Abstract
Background
Changes of hepatitis B core antigen antibody (anti-HBc) in liver pathological involvement in patients with chronic hepatitis B virus (HBV) infection have not been investigated in detail. This study aimed to explore evolving patterns of anti-HBc following liver pathological states and to investigate validities of anti-HBc for predicting liver pathological states.
Methods
254 HBeAg-positive and 237 HBeAg-negative patients with chronic HBV infection were enrolled. Liver pathological diagnoses referred to Scheuer standard, and anti-HBc was measured using chemiluminescence microparticle immunoassay.
Results
Anti-HBc was significantly positively correlated with pathological grades and stages in both HBeAg-positive (rs = 0.312, P < 0.0001, and rs = 0.268, P < 0.0001) and HBeAg-negative (rs = 0.270, P < 0.0001, and rs = 0.147, P=0.0237) patients. The medians of anti-HBc in pathological grades of G1, G2, and G3 and stages of S1, S2, S3, and S4 in HBeAg-positive patients were all significantly lower than those in HBeAg-negative patients (all P < 0.005). The areas under receiver-operating characteristic curves (95% confidence interval) of anti-HBc for predicting pathological grades ≥G2 and ≥G3, and stages ≥S2 and =S4 in HBeAg-positive patients were 0.683 (0.622–0.740) and 0.662 (0.601–0.720), and 0.627 (0.564–0.687) and 0.683 (0.622–0.740), respectively, and in HBeAg-negative patients were 0.681 (0.618–0.740) and 0.702 (0.639–0.760), and 0.569 (0.503–0.633) and 0.630 (0.565–0.691), respectively.
Conclusion
Following hepatic aggravation of necroinflammation and progression of fibrosis, anti-HBc increases gradually in HBeAg-positive patients and continues to increase gradually in HBeAg-negative patients, which is a useful but unsatisfactory marker for monitoring pathological states.
1. Background
Based on dynamic observation of hepatitis B e antigen (HBeAg) status, serum hepatitis B virus (HBV) DNA, and alanine transferase (ALT) levels, the natural history of chronic HBV infection can typically be divided into four successive phases: HBeAg-positive chronic infection, HBeAg-positive chronic hepatitis, HBeAg-negative chronic infection, and HBeAg-negative chronic hepatitis [1]. With reference to the similar criterion, the differences in some quantitative serum HBV markers such as hepatitis B surface antigen (HBsAg), hepatitis B core-related antigen (HBcrAg), hepatitis B core antigen antibody (anti-HBc), and HBV RNA during different phases of the natural history have been explored [2–10]. However, to date, most clinical practice guidelines on the management of chronic HBV infection with important international influence have not included these quantitative HBV markers as key parameters in the phase criteria of the natural history [11–14].
In fact, the disease evolvements during chronic HBV infection are often oscillatory. Whether HBeAg-positive or HBeAg-negative, the inactive chronic infection can occur with transient active chronic hepatitis; on the contrary, the active chronic hepatitis can occur with transient inactive chronic infection [1, 11–14]. Furthermore, influenced by the concept of “preconceptions,” the results of studies based on the criterion of the phases containing serum HBV DNA, due to potential relevance of HBV DNA with other HBV markers, cannot truly reflect the evolving patterns of other HBV markers during chronic HBV infection. Therefore, the rational investigation on dynamic changes of HBV markers including serum HBV DNA during chronic HBV infection should only be referring to liver pathological states and serum biochemical changes.
Recently, some studies have explored the predictive value of anti-HBc for liver pathological states in patients with chronic HBV infection from a practical perspective, but the performance of this has not been investigated in detail [15–20]. To further characterize the theoretical and practical value of anti-HBc, we comparatively depicted the evolving patterns of anti-HBc versus serum HBsAg and HBV DNA following liver inflammation intensities and fibrosis levels and evaluated the performance of anti-HBc versus serum HBsAg and HBV DNA in predicting liver inflammation intensities and fibrosis levels in patients with chronic HBV infection.
