Abstract
Background
This prospective pilot study explored same-day point-of-care viral load testing in a setting in Ghana that has yet to implement virological monitoring of antiretroviral therapy (ART).
Methods
Consecutive patients accessing outpatient care while on ART underwent HIV-1 RNA quantification by Xpert. Those with viraemia at the first measurement (T0) received immediate adherence counselling and were reassessed 8 weeks later (T1). Predictors of virological status were determined by logistic regression analysis. Drug resistance-associated mutations (RAMs) were detected by Sanger sequencing.
Findings
At T0, participants had received treatment for a median of 8·9 years; 297/333 (89·2%) were on NNRTI-based ART. The viral load was ≥40 copies/mL in 164/333 (49·2%) patients and ≥1000 copies/mL in 71/333 (21·3%). In the latter group, 50/65 (76·9%) and 55/65 (84·6%) harboured NRTI and NNRTI RAMs, respectively, and 27/65 (41·5%) had ≥1 tenofovir RAM. Among 150/164 (91·5%) viraemic patients that reattended at T1, 32/150 (21·3%) showed resuppression <40 copies/mL, comprising 1/65 (1·5%) subjects with T0 viral load ≥1000 copies/mL and 31/85 (36·5%) subjects with lower levels. A T0 viral load ≥1000 copies/mL and detection of RAMs predicted ongoing T1 viraemia independently of self-reported adherence levels. Among participants with T0 viral load ≥1000 copies/mL, 23/65 (35·4%) showed resuppression <1000 copies/mL; the response was more likely among those with higher adherence levels and no RAMs.
Interpretation
Same-day point-of-care viral load testing was feasible and revealed poor virological control and suboptimal resuppression rates despite adherence counselling. Controlled studies should determine optimal triaging modalities for same-day versus deferred viral load testing.
Funding
University of Liverpool, South Tees Infectious Diseases Research Fund
Keywords: HIV, Virological monitoring, Point-of-care, Adherence, Resuppression, Drug resistance
Research in context.
Evidence before this study
We searched PubMed for studies that used the Cepheid Xpert HIV-1 viral load assay for monitoring HIV-positive patients receiving antiretroviral therapy (ART) in sub-Saharan Africa. The search terms were “Cepheid”, “Xpert”, “HIV”, “viral load” and “Africa”. We found five published studies that investigated the technical performance of the assay when compared to centralised testing by standard laboratory-based assays. One study used the Xpert assay for on-site testing of patients deemed to be at increased risk of virological failure; in this study, viral load testing and subsequent adherence counselling were deferred by 1–3 weeks. No previous published study used the Xpert assay to provide same-day viral load testing followed by immediate, viral load-informed adherence counselling, and no previous published study used the Xpert assay to assess rates of resuppression after adherence counselling. Previous published studies often suffered from high rates of loss to follow-up, which limits the interpretation of the findings.
Added value of this study
The study demonstrates the feasibility of viral load testing at point of care in a HIV programmatic setting in sub-Saharan Africa where ART is available free of charge but there is no routine provision of viral load testing. Results were delivered to patients on the same day and proved helpful in guiding immediate adherence counselling. The results provide novel data for Ghana and raise concern. In a HIV cohort established on long-term, predominantly NNRTI-based ART without access to virological monitoring, nearly half had a detectable viral load at the first test and 1 in 5 had a viral load ≥1000 copies/mL. One important aspect was the high retention of viraemic patients into follow-up, with 91·5% reattending after 8 weeks. Despite improvements in self-reported adherence, none of the patients with viral load ≥1000 copies/mL achieved resuppression <40 copies/mL while continuing NNRTI-based ART (and only a subset resuppressed <1000 copies/mL), and the virological outcomes were related to the presence of drug resistance.
Implications of all the available evidence
In an epidemiological context where patients have been receiving long-term NNRTI-based ART in the absence of virological monitoring, detection of a viral load ≥1000 copies/mL can serve as a trigger for an immediate change of therapy both to limit the risk of disease progression and prevent onward transmission of highly drug-resistant strains. There is a need to survey whether the complex resistance patterns observed in patients with viral load ≥1000 copies/mL, including a high prevalence of tenofovir RAMs, affect the efficacy of novel HIV treatment regimens. There is also a need to determine the long-term outcomes of the subset of patients that resuppressed <1000 copies/mL but remained viraemic while continuing NNRTI-based ART. To prove effective for sub-Saharan Africa, viral load testing at point of care requires operational refinements, including modalities for effective triaging of patients to same-day versus deferred testing, which should be evaluated in controlled studies.
Alt-text: Unlabelled box
1. Introduction
Plasma HIV-1 RNA levels provide a direct measure of the efficacy of antiretroviral therapy (ART), predicting immunological and clinical outcomes and the risk of transmission [1]. Modelling indicates that differentiating care based on the viral load is cost-effective for low-income settings [2], whereby suppressed patients attend clinic visits less frequently and more resources are focused on patients with viraemia. The approach is endorsed by the WHO, whose guidelines indicate viraemic patients should receive adherence counselling, followed by a repeat viral load measurement taken 3–6 months later [3]. There is evidence indicating that resuppression is common after interventions to re-enforce adherence, especially in patients with a low viral load [4,5]. A change of the treatment regimen is only recommended once a viral load ≥1000 copies/mL is confirmed [3].
Access to ART has been expanding in sub-Saharan Africa. Of the estimated 25·7 million HIV-positive individuals, 15·4 million (60%) were receiving treatment in 2018, aiming for 95% by 2030 [6]. Virological monitoring of patients receiving ART would be expected to gradually expand; however, routine access faces significant barriers due to overburdened healthcare systems, financial constraints, poor training, and weak transport and laboratory infrastructure [7]. Implementation of viral load testing in sub-Saharan Africa may benefit from solutions that reduce the number of clinic visits, which in turn is likely to promote retention into care and improved clinical outcomes.
There is growing interest in novel platforms that enable viral load testing outside specialised laboratories. The Cepheid Xpert HIV-1 viral load assay was the first to receive WHO endorsement for use in resource-limited settings [8], and has comparable performance to laboratory-based assays [9]. Xpert is widely available across sub-Saharan Africa for the diagnosis of tuberculosis. Its modular, cartridge-based self-contained system can be used by non-specialised personnel and offers a low risk of contamination, a fast turn-around time for results, and no requirement for sample batching, features that make the platform suitable for same-day testing at point of care [10]. In comparative studies in Botswana, Malawi, South Africa, and Kenya, the Xpert HIV-1 viral load assay showed a high level of agreement with standard laboratory-based real-time PCR assays [11], [12], [13], [14], [15].
This prospective pilot study reports on same-day point-of-care (POC) HIV-1 RNA quantification by Xpert in a typical programmatic HIV centre in Ghana where ART is provided free of charge but virological monitoring of treated patients is yet to become part of routine care. POC viral load results were used to fast track viraemic patients to immediate adherence counselling and POC viral load testing was used to measure resuppression at the follow-up visit 8 weeks later. Factors associated with virological suppression and resuppression were identified, producing data that may help guide policy and inform the design of larger controlled studies.
2. Methods
2.1. Setting and sample size
The setting was the HIV clinic of the Komfo Anokye Teaching Hospital (KATH), a 1200-bed facility in the city of Kumasi and the second-largest hospital in Ghana, serving a population of around 10 million people in the Ashanti Region. Approval was granted by the Ethics Committee of the Kwame Nkrumah University of Science and Technology in Kumasi. Eligible participants were HIV-positive adults (≥18 years) attending for routine HIV care. Sample size was determined by estimating a total KATH HIV cohort of 4500 patients and a rate of viraemia (>40 copies/mL) of 40% (5% precision) [16].
