in a heterogeneous Pi supply was inhibited by the auxin transporter inhibitor 1-N-naphthylphthalamic acid (NPA). The proliferation of lateral roots was accompanied by an enhanced auxin response in the apical meristem and vascular tissues at the root tip, as demonstrated in a DR5::RFP marker line.
Conclusions
It is concluded that the response of maize root morphology to a heterogeneous Pi supply is modulated by local signals of Pi availability and systemic signals of plant P nutritional status, and is mediated by auxin redistribution.
Keywords: Auxin, heterogeneous phosphate supply, lateral-root development, maize, root morphology
INTRODUCTION
Phosphorus (P) is an essential macronutrient for plant growth and development (Hawkesford et al., 2012). Plant roots take up P from the soil solution mainly in the form of inorganic phosphate (Pi). In the soil, Pi is easily precipitated and adsorbed to soil particles, resulting in low Pi concentrations in the soil solution and a low diffusion coefficient (Tinker and Nye, 2000; Vance et al., 2003). The application of fertilizer, agricultural cultivation, soil biochemical processes and other factors lead to a heterogeneous distribution of Pi in soil (Robinson, 1996). In order to capture the unevenly distributed Pi plants have developed various strategies, including topsoil foraging (White et al., 2013) and root proliferation in Pi-rich patches (Hodge, 2004), rapid nutrient acquisition (Jackson et al., 1990), and modification of the rhizosphere through acidification (Jing et al., 2012) or release of organic acids (White et al., 2013). These adaptations play an important role in nutrient capture and enhanced nutrient-use efficiency in environments with a heterogeneous Pi supply (Shen et al., 2011).
Due to the heterogeneous distribution and low diffusion rate of Pi in soil, root architectural and morphological plasticity are central to the efficient acquisition of Pi by plant roots (Hodge, 2004; White et al., 2013). Root architectural responses, such as shallower root growth angle, enhance topsoil foraging, thereby increasing P acquisition in both cereal (maize) and leguminous species (bean and soybean) (Lynch, 2011). Root proliferation in Pi-rich patches is an effective way to access the poorly mobile and heterogeneously distributed Pi in soil. Numerous studies have observed that plant roots respond to Pi-rich patches by altering their morphology, especially through lateral root (LR) development (Linkohr et al., 2002; Sun et al., 2002; Shen et al., 2005; Yano and Kume, 2005; Thibaud et al., 2010; Liu et al., 2013). Early experiments with barley (Hordeum vulgare) showed that localized Pi supply stimulated LR growth within Pi-rich zones, while LR formation was suppressed in Pi-depleted zones (Drew, 1975). In common bean (Phaseolus vulgaris), fine-root proliferation exploiting Pi-rich patches was observed in plants with restricted P nutrition (Snapp et al., 1995). The LR development of different species appears to respond differently to heterogeneous Pi supply. In Arabidopsis thaliana, a heterogeneous Pi supply led to greater LR length in the Pi-rich zones, whereas LR density, defined as the LR number on each unit length of axial root, was not affected (Linkohr et al., 2002), or was even lower than in plants with a uniform Pi supply (Liu et al., 2013). In faba bean (Vicia faba), no significant change in root morphology was observed in response to a heterogeneous Pi supply, although this might have been a consequence of the P-replete nutritional status of the plants (Li et al., 2014). In white lupin (Lupinus albus), the formation of cluster roots occurs in response to a heterogeneous Pi supply (Shen et al., 2005; Shu et al., 2007). In wheat (Triticum aestivum), the densities of first-order and second-order LRs were significantly increased in the presence of a heterogeneous Pi supply (Sun et al., 2002). A field study of maize demonstrated that localized application of Pi and ammonium increased LR development and acidification in the nutrient-rich zones, thereby enhancing nutrient acquisition (Jing et al., 2012; Ma et al., 2014). Nevertheless, the physiological mechanisms controlling the dynamic responses of root morphology to a heterogeneous Pi supply are still largely unknown.
Auxin plays an important role at many stages of LR development (Lavenus et al., 2013). For example, it promotes pericycle cell cycle reactivation for LR initiation (Casimiro et al., 2001; Berleth et al., 2004; De Smet et al., 2006) and maintains subsequent root apical meristem activity (Ruzicka et al., 2009). It has been widely reported that auxin is a key regulator of LR development in response to Pi supply (Al-Ghazi et al., 2003; Peret et al., 2014). Previous studies have shown that the application of exogenous auxin enhances LR development in response to Pi deficiency (Gilbert et al., 2000; Tang et al., 2013), whereas auxin inhibitors curtail LR development in response to Pi deficiency (Lopez-Bucio et al., 2002). In Arabidopsis, studies using mutants have shown that Pi availability regulates LR development by altering auxin biosynthesis (Liu et al., 2013), signalling (Lopez-Bucio et al., 2005), sensitivity (Perez-Torres et al., 2008) and polar transport (Nacry et al., 2005; Talboys et al., 2014). In rice, overexpression of a Pi transporter, OsPht1;8, upregulated the expression of auxin-related genes and enhanced LR formation, but the mechanisms underlying this response are still to be determined (Jia et al., 2017). In maize, transcriptomic analyses have shown that auxin biosynthesis and transport participate in the development of LRs in response to Pi deficiency (Li et al., 2012). Heterogeneous Pi supply increased root proliferation in Pi-rich patches to capture the unevenly distributed Pi, but the underlying control of root proliferation is unclear. Here, we tested the hypothesis that heterogeneous Pi supply influences maize LR proliferation by regulating auxin redistribution. To understand better the morphological responses of the maize root system to heterogeneous Pi supply, we investigated the changes in root morphology and Pi acquisition in response to a heterogeneous Pi supply. We observed that LRs proliferated in the presence of a heterogeneous Pi supply. Since auxin plays a central role in LR development, we studied the effect of an auxin polar transport inhibitor [1-N-naphthylphthalamic acid (NPA)], examined auxin distribution using an auxin-responsive maize marker line expressing red fluorescent protein (RFP) under the control of a DR5 auxin-responsive promoter (DR5::RFP line), and determined the expression of auxin-related genes in the response of maize roots to a heterogeneous Pi supply.
MATERIALS AND METHODS
Plant material and growth conditions
Seeds of Zea mays B73 were surface-sterilized in 10 % H2O2 solution for 30 min, rinsed five times with water, and then soaked in saturated CaSO4 solution with continuous aeration for about 12 h. Thereafter, seeds were germinated in a filter paper roll system in distilled water at 25 °C (Woll et al., 2005). When two leaves were visible, the endosperm was removed and uniform seedlings were transferred to a Hoagland solution lacking Pi (LP) containing 2 mm Ca(NO3)2, 0.75 mm K2SO4, 0.65 mm MgSO4, 0.1 mm KCl, 1.0 × 10−3 mm H3BO3, 1.0 × 10−3 mm MnSO4, 1.0 × 10−4 mm CuSO4, 1.0 × 10−3 mm ZnSO4, 5.0 × 10−6 mm (NH4)6Mo7O24 and 0.1 mm Fe-EDTA. After 6 d of pre-culture in LP, uniform maize seedlings were selected and the primary root and seminal roots were carefully removed so that only two nodal roots of equal length (~15 cm long) remained on each plant. Both of these two remaining roots originated from the first node just above the mesocotyl, and were ~120° apart in the horizontal direction with respect to their origins at the stem. After 2–3 d of further pre-culture in LP solution to recover, these two remaining nodal roots were then placed in separate chambers of a double-chambered container for split-root experiments (Supplementary Data Fig. S1A). Three maize seedlings shared one double-chambered container and each chamber contained 1.7 L of nutrient solution. Three Pi treatments were applied. Phosphate was added as 500 μm KH2PO4 to the Hoagland solution in both compartments (HP treatment), only one compartment (Hetero-HP/Hetero-LP treatment) or neither compartment (LP treatment) (Supplementary Data Fig. S1A). To maintain an equimolar K concentration, KCl was added to the Hetero-LP compartment and in the LP treatment. The pH of the solution was adjusted daily to 6.0, and all solutions were renewed every 3 d. The nutrient solution was aerated continuously. Plants were grown in a controlled environment with a light:dark regime of 16:8 h, a temperature of 25:18 °C and a light intensity of 230 μmol m−2 s−1. During split-root experiments, the two original nodal roots were the only roots accessing nutrient solution. Plants were harvested after 2, 4 and 6 d of treatment (DAT), and shoot and root parameters were measured. For the experiment employing NPA, NPA was dissolved in DMSO before adding to the Hoagland solution. The final concentration of DMSO was 0.1 % (v/v) and the final concentration of NPA was 25 μm. In the control, only DMSO was supplied to the Hoagland solution, at a final concentration of 0.1 % (v/v). To investigate the effects of inhibition of auxin transport on root responses to contrasting Pi supply, NPA was applied to nutrient solutions in the split-root container. In addition, to test the hypothesis that auxin transport and distribution are altered in response to a heterogeneous Pi supply, NPA was applied during a pre-culture stage but subsequently withdrawn in the split-root system. For the NPA pre-treatment, after 6 d of pre-culturing in LP solution, the primary root and seminal roots were carefully removed, and the seedlings were allowed to grow for a further 2 d in LP solution with NPA. Seedlings with two similar nodal roots were then transferred to a split-root system containing nutrient solutions without NPA. All the experiments were conducted under the same growth conditions in the same environment-controlled growth chamber. The root responses to heterogeneous Pi were the same in the main experiment investigating Pi acquisition, biomass and root architecture, as well as in the experiment in which gene expression was studied, when DMSO alone was present in the nutrient solutions, and in the DR5::RFP line.
