Abstract
Background
MicroRNA (miR)-106a was involved in the tumorigenesis and highly expressed in gastric cancer. Required apatinib resistance greatly limits its efficacy in patients. Thus, the aim of the present study was to investigate the potential role of miR-106a-3p in gastric cancer cells with apatinib-resistance.
Material/Methods
The expression of miR-106a-3p was quantified by real-time quantitative polymerase chain reaction (RT-qPCR). Cell Counting Kit-8 (CCK-8) assay was performed to analyze the sensitivity of gastric cancer cells to apatinib. The expression of relevant drug-resistant proteins was detected by western blot. We searched Targetscan6.2 to find out the target gene of miR-106a-3p. Luciferase reporter assay was used to analyze whether miR-106a-3p bound to relevant gene of SOCS family. The SOCS2, SOCS4, and SOCS5 were qualified by western blot, and their mRNA levels were detected by RT-qPCR. Further, JAK2, STAT3, and their phosphorylation levels were detected by western blot.
Results
The results showed that the expression of miR-106a-3p was increased in apatinib-resistant gastric cancer, while miR-106a-3p inhibitor reduced the drug-resistance of SGC-7901-AP cells to apatinib. Dual luciferase reporter gene assay suggested that SOCS2, SOCS4, and SOCS5 were target genes of miR-106a-3p. The relevant SOCS genes silencing reversed the effects of miR-106a-3p inhibitor on decreasing the apatinib resistance of SGC-7901-AP cells, while the phosphorylation level of JAK and STAT reduced by miR-106a-3p inhibitor were increased.
Conclusions
miR-106a-3p induces apatinib resistance and activates JAK2/STAT3 by targeting SOCS system in gastric cancer. miR-106a-3p/SOCS plays a potent role in gastric cancer cell resistance to apatinib.
MeSH Keywords: Drug Resistance, Janus Kinase 2, MicroRNAs, STAT3 Transcription Factor, Suppressor of Cytokine Signaling Proteins
Background
Gastric cancer remains one of the most common cause of cancer death in humans worldwide. Gastric cancer seriously affects the survival rate and life condition of patients [1]. In clinical studies, apatinib is safe and efficient in the treatment of gastric cancer, which inhibits angiogenesis in cancer tissues by highly selectively competing for cellular binding site of ATP (adenosine triphosphate) in VEGFR-2 (Vascular endothelial growth factor receptor 2) [2,3]. However, gastric cancer cells would acquire the phenotype of drug-resistant continuously exposed to chemotherapy. The occurrence of drug-resistant cells was related to some signaling pathways. For example, the apatinib resistance of gastric carcinoma could be induced by the oncogene, dual-specificity phosphatase-1, via the activation of MAPK [4].
MicroRNA (miRNA) is involved in post-transcriptional regulation of DNA by binding to the 3′UTR region of the transcript sequence [5]. MiRNA-106a-3p (MiR-106a-3p) belongs to one of poorly conserved microRNA family members by Targetscan6.2. Even so, its effects cannot be neglected in gastric cancer as a circulatory oncogenic miRNA, the upregulation of miR-106a appeared in many cancers, especially stomach cancer. Furthermore, the study has found that the expression of miR-106a in gastric cancer tissues have a close relationship with the size, stage, and progression of tumor. It is still elusive how miR-106a exerts its functions in the progression of the apatinib resistance in gastric carcinoma [6]. Glutathione S-transferase π (GST-π), transports toxicant from tumor and mediates drug-resistant cell [7]. Multi-drug resistance-associated protein 1 (MRP1) decreases cellular drug concentration [8]. The ATP-binding cassette transporters P-glycoprotein/ABCB1 transport multiple drugs across membranes [9]. The signal of cytokines from the membrane is transferred into cytoplasm by activation of signal transduction pathways, in the process of which (Janus-activated kinase) JAK2/signal transducer and activator of transcription 3 (START3) pathway is widely involved. Continuous activation of JAK2/START3 pathway could cause the aberrant proliferation and vicious transformation of cells and widely participate in the development of malicious cancer [10,11]. The SOCS system could negatively regulate JAK/STAT system signal transduction. SOCS, suppressor of cytokine signaling, was characterized by the presence of a C-terminal SOCS box and a central SH2 domain [12]. SOCS-box-containing proteins probably function as E3 ubiquitin ligases and mediate the degradation of proteins associated with their N-terminal regions by NCBI. Some miRNA targeted SOCS gene, and then modulated STAT3 pathway [13]. MiR-106a as a vital oncogenic miRNA regulated the expression of many proteins involved in physiological processes in tumorigenesis [14]. Thus, the study focused on the role of miR-106a-3p in the apatinib resistance of gastric cancer, then discussed the potential biological mechanisms of apatinib resistance in gastric cancer.