2.2. Patients’ flow
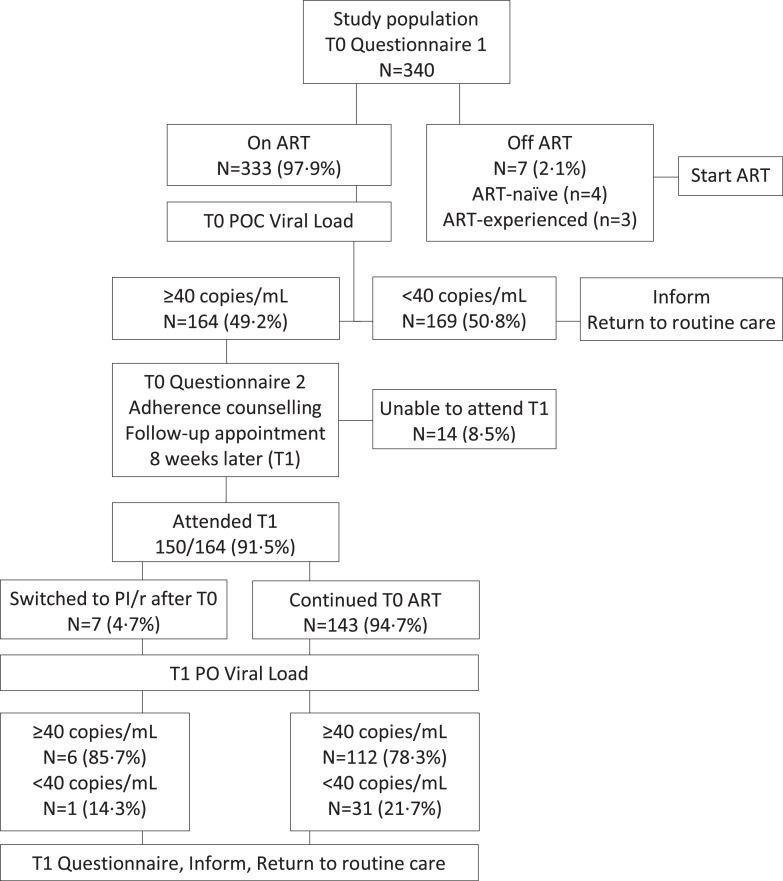
In February 2018 (T0), consecutive patients attending 4 out-patient clinics over 2 weeks were offered participation in the study and all accepted (Fig. 1). At T0, participants underwent blood sampling and completed a simple structured questionnaire, which was administered by trained local interpreters to overcome literacy barriers. The questionnaires collected information on adherence (T0 adherence questionnaire 1, see below) and socio-economic and life-style parameters: having enough food to be able to eat regular meals (always; most days; some of the time; never), alcohol consumption (never; occasionally [not more than once a week]; regularly [more than once a week]), and any use of traditional or herbal remedies. After completing the questionnaires, ART-naïve patients and subjects who had discontinued ART were directed to initiating or reinitiating treatment. Participants taking ART were invited to wait for the viral load result. Patients with virological suppression <40 copies/mL were informed and returned to routine care. Patients with viraemia were fast-tracked to immediate adherence review with the local, trained clinic nurses; counselling was informed by the viral load result and used a simple structured questionnaire (T0 adherence questionnaire 2, see below); potential reasons for poor adherence were addressed by the clinic nurses using the standard local format for adherence counselling, which includes eliciting any problem with tolerability or issues of forgetfulness, personal belief about the benefits of treatment, available support and disclosure of HIV status, and suggested strategies to improve adherence such as use of memory aids (e.g., setting an alarm on a mobile phone). Following the adherence review, patients were invited to attend a follow-up visit 8 weeks later (May 2018; T1), when POC viral load testing was repeated and adherence reassessed with a simple structured questionnaire (T1 adherence questionnaire, see below). CD4 cell counts and full blood counts were measured at KATH. All patients with CD4 count <200 cells/mm3 underwent urgent clinical review. Available clinical data were collected from the medical records.
Fig. 1.
Patients’ flow.
2.3. Adherence measures
The T0 questionnaire 1, which was administered at study entry prior to receipt of the viral load result, asked participants about any previous treatment interruption, defined as discontinuation of all antiretroviral drugs for ≥ 3 consecutive days since first starting ART, and how the patient would describe adherence in the previous 3 months on an ordinal visual analogue scale (VAS) ranging from 0% (complete non-adherence) to 100% (complete adherence) in 10% increments. The T0 questionnaire 2 was administered at adherence review to patients with viraemia, after they had received their viral load result; it asked about the number of doses missed in the previous month (none; 1; 2 to 3; >3). The T1 questionnaire, which was administered at follow-up after the patients had received their repeat viral load result, collected the VAS and the number of missed doses in the previous month (none; 1; 2 to 3; >3). The number of doses missed in the previous month according to the T0 questionnaire 2 and the T1 questionnaire were used to calculate an adherence score ranging from 3 points (no missed doses, best adherence) to 0 points (>3 missed doses, worst adherence).
2.4. Viral load testing
Plasma was separated from whole blood in EDTA by centrifugation at 2000 g for 10 min and tested by the Xpert HIV-1 viral load assay (Cepheid, Sunnyvale, USA). The assay quantifies HIV-1 Group M, N and O with a range of 40 to 107 copies/mL and provides results within 90 min [17]. The Xpert was located in a room adjacent to the clinical area. All patient received the result on the same day.
2.5. Resistance testing
If the T0 viral load was ≥200 copies/m, plasma virus was retrospectively tested for the presence of resistance-associated mutations (RAMs) in reverse transcriptase (RT, amino acids 1–335) and protease (amino acids 1–99) using Sanger sequencing as previously described [18]. Major RAMs and genotypic susceptibility scores (GSS) were determined using the Stanford HIV Drug Resistance database (v8·8); each drug in the regimen scored 0 with high-level resistance, 0·25 with intermediate resistance, 0·5 with low level resistance and 1 with potential low-level resistance or full susceptibility. HIV-1 subtypes were determined by phylogenetic analysis.
2.6. Analyses
Fisher's, chi-squared, or Mann-Whitney tests were used to compare the characteristics of study participants according to the T0 viral load. The correlation between number of treatment interruptions and T0 VAS score and between T0 viral load and GSS were assessed by Spearman's correlation analysis. Factors associated with viral load suppression <40 and <1000 copies/mL at T0 and with resuppression <40 and <1000 copies/mL at T1 were explored by multivariable logistic regression analysis. Variables with p < 0·1 in the univariable models were included in the multivariable models. The analysis of factors associated with viral load suppression and resuppression did not include the T0 CD4 cell count, which was analysed separately for its association with the T0 viral load using univariable linear regression analysis. The analysis of factors associated with viral load resuppression did not include detection of NNRTI RAMs as these were part of the GSS calculation. Collinearity was assessed by calculating the variance inflation factor. Changes in adherence scores and viral load between T0 and T1 were analysed by Wilcoxon signed rank test. Statistical analyses were performed with STATA, version 14 (StataCorp Inc, College Station, USA).
3. Results
3.1. Study population at T0
The characteristics of the 333 patients who were on ART at T0 are shown in Table 1. The cohort included a majority of women (246/333, 73·9%), was long established on ART (median 8·9 years) and showed a median CD4 count of 626 cells/mm3. Most participants (297/333, 89·2%) were receiving an NNRTI (predominantly efavirenz) whereas 36/333 (10·8%) were on a ritonavir-boosted protease inhibitor (PI/r, predominantly lopinavir/ritonavir), each usually combined with the NRTIs tenofovir disoproxil fumarate (TDF)/lamivudine (3TC) (187/333, 56·2%) or zidovudine (AZT)/3TC (141/333, 42·3%). Overall, 164/333 (49·2%) patients showed a viral load ≥40 copies/mL, with median levels in this group of 2·6 log10 copies/mL (IQR 2·0–4·4); 71/333 (21·3%) had a viral load ≥1000 copies/mL. The CD4 count was 134 cells/mm3 lower for each 1 log10 copies/mL increase in viral load (95% CI −155 to −113; p < 0·0001).
Table 1.