Shoot biomass and P concentration
After harvesting, shoot material was heated to 105 °C for 30 min then dried at 70 °C to a constant weight. Oven-dried shoots were cut into small pieces with scissors and ground into powder with mortars and pestles. The ground shoot material was ashed at 580 °C for 10 h, and then dissolved in 2 mL of 1:1 (v/v) HNO3 and 18 mL of deionized water. The solution was filtered through filter paper, and shoot P concentration was assayed using the vanado-molybdate method (Westerman, 1990). The shoot P content was calculated as the product of shoot P concentration and biomass.
Root parameter analysis
Roots from each compartment were analysed separately. Fresh roots were scanned using an Epson V800 scanner at a resolution of 400 dpi. Images were analysed using WinRhizo software (Regent Instruments, Quebec, QC, Canada) to obtain total root length and root surface area. The numbers of first- and second-order LRs were counted. The lengths of nodal roots and first- and second-order LRs were measured on scanned images using ImageJ software (version 1.4, http://rsb.info.nih.gov/ij). The average first-order LR length was assessed in the middle part of the nodal root, 5–10 cm from the base of the root, where the maximum length was found. The first-order LR density was determined by dividing the number of first-order LRs by the length of the branched zone of the nodal root. Feulgen staining (Woll et al., 2005) was used to determine the number of LR primordia and the length of LR meristems. After staining, roots were photographed using a stereomicroscope (Olympus BX51, Tokyo, Japan). The numbers of first- and second-order LR primordia were counted along the entire length of nodal roots, and the first-order LR primordium density was calculated by dividing the number of first-order LR primordia by the length of the primordium zone of the nodal root. Meristem length was assessed as the distance between the quiescent centre and the first elongating cell (Giehl et al., 2012). The lengths of root meristems and epidermal cells were quantified using ImageJ software (version 1.4). Root vitality (dehydrogenase activity) was analysed by the triphenyl tetrazolium chloride (TTC) method (Clemensson-Lindell, 1994). The entire nodal root system, including the axial root and the first- and second-order LRs, was taken to measure root vitality. The results presented are average values of six seedlings. For estimates of the length of the first-order LR meristem, each replicate consisted of more than ten first-order LRs.
Visualization of auxin response
Auxin responses were visualized using a DR5::RFP marker line (Gallavotti et al., 2008). Roots were fixed in 4 % paraformaldehyde in phosphate buffer for at least 1 h at 4 °C. First-order LR segments were observed using a laser confocal scanning microscope (Te2000-E; Nikon, Tokyo, Japan) with a filter set for rhodamine (excitation 546/12 nm, beamsplitter 560 nm, emission 575/64 nm). The intensity of fluorescence was quantified using the Te2000-E Nikon software.
Gene expression analysis
Root systems from each individual compartment were sampled to analyse the expression of Pi response genes. The first-order LRs in each compartment were sampled to analyse auxin and cell-cycle-related genes. After washing with distilled water three times, root samples were frozen in liquid nitrogen and kept in a −80 °C freezer. Total RNA was extracted using the Plant RNeasy Mini Kit from Qiagen according to the manufacturer’s protocol. Thereafter, 1 μg of total RNA was used for cDNA synthesis using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara) according to the product manual. Real-time quantitative PCR (qRT–PCR) was performed using the iQTM5 Real-Time PCR Detection Systems (Bio-Rad) with the SYBR Premix Ex Taq II (Tli RNaseH Plus) from Takara. Gene expression was determined on six biological replicates. The reference genes were Zmβ-tubulin (Wang et al., 2005) and ZmUBCP (Melida et al., 2015). The primers used to quantify gene expression are listed in Supplementary Data Table S1.
Statistical analysis
Data were analysed using IBM Statistics SPSS 21 (SPSS, Chicago, IL, USA). Following one-way ANOVA, Tukey’s HSD post hoc test was used to compare differences between means at the 0.05 probability level.
RESULTS
Shoot growth and P uptake
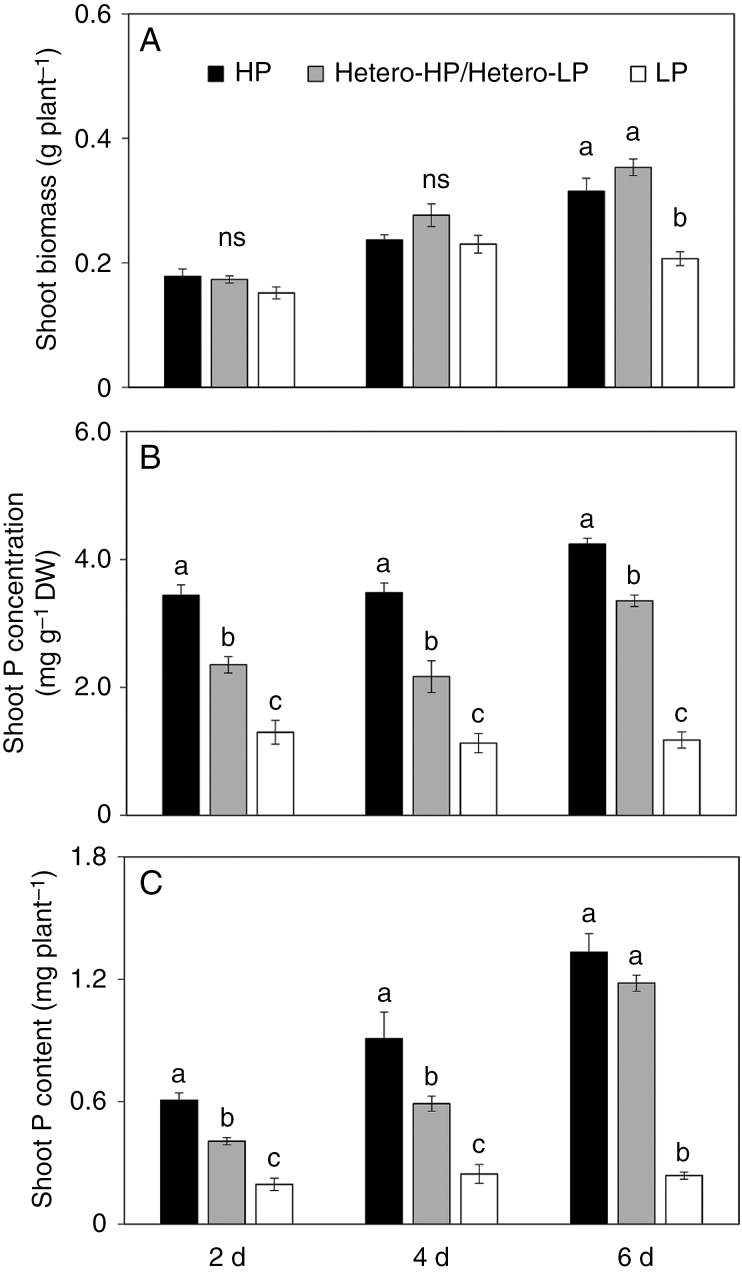
The consequences of heterogeneous Pi supply for maize root architecture were determined by growing plants in a split-root system in which one compartment received high Pi (Hetero-HP) and the other low Pi (Hetero-LP) solutions (Supplementary Data Fig. S1A). By 2 and 4 DAT, plants grown with a heterogeneous Pi supply (Hetero-HP/Hetero-LP) had similar shoot biomass (Fig. 1A), but lower shoot P concentration (Fig. 1B) and shoot P content (Fig. 1C) than plants receiving a homogeneous high Pi (HP) supply. Shoot P concentration (Fig. 1B) and shoot P content (Fig. 1C) were greater in the Hetero-HP/Hetero-LP treatment than in the treatment with a homogeneous low Pi (LP) supply. At 6 DAT, shoot biomass (Fig. 1A) and shoot P content (Fig. 1C) in plants receiving the Hetero-HP/Hetero-LP supply did not differ from plants receiving the HP treatment, but were greater than those in plants receiving the LP treatment. The shoot P concentration (Fig. 1B) of plants receiving the Hetero-HP/Hetero-LP treatment was 21 % lower than that of those receiving the HP treatment, but 183 % greater than that of those receiving the LP treatment.