2. Patients and Methods
2.1. Study Population
A total of 577 Chinese patients with chronic HBV infection who underwent liver biopsy at Shanghai Public Health Clinical Center of Fudan University between January 2015 and December 2017 were retrospectively screened, among whom 86 patients with the following conditions were excluded: 23 with poor quality of biopsy specimens (biopsy length <10 mm), 8 with incomplete laboratory data, 5 coinfected with other forms of viral hepatitis (2 with hepatitis C, 1 with hepatitis D, and 2 with hepatitis E), 9 with nonalcoholic fatty liver disease (steatosis >5% of hepatocytes), 2 with significant alcohol consumption (>20 g per day), 6 with drug-induced liver injuries, and 33 receiving treatment with nucleosides/nucleotides, interferon alphas, and glycyrrhizinates in the last 6 months.
After exclusions, 491 patients were enrolled in this study. The diagnoses of all patients were in accordance with the standard elaborated in the EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection [1].
2.2. Pathological Diagnoses
Ultrasound-guided liver biopsy was performed using a one-second liver biopsy needle (16G). Biopsies collected were immediately transferred into plastic tubes and snap frozen. Biopsies were fixed in neutral formaldehyde, dehydrated in an ethanol gradient, made transparent with xylene, immersed in paraffin, sliced, and stained with hematoxylin and eosin and Masson trichrome. One experienced pathologist who was blinded to any clinical and laboratory information was assigned to interpret all biopsies. Liver pathological diagnoses referred to Scheuer standard [21], in which grade is used to depict the intensity of necroinflammation and stage is used to describe the level of fibrosis and architectural alteration; the grades include five levels from G0 to G4, and the stages include five levels from S0 to S4.
Intrahepatic HBsAg and hepatitis B core antigen (HBcAg) were detected by immunohistochemistry. Formalin-fixed paraffin-embedded sections were routinely dewaxed and rehydrated. After heat-induced antigen retrieval in sodium citrate (pH 6.0) buffer, sections were incubated with primary monoclonal antibody against HBsAg (clone1044/341, Novocastra) and rabbit polyclonal antibodies against HBcAg (Dako). After washing, the polymer detection system (Polink-1 HRP, GBI Labs) was incubated for 30 min at room temperature and developed with 3, 3′-diaminobenzidine (DAB). Expression of intrahepatic HBsAg and HBcAg was assessed by the semiquantitative scoring method. The scores include four levels: 0 (no positive cells), 1 (positive cells <25%), 2 (positive cells 25%–49%), and 3 (positive cells ≥50%).
2.3. Laboratory Assays
Serum samples used for measurements were taken within 1 day before and 1 day after liver biopsy and stored at −40°C. Anti-HBc was measured using chemiluminescence microparticle immunoassay (CMIA) in a UMIC Caris200 automated analyzer (United Medical Instruments Co., Ltd, Xiamen, China), and anti-HBc kits were kindly provided by Innodx Biotech Co. Ltd. (Xiamen, China); the detection range was 1.0 × 102 to 1.0 × 105 IU/mL. HBsAg and HBeAg were measured using CMIA in an Abbott Architect I2000 automated analyzer (Abbott Laboratories, Chicago, USA), and the reagents were purchased from Abbott Laboratories (Chicago, USA); the detection range of HBsAg was 0.05 to 250 IU/mL, and if the serum exceeded the upper limit of detection, it was diluted 500 times and retested; the lower detection limit of HBeAg was 1.0 S/CO. HBV DNA was measured using PCR-fluorescence probing assay in a Roche LightCycler480 qPCR system (Roche Diagnostics Ltd., Rotkreuz, Switzerland), and the HBV DNA kits were purchased from Sansure Biotech Inc. (Changsha, China); the detection range was 5.0 × 102 to 2.0 × 109 IU/mL.
Serum ALT, aspartate transferase (AST), gamma-glutamyl transpeptidase (GGT), and cholinesterase (ChE) of biochemical parameters were measured using a Hitachi 7600 automated biochemist analyzer (Hitachi, Tokyo, Japan); the normal ranges were 9 to 50 IU/L, 15 to 40 IU/L, 10 to 60 IU/L, and 4.000 to 15.000 kU/L, respectively. Blood platelet (PLT) was measured using a Sysmex-XT 4000i automated hematology analyzer (Mundelein, IL, USA); the normal range was 125 × 109/L to 350 × 109/L.