Baseline characteristics of the study population according to the T0 viral load.
| Characteristics | Total | T0 viral load (copies/mL) |
p | ||||
|---|---|---|---|---|---|---|---|
| <40 | 40–199 | 200–999 | ≥1000 | ||||
| Total number (%) | 333 (100) | 169 (100) | 63 (100) | 30 (100) | 71 (100) | – | |
| Female gender, n (%) | 246 (73·9) | 135 (79·9) | 41 (65·1) | 20 (66·7) | 50 (70·4) | 0·07 | |
| Age, median years (IQR) | 48 (42–54) | 49 (42–55) | 48 (42–54) | 46 (42–52) | 47 (41–50) | 0·11 | |
| Time since HIV diagnosis, median years (IQR) | 9·5 (6·3–12·0) | 10·1 (6·6–12·4) | 9·3 (6·7–11·7) | 7·1 (2·3–10·9) | 9·2 (6·4–11·8) | 0·20 | |
| In stable partnership, n (%) | 165 (49·6) | 73 (43·2) | 37 (58·7) | 12 (40·0) | 43 (60·6) | 0·02 | |
| Children in the household, median number (IQR) | 3 (2–4) | 3 (1–4) | 3 (1–4) | 3 (1–4) | 3 (2–4) | 0·86 | |
| Education level, n (%) | none/primary | 171 (51·4) | 95 (56·2) | 24 (38·1) | 11 (36·7) | 41 (57·8) | 0·02 |
| secondary/post-secondary | 162 (48·7) | 74 (43·8) | 39 (61·9) | 19 (63·3) | 30 (42·3) | ||
| Enough food, n (%) | always/most days | 267 (80·2) | 131 (77·5) | 51 (81·0) | 26 (86·7) | 59 (83·1) | 0·48 |
| some of the time/never | 61 (18·3) | 37 (21·9) | 9 (14·3) | 4 (13·3) | 11 (15·5) | ||
| no data | 5 (1·5) | 1 (0·6) | 3 (4·8) | 0 (0) | 1 (1·4) | ||
| Alcohol consumption, n (%) | never | 317 (95·2) | 164 (97·0) | 61 (96·8) | 28 (93·3) | 64 (90·1) | 0·07 |
| occasionally | 13 (3·9) | 5 (3·0) | 1 (1·6) | 1 (3·3) | 6 (8·5) | ||
| regularly | 3 (0·9) | 0 (0) | 1 (1·6) | 1 (3·3) | 1 (1·4) | ||
| Traditional or herbal remedies, n (%) | 11 (3·3) | 4 (2·4) | 0 (0) | 1 (3·3) | 6 (8·5) | 0·04 | |
| Duration of ART, median years (IQR) | 8·9 (5·7–11·3) | 9·5 (5·9–11·3) | 8·3 (4·5–11·3) | 8·0 (2·1–10·8) | 8·9 (6·3–11·2) | 0·47 | |
| Third agent, n (%) | NNRTIa | 297 (89·2) | 155 (91·7) | 55 (87·3) | 21 (70·0) | 66 (93·0) | 0·01 |
| PI/rb | 36 (10·8) | 14 (8·3) | 8 (12·7) | 9 (30·0) | 5 (7·0) | ||
| NRTI backbone, n (%) | TDF/3TC | 187 (56·2) | 92 (54·4) | 43 (68·3) | 18 (60·0) | 34 (47·9) | 0·05 |
| AZT/3TC | 141 (42·3) | 76 (45·0) | 18 (28·6) | 10 (33·3) | 37 (52·1) | ||
| Treatment interruptions since first starting ART, n (%) | none | 250 (75·1) | 147 (87·0) | 41 (65·1) | 21 (70·0) | 41 (57·8) | <0·001 |
| 1 | 42 (12·6) | 8 (4·7) | 11 (17·5) | 5 (16·7) | 18 (25·4) | ||
| 2–3 | 36 (10·8) | 12 (7·1) | 10 (15·9) | 4 (13·3) | 10 (14·1) | ||
| > 3 | 5 (1·5) | 2 (1·2) | 1 (1·6) | 0 (0) | 2 (2·8) | ||
| VAS score, median% (IQR) | 100 (100–100) | 100 (100–100) | 100 (90–100) | 100 (90–100) | 100 (85–100) | 0·001 | |
| VAS score category, n (%) | 100% | 258 (77·5) | 146 (86·4) | 45 (71·4) | 20 (66·7) | 47 (66·2) | <0·001 |
| 90–100% | 37 (11·1) | 13 (7·7) | 9 (14·3) | 9 (30·0) | 6 (8·5) | ||
| 80–90% | 23 (6·9) | 7 (4·1) | 6 (9·5) | 1 (3·3) | 9 (12·7) | ||
| <80% | 15 (4·5) | 3 (1·8) | 3 (4·8) | 0 (0) | 9 (12·7) | ||
| CD4 count, median cells/mm3 (IQR) | 626 (373–840) | 757 (575–970) | 640 (445–774) | 611 (390–799) | 234 (155–410) | <0·001 | |
| CD4 count <200 cells/mm3, n (%) | 34 (10·2) | 6 (3·6) | 1 (1·6) | 1 (3·3) | 26 (36·6) | <0·001 | |
| HIV-1 RNA, median log10 copies/mL | 1·3 (0·70–2·6) | 1·3 (0·70–1·3) | 1·9 (1·8–2·1) | 2·5 (2·4–2·7) | 4·6 (4·0–5·3) | – | |
Abbreviations: ABC=abacavir; ART=antiretroviral therapy; ATV/r=ritonavir-boosted atazanavir; AZT=zidovudine; EFV=efavirenz; IQR=interquartile-range; LPV/r=ritonavir-boosted lopinavir; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleos(t)ide reverse transcriptase inhibitor; NVP=nevirapine; PI/r=ritonavir-boosted protease inhibitor; 3TC=lamivudine; TDF=tenofovir disoproxil fumarate; VAS=visual analogue scale.
NNRTI-based regimens: TDF/3TC EFV n = 155 (46·6%), TDF/3TC NVP n = 12 (3·6%), AZT/3TC EFV n = 60 (18·0%), AZT/3TC NVP n = 70 (21·0%).
PI/r-based regimens: TDF/3TC LPV/r n = 18 (5·4%), TDF/3TC ATV/r n = 2 (0·6%), AZT/3TC LPV/r n = 10 (3·0%), AZT/3TC ATV/r n = 1 (0·3%), ABC 3TC ATV/r n = 1 (0·3%), Other n = 4 (1·2%).
3.2. Factors associated with the T0 viral load
The analysis of factors associated with the T0 viral load is shown in Table 2. The number of treatment interruptions and VAS score were significantly correlated (Spearman's rho −0·45; p < 0.0001) and were therefore modelled separately. After adjustment, viral load suppression <40 copies/mL was more likely in females, patients with sufficient food at least some of the time, those that either did not report treatment interruptions or had a higher VAS score, and (marginally) among older patients. Viral load suppression <1000 copies/mL was more likely among older patients, those receiving TDF/3TC rather than AZT/3TC, and those that either did not report treatment interruptions or had a higher VAS score (Table 3).
Table 2.
Univariable and multivariable logistic regression analysis of factors associated with a T0 viral load <40 copies/mL.
| Variable | Univariable analysis |
Multivariable analysisa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
|||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||
| Gender | female vs male | 1·90 | 1·15–3·12 | 0·01 | 1·92 | 1·09–3·36 | 0·02 | 1·91 | 1·10–3·32 | 0·02 |
| Age | per 5 years older | 1·13 | 0·99–1·29 | 0·06 | 1·13 | 0·98–1·30 | 0·09 | 1·15 | 1·00–1·32 | 0·06 |
| Enough food | never vs at least some of the time | 0·30 | 0·09–0·95 | 0·04 | 0·25 | 0·07–0·95 | 0·04 | 0·22 | 0·06–0·79 | 0·02 |
| Alcoholb | yes vs no | 0·19 | 0·02–1·64 | 0·13 | ||||||
| Traditional or herbal remedies | yes vs no | 0·54 | 0·16–1·89 | 0·34 | ||||||
| Third agent | PI/r vs NNRTI | 0·58 | 0·29–1·18 | 0·14 | ||||||
| NRTI backbone | AZT/3TC vs TDF/3TC | 0·83 | 0·53–1·28 | 0·40 | ||||||
| Duration of ART | per 1 year longer | 1·03 | 0·98–1·09 | 0·29 | ||||||
| Treatment interruptions | ≥1 vs none | 0·25 | 0·15–0·44 | <0·001 | 0·29 | 0·16–0·52 | <0·001 | – | ||
| VAS score | per 10% score higher | 1·69 | 1·26–2·26 | <0·001 | – | 1·52 | 1·13–2·06 | 0·01 | ||
| Time since HIV diagnosis | per year longer | 1·05 | 0·99–1·11 | 0·12 | ||||||
| T0 CD4 count | per 100 cells/mm3 higher | 1·38 | 1·27–1·51 | <0·001 | – | – | ||||
Abbreviations: ART=antiretroviral therapy; AZT=zidovudine, CI=confidence interval; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleos(t)ide reverse transcriptase inhibitor; OR=odds ratio; PI/r=ritonavir-boosted protease inhibitor; 3TC=lamivudine; TDF=tenofovir disoproxil fumarate; VAS=visual analogue scale.