Fig. 1.
Effect of homogeneous and heterogeneous Pi supply on plant growth and P status. (A) Shoot biomass, (B) shoot P concentration and (C) shoot P content of maize plants in a split-root system with different Pi supplies: both compartments receiving Pi (HP, black bars); one compartment receiving Pi and the other receiving no Pi (Hetero-HP/Hetero-LP, grey bars); and neither compartment receiving Pi (LP, white bars). Bars indicate means ± s.e. (n = 6 individual plants). Different letters indicate significant differences among Pi treatments on each given day (P < 0.05).
Root growth and development
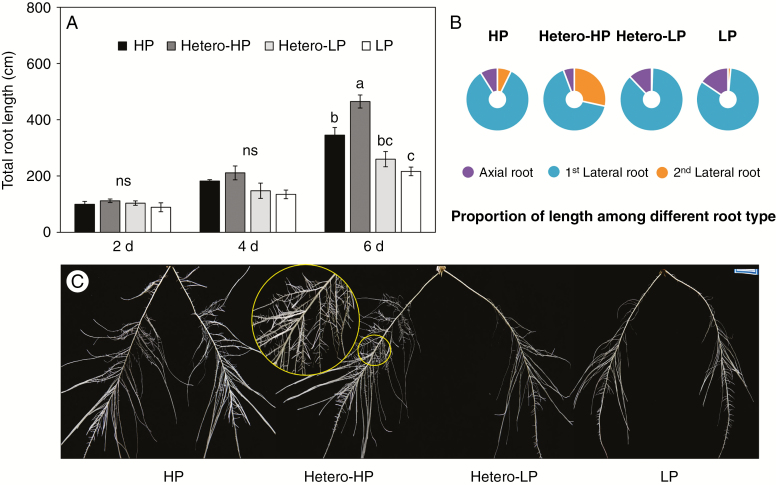
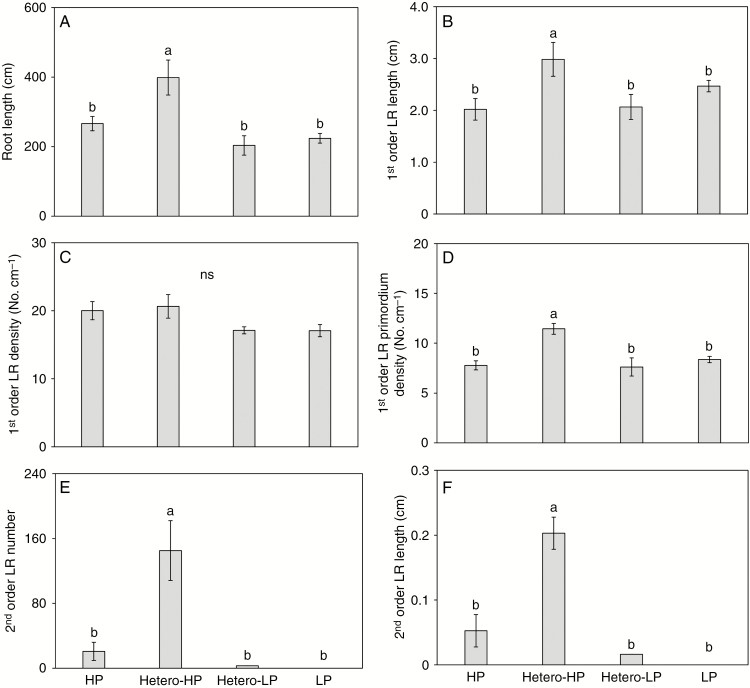
Roots in the Hetero-HP compartment proliferated more than roots in the Hetero-LP compartment and roots of plants receiving the HP or LP treatments (Fig. 2C). At 6 DAT, the total root length in the Hetero-HP compartment was 79 % greater than that in the Hetero-LP compartment (Fig. 2A). In addition, the total root length in the Hetero-HP compartment was 35 % greater than that in a single root compartment in plants receiving the HP treatment (Fig. 2A). Furthermore, LR development was greatly promoted in the Hetero-HP compartment (Fig. 2B, C). The proportion of second-order LRs to total root length was 57 and 4 times greater in the Hetero-HP compartment than in the Hetero-LP compartment and in the root compartments of plants receiving the HP treatment, respectively (Fig. 2B), while the proportion of first-order LRs to total root length was 24 and 21 % lower in the Hetero-HP compartment than in the Hetero-LP compartment and the root compartments of the HP treatment, respectively (Fig. 2B). Root vitality, as indicated by dehydrogenase activity, was analysed with the TTC method. Roots showed greater vitality in Hetero-HP than HP, LP and Hetero-LP (Supplementary Data Fig. S2). The vitality of roots in the Hetero-HP compartment and in the HP treatment was greater than that of roots in the Hetero-LP compartment and the LP treatment (Supplementary Data Fig. S2).
Fig. 2.
Effect of homogeneous and heterogeneous Pi supply on (A) total root length, (B) the proportion of different root types in total root length and (C) root phenotype of maize plants at 6 DAT. Maize plants receiving different Pi supplies were grown in a split-root system with both compartments receiving Pi (HP), one compartment receiving Pi (Hetero-HP) and the other receiving no Pi (Hetero-LP), or neither compartment receiving Pi (LP). In the homogeneous treatments, only one randomly chosen compartment was compared. Bars indicate means ± s.e. (n = 6 individual plants). Different letters indicate significant differences among treatments on each given day (P < 0.05). ns, not significant.