Material and Methods
Cell culture
Human gastric cancer cell line including SGC-7901 and BGC-823 were purchased from the Cell Bank of Chinese Academy of Science (Shanghai, China). The cells were seeded into cell culture plate with RPMI-1640 medium containing 10% fetal bovine serum (FBS), 100 mg/L streptomycin, and 100 U/mL penicillin, and put into an incubator with 5% CO2 at 37°C (FBS, RPMI-1640, streptomycin, penicillin; Gibco, USA).
Cell line of apatinib-resistance
SGC-7901/BGC-823 cells of ogarithmic phase were respectively treated with apatinib at the concentration of 0.001 μg/mL for 48 hours. Then, the refresh RPMI-1640 medium was replaced for subsequent culture. Cells of ogarithmic phase without evident death, were continuously cultured by gradually increasing the concentration of apatinib. Finally, cells that could survive stably at 1 μg/mL of apatinib, were regarded as drug-resistant SGC-7901 or BGC-823 (namely SGC-7901-AR, BGC-823-AR).
Plasmids, miRNA, and cell transfection
MiR-106a-3p mimic and corresponding negative control (miR-106a-3p NC), and miR-106a-3p inhibitor and corresponding negative control were purchased from GenePharma (Shanghai, China). The ShRNA plasmid vector anti-SOCS2, SOCS4 or SOCS5 were constructed by GenePharma (Shanghai, China), and referred as ShRNA-SOCS2, ShRNA-SOCS4, and ShRNA-SOCS5 respectively. The plasmid and oligonucleotide were transfected into cells by Lipofectamine 2000 reagent (Invitrogen, USA). Then, the cells were continuously cultured for 48 hours.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted by TRIzol reagent (Invitrogen, USA). Then, the concentration of total RNA was measured by ultraviolet spectrophotometer. RNA was reverse transcribed into cDNA in the miScript Reverse Transcription Kit (TaKaRa, Japan) according to the manufacturer’s instructions. Small RNA, U6, was used as a reference gene.
Cell Counting Kit-8 (CCK-8)
The cells were inoculated into 96-well plates and cultured for 24 hours. Next, Cell Counting Kit-8 (CCK8) solution (KeyGen, Nanjing, China) was added, and the cells were continually cultured for 4 hours in the incubator. The absorbance was detected by microplate reader (Bio-Rad, USA).
Western blot
The cells transfected for 48 hours were cleaved using RIRP Lysis Buffer (Thermo Fisher Scientific). Then, the supernatant was collected after centrifugation 4 times at 14 000 rpm for 15 minutes. The protein concentration was detected by bicinchoninic acid (BCA) kit (Bio-Rad, USA). The target protein was separated by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and transferred into PVDF (polyvinylidene difluoride) membrane overnight. After the blots were sealed using 10% skim milk, the primary antibody was used to hybridize with the target protein overnight at 4°C (anti-MRP1, 1: 1000; anti-GST-π, 1: 2000; anti-ABCB1, 1: 1000; anti-GAPDH, 1: 5000; anti-SOCS2, 1: 1000; anti-SOCS4, 1: 1000; anti-SOCS5, 1: 1000; anti-p-JAK2, 1: 2000; anti-JAK2, 1: 2000; anti-p-STAT3, 1: 2000; anti-STAT3, 1: 2000; Thermo Fisher Scientific). Subsequently, the blots were incubated with horseradish peroxidase conjugated secondary antibody (1: 10 000) after washing in TBST. We used an ECL (enhanced chemiluminescence) kit (Abcam, USA) developed the blots.