Model 1 includes the reported history of treatment interruption whereas Model 2 includes the VAS score; neither model includes the CD4 cell count, which was analysed separately.
Occasional or regular use.
Table 3.
Univariable and multivariable logistic regression analysis of predictors of a T0 viral load <1000 copies/mL.
| Variable | Univariable analysis |
Multivariable analysisa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
|||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||
| Gender | female vs male | 1·25 | 0·70–2·23 | 0·46 | ||||||
| Age | per 5 years older | 1·18 | 1·01–1·39 | 0·04 | 1·20 | 1·02–1·43 | 0·03 | 1·21 | 1·02–1·43 | 0·03 |
| Enough food | never vs at least some of the time | 0·43 | 0·15–1·23 | 0·12 | ||||||
| Alcoholb | yes vs no | 0·26 | 0·05–1·33 | 0·11 | ||||||
| Traditional or herbal remedies | yes vs no | 0·21 | 0·06–0·71 | 0·01 | 0·34 | 0·09–1·32 | 0·12 | 0·35 | 0·09–1·35 | 0·13 |
| Third agent | PI/r vs NNRTI | 1·77 | 0·66–4·74 | 0·25 | ||||||
| NRTI backbone | AZT/3TC vs TDF/3TC | 0·62 | 0·37–1·05 | 0·08 | 0·44 | 0·24–0·79 | 0·01 | 0·42 | 0·23–0·77 | 0·01 |
| Duration of ART | per 1 year longer | 0·99 | 0·92–1·05 | 0·69 | ||||||
| Treatment interruptions | ≥1 vs none | 0·35 | 0·20–0·61 | <0·001 | 0·42 | 0·23–0·79 | 0·01 | – | ||
| VAS score | per 10% higher | 1·60 | 1·24–2·07 | <0·001 | – | 1·48 | 1·12–1·96 | 0·01 | ||
| Time since HIV diagnosis | per year longer | 1·00 | 0·93–1·07 | 0·98 | ||||||
| T0 CD4 count | per 100 cells/mm3 higher | 1·95 | 1·66–2·29 | <0·001 | – | – | ||||
Abbreviations: ART=antiretroviral therapy; AZT=zidovudine, CI=confidence interval; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleos(t)ide reverse transcriptase inhibitor; OR=odds ratio; PI/r=ritonavir-boosted protease inhibitor; 3TC=lamivudine; TDF=tenofovir disoproxil fumarate; VAS=visual analogue scale.
Model 1 includes the reported history of treatment interruption whereas Model 2 includes the VAS score; neither model includes the CD4 cell count, which was analysed separately.
b Occasional or regular use.
3.3. T1 viral load
Among T0 participants with a viral load ≥40 copies/mL, 150/164 (91·5%) returned at T1. The distribution of T1 viral load according to the T0 viral load and ART regimen is shown in Table 4. Between T0 and T1, the median viral load (in log10 copies/mL) changed from 2·6 (IQR 2·0–4·4) to 2·3 (IQR 1·7–3·6) (p = 0·0017) and 32/150 (21·3%) patients achieved resuppression <40 copies/mL. Among the 122/150 (81·3%) patients continuing NNRTI-based ART, the viral load changed from 2·5 (IQR 1·9–4·2) to 2·4 (IQR 1·7–4·1) (p = 0·07); 25/122 (20·5%) achieved resuppression <40 copies/mL. Among the 21/150 (14·0%) patients continuing PI/r-based ART, the viral load changed from 2·6 (IQR 2·1–3·0) to 2·2 (IQR 1·3–2·8) (p = 0·042); 6/21 (28·6%) achieved resuppression <40 copies/mL. There were 7/150 (4·7%) patients who changed from NNRTI-based to PI/r-based ART after T0 owing to low CD4 cell counts; in this subset, the viral load changed from 5·3 (IQR 4·9–5·8) to 2·2 (IQR 1·6–2·5) (p = 0·018); 1/7 (14·3%) achieved resuppression <40 copies/mL. Among subjects with T0 viral load 40–999 copies/mL, 31/85 (36·5%) achieved resuppression <40 copies/mL. Only 1 subject among the 65 (1·5%) with T0 viral load ≥1000 copies/mL achieved resuppression <40 copies/mL; this subject was in the subset that changed to PI/r-based ART after T0. Among participants with T0 viral load ≥1000 copies/mL, 23/65 (35·4%) had a T1 viral load ≥40 but <1000 copies/mL, comprising 19/60 (31.7%) patients on NNRTI-based ART and 4/5 (80·0%) on PI/r-based ART.
Table 4.
The T1 viral load among patients with T0 viraemia, by T0 viral load and ART regimen.
| T0 ART regimen | T0 viral load (copies/mL) | T1 viral load (copies/mL) |
|||
|---|---|---|---|---|---|
| <40 | 40–199 | 200–999 | ≥1000 | ||
| NNRTI-based, n (%) | 40–199 | 18 (36%) | 24 (48%) | 8 (16%) | 0 (0%) |
| 200–999 | 7 (37%) | 5 (26%) | 6 (32%) | 1 (5%) | |
| ≥1000 | 1 (2%)a | 7 (12%)b | 11 (18%)b | 41 (68%) | |
| PI-based, n (%) | 40–199 | 3 (38%) | 4 (50%) | 0 (0%) | 1 (13%) |
| 200–999 | 3 (38%) | 1 (13%) | 2 (25%) | 2 (25%) | |
| ≥1000 | 0 (0%) | 2 (40%) | 2 (40%) | 1 (20%) | |
| Total | All strata | 32 (21%) | 43 (29%) | 29 (19%) | 46 (31%) |
Abbreviations: ART=antiretroviral therapy; NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor.
The patient switched to a PI/r-based regimen between T0 and T1.
Three patients switched to a PI/r-based regimen between T0 and T1.
3.4. Factors associated with the T1 viral load
The analysis of factors associated with the T1 viral load are shown in Table 5. The T0 viral load and GSS were significantly correlated (Spearman's rho −0·66; p<0·0001) and were therefore modelled separately. After adjustment, the likelihood of resuppression <40 copies/mL decreased by nearly 3-fold for each 1 log10 copies/mL increase in T0 viral load. When the T0 viral load was analysed as a categorical variable, resuppression <40 copies/mL was over 30-fold less likely with a T0 viral load ≥1000 copies/mL. In the model including the GSS, the likelihood of resuppression <40 copies/mL almost halved with each 0·5 unit decrease in GSS; the presence of NNRTI RAMs reduced by almost 15-fold the likelihood of resuppression <40 copies/mL. A separate analysis considered the likelihood of viral load resuppression <1000 copies/mL among patients with T0 viral load ≥1000 copies/mL (Table 6). The T1 VAS and the GSS independently predicted viral load resuppression <1000 copies/mL in this group.
Table 5.