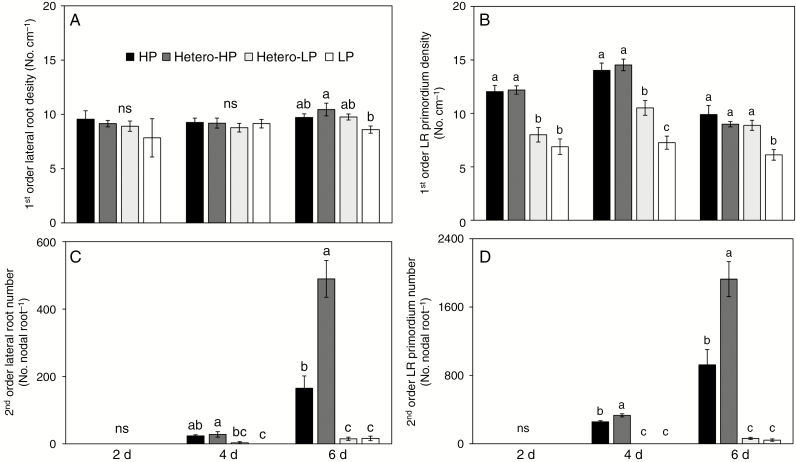
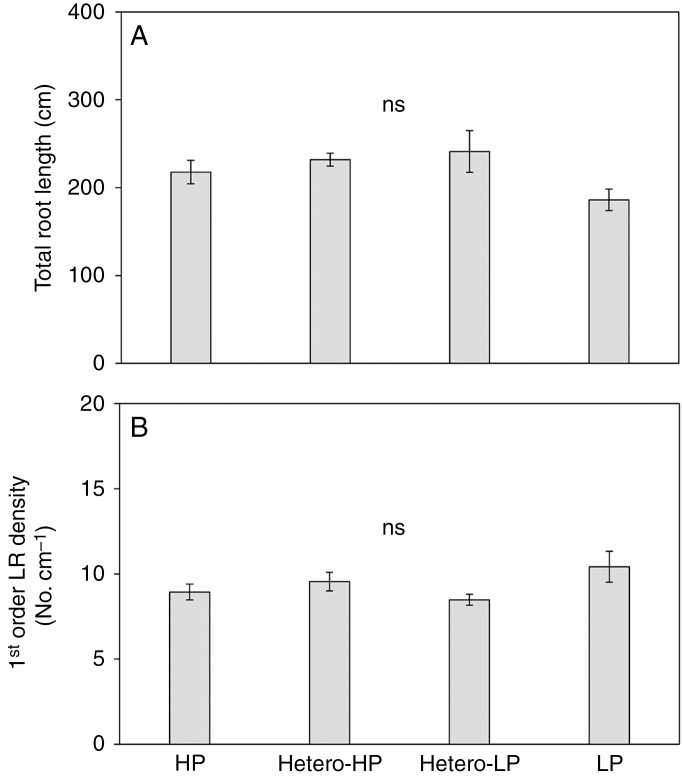
The first-order LR density of roots in the Hetero-HP compartment did not differ from that of roots in either the Hetero-LP compartment or in the root compartments of plants receiving the HP treatment, but was greater than that in roots of plants receiving the LP treatment at 6 DAT (Fig. 3A). By 2 and 4 DAT, there was a greater density of first-order LR primordia in roots in the Hetero-HP compartment than in the Hetero-LP compartment (Fig. 3B), but there was no difference between them at 6 DAT (Fig. 3B). No second-order LRs or second-order LR primordia were observed at 2 DAT (Fig. 3C, D). At 4 DAT, the number of second-order LRs of roots in the Hetero-HP compartment was 7.5 times greater than that of roots in the Hetero-LP compartment, but did not differ from the number of second-order LRs of roots of plants receiving the HP treatment (Fig. 3D). The number of second-order LR primordia in roots in the Hetero-HP compartment was 332 and 1.3 times higher than in roots in the Hetero-LP compartment and roots of plants receiving the HP treatment, respectively (Fig. 3D). At 6 DAT, roots in the Hetero-HP compartment had 32 times more second-order LRs (Fig. 3C) than roots in the Hetero-LP compartment. The number of second-order LRs in the Hetero-HP compartment was also significantly greater (3.2 times) than that in roots of plants receiving the HP treatment (Fig. 3C). The number of second-order LR primordia showed a similar pattern to that of second-order LR number among treatments (Fig. 3D).
Fig. 3.
Effect of homogeneous and heterogeneous Pi supply on (A) first-order LR density and (B) primordium number, and (C) second-order LR number and (D) primordium number of maize plants at 2, 4 and 6 DAT. For explanation of the treatments see the legend of Fig. 2. Bars indicate means ± s.e. (n = 6 individual plants). Different letters indicate significant differences among treatments on each given day (P < 0.05).
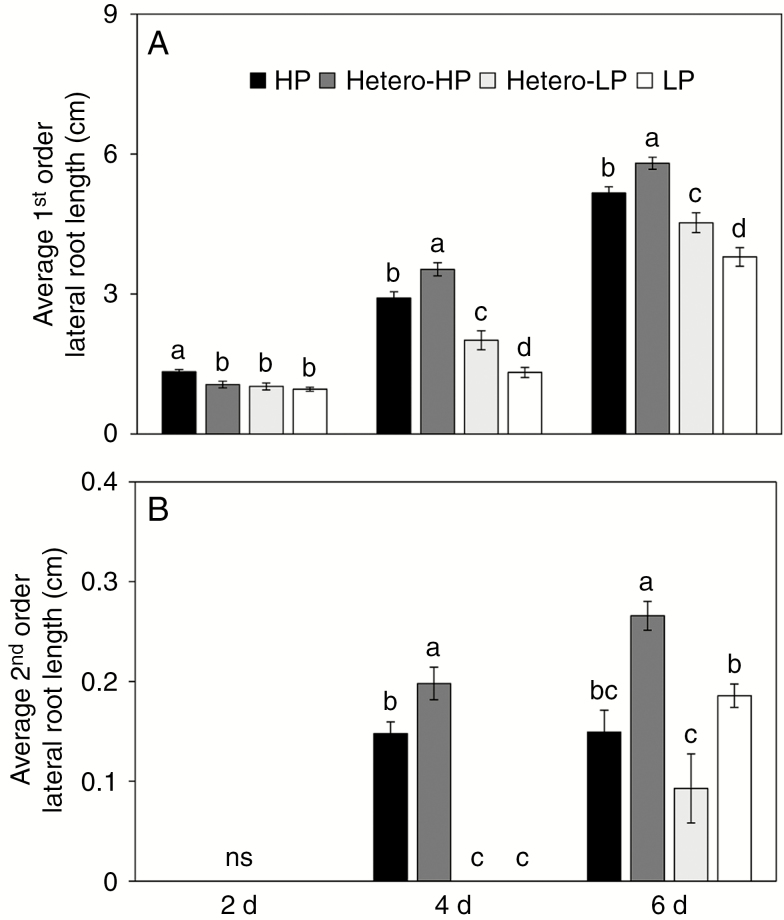
The average LR length in the middle part of the nodal root (5–10 cm from the base of the root) was also greater in the presence of a heterogeneous Pi supply. At 2 DAT the average length of first-order LRs in the Hetero-HP compartment was similar to that in the Hetero-LP compartment, but less than that in roots of plants receiving the HP treatment (Fig. 4A). Subsequently, the lengths of both first- and second-order LR in the Hetero-HP compartment were greater than in the Hetero-LP compartment and roots of plants receiving HP or LP treatments at 4 and 6 DAT (Fig. 4A, B). Microscopic analysis indicated that the length of LRs in the Hetero-HP compartment was stimulated by increasing the size of the LR meristem (Supplementary Data Fig. S3A) rather than the length of epidermal cells (Supplementary Data Fig. S3B).
Fig. 4.
Effect of homogeneous and heterogeneous Pi supply on the (A) lengths of first-order LRs and (B) second-order LRs of maize plants at 2, 4 and 6 DAT. For explanation of the treatments see the legend of Fig. 2. Bars indicate means ± s.e. (n = 6 individual plants); each replicate comprised more than ten LRs. Different letters indicate significant differences among treatments on each given day (P < 0.05).
Effects of auxin on root proliferation
The addition of the auxin polar transport inhibitor NPA to growth media reduced LR formation and elongation, and inhibited root proliferation in response to a heterogeneous Pi supply (Supplementary Data Fig. S4A). The total root length (Fig. 5A) and first-order LR density (Fig. 5B) in roots treated with NPA did not differ among Pi treatments. In addition, the development of second-order LRs was totally inhibited in all Pi treatments in the presence of NPA (Supplementary Data Fig. S4A).
Fig. 5.
Effect of the auxin polar transport inhibitor NPA on (A) total root length and (B) first-order LR density of maize plants at 6 DAT. NPA was applied to all solutions in the split-root container. For explanation of treatments see the legend of Fig. 2. Bars indicate means ± s.e. (n = 6 individual plants). Different letters indicate significant differences among treatments (P < 0.05).
For the seedlings that had been pre-cultured with NPA but were subsequently not exposed to NPA, LR proliferation was observed in roots in the Hetero-HP compartment at 6 DAT (Supplementary Data Fig. S4B). The total length of roots in the Hetero-HP compartment was 96 and 50 % greater than that in the Hetero-LP compartment and in roots of plants receiving the HP treatment, respectively (Fig. 6A). It is noteworthy that the total length of roots in the Hetero-HP compartment relative to that in the HP treatment was much greater when plants were pre-cultured with NPA (50 %, Fig. 6A) than when plants were not pre-cultured with NPA (35 %, Fig. 2A). There was no difference in first-order LR density among treatments (Fig. 6C). The first-order LR density in the roots of plants pre-cultured with NPA (Fig. 6C) was greater than that in the roots of plants that had not been pre-cultured with NPA (Fig. 3A) at 6 DAT, which is related to the reduced axial length of the nodal roots following the NPA pre-treatment (Supplementary Data Fig. S5). Roots in the Hetero-HP compartment had greater first-order LR length (Fig. 6B), first-order LR primordium density, second-order LR number and second-order LR length than roots in the Hetero-LP compartment and roots of plants receiving HP or LP treatments (Fig. 6D–F). Like total root length, LR formation and elongation of roots in the Hetero-HP compartment relative to roots in the HP treatment were greater in plants pre-cultured with NPA (Fig. 6B, D–F) than in plants pre-cultured without NPA (Figs 3 and 4). Heterogeneous Pi supply rescued second-order LR formation, which was strongly inhibited after pre-culturing with NPA (Fig. 6E, F).