Luciferase reporter assay
SOCS2, SOCS4, or SOCS5 Luciferase reporter plasmid were constructed by GenePharma (Shanghai, China), the 3′UTR site of which was constructed downstream of the luciferase gene. WT SOCS was defined by the 3′UTR site binding to miR-106a-3p, while MUT SOCS was defined by the binding domain of 3′UTR where all base mutant occurred. SOCS2, SOCS4, or SOCS5 Luciferase reporter plasmid (wild type [WT] or mutant [MUT]) and miR-106a-3p NC or miR-106a-3p mimic were cotransfected into SGC-7901 cells using Lipofectamine 2000 according to manufacturer’s protocols. The transfection effects were detected after 48 hours.
Statistical analysis
Multiple comparison was analyzed by ANOVA. The post t-test was used for pairwise comparison. The statistical analysis of experimental data was performed using Prism. P<0.05 was considered to indicate statistically significant difference.
Results
MiR-106a-3p inhibitor reduced SGC-7901 resistance to apatinib
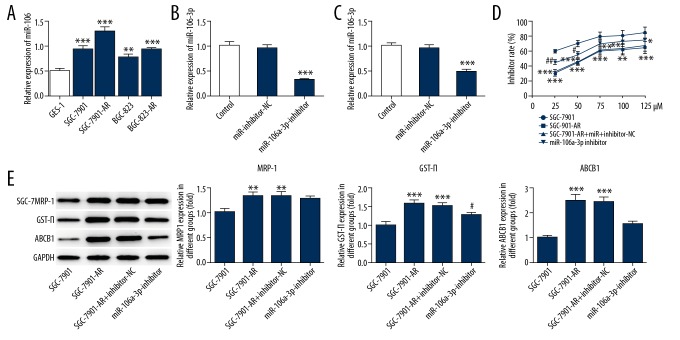
RT-qPCR was used to detect the miR-106a-3p levels. The miR-106a-3p levels presented significant increase in SGC-7901-AR and BGC-823-AR (Figure 1A). There were higher levels of miR-106a-3p in SGC-7901 cells compared to BGC-823 cells. MiR-106a-3p inhibitor significantly decreased the expression of miR-106a-3p in SGC-7901 and SGC-7901-AR respectively compared to miR-inhibitor-NC group. (Figure 1B, 1C). Furthermore, the cell viability was assessed by CCK8 assay. miR-106a-3p inhibitor significantly increased the suppression of apatinib to SGC-7901-AR cells (Figure 1D). Western blot was used to detect the expression of drug-resistant related protein. The expression of GST-π and ABCB1 were significantly decreased in SGC-7901-AR by miR-106a-3p, while MRP1 levels had no obvious changes (Figure 1E, 1F).
Figure 1.
The effect of miR-106a-3p inhibitor in gastric cancer cells resistance to apatinib. (A) The relative expression of miR-106a-3p in different cell lines. (B) The effect of miR-106a-3p inhibitor in SGC-7901 cells. (C) The effect of miR-106a-3p inhibitor in SGC-7901-AR cells. (D) The effects of different concentrations of apatinib on cell viability. (E) The effects of miR-106a-3p inhibitor on drug-resistance related proteins. * P<0.05 comparing to SGC-7901. # P<0.05 comparing to miR-106a-3p-inhibitor-NC.