Univariable and multivariable logistic regression analysis of factors associated with lack of resuppression <40 copies/mL at T1.
| Variable | Univariable analysis |
Multivariable analysisa |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
|||||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||
| Gender | female vs male | 0·96 | 0·41–2·22 | 0·92 | |||||||||
| Age | per 5 years older | 0·98 | 0·77–1·25 | 0·89 | |||||||||
| Enough food | never vs at least some of the time | 0·53 | 0·15–1·88 | 0·33 | |||||||||
| Alcoholb | yes vs no | 1·09 | 0·12–10·1 | 0·94 | |||||||||
| Traditional or herbal remedies | yes vs no | 1·66 | 0·19–14·3 | 0·64 | |||||||||
| Third agent | PI/r vs NNRTI | 0·63 | 0·22–1·79 | 0·39 | |||||||||
| NRTI backbone | AZT/3TC vs TDF/3TC | 0·55 | 0·25–1·23 | 0·15 | |||||||||
| Duration of ART | per 1 year longer | 1·01 | 0·91–1·12 | 0·83 | |||||||||
| Treatment interruptions | ≥1 vs never | 0·65 | 0·30–1·43 | 0·28 | |||||||||
| T1 VAS | per 10% higher | 0·67 | 0·44–1·00 | 0·05 | 0·76 | 0·45–1·29 | 0·31 | 0·75 | 0·43–1·29 | 0·30 | 0·73 | 0·44–1·20 | 0·21 |
| Change in adherence score | per unit increase | 1·02 | 0·76–1·37 | 0·89 | |||||||||
| Time since HIV diagnosis | per year longer | 0·99 | 0·89–1·09 | 0·81 | |||||||||
| T0 CD4 count | per 100 cells/mm3 higher | 0·80 | 0·69–0·92 | 0·001 | – | – | – | ||||||
| T0 viral load | per 1 log10 copies/mL higher | 3·02 | 1·71–5·32 | <0·001 | 2·81 | 1·59–4·98 | <0·001 | – | – | ||||
| T0 viral load | ≥1000 vs <1000 copies/mL | 36·7 | 4·85–278 | <0·001 | – | 31·6 | 4·15–240 | 0·001 | – | ||||
| T0 GSS scorec | per 0·5 higher | 0·55 | 0·39–0·79 | 0·001 | – | – | 0·58 | 0·41–082 | 0·002 | ||||
| T0 NNRTI RAMs | yes vs no | 14·5 | 3·31–63·5 | <0·001 | – | – | – | ||||||
Abbreviations: ART=antiretroviral therapy; AZT=zidovudine; CI=confidence interval; GSS=genotypic susceptibility score; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleos(t)ide reverse transcriptase inhibitor; OR=odds ratio; PI/r=ritonavir-boosted protease inhibitor; RAMs=resistance-associated mutations; 3TC=lamivudine; TDF=tenofovir disoproxil fumarate; VAS=visual analogue scale.
Model 1 and Model 2 include the T0 viral load as a continuous and categorical variable respectively, and do not include the T0 GSS score; Model 3 includes the T0 GSS and does not include the T0 viral load.
Occasional or regular use.
A GSS of 3 was arbitrarily assigned to patients with viral load <200 copies/mL and to 5 patients with viral load 200–400 copies/mL that did not yield a sequencing amplicon in 2 attempts (based on the absence of RAMs in other samples with viral load 200–400 copies/mL).
Table 6.
Univariable and multivariable logistic regression analysis of factors associated with lack of resuppression <1000 copies/mL at T1 among subjects with T0 viral load ≥1000 copies/mL.
| Variable | Univariable analysis |
Multivariable analysisa |
|||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | ||
| Gender | female vs male | 1.19 | 0.40–3.50 | 0.75 | |||
| Age | per 5 years older | 1.00 | 0.69–1.44 | 1.00 | |||
| Enough food | never vs at least some of the time | 0.24 | 0.04–1.45 | 0.12 | |||
| Alcoholb | yes vs no | 1.10 | 0.09–12.8 | 0.94 | |||
| Traditional or herbal remedies | yes vs no | 2.97 | 0.33–27.1 | 0.33 | |||
| Third agent | PI/r vs NNRTI | 0.12 | 0.01–1.11 | 0.06 | 0.34 | 0.03–4.24 | 0.40 |
| NRTI backbone | AZT/3TC vs TDF/3TC | 1.88 | 0.67–5.30 | 0.23 | |||
| Duration of ART | per 1 year longer | 1.04 | 0.90–1.20 | 0.60 | |||
| Treatment interruptions | ≥1 vs never | 1.29 | 0.46–3.62 | 0.64 | |||
| T1 VAS | per 10% higher | 0.67 | 0.47–0.98 | 0.036 | 0.54 | 0.29–0.99 | 0.047 |
| Change in adherence score | per unit increase | 1.26 | 0.83–1.89 | 0.28 | |||
| Time since HIV diagnosis | per year longer | 1.02 | 0.88–1.19 | 0.75 | |||
| T0 CD4 count | per 100 cells/mm3 higher | 0.95 | 0.85–1.07 | 0.42 | |||
| T0 viral load | per 1 log10 copies/mL higher | 1.14 | 0.62–2.06 | 0.68 | |||
| T0 GSS scorec | per 0·5 higher | 0.47 | 0.32–0.69 | <0.001 | 0.43 | 0.27–0.69 | <0.001 |
| T0 NNRTI RAMs | yes vs no | 21.9 | 2.52–190 | 0.005 | – | – | – |
Abbreviations: ART=antiretroviral therapy; AZT=zidovudine; CI=confidence interval; GSS=genotypic susceptibility score; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleos(t)ide reverse transcriptase inhibitor; OR=odds ratio; PI/r=ritonavir-boosted protease inhibitor; RAMs=resistance-associated mutations; 3TC=lamivudine; TDF=tenofovir disoproxil fumarate; VAS=visual analogue scale.
Model 1 and Model 2 include the T0 viral load as a continuous and categorical variable respectively, and do not include the T0 GSS score; Model 3 includes the T0 GSS and does not include the T0 viral load.
Occasional or regular use.
A GSS of 3 was arbitrarily assigned to patients with viral load <200 copies/mL and to 5 patients with viral load 200–400 copies/mL that did not yield a sequencing amplicon in 2 attempts (based on the absence of RAMs in other samples with viral load 200–400 copies/mL).
3.5. Change in adherence score between T0 and T1
Among patients with T0 viraemia, the adherence score based on the number of pills missed in the previous month improved from a median of 1·0 (IQR 1·0–3·0) at T0 to a median of 3·0 (IQR 1·0–3·0) at T1 (p<0·0001). Among patients with T0 viral load ≥1000 copies/mL, the median adherence score improved from 0·0 (IQR 0·0–1·0) to 3·0 (IQR 1·0–3·0) (p<0·0001).
3.6. Resistance analysis
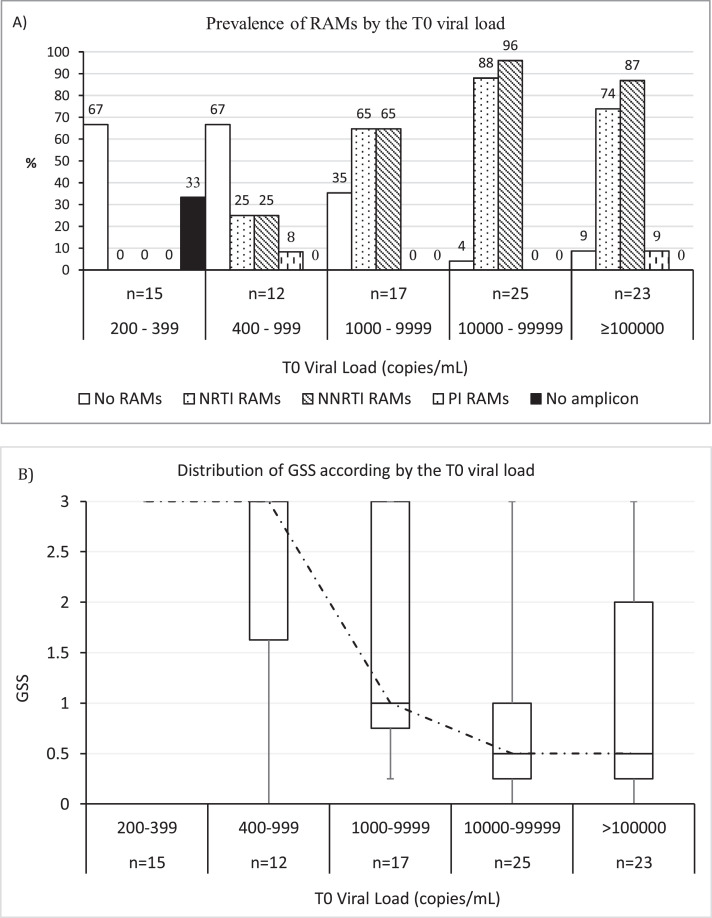
A total of 87 patients with sufficient sample underwent resistance testing, including 65/71 (91·5%) with T0 viral load ≥1000 copies/mL and 22/30 (73·3%) with T0 viral load 200–999 copies/mL. Prevalence of RAMs according to the T0 viral load and ART regimen is summarised in Table 7. Prevalence of ≥ 1 NRTI, NNRTI or PI RAM was 53/87 (60·9%), 59/87 (67·8%) and 3/87 (3·4%), respectively. Prevalence of ≥ 1 RAM increased by T0 viral load stratum (Fig. 2A) and the GSS decreased in parallel (Fig. 2B). No RAMs were detected in 15 patients with viral load 200–400 copies/mL. Among 12 patients with viral load 400–999 copies/mL, 4 (33·3%) had ≥ 1 RAM, most commonly M184V/I and K103N/S (Table 8). Patients with viral load ≥1000 copies/mL had more extensive resistance (median 2 RAMs; [IQR 1–3], and more complex resistance patterns: 35/65 (53·8% had M184V/I and K103N/S, 20/65 (30·8%) had ≥ 1 discriminatory RAM (other than M184V/I), and 24/65 (36·9%) had ≥ 1 TAM. CRF02_AG was the most prevalent subtype (66/87, 75·9%).