Fig. 6.
Effect of homogeneous and heterogeneous Pi supply on (A) total root length, (B) length of first-order LRs, (C) first-order LR density, (D) first-order LR primordium density, (E) second-order LR number and (F) second-order LR length of maize plants pre-cultured with the auxin polar transport inhibitor NPA for 2 d but subsequently grown in the absence of NPA for 6 d. For explanation of treatments see the legend of Fig. 2. Bars indicate means ± s.e. (n = 6 individual plants). Different letters indicate significant differences among treatments (P < 0.05).
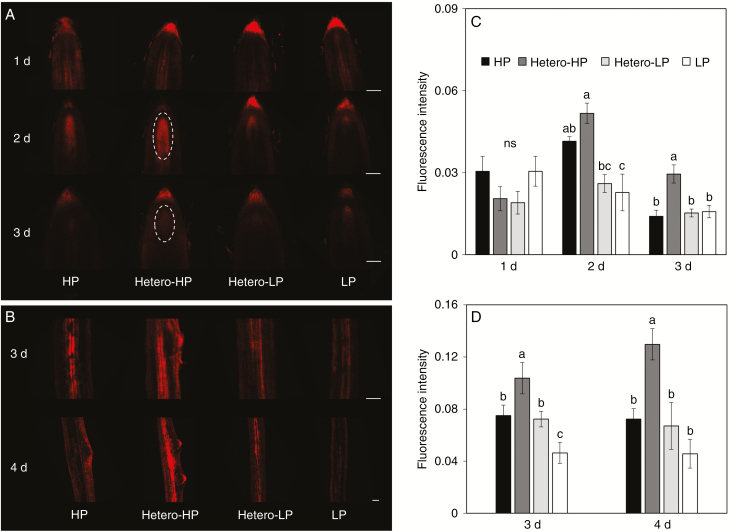
Auxin response and distribution were examined in first-order LRs using a DR5::RFP marker line. Laser confocal images indicated that there was greater auxin response around the root meristem zone at 2 and 3 DAT (Fig. 7A). At 3 DAT the fluorescence intensity around the meristem zone of roots was significantly greater in the Hetero-HP compartment than in the Hetero-LP compartment or in roots of plants receiving HP or LP treatments (Fig. 7C). In addition, the fluorescence signal around the vasculature where LR primordia are formed was much stronger than in other regions of roots in the Hetero-HP compartment (Fig. 7B) and was significantly greater than in roots receiving other Pi treatments at 3 and 4 DAT (Fig. 7D).
Fig. 7.
Effect of homogeneous and heterogeneous Pi supply on tissue-specific auxin distribution at 1, 2, 3 and 4 DAT. Auxin response reporter activity in DR5::RFP-expressing transgenic maize lines was observed in longitudinal orientation close to (A) the first-order LR tip meristematic zone and (B) the second-order LR initiation zone. Scale bars = 100 µm. The fluorescence signal intensity was measured (C) in the first-order LR apical meristematic zone at 1, 2 and 3 DAT, and (D) in the second-order LR initiation zone at 3 and 4 DAT. For explanation of treatments see the legend of Fig. 2. Each value represents the average fluorescence intensity of 10–15 pictures. Bars indicate means ± s.e. for four biological replicates and each replicate comprised six to ten individual root segments. Different letters indicate significant differences among treatments on each given day (P < 0.05).
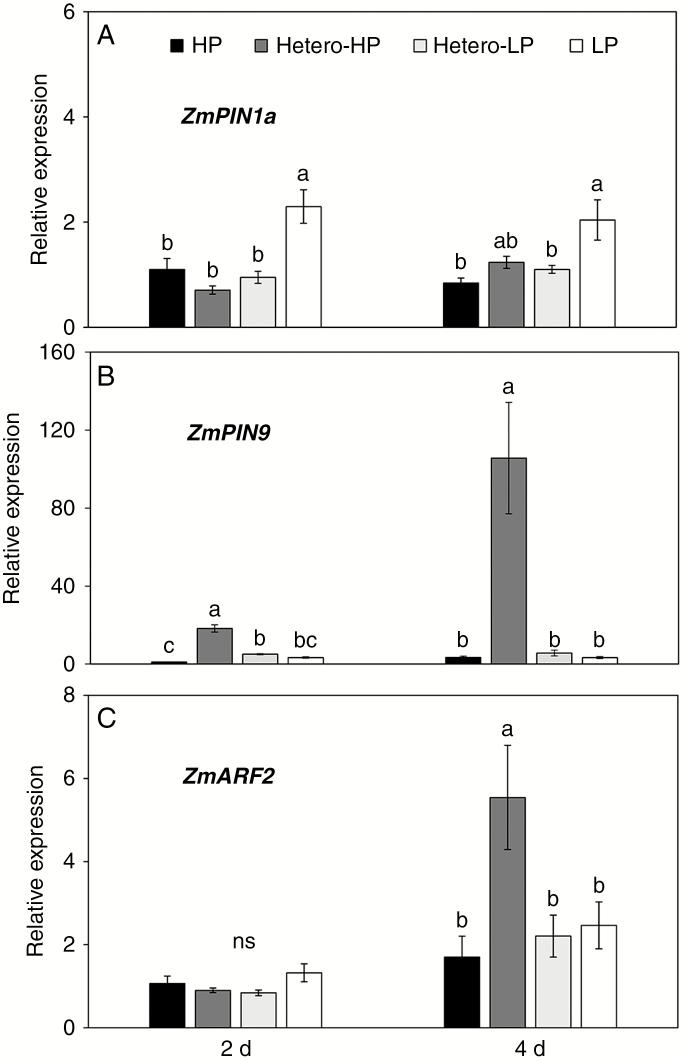
Gene expression analysis in the first-order LRs was performed using qRT–PCR at 2 and 4 DAT. At 2 DAT the expression of the auxin polar transporter ZmPIN9 (Fig. 8B) was greater in roots in the Hetero-HP compartment than in roots in the Hetero-LP compartment (3.6-fold) and in roots of plants receiving the HP treatment (18-fold), while another auxin polar transporter, ZmPIN1a (Fig. 8A), the auxin response genes ZmARF2 (Fig. 8C) and ZmTIR1 (Supplementary Data Fig. S6B) and the auxin biosynthesis gene ZmGH3 (Supplementary Data Fig. S6A) showed no significant differences among treatments. By 4 DAT, ZmPIN9 expression in the Hetero-HP compartment was 18 times greater than that in the Hetero-LP compartment and 33 times greater than that in the HP treatment (Fig. 8B). The expression of ZmARF2 (Fig. 8C), ZmTIR1 (Supplementary Data Fig. S6B), ZmGH3 (Supplementary Data Fig. S6A) and cell cycle genes ZmCYCB1;1 (Supplementary Data Fig. S6C) and ZmCDKB1;1 (Supplementary Data Fig. S6D) in the first-order LRs was also greater in roots in the Hetero-HP compartment than in roots in the Hetero-LP compartment or roots of plants receiving HP or LP treatments at 4 DAT. The expression of ZmPIN9 (Fig. 8B), ZmGH3, ZmCYCB1;1 and ZmCDKB1;1 (Supplementary Data Fig. S6A, C, D) increased between 2 and 4 DAT in roots in the Hetero-HP compartment, and that of ZmARF2 (Fig. 8C) and ZmTIR1 (Supplementary Data Fig. S6B) increased between 2 and 4 DAT in all treatments, while ZmPIN1a (Fig. 8A) did not change between 2 and 4 DAT in any treatment.
Fig. 8.
Effect of homogeneous and heterogeneous Pi supply on the expression patterns of auxin-related genes in first-order LRs. Expression of (A) ZmPIN1a, (B) ZmPIN9 and (C) ZmARF2 relative to that observed in first-order LRs of the HP treatment at 2 DAT was determined by qRT–PCR. For explanation of treatments see the legend of Fig. 2. Bars indicate means ± s.e. (n = 6 individual plants). Different letters indicate significant differences among treatments on each given day (P < 0.05).