MiR-106a-3p targeted relevant genes of SOCS family
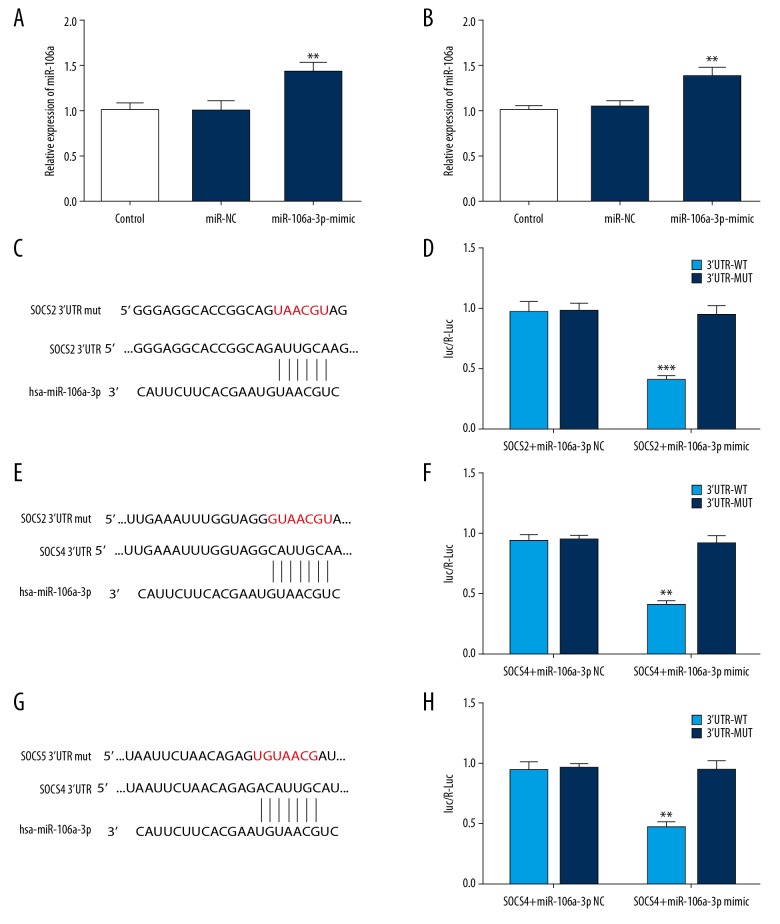
The miR-106a-3p levels were significantly increased by miR mimic in SGC-7901 and SGC-7901-AR cells (Figure 2A, 2B). Targetscan6.2 was used to detect the relevant target miRNA. The binding sites of SOCS 3′UTR and miR-106a-3p, and the mutant sites were shown in Figure 2C, 2E, 2G. Luciferase reporter assay showed that the group SOCS 3′UTR WT+ miR-106a-3p mimic, presented reduced luciferase activity (Figure 2D, 2F, 2H). Thus, SOCS2, SOCS4, and SOCS5 were target genes of miR-106a-3p.
Figure 2.
(A, B) The effects of miR-106a-3p mimic on the SGC-7901 and SGC-7901-AR cells. (C, E, G) The target genes of miR-106a-3p were analyzed by Luciferase reporter assay. (D, F, H) Luciferase reporter assay analyzed whether miR-106a-3p bound to SOCS 3′UTR. * P<0.05.
MiR-106a-3p inhibitor decreased the relevant gene expression of SOCS family
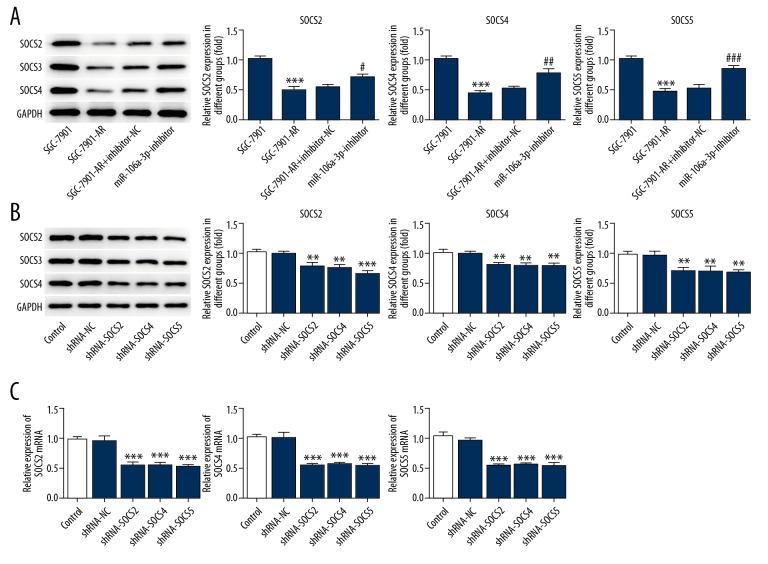
MiR-106a-3p inhibitor significantly increased the expression of SOCS2, SOCS4, and SOCS5 (Figure 3A). ShRNA-SOCS significantly decreased the expression of corresponding SOCS (Figure 3B, 3C).
