Highlights
-
•
Reports 4th case of DFSP like tumor of the uterus or cervix, and 2nd in fundus.
-
•
These tumors may be misdiagnosed as leiomyosarcomas.
-
•
These tumors are CD34 positive and have the COL1A1-PDGFB translocation.
-
•
These tumors may respond to targeted Imatinib therapy.
-
•
Targeted therapies, including Imatinib, are available for DFSP like tumors.
Keywords: Uterine sarcoma, Dermatofibrosarcoma protuberans (DFSP), COL1A1-PDGFB fusion, Imatinib
Abstract
COL1A1-PDGFB gene fusion associated uterine sarcoma, so called dermatofibrosarcoma-like tumor, a recently reported entity in the uterine corpus, morphologically appears as high grade sarcoma with some features of dermatofibrosarcoma. So far only one other case has been reported in the uterine corpus and two in the uterine cervix. Identification of this gene fusion allows greater choice of targeted therapy in these patients. All the reported cases in the mullerian system are found to be CD34 positive by immunohistochemistry, a commonly used antibody in most immunohistochemistry laboratories. We would, therefore, propose routine CD34 immunohistochemical staining in all high grade uterine sarcomas which have failed other common immunohistochemical markers.
1. Introduction
Uterine sarcomas are a rare group of tumors which comprise about 3–7% of uterine malignancies (Wen, 2016). Subtypes include leiomyosarcoma, adenosarcoma, endometrial stromal sarcoma, and undifferentiated sarcoma (Wen, 2016, Horng, 2016, Kurman, 2014). Some sarcomas of the uterus are not easily classifiable including some which have a histologic appearance similar to fibrosarcoma (Croce, 2019). Gene fusion study is increasingly being used, not only for subcategorization, but also for expanding treatment options. In a recent review of 13 cases of spindle cell sarcomas of the vagina, cervix, and uterine fundus with features resembling fibrosarcoma, 3 tumors with COL1A1-PDGFB (17q21.33 and 22q13.1) fusion were identified for the first time (Croce, 2019). Two of these reported tumors were found in the cervix and one within the uterine corpus (Croce, 2019). COL1A1-PDGFB fusion is commonly associated with dermatofibrosarcoma protuberans (DFSP), seen most commonly in the skin (Takahira, 2007, Network, 2018).
We are reporting the fourth case of DFSP like tumor of the gynecologic tract with COL1A1-PDGFB fusion. Identification of this unique gene fusion allows greater choices for therapy, especially targeted therapies which are now available.
2. Case presentation
A 43-year-old female with no significant past medical history was noted to have a large irregular uterus on exam at her annual physical. A pelvic ultrasound identified a 12 cm fibroid uterus. At the time, she was asymptomatic. Over the subsequent six months she developed progressively heavier menses resulting in significant anemia. A repeat ultrasound demonstrated a significantly larger uterus measuring 21x17cm with multiple fibroids and heterogeneous appearance (Fig. 1A). She desired hysterectomy, and was scheduled for total abdominal hysterectomy with her primary OBGYN. At surgery, she was noted to have a large necrotic mass emanating from the anterior fundus and invading into the bladder and omentum. Gynecology oncology was consulted intraoperatively and removed the mass with no gross residual disease. Post operatively, she underwent CT of the chest, abdomen, and pelvis which demonstrated no evidence of metastatic disease.
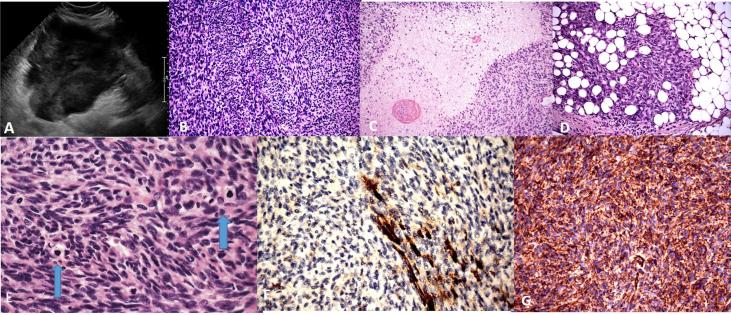
Fig. 1.