DISCUSSION
Root foraging contributes to Pi acquisition and shoot growth in plants with a heterogeneous Pi supply
Nutrients often show a heterogeneous distribution in the soil, and root proliferation in nutrient-rich patches is an important foraging strategy, especially for elements, such as P, that are relatively immobile in the soil (Robinson, 1996; Yano and Kume, 2005; Hodge, 2010; Lynch, 2011; White et al., 2013; Li et al., 2014). In our split-root system, the total root length in the Hetero-HP compartment was 79 % greater than in the Hetero-LP compartment, illustrating the preferential partitioning of biomass to the place with greater Pi availability (Fig. 2A). This result is consistent with studies on other plant species showing a greater proportion of root biomass being apportioned to nutrient-rich zones (Adams et al., 2002; Yano and Kume, 2005). In addition, total length (Fig. 2A) and vitality (Supplementary Data Fig. S2) were greater in roots in the Hetero-HP compartment than in roots of plants receiving the HP treatment. It has been reported that root density (He et al., 2003; Thibaud et al., 2010) and Pi uptake capacity (Jackson et al., 1990) are increased in Pi-rich patches, which allows plants to compensate for restricted Pi acquisition by other parts of the root system.
The shoots of the plants receiving a heterogeneous HP supply achieved a similar biomass and P content to plants receiving a homogeneous HP supply, despite having access to only half the Pi supply (Fig. 1). These results suggest that, in comparison with a homogeneous HP supply, roots receiving a heterogeneous Pi supply had enhanced P acquisition efficiency (i.e. Pi acquisition per unit root length), which contributed to P uptake and biomass production. Previous field studies have also shown that greater root proliferation and enhanced root uptake capacity in Pi-rich patches maintains Pi uptake and biomass production (Shen et al., 2005; Yano and Kume, 2005; Jing et al., 2012; Ma et al., 2013). The proportion of second-order LRs to total root length in the Hetero-HP compartment was much greater than in roots of plants receiving the HP treatment (Fig. 2B). Lateral roots are thinner and have larger specific surface area than the main root, which increases nutrient acquisition efficiency (Wen et al., 2019). Thus, increased LR proliferation in Pi-rich zones provides an effective Pi foraging strategy in plants with a heterogeneous Pi supply.
Changes in root architecture in response to heterogeneous Pi supply
The development of LRs is a crucial component of root morphological responses to heterogeneous Pi supply, and LR proliferation greatly enhances soil exploration and acquisition in Pi from Pi-rich patches (Lynch, 2011). Our results showed that second-order LR formation was stimulated in the Hetero-HP compartment (Fig. 3C, D). The formation of LRs appears to be affected differently by a heterogeneous Pi supply depending on the way the heterogeneous Pi is applied, the growth stage of the plant and the plant species studied (Snapp et al., 1995; Sun et al., 2002; Liu et al., 2013). Different plant species exhibit different strategies in response to heterogeneous distribution of nutrients (Robinson, 1994). In general, monocot species place a larger proportion of roots in nutrient-rich patches than dicots (Cahill and McNickle, 2011). Regarding root morphology, previous studies have shown that cereals are more responsive to P supply than leguminous species (Li et al., 2014; Liu et al., 2016; Lyu et al., 2016). In the present study, second-order LR development in maize was stimulated significantly in response to heterogeneous HP supply, which is consistent with previous studies in barley (Drew, 1975) and wheat (Sun et al., 2002), indicating the strong morphological plasticity of cereal roots in response to heterogeneous Pi supply. Root developmental processes of monocot cereals such as maize are more complex than in the model plant Arabidopsis (Jansen et al., 2012). Maize has nodal roots, which do not exist in Arabidopsis (Hochholdinger and Zimmermann, 2008; Yu et al., 2016). Furthermore, LR initiation in maize is associated with the radial positioning of phloem-pole pericycle cells rather than the xylem-pole pericycle cells in Arabidopsis (Jansen et al., 2012; Yu et al., 2015). In the present study, the numbers of second-order LRs and second-order LR primordia were increased, but those of first-order LRs were barely altered by a heterogeneous Pi supply (Fig. 3). There were no significant differences in first-order LR density among treatments at 2 or 4 DAT, but decreased slightly in the LP treatment at 6 DAT (Fig. 3A), which was probably related to the reduced primordium density in this treatment (Fig. 3B). As expected, primordium density (Fig. 3B) showed more pronounced responses to Pi supply than LR density (Fig. 3A). The different response of first-order LRs and second-order LRs to heterogeneous Pi application might be explained as follows: (1) second-order LRs exhibit finer diameter and more rapid rates of production and mortality than first-order LRs, which are regarded as the sign of stronger plasticity in ecological studies (Hodge, 2004; Ito et al., 2006); (2) at the beginning of the split-root treatment, some of the first-order LR primordia have already formed, which may conceal any stimulating effect of heterogeneous Pi supply on first-order LR formation. Thus, it is more meaningful to focus on the responses of new second-order LRs rather than first-order LRs to heterogeneous Pi supply in the experiment reported here.
Roots in the Hetero-HP compartment had greater average length of both first- and second-order LRs compared with roots in the Hetero-LP compartment or roots of plants receiving HP or LP treatments (Fig. 4A), which is consistent with results in Arabidopsis (Linkohr et al., 2002). Microscopic analysis of root anatomy indicated that this was a consequence of enlargement of the apical meristem of LRs in the Hetero-HP compartment (Supplementary Data Fig. S3). In addition, the length of axial roots in the LP treatment was greater than that in the HP treatment or in the Hetero-HP compartment (Supplementary Data Fig. S5). This result is consistent with a previous study on maize in a hydroponic system, which also reported that low Pi promoted axial root elongation compared with a sufficient Pi supply (Li et al., 2012). Work on Arabidopsis in gel medium showed arrested axial root elongation under Pi deficiency (Svistoonoff et al., 2007), which was related to the excess Fe availability in the medium (Ward et al., 2008; Müller et al., 2015). Hence, the response pattern of axial root elongation to low Pi availability is dependent on the experimental system and plant species. The regulatory mechanisms of root elongation in response to heterogeneous Pi availability in maize require further examination.
Role of auxin in root proliferation in response to a heterogeneous Pi supply
Auxin plays an essential role in LR development (Lavenus et al., 2013). In the present study, the auxin polar transport inhibitor NPA reduced the amount of LR proliferation induced by a heterogeneous Pi supply (Supplementary Data Fig. S4A), suggesting that auxin polar transport was involved in the regulation of LR development in response to heterogeneous Pi supply. Similarly, NPA application restricted LR formation in Arabidopsis with low Pi supply (Lopez-Bucio et al., 2002). Furthermore, the increase in total root length in the Hetero-HP treatment was enhanced in comparison with that in the HP treatment when pre-cultured with NPA (Figs 2A and 6A). Studies using the DR5::RFP maize line revealed a strong auxin response in root apical and pericycle cells of first-order LRs in the Hetero-HP compartment (Fig. 7), suggesting that a heterogeneous Pi supply promoted auxin redistribution. In Arabidopsis and wheat, Pi deficiency has also been shown to alter auxin distribution in roots and to affect LR branching (Nacry et al., 2005; Talboys et al., 2014). In addition, the correlation of auxin response maxima with the pattern of LR proliferation highlights the involvement of auxin distribution in regulating LR development in response to heterogeneous Pi supply. Previous studies have revealed the key role of auxin in maintaining apical meristem activity (Ruzicka et al., 2009), and indicated that local auxin response maxima in pericycle cells are essential for LR initiation (De Smet et al., 2006; Jansen et al., 2012). It is possible that the auxin response maxima in the pericycle cells of first-order LRs contributed to the formation of second-order LRs observed in the present study. In addition, we noticed that the red fluorescence was greater in the cap of roots in the Hetero-LP compartment and in the LP treatment (Fig. 7A). In Arabidopsis, the programmed death of root cap cells can release auxin (Xuan et al., 2016). However, there is no information about the relationship between root cap cells and auxin responses in maize, which should be investigated further.