Figure 3.
MiR-106a-3p inhibitor decreased the relevant gene expression of SOCS family. (A) MiR-106a-3p inhibitor reduced the expression of SOCS. (B) The expression of SOCS was detected by western blot. (C) The expression of SOCS mRNA was detected by RT-qPCR. * P<0.05 comparing to SGC-7901 and # P<0.05 comparing to miR-106a-3p-inhibitor-NC in (A). * P<0.05 comparing to shRNA-NC.
Sh-SOCS reversed the effect of miR-106a-3p inhibitor to p-JAK2, p-STAT3 and cell viability
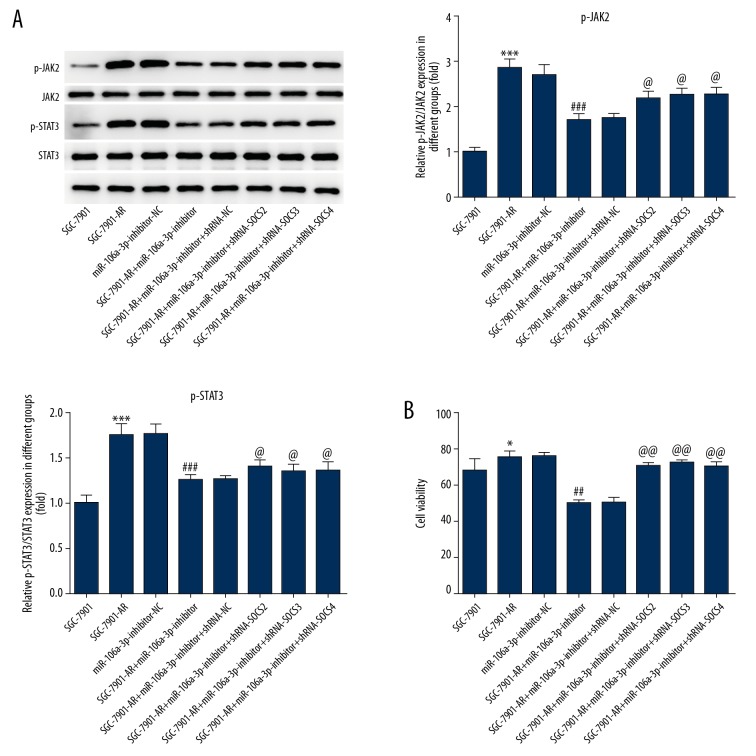
MiR-106a-3p inhibitor significantly reduced the phosphorylation levels of JAK2 and STAT3, while Sh-SOCS significantly reversed the effects (Figure 4A). The cell viability also presents this effect (Figure 4B).
Figure 4.
The effects of sh-SOCS on JAK2 and STAT3 phosphorylation, and cell viability. (A) Sh-SOCS reversed the effect of miR-106a-3p inhibitor to p-JAK2, p-STAT3. (B) The effect of miR-106a-3p inhibitor on cell viability was reversed by Sh-SOCS. * P<0.05 comparing to SGC-7901. # P<0.05 comparing to miR-106a-3p-inhibitor-NC. @ P<0.05 comparing to miR-106a-3p-inhibitor.
Conclusions
Gastric cancer is a malignant tumor originating from gastric mucosa epithelium. Apatinib resistance in cancer cells severely limited the clinical application of apatinib. This study showed that miR-106a-3p affected the resistance of gastric cancer cell to apatinib in vitro. The mechanism involved in the process was that miR-106a-3p targeted the genes of SOCS family to induce apatinib resistance in gastric cancer and JAK/STAT also played a vital role and could help us to understand how miRNA exerts functions in gastric cancer cell resistance to apatinib.