A. Preoperative ultrasound, with a sagittal view of the uterus. B. Tumor with interlacing fascicles of spindle cells. (200×) C. Focal areas of geographic, tumor cell necrosis (200×). D. Tumor enveloping periuterine adipose tissue, reminiscent of DFSP of the skin (200×). E. Mildly atypical spindle cells arranged in interlacing fascicles with mitotic figures (arrows) (600×). F. SMA showing patchy positivity within tumor (400×). G. CD34 showing strong diffuse positivity within the tumor (400×).
The hysterectomy specimen demonstrated a uterus and cervix with a fragmented lesion on the anterior surface of the uterine fundus measuring 21.5 × 15.0 × 14.0 cm. On gross examination the lesion had a pink-tan-white whorled appearance with areas of necrosis and congestion. Additional white whorled lesions consistent with fibroids were also identified.
Histologic sections demonstrated a spindle cell neoplasm with unusual morphologic characteristics. Some areas of the tumor were very bland and had the appearance of a typical leiomyoma with rare mitotic figures while other areas were very cellular with interlacing fascicles of spindle cells with numerous mitotic figures (45 per 10 high powered fields). Ischemic and tumor cell necrosis were identified. Focal areas of myxoid changes were noted. (Fig. 1B–G) The tumor extended into the peri-uterine soft tissues, focally encompassing and entrapping adipose tissue, similar to DFSPs at other locations (Fig. 1D).
Immunohistochemical stains were performed at the time of diagnosis and included CD10, Caldesmon, Smooth Muscle Actin (SMA), Ki-67, p53, desmin and p16 (see Table 1). Despite the unusual characteristics the features were considered most consistent with a diagnosis of leiomyosarcoma of the uterus, FIGO Stage IVA and AJCC Stage pT4Nx.
Table 1.
Immunohistochemical stains performed at time of initial diagnosis.
| Immunohistochemical Stain | Clone | Results |
|---|---|---|
| CD10 | M; 56C6; Dako | Focally positive |
| Caldesmon | M; h-CD; Dako | Negative |
| Smooth muscle actin (SMA) | M; 1A4: Dako | Focally positive |
| Ki-67 antigen | M; MIB-1; Dako | Extremely high |
| p53 protein | M; DO-7; Dako | Mutated positive |
| Desmin | M; D33; Dako | Negative |
| p16 INK4a antigen | M; E6H4; Ventana | Negative |
She underwent six cycles of Docetaxel (Day 8 of 21-day cycle) and Gemcitabine (Day 1 and 8 of 21-day cycle). After completion of six cycles, CT imaging demonstrated progression of disease with multiple nodules within the pelvic peritoneum. She was switched to an alternative regimen of Doxorubicin (Day 1 of 21-day cycle) and Olaratumab, a platelet derived growth factor inhibitor (Day 1 and 8 of 21-day cycle). She was found to have stable disease on repeat imaging after seven cycles, and was continued on Olaratumab maintenance alone. However, after cycle ten, she was noted to have significant progression of disease with multiple pelvic masses.
Genomic testing of the tumor revealed no actionable mutations. The tumor was microsatellite stable. She was started on Trabectedin (Day 1 of a 21 day cycle) however the patient developed severe abdominal pain after two cycles and was again noted to have marked progression of disease on imaging with multiple pelvic masses measuring 4–22 cm. At this time, she had progressed through three lines of chemotherapy. She was transitioned to 800 mg daily of Pazopanib, a small molecule inhibitor in the angiogenesis pathway, with improvement in her abdominal symptoms. She completed nine cycles until she presented with symptoms of progression.
Gene fusion study was subsequently performed because of the lack of treatment response and COL1A1-PDGFB fusion was identified, an anomaly commonly found in DFSPs. Subsequently a CD34 immunohistochemical stain (clone QBEnd10, Dako) was performed on the tumor, and was strongly positive (Fig. 1G). A review of the literature found a recent, March of 2019, case series by Croce et al. that reported 3 cases of spindle cell sarcomas with features resembling fibrosarcoma in the gynecologic tract that were positive for COL1A1-PDGFB fusion (Croce, 2019). All of the reported cases, including the current case, were strongly CD34 positive (Croce, 2019). She was started on treatment for this actionable COL1A1-PDGFB fusion with 400 mg BID of Imatinib, a tyrosine kinase inhibitor.