Table 7.
Prevalence of resistance-associated mutations (RAMs) according to the T0 viral load and ART regimen.
| ART regimen | RAMs | HIV-1 viral load (copies/mL) |
|
|---|---|---|---|
| 200–999 | ≥1000 | ||
| NNRTI-based | |||
| Number tested | 79 | 19 | 60 |
| RAMs, n (%) | None | 14 (73.7) | 7 (11.7) |
| NNRTI only | 1 (5.3) | 6 (10.0) | |
| NRTI only | 0 (0) | 0 (0) | |
| NRTI+NNRTI | 0 (0) | 47 (78.3) | |
| NRTI+NNRTI+PI | 0 (0) | 1 (1.7) | |
| No amplicon | 4 (21.1) | 0 (0) | |
| PI-based | |||
| Number tested | 13 | 8 | 5 |
| RAMs, n (%) | None | 4 (50.0) | 2 (40.0) |
| NNRTI only | 0 (0) | 0 (0) | |
| NRTI only | 1 (12.5) | 1 (20.0) | |
| NRTI+NNRTI | 2 (25.0) | 2 (40.0) | |
| NRTI+NNRTI+PI | 1 (12.5) | 1 (20.0) | |
| No amplicon | 1 (12.5) | 0 (0) | |
Abbreviation: ART=antiretroviral treatment; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleoside reverse transcriptase inhibitor; PI=protease inhibitor.
Fig. 2.
A) Prevalence of ≥ 1 resistance-associated mutation (RAM) by drug class and according to the T0 viral load; B) Box and whiskers of the genotypic susceptibility score (GSS) according to the T0 viral load; for each viral load stratum, the box indicates the distribution of the score (median and IQR); the dotted line connects the median values of the score.
Table 8.
Types of resistance-associated mutations (RAMs) stratified by the T0 viral load.
| Group | T0 HIV-1 RNA (copies/mL) |
||||
|---|---|---|---|---|---|
| 200–999 | ≥1000 | ||||
| n | % | n | % | ||
| Total number testeda | 22 | 100 | 65 | 100 | |
| NRTI RAMs | Any | 3 | 13·6 | 50 | 76·9 |
| Discriminatory | M184V/I | 3 | 13·6 | 48 | 73·8 |
| K65R | – | – | 12 | 18·5 | |
| K70E/G/N | – | – | 7 | 10·8 | |
| L74I/V | 1 | 4·5 | 5 | 7·7 | |
| Y115F | – | – | 3 | 4·6 | |
| TAMs type 1 | M41L | 2 | 9·1 | 12 | 18·5 |
| L210W | 1 | 4·5 | 6 | 9·2 | |
| T215Y | 2 | 9·1 | 9 | 13·8 | |
| TAMs type 2 | D67N/G | 1 | 4·5 | 7 | 10·8 |
| K70R | – | – | 8 | 12·3 | |
| T215F | – | – | 4 | 6·2 | |
| K219Q/E/R | 1 | 4·5 | 14 | 21·5 | |
| T215 revertants | T215D/I/V | – | – | 3 | 4·6 |
| Miscellaneous | E44D | 1 | 4·5 | 2 | 3·1 |
| T69G | – | – | 1 | 1·5 | |
| NNRTI RAMs | Any | 4 | 18·2 | 55 | 84·6 |
| A98G | – | – | 10 | 15·4 | |
| L100I | – | – | 1 | 1·5 | |
| K101E/H/P | – | – | 9 | 13·8 | |
| K103N/S | 3 | 13·6 | 43 | 66·2 | |
| V108I | 1 | 4·5 | 10 | 15·4 | |
| E138G/Q | – | – | 5 | 7·7 | |
| G190A/S | – | – | 8 | 12·3 | |
| Y181C | – | – | 10 | 15·4 | |
| Y188L | – | – | 3 | 4·6 | |
| H221Y | – | – | 4 | 6·2 | |
| P225H | – | – | 16 | 24·6 | |
| F227L | – | – | 3 | 4·6 | |
| M230L | – | – | 5 | 7·7 | |
| K238T | 1 | 4·5 | 6 | 9·2 | |
| Y318F | – | – | 1 | 1·5 | |
| PI RAMsb | Any | 1 | 4·5 | 2 | 3·1 |
| L33F | – | – | 1 | 1·5 | |
| M46I | 1 | 4·5 | 1 | 1·5 | |
| L76V | 1 | 4·5 | – | – | |
| V82A | – | – | 1 | 1·5 | |
| I84V | 1 | 4·5 | – | – | |
Abbreviations: IQR=interquartile range; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleos(t)itide reverse transcriptase inhibitor; RAMs=resistance-associated mutations; PI=protease inhibitor; TAMs=thymidine analogue mutations.
HIV-1 subtypes comprised CRF02_AG (66/87, 75·9%), CRF06_cpx (9/87, 10·3%), A (5/87, 5·7%), CRF09_cpx (4/87, 4·6%), G (2/87, 2·3%), and D (1/87, 1·1%).
The 3 subjects with PI RAMs had received lopinavir/ritonavir for a median of 0·74 years (IQR 0·08–1·34).
4. Discussion
In this prospective study of a mature HIV cohort accessing treatment in a real-life setting in Ghana, POC viral load testing was technically feasible and effectively informed immediate adherence counselling for patients with viraemia. After long-term, mainly NNRTI-based ART in the absence of routine access to virological monitoring, nearly half of the patients had a detectable viral load, and 1 in 5 had a viral load ≥1000 copies/mL, the WHO-endorsed threshold for defining virological failure [3]. Eight weeks later, there was high retention into follow-up. Although self-reported adherence levels improved post-counselling, none of the patients with a viral load ≥1000 copies/mL achieved resuppression <40 copies/mL while continuing NNRTI-based ART, whereas a substantial proportion of those with lower viral load levels did. We observed co-existence of high viral loads and complex drug resistance patterns in the group with viral load ≥1000 copies/mL, which suggests that delaying a switch to second-line ART might be counterproductive in terms of risk of disease progression and potential transmission of drug-resistant strains. Conversely, patients with viral load <1000 copies/mL had limited drug resistance, increasing the likelihood of improved virological control after adherence counselling. Of note, around a third of patients with viral load ≥1000 copies/mL showed a subsequent viral load <1000 copies/mL while continuing NNRTI-based ART. There remains uncertainty about the durability of partial virological responses given the high risk of emerging drug resistance during NNRTI-based therapy [32].