Several key elements of the auxin pathway appear to play an important role in root proliferation in response to heterogeneous Pi supply in maize. Gene expression analysis of first-order LRs in the Hetero-HP compartment had greater ZmPIN9 (auxin transporter facilitates auxin efflux) expression than in roots in other treatments at an early stage of development when other auxin-related genes were not affected (Fig. 8, Supplementary Data Fig. S6). ZmPIN9 is a monocot-specific PIN gene, expressed in the root epidermis, pericycle and phloem in maize (Forestan and Varotto, 2012; Balzan et al., 2014). A previous study in maize has shown that a heterogeneous nitrate supply triggers ZmPIN9-mediated auxin efflux and subsequent cell cycle activation, which leads to initiation of LR primordia (Yu et al., 2015). In the present study, the expression of the auxin biosynthesis gene ZmGH3, the auxin response genes ZmARF2 and ZmTIR1 and the cell cycle gene ZmCYCB1;1 were subsequently upregulated in roots in the Hetero-HP compartment (Supplementary Data Fig. S6). It has been widely reported that polar auxin transport plays a critical role in auxin distribution and LR formation in response to Pi availability (Nacry et al., 2005; Talboys et al., 2014). Polar auxin transport results from the expression and subcellular localization of auxin carrier protein families AUXIN TRANSPORTER PROTEIN 1 (AUX1) and PIN-FORMED (PIN) (Laskowski, 2013). Combining the results from the NPA experiment, the fluorescence analysis of DR5::RFP and the gene expression of auxin-related genes, our results suggest that upregulation of ZmPIN9 expression might direct auxin redistribution in response to sensing a heterogeneous Pi supply, and auxin response genes then play a role in root proliferation in the Pi-rich zone.
Local and systemic regulation in heterogeneous Pi conditions
In general, root responses to a heterogeneous Pi supply are controlled by an integrated regulatory system responding to both local Pi availability and plant P status (Hammond and White, 2008; White and Hammond, 2008). In this study it is likely that root proliferation and gene expression in response to a heterogeneous Pi supply are controlled by an integrated regulatory system, which is dependent on both local environmental Pi sensing and systemic P regulation. Total root length (Fig. 2A), second-order LR formation (Fig. 3C, D) and LR elongation (Fig. 4A, B) in roots in the Hetero-HP compartment were greater than in roots in the Hetero-LP compartment, indicating that roots in the Hetero-HP compartment respond to the local P status. Roots in the Hetero-HP compartment also showed a greater proliferation of LRs than roots of plants receiving the HP treatment (Figs 2–4), implying that the root response to heterogeneous Pi supply also depends on a systemic signal, which might be related to the lower shoot P concentration of plants receiving a heterogeneous Pi supply than that of plants from the HP treatment (Fig. 1B). Previous studies have demonstrated the existence of systemic P regulation of root development with the split-root system (Shane et al., 2003; Thibaud et al., 2010), and the systemic signalling relies on communication among organs mediated by long-distance signalling pathways (Lin et al., 2014; Liu et al., 2014; Puga et al., 2017). Thus, both systemic and local signals appear to direct gene expression and the root morphological development of maize roots in response to a heterogeneous Pi supply. Nevertheless, further research on the integration of local and systemic signals in response to a heterogeneous Pi supply is required.
Conclusions
The present study describes the dynamic responses of maize roots in response to a heterogeneous Pi supply. Both LR formation and LR length were increased by a heterogeneous Pi supply and these responses are likely to be regulated by both local Pi availability and plant P status. The second-order LR proliferation in Pi-rich zones might be triggered by the modification of auxin distribution in first-order LRs in response to a heterogeneous Pi supply, which may be mediated by the monocot-specific ZmPIN9. Thus, manipulation of auxin signalling could be a means to improve root system architecture and increase Pi efficiency of crops in soils with spatially heterogeneous Pi supply.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Fig. S1: diagrammatic representation of the experimental setup and plant growth. Fig. S2: root vitality of plants receiving homogeneous and heterogeneous Pi supplies for 6 d. Fig. S3: effect of homogeneous and heterogeneous Pi supply on first-order lateral root apical meristem length and epidermis cell length of maize plants. Fig. S4: effect of inhibition of polar auxin transport on the root phenotype of plants receiving homogeneous and heterogeneous Pi supplies. Fig. S5: axial root length of maize plants in response to homogeneous and heterogeneous Pi supplies without or with 1-N-naphthylphthalamic acid pre-treatment. Fig. S6: relative expression of auxin-related and cell cycle-related genes in first-order LRs in plants receiving homogeneous and heterogeneous Pi supplies. Table S1: primers used for qPCR analysis.
FUNDING
This work was supported financially by the National Science Foundation of China (No. 31572190, 31772402, 31330070), the National Key R&D Program of China (2017YFD0200204, 2017YFD0200200) and the Rural and Environment Science and Analytical Services Division (RESAS) of the Scottish Government.
ACKNOWLEDGEMENTS
We thank Prof. David Jackson (Cold Spring Harbor) for the supply of the maize DR5::RFP marker line. X.W., J.S. and L.C. designed the study; X.W. and J.F. performed the experiments and collected the data; X.W. analysed the data; X.W., P.W., J.S. and L.C. interpreted the data and wrote the manuscript.
LITERATURE CITED
- Adams MA, Bell TL, Pate JS. 2002. Phosphorus sources and availability modify growth and distribution of root clusters and nodules of native Australian legumes. Plant, Cell and Environment 25: 837–850. [Google Scholar]
- Al-Ghazi Y, Muller B, Pinloche S, et al. 2003. Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signalling. Plant, Cell and Environment 26: 1053–1066. [Google Scholar]
- Balzan S, Johal GS, Carraro N. 2014. The role of auxin transporters in monocots development. Frontiers in Plant Science 5: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleth T, Krogan NT, Scarpella E. 2004. Auxin signals – turning genes on and turning cells around. Current Opinion in Plant Biology 7: 553–563. [DOI] [PubMed] [Google Scholar]
- Cahill JF Jr, McNickle GG. 2011. The behavioral ecology of nutrient foraging by plants. Annual Review of Ecology, Evolution, and Systematics 42: 289–311. [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, et al. 2001. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemensson-Lindell A. 1994. Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental stress in coniferous forest stands: applications and limitations. Plant and Soil 159: 297–300. [Google Scholar]
- Drew M. 1975. Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist 75: 479–490. [Google Scholar]
- Forestan C, Varotto S. 2012. The role of PIN auxin efflux carriers in polar auxin transport and accumulation and their effect on shaping maize development. Molecular Plant 5: 787–798. [DOI] [PubMed] [Google Scholar]
- Gallavotti A, Yang Y, Schmidt RJ, Jackson D. 2008. The relationship between auxin transport and maize branching. Plant Physiology 147: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl RFH, Lima JE, von Wirén N. 2012. Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. Plant Cell 24: 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL. 2000. Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Annals of Botany 85: 921–928. [Google Scholar]
- Hammond JP, White PJ. 2008. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany 59: 93–109. [DOI] [PubMed] [Google Scholar]
- Hawkesford M, Horst W, Kichey T, et al. 2012. Functions of macronutrients. In: Marschner P, ed. Marschner’s Mineral nutrition of higher plants, 3rd edn London, UK: Academic Press, 135–189. [Google Scholar]
- He Y, Liao H, Yan X. 2003. Localized supply of phosphorus induces root morphological and architectural changes of rice in split and stratified soil cultures. Plant and Soil 248: 247–256. [Google Scholar]
- Hochholdinger F, Zimmermann R. 2008. Conserved and diverse mechanisms in root development. Current Opinion in Plant Biology 11: 70–74. [DOI] [PubMed] [Google Scholar]
- Hodge A. 2004. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist 162: 9–24. [Google Scholar]
- Hodge A. 2010. Roots: the acquisition of water and nutrients from the heterogeneous soil environment. Progress in Botany 71: 307–338. [Google Scholar]
- Ito K, Tanakamaru K, Morita S, Abe J, Inanaga S. 2006. Lateral root development, including responses to soil drying, of maize (Zea mays) and wheat (Triticum aestivum) seminal roots. Physiologia Plantarum 127: 260–267. [Google Scholar]
- Jackson RB, Manwaring JH, Caldwell MM. 1990. Rapid physiological adjustment of roots to localized soil enrichment. Nature 344: 58–60. [DOI] [PubMed] [Google Scholar]
- Jansen L, Roberts I, De Rycke R, Beeckman T. 2012. Phloem-associated auxin response maxima determine radial positioning of lateral roots in maize. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Zhang S, Wang L, et al. 2017. OsPht1;8, a phosphate transporter, is involved in auxin and phosphate starvation response in rice. Journal of Experiment Botany 68: 5057–5068. [DOI] [PubMed] [Google Scholar]