MiR-106a-3p inhibitor suppressed the gastric cancer cell viability and reduced drug-resistant related proteins expression including GST-π and ABCB1. However, the expression of MRP1 presented no significant changes. The demethylation of miRNA promoter region mediated multi-drug resistance by targeting drug-resistant related ABC transporters including ABCB1 and ABCC5 [15]. A previous study showed that miR-508-5p could combine the 3′UTR region of ABCB1 and inhibited its levels to modulate multi-drug resistance in gastric cancer [16].
MiRNA presented abnormal expression in gastric cancer through comparing cancer tissues to adjacent noncancerous tissues [17]. A previous study showed that miRNA was involved in the regulation of SOCS on certain pathological processes [18]. The SOCS system is a common molecular regulation mechanism of cell to external stimulus. Targeting SOCS system might give rise to certain subsequent reactions, such as affecting the drug resistance process of cells. Furthermore, the levels of SOCS proteins present significantly decrease in many cancers [19]. The increasing SOCS-1 played a protective role in suppressing the proliferation in gastric cancer by JAK/STAT and p38 MAPK [20]. The hypermethylated promoter of SOCS1 and SOCS6 was found in human with gastric cancer, which caused downregulation of SOCS1 expression. However, the mRNA of SOCS6 also decreased possibly due to its allelic loss and promoter hypermethylation in gastric cancer [21–23]. MiRNA suppresses the expression of gene through binding to mRNA. Thus, it has been suggested that miRNA is less involved in posttranscriptional modification of the genes. Our study indicated that miR-106a-3p could induce drug-resistance by targeting 3′UTR region of SOCS2, SOCS4, and SOCS5. A previous study found that the miRNA expression was negatively linked to SOCS5 in pancreatic cancer in vivo. Furthermore, miRNA affected cell metastasis by targeting SOCS5 in vivo [24]. miR-101/SOCS2 was involved in cell growth and tumorigenesis of gastric cancer [25].
MiR-106a-3p activated JAK/STAT3 by targeting SOCS family in our study. The study has found that miR/JAK is involved in the progression of cancer. For example, the feedback loop between miR-301a and JAK/STAT3 pathway mediated the metastasis of pancreatic cancer [24]. Furthermore, miRNA altered the metastasis of pancreatic cancer by targeting SOCS5/STAT3 [26]. Thus, miRNA/SOCS/JAK could partly explain the relevant mechanism in the gastric cancer cells resistance to apatinib. Furthermore, targeting miR/SOCS plays a vital role in reducing cells resistance to apatinib in gastric cancer.
Footnotes
Conflict of interest
None.
Source of support: The study is support by Shanxi Province Service Industry Innovation Discipline Group Construction Plan (No. 201809)
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374–80. doi: 10.1111/j.1349-7006.2011.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 4.Teng F, Xu Z, Chen J, et al. DUSP1 induces apatinib resistance by activating the MAPK pathway in gastric cancer. Oncol Rep. 2018;40:1203–22. doi: 10.3892/or.2018.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao B, Guo J, Miao Y, et al. Detection of miR-106a in gastric carcinoma and its clinical significance. Clin Chim Acta. 2009;400:97–102. doi: 10.1016/j.cca.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Leslie G, Gentry-Maharaj A, et al. Evaluation of polygenic risk scores for ovarian cancer risk prediction in a prospective cohort study. J Med Genet. 2018;55:546–54. doi: 10.1136/jmedgenet-2018-105313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdallah EA, Fanelli MF, Souza ESV, et al. MRP1 expression in CTCs confers resistance to irinotecan-based chemotherapy in metastatic colorectal cancer. Int J Cancer. 2016;139:890–98. doi: 10.1002/ijc.30082. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Tibben M, Wang Y, et al. P-glycoprotein (MDR1/ABCB1) controls brain accumulation and intestinal disposition of the novel TGF-beta signaling pathway inhibitor galunisertib. Int J Cancer. 2019 doi: 10.1002/ijc.32568. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat Rev Cancer. 2014;14:736–46. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 11.Menheniott TR, Judd LM, Giraud AS. STAT3: A critical component in the response to Helicobacter pylori infection. Cell Microbiol. 2015;17:1570–82. doi: 10.1111/cmi.12518. [DOI] [PubMed] [Google Scholar]