In pooled analyses of studies from sub-Saharan Africa, 65% and 62% of patients had a suppressed viral load (by intention to treat) after 24 months of first-line NNRTI-based ART and second-line PI/r-based ART, respectively [19,20]. Rates of virological suppression differ by region, and tend to be higher in randomised clinical trials than in observational cohorts [20]. Long-term data are scarce. In this study, after a median of 8·9 years of predominantly NNRTI-based ART, 49·2% had a detectable viral load and 21·3% had ≥1000 copies/mL. Similar alarming data were reported from Togo: after a median of 6 years of predominantly NNRTI-based ART, nearly 60% of patients had viraemia, and 40% had a viral load ≥1000 copies/mL [21]. New strategies are needed to improve management of HIV-positive cohorts in West Africa, where care continues to be delivered largely in the absence of virological monitoring.
In sub-Saharan Africa, POC assays were previously used effectively for diagnosing HIV-positive infants, measuring CD4 cell counts, and detecting tuberculosis [22], [23], [24]. A trial from South Africa - a setting with established virological monitoring capacity – recently reported preliminary data showing that soon after ART initiation, POC viral load testing by Xpert may promote higher retention in care and greater virological suppression relative to standard laboratory testing [25]. A previous multicentre study in rural Zimbabwe used Xpert for on-site HIV-1 RNA testing of a selected population, but patients did not wait for the results [26]. Adherence counselling was planned for subjects with a viral load ≥1000 copies/mL; however, 1–3 weeks after the initial viral load test, about half (53/96, 55%) of those with viral load ≥1000 copies/mL were lost to follow-up. In our study, all patients waited for their results, and all patients with any level of viraemia received adherence counselling. Furthermore, perhaps anecdotally aided by the first adherence counselling and knowledge that results would be available on the same day, attendance at the follow-up visit was 91·5% among viraemic patients.
POC viral load testing was technically successful, and knowledge of the result often unmasked problems with adherence that had not emerged at the first interview a few hours earlier. However, implementation on a larger, routine scale requires a number of operational solutions. Firstly, back-up batteries of sufficient potency are required to ensure continuous supply of electricity and avoid assay failure. Second, the size of the Xpert unit dictates the number of tests that can be run within a typical clinic day. Where larger or multiple units are not available, strategies must be defined to triage patients according to their risk of viraemia. In our study, both a history of treatment interruption and the VAS score were associated with the viral load. Other studies from Mozambique [27], Nigeria, Uganda, Kenya and Tanzania [28] similarly indicated that measured or self-reported adherence independently predicted a viral load ≥1000 copies/mL. Thus, simple measures of adherence could be applied to fast track patients to POC vs. deferred viral load testing, a hypothesis that needs investigating in controlled studies. Additional factors could be used to develop a triaging score. For example, age, gender, food availability and use of AZT/3TC versus TDF/3TC were predictors of viraemia in this study.
A meta-analysis of 5 studies conducted between 2004 and 2013 explored rates of resuppression after adherence counselling in high and middle-low income countries, including Mali, Burkina Faso, Swaziland, and South Africa [29]. A pooled estimate of 70% was derived from a total population of 406 patients that differed in terms of treatment history and definition of viraemia and resuppression. Resuppression rates following adherence counselling in patients with a viral load ≥1000 copies/mL while receiving first-line NNRTI-based ART were reported from a trial in Uganda (resuppression rate 19/70, 27%) [4], a multicentre study from Burkina Faso, Cameroon, and Senegal (81/584, 14%) [30], and a prospective study from rural Lesotho (39/110, 35%) [31]. One important limitation of these studies was that viral load testing was deferred and rates of loss to follow-up ranged up to 33%. A study from Uganda also reported that adherence levels (measured by electronic pill count) predicted the likelihood of resuppression for patients with viral load 500–1000 copies/mL, but not for those with viral load ≥1000 copies/mL [5]. The available data are in line with our findings. We found that the T0 viral load predicted the likelihood of resuppression <40 copies/mL independently of self-reported adherence, whereas adherence levels (as well as detection of RAMs) were independently predictive of resuppression <1000 copies/mL among patients with viral load ≥1000 copies/mL. Although the predictive effect of the T0 viral load moved along a continuum, a viral load ≥1000 copies/mL identified patients with extensive drug resistance and reduced likelihood of resuppression while continuing NNRTI-based ART.
In previous studies, prevalence of RAMs in NNRTI-treated patients with viral load ≥1000 copies/mL was 89% (54/61) after a median of 3 years in Mozambique [27], 99% (440/446) after 4 years in Burkina Faso, Senegal and Cameroon [30], 92% (77/84) after 5 years in Mali [33], and 99% (163/164) after 6 years in Togo [21]. After a median of 8·9 years of predominantly NNRTI-based ART in this study, 55/65 (85%) patients with viral load ≥1000 copies/mL had ≥1 RAM, usually including multiple NRTI and NNRTI RAMs. We noted a relatively high prevalence of tenofovir RAMs in this group, with 21/65 (32%) patients showing K65R, K70E/G/N, L74I/V, or Y115F. As previously observed by us [34] and others [30], the highly mutated virus strains did not show evidence of impaired fitness given the high viral loads and low CD4 cell counts. Tenofovir remains a key component of first- and second-line ART in sub-Saharan Africa, including forthcoming regimens with dolutegravir. It will be important to monitor the impact of tenofovir RAMs in treated populations and assess the risk of transmission and impact on the efficacy of pre-exposure prophylaxis.
There are limitations to this study. For most patients, there had been no previous viral load measurement to guide treatment decisions, and the findings may not directly apply to different epidemiological contexts where virological monitoring is already established. As the cohort had no routine access to viral load testing, no randomised comparison with standard of care was possible. Given the pilot nature of the study and the need to obtain initial data on rates of viraemia and associated risk factors, no attempt was made at exploring same-day versus deferred POC viral load testing; controlled studies are planned to determine the optimal strategy for triaging patients. Whilst it may be argued that longer follow-up or a different modality of adherence counselling might have improved responses in the group with viral load ≥1000 copies/mL, the high prevalence of NRTI and NNRTI RAMs makes such outcomes unlikely. Direct measures such as pill-counts could have enhanced the evaluation of adherence, although there is evidence that self-reported measures may perform better than pill-counts in these settings [35]. Detection of RAMs by population sequencing might have failed to detect low-frequency variants. However, we previously documented that in a population long established on NNRTI-based ART deep sequencing affords a rather modest increase in yield [34].
In conclusion, the current WHO-endorsed two-stage algorithm, whereby a switch to a second-line regimen is recommended only if a viral load measurement ≥1000 copies/mL is confirmed 3–6 months after adherence counselling, may be regarded as most effective in settings with a low prevalence of NNRTI resistance. In settings with high population-level estimates of NNRTI resistance, and especially when testing patients who have received long-term NNRTI-based ART without virological monitoring, a viral load measurement ≥1000 copies/mL is typically accompanied by high rates of NRTI and NNRTI drug resistance and can be used to trigger a switch to second-line ART without waiting for the confirmatory measurement.
5. Contributors
AMG designed the study; GV, DO, MA, DC, RP and AMG were involved in the study management; GV, AA, DO, LS, HA, DA, AB, DC, RP and AMG collected the data; GV, AA, RP and AMG analysed the data; CS performed the statistical analysis; GV, RP and AMG interpreted the data; GV and AMG wrote the manuscript, with the critical input of all co-authors.
Declaration of competing interest
Dr. Smith reports personal fees from Gilead Sciences and ViiV, outside the submitted work; Dr. Chadwick reports personal fees from Gilead, outside the submitted work; Dr. Geretti reports grants from BMS, Gilead, Janssen, and ViiV, and personal fees from Roche Pharma Research & Early Discovery, outside the submitted work. All other authors have nothing to disclose.