2.4. Statistical Analyses
Quantitative variables were expressed as median (interquartile range (IQR)), and categorical variables were expressed as proportions. Fisher's Z test was used to compare the differences in Spearman correlation coefficients between anti-HBc, serum HBsAg and HBV DNA, and intrahepatic HBsAg and HBcAg with pathological grades and stages. The Kruskal–Wallis test was used to compare the differences in medians of anti-HBc among different liver pathological grades and stages. The Mann–Whitney U test was used to compare the differences in medians of anti-HBc of the same pathological grades and stages between HBeAg-positive and HBeAg-negative patients. Receiver-operating characteristic (ROC) curve was used to evaluate the validities of anti-HBc, serum HBsAg, and HBV DNA in predicting liver necroinflammation intensities and fibrosis levels. The paired-samples Delong Z test was used to compare the differences in areas under ROC curves (AUCs) between anti-HBc, serum HBsAg, and HBV DNA in predicting the same hepatic necroinflammation intensities and fibrosis levels. Medcalc version 15.8 (MedCalc Software, Broekstraat, Mariakerke, Belgium) was used for statistical analyses and graphic productions. A two-sided P value of <0.05 was considered statistically significant.
3. Results
3.1. Clinical, Laboratory, and Pathological Characteristics of Study Population
The clinical, laboratory, and pathological data of the study population based on HBeAg status are summarized in Table 1.
Table 1.
Clinical, laboratory, and pathological characteristics of study population.
| Variables | HBeAg-positive (n = 254) | HBeAg-negative (n = 237) | χ 2a /Z b | P # |
|---|---|---|---|---|
| Gender (male : female) | 163 : 91 | 145 : 92 | 0.350a | 0.5540 |
| Age (years), median (IQR) | 33 (28–39) | 42 (35–49) | 9.475b | <0.0001 |
| Serum ALT (IU/L), median (IQR) | 67.5 (40.0–154.0) | 35.0 (20.0–93.5) | 6.587b | <0.0001 |
| Serum AST (IU/L), median (IQR) | 46.0 (29.0–89.0) | 28.0 (19.0–56.3) | 6.161b | <0.0001 |
| Serum GGT (IU/L), median (IQR) | 31.0 (18.0–71.0) | 26.0 (16.8–55.3) | 1.751b | 0.0799 |
| Serum ChE (kU/L), median (IQR) | 7.525 (6.493–9.086) | 8.378 (6.785–9.669) | 3.177b | 0.0015 |
| Blood PLT (×109/L), median (IQR) | 171 (139–207) | 169 (127–208) | 1.017b | 0.3091 |
| Serum anti-HBc (log10 IU/L), median (IQR) | 3.526 (3.137–3.806) | 4.222 (3.668–4.641) | 10.747b | <0.0001 |
| Serum HBsAg (log10 IU/L), median (IQR) | 4.010 (3.446–4.596) | 3.282 (2.762–3.615) | 11.902b | <0.0001 |
| Serum HBV DNA (log10 IU/L), median (IQR) | 7.228 (6.336–7.806) | 3.565 (<2.699–5.235) | 15.327b | <0.0001 |
| Intrahepatic HBsAg (0 : 1 : 2 : 3) | 3 : 54 : 95 : 102 | 17 : 104 : 82 : 34 | 60.061a | <0.0001 |
| Intrahepatic HBcAg (0 : 1 : 2 : 3) | 103 : 70 : 64 : 17 | 207 : 27 : 3 : 0 | 126.052a | <0.0001 |
| Pathological grade (0 : 1 : 2 : 3 : 4) | 0 : 153 : 85 : 16 : 0 | 0 : 182 : 39 : 16 : 0 | 19.009a | 0.0001 |
| Pathological stage (0 : 1 : 2 : 3 : 4) | 0 : 115 : 89 : 16 : 34 | 0 : 140 : 47 : 16 : 34 | 14.851a | 0.0019 |
IQR, interquartile range; ALT, alanine transferase; AST, aspartate transferase; GGT, gamma-glutamyl transpeptidase; ChE, cholinesterase; PLT, platelet; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBcAg, hepatitis B core antigen; anti-HBc, antibodies to hepatitis B core antigen; HBV DNA, hepatitis B virus DNA. #Comparison between HBeAg-positive and HBeAg-negative patients; aPearson chi-square test; bMann–Whitney U test.