- 12.Kazi JU, Kabir NN, Flores-Morales A, Ronnstrand L. SOCS proteins in regulation of receptor tyrosine kinase signaling. Cell Mol Life Sci. 2014;71:3297–310. doi: 10.1007/s00018-014-1619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Q, Li YY, He WF, et al. Interplay between microRNAs and the STAT3 signaling pathway in human cancers. Physiol Genomics. 2013;45:1206–14. doi: 10.1152/physiolgenomics.00122.2013. [DOI] [PubMed] [Google Scholar]
- 14.Dong S, Zhang X, Liu D. Overexpression of long noncoding RNA GAS5 suppresses tumorigenesis and development of gastric cancer by sponging miR-106a-5p through the Akt/mTOR pathway. Biol Open. 2019;8(6) doi: 10.1242/bio.041343. pii: bio041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q, Yang Z, Xia L, et al. Methylation of miR-129-5p CpG island modulates multi-drug resistance in gastric cancer by targeting ABC transporters. Oncotarget. 2014;5:11552–63. doi: 10.18632/oncotarget.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang Y, Zhang Z, Liu Z, et al. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267–76. doi: 10.1038/onc.2013.297. [DOI] [PubMed] [Google Scholar]
- 17.Petrocca F, Visone R, Onelli MR, et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–86. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Fu M, Wang B, Chen X, et al. MicroRNA gga-miR-130b suppresses infectious bursal disease virus replication via targeting of the viral genome and cellular suppressors of cytokine signaling 5. J Virol. 2017;92(1) doi: 10.1128/JVI.01646-17. pii: e01646–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabir NN, Sun J, Ronnstrand L, Kazi JU. SOCS6 is a selective suppressor of receptor tyrosine kinase signaling. Tumour Biol. 2014;35:10581–89. doi: 10.1007/s13277-014-2542-4. [DOI] [PubMed] [Google Scholar]
- 20.Souma Y, Nishida T, Serada S, et al. Antiproliferative effect of SOCS-1 through the suppression of STAT3 and p38 MAPK activation in gastric cancer cells. Int J Cancer. 2012;131:1287–96. doi: 10.1002/ijc.27350. [DOI] [PubMed] [Google Scholar]
- 21.Chan MW, Chu ES, To KF, Leung WK. Quantitative detection of methylated SOCS-1, a tumor suppressor gene, by a modified protocol of quantitative real time methylation-specific PCR using SYBR green and its use in early gastric cancer detection. Biotechnol Lett. 2004;26:1289–93. doi: 10.1023/B:BILE.0000044922.43572.2d. [DOI] [PubMed] [Google Scholar]
- 22.Galm O, Yoshikawa H, Esteller M, et al. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood. 2003;101:2784–88. doi: 10.1182/blood-2002-06-1735. [DOI] [PubMed] [Google Scholar]
- 23.Lai RH, Hsiao YW, Wang MJ, et al. SOCS6, downregulated in gastric cancer, inhibits cell proliferation and colony formation. Cancer Lett. 2010;288:75–85. doi: 10.1016/j.canlet.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Hu H, Zhang Q, Chen W, et al. MicoRNA-301a Promotes pancreatic cancer invasion and metastasis through the JAK/STAT3 signaling pathway by targeting SOCS5. Carcinogenesis. 2019 doi: 10.1093/carcin/bgz121. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Xia Y, Li L, Zhang G. MiR-101 inhibits cell growth and tumorigenesis of Helicobacter pylori related gastric cancer by repression of SOCS2. Cancer Biol Ther. 2015;16:160–69. doi: 10.4161/15384047.2014.987523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Li J, Guo H, et al. BRM transcriptionally regulates miR-302a-3p to target SOCS5/STAT3 signaling axis to potentiate pancreatic cancer metastasis. Cancer Lett. 2019;449:215–25. doi: 10.1016/j.canlet.2019.02.031. [DOI] [PubMed] [Google Scholar]