Acknowledgements
We gratefully acknowledge the support received by the KATH personnel that made this research possible, namely interpreters, doctors, nurses, laboratory technicians, and administrative staff. We are very grateful for the help received from Mr Fred Barker and Mr David Stewart with retrieving data from the medical records. Above all we are very thankful to the patients who participated in the study. The funding sources were not involved in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
References
- 1.Rodger AJ, Cambiano V, Bruun T. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316:171–181. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 2.Phillips A, Shroufi A, Vojnov L. Sustainable hiv treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528:S68. doi: 10.1038/nature16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation . 2nd ed. Switzerland; Geneva,: 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [PubMed] [Google Scholar]
- 4.Gupta RK, Goodall RL, Ranopa M. High rate of HIV resuppression after viral failure on first-line antiretroviral therapy in the absence of switch to second-line therapy. Clin Infect Dis. 2014;58:1023–1026. doi: 10.1093/cid/cit933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCluskey SM, Boum Y, 2nd, Musinguzi N. Appraising viral load thresholds and adherence support recommendations in the World Health Organization guidelines for detection and management of virologic failure. J Acquir Immune Defic Syndr. 2017;76:183–187. doi: 10.1097/QAI.0000000000001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joint United Nations Programme on HIV/AIDS. Global HIV & AIDS statistics — 2018 fact sheet. 2018.
- 7.Roberts T., Cohn J., Bonner K., Hargreaves S. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis. 2016;62:1043–1048. doi: 10.1093/cid/ciw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation. HIV diagnostic tests in low and middle-income countries: forecasts of global demand for 2014-2018. Geneva; 2015.
- 9.Sacks JA, Fong Y, Gonzalez MP. Performance of Cepheid Xpert HIV-1 viral load plasma assay to accurately detect treatment failure. AIDS. 2019;33:1881–1889. doi: 10.1097/QAD.0000000000002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorward J, Drain PK, Garrett N. Point-of-care viral load testing and differentiated HIV care. The Lancet HIV. 2018;5:e8–e9. doi: 10.1016/S2352-3018(17)30211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyo S, Mohammed T, Wirth KE. Point-of-care Cepheid Xpert HIV-1 viral load test in rural African communities is feasible and reliable. J Clin Microbiol. 2016;54:3050. doi: 10.1128/JCM.01594-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gous N, Scott L, Berrie L, Stevens W. Options to expand HIV viral load testing in South Africa: evaluation of the GeneXpert(R) HIV-1 viral load assay. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrett NJ, Drain PK, Werner L. Diagnostic accuracy of the point-of-care Xpert HIV-1 viral load assay in a South African HIV clinic. J AIDS. 2016;72:e45–e48. doi: 10.1097/QAI.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceffa S, Luhanga R, Andreotti M. Comparison of the Cepheid GeneXpert and Abbott M2000 HIV-1 real time molecular assays for monitoring HIV-1 viral load and detecting HIV-1 infection. J Virol Meth. 2016;229:35–39. doi: 10.1016/j.jviromet.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Bwana P, Ageng’o J, Mwau M. Performance and usability of Cepheid GeneXpert HIV-1 qualitative and quantitative assay in Kenya. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0213865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boender TS, Sigaloff KCE, McMahon JH. Long-term virological outcomes of first-line antiretroviral therapy for HIV-1 in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2015;61:1453–1461. doi: 10.1093/cid/civ556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cepheid. Xpert® HIV-1 Viral Load. 2018. http://www.cepheid.com/en/cepheid-solutions/clinical-ivd-tests/virology/xpert-hiv-1-viral-load (accessed 11/05/2019).
- 18.Stockdale AJ, Phillips RO, Beloukas A. Liver fibrosis by transient elastography and virologic outcomes after introduction of tenofovir in lamivudine-experienced adults with HIV and hepatitis B virus coinfection in Ghana. Clin Infect Dis. 2015;61:883–891. doi: 10.1093/cid/civ421. [DOI] [PubMed] [Google Scholar]
- 19.Boender T.S., Sigaloff K.C.E., McMahon J.H. Long-term virological outcomes of first-line antiretroviral therapy for HIV-1 in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2015;61:1453–1461. doi: 10.1093/cid/civ556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stockdale AJ, Saunders MJ, Boyd MA. Effectiveness of protease inhibitor/nucleos(t)ide reverse transcriptase inhibitor-based second-line antiretroviral therapy for the treatment of human immunodeficiency virus type 1 infection in sub-Saharan Africa: a systematic review and meta-analysis. Clin Infect Dis. 2018;66:1846–1857. doi: 10.1093/cid/cix1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konou AA, Dagnra AY, Vidal N. Alarming rates of virological failure and drug resistance in patients on long-term antiretroviral treatment in routine HIV clinics in Togo. AIDS. 2015;29:2527–2530. doi: 10.1097/QAD.0000000000000906. [DOI] [PubMed] [Google Scholar]
- 22.Frank SC, Cohn J, Dunning L. Clinical effect and cost-effectiveness of incorporation of point-of-care assays into early infant HIV diagnosis programmes in Zimbabwe: a modelling study. Lancet HIV. 2019;6:e182–e190. doi: 10.1016/S2352-3018(18)30328-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai MA, Okal DO, Rose CE. Clinical effect and cost-effectiveness of incorporation of point-of-care assays into early infant HIV diagnosis programmes in Zimbabwe: a modelling study. Lancet HIV. 2017;4:e393–e401. doi: 10.1016/S2352-3018(18)30328-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pooran A, Theron G, Zijenah L. Point of care Xpert MTB/RIF versus smear microscopy for tuberculosis diagnosis in southern African primary care clinics: a multicentre economic evaluation. Lancet Global Health. 2019;7:e798–e807. doi: 10.1016/S2214-109X(19)30164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drain PK, Dorward J, Violette L. Point-of-care viral load testing improves HIV viral suppression and retention in care. Conference on Retroviruses and Opportunistic Infections; Seattle; 2019. Abstract n.53. [Google Scholar]
- 26.Ndlovu Z, Fajardo E, Mbofana E. Multidisease testing for HIV and TB using the GeneXpert platform: a feasibility study in rural Zimbabwe. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0193577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rupérez M, Pou C, Maculuve S. Determinants of virological failure and antiretroviral drug resistance in Mozambique. J Antimicrob Chemother. 2015;70:2639–2647. doi: 10.1093/jac/dkv143. [DOI] [PubMed] [Google Scholar]
- 28.Kiweewa F, Esber A, Musingye E. HIV virologic failure and its predictors among HIV-infected adults on antiretroviral therapy in the African cohort study. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0211344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonner K., Mezochow A., Roberts T., Ford N., Cohn J. Viral load monitoring as a tool to reinforce adherence: a systematic review. J AIDS. 2013;64:74–78. doi: 10.1097/QAI.0b013e31829f05ac. [DOI] [PubMed] [Google Scholar]
- 30.Guichet E, Aghokeng A, Serrano L. High viral load and multidrug resistance due to late switch to second-line regimens could be a major obstacle to reach the 90-90-90 UNAIDS objectives in sub-Saharan Africa. AIDS Res Hum Retroviruses. 2016;32:1159–1162. doi: 10.1089/AID.2016.0010. [DOI] [PubMed] [Google Scholar]
- 31.Labhardt ND, Ringera I, Lejone TI. When patients fail UNAIDS’ last 90 - the “failure cascade” beyond 90-90-90 in rural Lesotho, Southern Africa: a prospective cohort study. J Int AIDS Soc. 2017;20:21803. doi: 10.7448/IAS.20.1.21803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swenson L.C., Min J.E., Woods C.K. HIV drug resistance detected during low-level viremia is associated with subsequent virologic failure. AIDS. 2014;28:1125–1134. doi: 10.1097/QAD.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcelin AG, Soulié C, Fofana DB. High level of HIV-1 resistance in patients failing long-term first-line antiretroviral therapy in Mali. J Antimicrob Chemother. 2014;69:2531–2535. doi: 10.1093/jac/dku153. [DOI] [PubMed] [Google Scholar]
- 34.Villa G, Phillips RO, Smith C. Drug resistance outcomes of long-term ART with tenofovir disoproxil fumarate in the absence of virological monitoring. J Antimicrob Chemother. 2018;73:3148–3157. doi: 10.1093/jac/dky281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciaffi L, Koulla-Shiro S, Sawadogo AB. Boosted protease inhibitor monotherapy versus boosted protease inhibitor plus lamivudine dual therapy as second-line maintenance treatment for HIV-1-infected patients in sub-Saharan Africa (ANRS12 286/MOBIDIP): a multicentre, randomised, parallel, open-label, superiority trial. Lancet HIV. 2017;4:e384–ee92. doi: 10.1016/S2352-3018(17)30069-3. [DOI] [PubMed] [Google Scholar]