The frequency of pathological grades ≥G2 and ≥G3 of HBeAg-positive patients (39.8% and 6.3%) was significant greater than and close to that of HBeAg-negative patients (23.2% and 6.8%) (χ2 = 14.750, P=0.0001, and χ2 = 0.000, P=0.9842), respectively; the frequency of pathological stages ≥S2 of HBeAg-positive patients (54.7%) was significantly greater than that of HBeAg-negative patients (40.9%) (χ2 = 8.804, P=0.0030), and the frequency of pathological stages ≥S3 and S4 of HBeAg-positive patients (19.7% and 13.4%) was both not statistically different from that of HBeAg-negative patients (21.1% and 14.3%) (χ2 = 0.076, P=0.782, and χ2 = 0.031, P=0.8595).
3.2. Correlation of Anti-HBc with Liver Pathological Grades and Stages
The Spearman correlation coefficients of anti-HBc, serum HBsAg and HBV DNA, and intrahepatic HBsAg and HBcAg with liver pathological grades and stages were summarized in Table 2. The differences in Spearman correlation coefficients among anti-HBc, serum HBsAg and HBV DNA, and intrahepatic HBsAg and HBcAg with liver pathological grades and stages were compared (Table 2).
Table 2.
Differences in Spearman correlation coefficients among serum and intrahepatic HBV markers with pathological grades and stages.
| Variable | HBeAg-positive (n = 254) | HBeAg-negative (n = 237) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pathological grade | Pathological stage | Pathological grade | Pathological stage | |||||
| r s | P | r s | P | r s | P | r s | P | |
| Serum anti-HBc | 0.312ab | <0.0001 | 0.268c | <0.0001 | 0.270d | <0.0001 | 0.147e | 0.0237 |
| Serum HBsAg | −0.248 | 0.0001 | −0.272 | <0.0001 | 0.183 | 0.0046 | 0.127 | 0.0503 |
| Serum HBV DNA | −0.073a | 0.2470 | −0.128 | 0.0410 | 0.493d | <0.0001 | 0.411e | <0.0001 |
| Intrahepatic HBsAg | 0.017b | 0.7825 | 0.030c | 0.6380 | 0.212 | 0.0010 | 0.221 | 0.0006 |
| Intrahepatic HBcAg | −0.270 | <0.0001 | −0.254 | <0.0001 | 0.220 | 0.0007 | 0.169 | 0.0093 |
a–ePairwise comparisons between different Spearman correlation coefficients, P < 0.05, by Fisher's Z test. a: Z = 2.797, P=0.0052; b: Z = 3.425, P=0.0006; c: Z = 2.741, P=0.0061; d: Z = 2.846, P=0.0044; e: Z = 3.123, P=0.0018.
3.3. Differences in Anti-HBc among Liver Pathological Grades and Stages
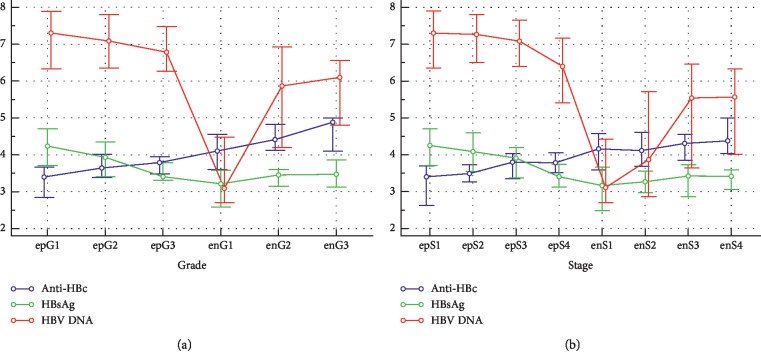
The differences in medians of anti-HBc among different liver pathological grades and stages were summarized in Table 3. The differences in medians of anti-HBc of the same pathological grades and stages between HBeAg-positive and HBeAg-negative patients were compared (Table 3). The evolving patterns of anti-HBc and serum HBsAg and HBV DNA following pathological grades and stages and HBeAg status were illustrated in Figure 1.
Table 3.