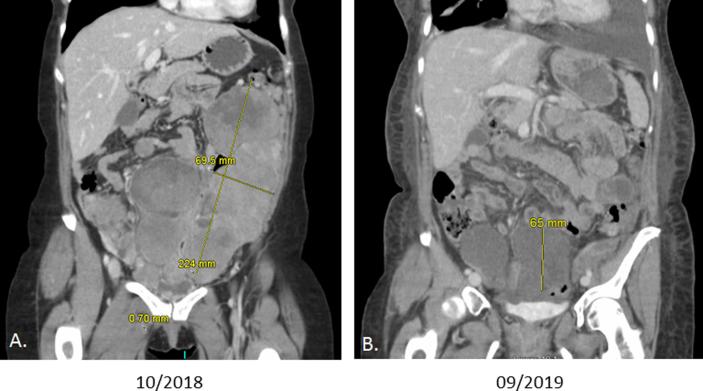
After 11 weeks on Imatinib, CT imaging noted interval resolution of pulmonary nodules and significant decrease in peritoneal deposits with the largest only measuring 6.5 cm (Fig. 2).
Fig. 2.
A. CT scan post treatment with Imantinib. B. CT scan pretreatment with Imantinib. Note the dramatic decrease in size of the tumor, 224 mm to 65 mm.
3. Discussion and review of literature
The diagnosis of uterine sarcomas may be challenging, as seen in this case. Extensive sampling and immunohistochemical workup demonstrated a spindle cell neoplasm, with mild to moderate nuclear atypia, with focal tumor cell necrosis and increased mitotic activity. Smooth muscle markers were ambiguous with some markers being negative (desmin and caldesmon) and others being focally positive (smooth muscle actin). Given the clinical presentation, gross examination, and histologic evaluation at the time of diagnosis, the diagnosis of “most consistent with leiomyosarcoma” was rendered, and the patient was treated accordingly. In retrospect, molecular testing would have been useful in this case. Unfortunately, the patient’s initial treatments were unsuccessful. Fortunately, in the search for targetable therapy, the patient’s tumor was found to have COL1A1-PDGFB fusion most commonly associated with DFSP, which may be treated with targeted therapy (Navarrete-Dechent, 2019).
DFSP is a rare tumor with 4.5 cases per million persons per year in the US. DFSP account for 18% of all cutaneous soft tissue sarcomas and most commonly occurs on the skin of the trunk (Rouhani, et al., 2008). The tumor is locally aggressive with recurrence rates of 10–60%, but overall five-year survival rates reach 98–100% (Network, 2018, Rouhani, et al., 2008). The tumor most commonly arises from the dermis, but also has been found to arise from the subcutaneous tissue of the breast (Pohlodek, 2017). Regional metastases are extremely rare; therefore surgical excision with adequate margins is largely sufficient treatment with close follow up surveillance (Network, 2018). Adjuvant radiation therapy is recommended for positive margins or recurrence. Molecular targeted therapy with Imatinib has a growing body of research for its use in DFSP and is currently approved for use in unresectable, recurrent, or metastatic DFSP. A 2019 systematic review of patients with locally advanced or metastatic DFSP found that 60% of patients had a complete or partial response to Imatinib treatment (Navarrete-Dechent, 2019).
All 4 of the DFSP like tumors of the gynecologic tract reported thus far have been positive for CD34 and have the COL1A1-PDGFB fusion (Croce, 2019), just like DFSPs at other locations including the breast and vulva (Takahira, 2007, Network, 2018, Gokden, 2003). In addition, these tumors have a similar histologic pattern to DFSPs at other locations including a cellular proliferation consisting of interlacing fascicles of spindle cells with mild atypia and increased mitotic activity (Network, 2018, Pohlodek, 2017, Gokden, 2003).