- Jing J, Zhang F, Rengel Z, Shen J. 2012. Localized fertilization with P plus N elicits an ammonium-dependent enhancement of maize root growth and nutrient uptake. Field Crops Research 133: 176–185. [Google Scholar]
- Laskowski M. 2013. Lateral root initiation is a probabilistic event whose frequency is set by fluctuating levels of auxin response. Journal of Experimental Botany 64: 2609–2617. [DOI] [PubMed] [Google Scholar]
- Lavenus J, Goh T, Roberts I, et al. 2013. Lateral root development in Arabidopsis: fifty shades of auxin. Trends in Plant Science 18: 450–458. [DOI] [PubMed] [Google Scholar]
- Li H, Ma Q, Li H, Zhang F, Rengel Z, Shen J. 2014. Root morphological responses to localized nutrient supply differ among crop species with contrasting root traits. Plant and Soil 376: 151–163. [Google Scholar]
- Li Z, Xu C, Li K, Yan S, Qu X, Zhang J. 2012. Phosphate starvation of maize inhibits lateral root formation and alters gene expression in the lateral root primordium zone. BMC Plant Biology 12: 89–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WY, Huang TK, Leong SJ, Chiou TJ. 2014. Long-distance call from phosphate: systemic regulation of phosphate starvation responses. Journal of Experiment Botany 65: 1817–1827. [DOI] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HMO. 2002. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant Journal 29: 751–760. [DOI] [PubMed] [Google Scholar]
- Liu H, White PJ, Li C. 2016. Biomass partitioning and rhizosphere responses of maize and faba bean to phosphorus deficiency. Crop and Pasture Science 67: 847–856. [Google Scholar]
- Liu Q, Zhou G, Xu F, Yan X, Liao H, Wang J. 2013. The involvement of auxin in root architecture plasticity in Arabidopsis induced by heterogeneous phosphorus availability. Biologia Plantarum 57: 739–748. [Google Scholar]
- Liu TY, Lin WY, Huang TK, Chiou TJ. 2014. MicroRNA-mediated surveillance of phosphate transporters on the move. Trends in Plant Science 19: 647–655. [DOI] [PubMed] [Google Scholar]
- Lopez-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. 2002. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiology 129: 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, et al. 2005. An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiology 137: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology 156: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y, Tang H, Li H, Zhang F, et al. 2016. Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Frontiers in Plant Science 7: 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Wang X, Li H, et al. 2014. Localized application of NH4+-N plus P enhances zinc and iron accumulation in maize via modifying root traits and rhizosphere processes. Field Crops Research 164: 107–116. [Google Scholar]
- Ma Q, Zhang F, Rengel Z, Shen J. 2013. Localized application of NH4+-N plus P at the seedling and later growth stages enhances nutrient uptake and maize yield by inducing lateral root proliferation. Plant and Soil 372: 65–80. [Google Scholar]
- Melida H, Largo-Gosens A, Novo-Uzal E, et al. 2015. Ectopic lignification in primary cellulose-deficient cell walls of maize cell suspension cultures. Journal of Integrative Plant Biology 57: 357–372. [DOI] [PubMed] [Google Scholar]
- Müller J, Toev T, Heisters M, et al. 2015. Iron-dependent callose deposition adjusts root meristem maintenance to phosphate availability. Developmental Cell 33: 216–230. [DOI] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B. 2005. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiology 138: 2061–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peret B, Desnos T, Jost R, Kanno S, Berkowitz O, Nussaume L. 2014. Root architecture responses: in search for phosphate. Plant Physiology 166: 1713–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Torres CA, Lopez-Bucio J, Cruz-Ramirez A, et al. 2008. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20: 3258–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga MI, Rojas-Triana M, de Lorenzo L, Leyva A, Rubio V, Paz-Ares J. 2017. Novel signals in the regulation of Pi starvation responses in plants: facts and promises. Current Opinion in Plant Biology 39: 40–49. [DOI] [PubMed] [Google Scholar]
- Robinson D. 1994. The responses of plants to non-uniform supplies of nutrients. New Phytologist 127: 635–74. [DOI] [PubMed] [Google Scholar]
- Robinson D. 1996. Variation, co-ordination and compensation in root systems in relation to soil variability. Plant and Soil 187: 57–66. [Google Scholar]
- Ruzicka K, Simaskova M, Duclercq J, et al. 2009. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proceedings of the National Academy of Sciences of the USA 106: 4284–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, De Vos M, De Roock S, Lambers H. 2003. Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system. Plant, Cell and Environment 26: 265–273. [Google Scholar]
- Shen J, Li H, Neumann G, Zhang F. 2005. Nutrient uptake, cluster root formation and exudation of protons and citrate in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Science 168: 837–845. [Google Scholar]
- Shen J, Yuan L, Zhang J, et al. 2011. Phosphorus dynamics: from soil to plant. Plant Physiology 156: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu L, Shen J, Rengel Z, Tang C, Zhang F, Cawthray GR. 2007. Formation of cluster roots and citrate exudation by Lupinus albus in response to localized application of different phosphorus sources. Plant Science 172: 1017–1024. [Google Scholar]
- De Smet I, Vanneste S, Inze D, Beeckman T. 2006. Lateral root initiation or the birth of a new meristem. Plant Molecular Biology 60: 871–887. [DOI] [PubMed] [Google Scholar]
- Snapp S, Koide R, Lynch J. 1995. Exploitation of localized phosphorus-patches by common bean roots. Plant and Soil 177: 211–218. [Google Scholar]
- Sun H, Zhang F, Li L, Tang C. 2002. The morphological changes of wheat genotypes as affected by the levels of localized phosphate supply. Plant and Soil 245: 233–238. [Google Scholar]
- Svistoonoff S, Creff A, Reymond M, et al. 2007. Root tip contact with low-phosphate media reprograms plant root architecture. Nature Genetics 39: 792–796. [DOI] [PubMed] [Google Scholar]
- Talboys PJ, Healey JR, Withers PJ, Jones DL. 2014. Phosphate depletion modulates auxin transport in Triticum aestivum leading to altered root branching. Journal of Experiment Botany 65: 5023–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Shen J, Zhang F, Rengel Z. 2013. Interactive effects of phosphorus deficiency and exogenous auxin on root morphological and physiological traits in white lupin (Lupinus albus L.). Science China 56: 313–323. [DOI] [PubMed] [Google Scholar]
- Thibaud MC, Arrighi JF, Bayle V, et al. 2010. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant Journal 64: 775–789. [DOI] [PubMed] [Google Scholar]
- Tinker PB, Nye PH. 2000. Solute movement in the rhizosphere. Oxford: Oxford University Press. [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 423–447. [DOI] [PubMed] [Google Scholar]
- Wang H, Nussbaum-Wagler T, Li B, et al. 2005. The origin of the naked grains of maize. Nature 436: 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JT, Lahner B, Yakubova E, Salt DE, Raghothama KG. 2008. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiology 147: 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman RL. 1990. Soil testing and plant analysis, 3rd edn Madison, WI:American Society of Agronomy and Soil Science Society of America. [Google Scholar]
- Wen Z, Li H, Shen Q, et al. 2019. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytologist 223: 882–895. [DOI] [PubMed] [Google Scholar]
- White PJ, Hammond JP. 2008. Phosphorus nutrition of terrestrial plants. In: White PJ, Hammond JP, eds. The ecophysiology of plant-phosphorus interactions. Dordrecht: Springer, 51–81. [Google Scholar]
- White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM. 2013. Matching roots to their environment. Annals of Botany 112: 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll K, Borsuk LA, Stransky H, Nettleton D, Schnable PS, Hochholdinger F. 2005. Isolation, characterization, and pericycle-specific transcriptome analyses of the novel maize lateral and seminal root initiation mutant rum1. Plant Physiology 139: 1255–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Band LR, Kumpf RP, et al. 2016. Cyclic programmed cell death stimulates hormone signaling and root development in Arabidopsis. Science 351: 384–387. [DOI] [PubMed] [Google Scholar]
- Yano K, Kume T. 2005. Root morphological plasticity for heterogeneous phosphorus supply in Zea mays L. Plant Production Science 8: 427–432. [Google Scholar]
- Yu P, Eggert K, von Wiren N, Li C, Hochholdinger F. 2015. Cell type-specific gene expression analyses by RNA sequencing reveal local high nitrate-triggered lateral root initiation in shoot-borne roots of maize by modulating auxin-related cell cycle regulation. Plant Physiology 169: 690–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Gutjahr C, Li C, Hochholdinger F. 2016. Genetic control of lateral root formation in cereals. Trends in Plant Science 21: 951–961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.