Differences in median of anti-HBc among different pathological grades and stages and between HBeAg-positive and HBeAg-negative patients.
| HBeAg-positive (n = 254) | HBeAg-negative (n = 237) | Z | P ∗ | |||
|---|---|---|---|---|---|---|
| n | Anti-HBc, median (IQR) | n | Anti-HBc, median (IQR) | |||
| Grade | ||||||
| G1 | 153 | 3.399 (2.849–3.669)ab | 182 | 4.104 (3.598–4.557)cd | 9.442 | <0.0001 |
| G2 | 85 | 3.643 (3.383–4.011)a | 39 | 4.413 (4.125–4.829)c | 6.213 | <0.0001 |
| G3 | 16 | 3.789 (3.478–3.948)b | 16 | 4.885 (4.100–>5.000)d | 3.138 | 0.0017 |
| χ 2 | 24.785 | χ 2 | 17.305 | |||
| P # | <0.0001 | P # | 0.0002 | |||
|
| ||||||
| Stage | ||||||
| S1 | 115 | 3.403 (2.624–3.697)ef | 140 | 4.160 (3.593–4.576) | 8.184 | <0.0001 |
| S2 | 89 | 3.491 (3.265–3.731)g | 47 | 4.116 (3.683–4.612) | 5.226 | <0.0001 |
| S3 | 16 | 3.801 (3.347–4.039)e | 16 | 4.308 (3.849–4.559) | 2.864 | 0.0042 |
| S4 | 34 | 3.785 (3.511–4.060)fg | 34 | 4.378 (4.034–>5.000) | 4.272 | <0.0001 |
| χ 2 | 19.822 | χ 2 | 6.886 | |||
| P # | 0.0002 | P # | 0.0756 | |||
IQR, interquartile range. #Comparison among different grades and stages, Kruskal–Wallis test. ∗Comparison between HBeAg-positive and HBeAg-negative patients, Mann–Whitney U test. a–gPairwise comparisons between different grades and stages, P < 0.05, by post hoc analysis.
Figure 1.
Multiple variables graph of anti-HBc and serum HBsAg and HBV DNA clustered by liver pathological grades (a) and stages (b) of HBeAg-positive and HBeAg-negative patients. The vertical axis represents anti-HBc and serum HBsAg and HBV DNA levels, the units of measurement of which are all log10 IU/mL; the small circles represent the medians, and the horizontal lines above and below the small circles represent the quartiles. Horizontal axis represents pathological grades and stages, where epG1, epG3, epG3 and epS1, epS2, epS3, epS4 represent G1, G3, G3 and S1, S2, S3, S4 of HBeAg-positive patients, respectively, and enG1, enG3, enG3 and enS1, enS2, enS3, enS4 represent G1, G3, G3 and S1, S2, S3, S4 of HBeAg-negative patients, respectively.
3.4. Performance of Anti-HBc in Predicting Liver Pathological States
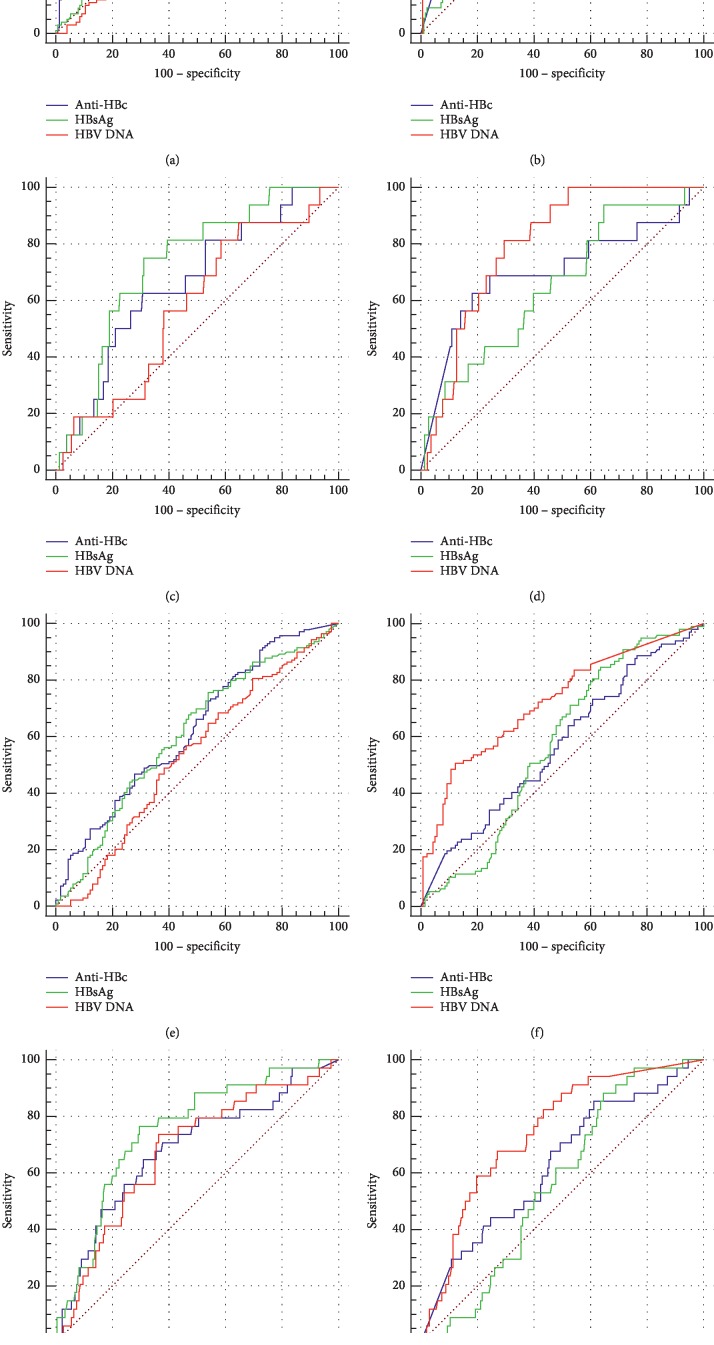