In this case the tumor was extremely aggressive, with local extension into the bladder and omentum at the time of diagnosis and marked progression of disease with lesions measuring up to 22 cm after treatment. Due to these treatment failures an extensive workup was performed that lead to the discovery of the COL1A1-PDGFB fusion. At the time of initial diagnosis this entity had yet to be described. An awareness of tumors similar to DFSP arising in the uterus or cervix will help speed the diagnosis and make available targeted treatment options.
4. Conclusions
The paper by Croce et al. has a detailed algorithm for handling difficult uterine sarcoma cases (Croce, 2019). This algorithm includes using immunohistochemical stains and molecular testing to classify these tumors, and we defer to their paper for a detailed discussion on the topic (Croce, 2019). We concur with their findings and recommend that CD34 positive tumors of the uterus be tested for the COL1A1-PDGFB fusion to confirm the diagnosis. This will help guide treatment, including giving patients additional targeted therapy options, such as use of Imatinib (Network, 2018), which has proven useful in this case.
Further studies will need to be performed to see how more of these tumors respond to Imatinib treatment; however this patient demonstrated a marked response after initial use which is encouraging. As more targeted therapies become available, identification of the actionable genetic alteration is important. Identifying DFSP like tumors of the gynecologic tract will make targeted therapies, like Imatinib, available to these patients.
CRediT authorship contribution statement
Samuel L. Grindstaff: Investigation, Writing - original draft, Writing - review & editing. Jessica DiSilvestro: Investigation, Writing - original draft, Writing - review & editing. Katrine Hansen: Writing - review & editing. Paul DiSilvestro: Writing - review & editing. C. James Sung: Writing - review & editing. M. Ruhul Quddus: Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Wen K.C. Uterine sarcoma part I-uterine leiomyosarcoma: the topic advisory group systematic review. Taiwan J. Obstet. Gynecol. 2016;55(4):463–471. doi: 10.1016/j.tjog.2016.04.033. [DOI] [PubMed] [Google Scholar]
- Horng H.C. Uterine sarcoma part II-uterine endometrial stromal sarcoma: the TAG systematic review. Taiwan J. Obstet. Gynecol. 2016;55(4):472–479. doi: 10.1016/j.tjog.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Kurman R.J. WHO classification of tumours of the female reproductive organs. lyon france: international agency for research on. Cancer. 2014;IARC:135–147. [Google Scholar]
- Croce S. Uterine and vaginal sarcomas resembling fibrosarcoma: a clinicopathological and molecular analysis of 13 cases showing common NTRK-rearrangements and the description of a COL1A1-PDGFB fusion novel to uterine neoplasms. Mod. Pathol. 2019;32(7):1008–1022. doi: 10.1038/s41379-018-0184-6. [DOI] [PubMed] [Google Scholar]
- Takahira T. Detection of COL1A1-PDGFB fusion transcripts and PDGFB/PDGFRB mRNA expression in dermatofibrosarcoma protuberans. Mod. Pathol. 2007;20(6):668–675. doi: 10.1038/modpathol.3800783. [DOI] [PubMed] [Google Scholar]
- Network, N.C.C. Dermatofibrosarcoma Protuberans (Version 1.2019) 2018 August 31,2018 [cited 2019 August 30, 2019]; Available from: https://www.nccn.org/professionals/physician_gls/PDF/dfsp.pdf.
- Navarrete-Dechent C. Imatinib treatment for locally advanced or metastatic dermatofibrosarcoma protuberans: a systematic review. JAMA Dermatol. 2019;155(3):361–369. doi: 10.1001/jamadermatol.2018.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhani P. Cutaneous soft tissue sarcoma incidence patterns in the U.S. : an analysis of 12,114 cases. Cancer. 2008;113(3):616–627. doi: 10.1002/cncr.23571. [DOI] [PubMed] [Google Scholar]
- Pohlodek K. Dermatofibrosarcoma protuberans of the breast: a case report. Oncol. Lett. 2017;14(1):993–998. doi: 10.3892/ol.2017.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokden N. Dermatofibrosarcoma protuberans of the vulva and groin: detection of COL1A1-PDGFB fusion transcripts by RT-PCR. J. Cutan. Pathol. 2003;30(3):190–195. doi: 10.1034/j.1600-0560.2003.00037.x. [DOI] [PubMed] [Google Scholar]