The ROC curves of anti-HBc, serum HBsAg, and HBV DNA for predicting liver pathological grades ≥G2 and ≥G3 and stages ≥S2 and =S4 were illustrated in Figure 2. AUCs of anti-HBc, serum HBsAg, and HBV DNA for predicting liver pathological grades ≥G2 and ≥G3 and stages ≥S2 and =S4 were summarized in Table 4. Of HBeAg-positive patients, the AUCs of anti-HBc and serum HBsAg for predicting liver pathological grades ≥G2 and ≥G3 and stages ≥S2 and =S4 and of serum HBV DNA for predicting pathological stages =S4 were all significantly greater than the area under diagonal reference line (all P < 0.05). Of HBeAg-negative patients, the AUCs of anti-HBc for predicting pathological grades ≥G2 and ≥G3 and stages =S4, and of serum HBsAg for predicting pathological grades ≥G2 and ≥G3, and of serum HBV DNA for predicting pathological grades ≥G2 and ≥G3 and stages ≥S2 and =S4 were all greater than the area under diagonal reference line (all P < 0.05) (Figure 2, Table 4).
Figure 2.
ROC curves of anti-HBc serum HBsAg and HBV DNA for predicting pathological grades ≥G2 (a, b), ≥G3 (c, d) and stages ≥S2 (e, f), =S4 (g, h) of HBeAg-positive (a, c, e, g) and HBeAg-negative (b, d, f, h) patients.
Table 4.
Performance of serum anti-HBc, HBsAg, and HBV DNA for predicting liver pathological states.
| Predicting pathological states of HBeAg-positive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| State variable | Test variable | ROC curve analyses | Tradeoff cutoffs (log10 IU/mL) and corresponding parameters | |||||||
| AUC | SE | 95% CI | Cutoff | Sen (%) | Spe (%) | Ppv (%) | Npv (%) | Acc | ||
| ≥G2 | Anti-HBc | 0.683a | 0.0341 | 0.622–0.740 | >3.558 | 59.4 | 62.8 | 51.3 | 70.1 | 0.606 |
| HBsAg | 0.635b | 0.0353 | 0.572–0.694 | ≤3.709 | 47.5 | 75.2 | 55.8 | 68.5 | 0.615 | |
| HBV DNA | 0.538ab | 0.0365 | 0.475–0.601 | ≤7.117 | 51.5 | 56.2 | 43.7 | 63.7 | 0.535 | |
| ≥G3 | Anti-HBc | 0.662 | 0.0683 | 0.601–0.720 | >3.558 | 62.5 | 55.0 | 8.5 | 95.6 | 0.424 |
| HBsAg | 0.729c | 0.0576 | 0.670–0.783 | ≤3.709 | 75.0 | 68.9 | 14.0 | 97.6 | 0.522 | |
| HBV DNA | 0.579c | 0.0704 | 0.516–0.640 | ≤7.117 | 56.3 | 53.8 | 7.6 | 94.8 | 0.410 | |
| ≥S2 | Anti-HBc | 0.627d | 0.0351 | 0.564–0.687 | >3.594 | 48.9 | 67.8 | 64.8 | 52.3 | 0.574 |
| HBsAg | 0.609e | 0.0359 | 0.546–0.670 | ≤3.732 | 43.2 | 73.9 | 66.7 | 51.8 | 0.567 | |
| HBV DNA | 0.535de | 0.0370 | 0.472–0.597 | ≤6.782 | 41.7 | 64.4 | 58.6 | 47.7 | 0.518 | |
| =S4 | Anti-HBc | 0.683 | 0.0523 | 0.622–0.740 | >3.594 | 67.7 | 62.7 | 21.9 | 92.6 | 0.539 |
| HBsAg | 0.751 | 0.0435 | 0.693–0.803 | ≤3.732 | 73.5 | 70.5 | 27.8 | 94.5 | 0.605 | |
| HBV DNA | 0.675 | 0.0496 | 0.613–0.732 | ≤6.782 | 64.7 | 65.0 | 22.2 | 92.3 | 0.547 | |
|
| ||||||||||
| Predicting pathological states of HBeAg-negative | ||||||||||
| State variable | Test variable | ROC curve analyses | Tradeoff cutoffs (log10 IU/mL) and corresponding parameters | |||||||
| AUC | SE | 95% CI | Cutoff | Sen (%) | Spe (%) | Ppv (%) | Npv (%) | Acc | ||
|
| ||||||||||
| ≥G2 | Anti-HBc | 0.681f | 0.0410 | 0.618–0.740 | >4.454 | 52.7 | 68.7 | 33.7 | 82.8 | 0.581 |
| HBsAg | 0.622g | 0.0401 | 0.557–0.684 | >3.411 | 56.4 | 62.6 | 31.3 | 82.6 | 0.557 | |
| HBV DNA | 0.833fg | 0.0287 | 0.780–0.878 | >4.637 | 67.3 | 78.0 | 48.1 | 88.7 | 0.696 | |
| ≥G3 | Anti-HBc | 0.702 | 0.0841 | 0.639–0.760 | >4.454 | 68.8 | 66.1 | 12.8 | 96.7 | 0.500 |
| HBsAg | 0.653h | 0.0718 | 0.589–0.713 | >3.411 | 62.5 | 59.7 | 10.1 | 95.7 | 0.455 | |
| HBV DNA | 0.800h | 0.0421 | 0.743–0.849 | >4.637 | 75.0 | 70.6 | 15.6 | 97.5 | 0.539 | |
| S2 | Anti-HBc | 0.569i | 0.0378 | 0.503–0.633 | >4.309 | 47.4 | 57.1 | 43.4 | 61.1 | 0.522 |
| HBsAg | 0.572j | 0.0371 | 0.506–0.635 | >3.320 | 52.6 | 55.7 | 45.1 | 62.9 | 0.538 | |
| HBV DNA | 0.723ij | 0.0338 | 0.661–0.779 | >4.152 | 60.8 | 70.7 | 59.0 | 72.3 | 0.657 | |
| S4 | Anti-HBc | 0.630k | 0.0521 | 0.565–0.691 | >4.309 | 55.9 | 57.1 | 17.9 | 88.5 | 0.483 |
| HBsAg | 0.566l | 0.0441 | 0.500–0.630 | >3.320 | 55.9 | 53.7 | 16.8 | 87.9 | 0.462 | |
| HBV DNA | 0.746kl | 0.0421 | 0.685–0.800 | >4.152 | 70.6 | 62.6 | 24.0 | 92.7 | 0.553 | |
AUC, areas under ROC curve; SE, standard error; 95% CI, 95% confidence interval; Sen, sensitivity; Spe, specificity; Ppv, positive predictive value; Npv, negative predictive value; Acc, accuracy. a–lPairwise comparisons between different AUCs, P < 0.05, by the paired-samples Delong Z test. a: Z = 3.481, P=0.0005; b: Z = 3.245, P=0.0012; c: Z = 2.365, P=0.0180; d: Z = 2.238, P=0.0252; e: Z = 2.547, P=0.0109; f: Z = 3.676, P=0.0002; g: Z = 5.072, P < 0.0001; h: Z = 2.590, P=0.0096; i: Z = 4.266, P < 0.0001; j: Z = 3.491, P=0.0005; k: Z = 2.219, P=0.0265; l: Z = 3.431, P=0.0006.
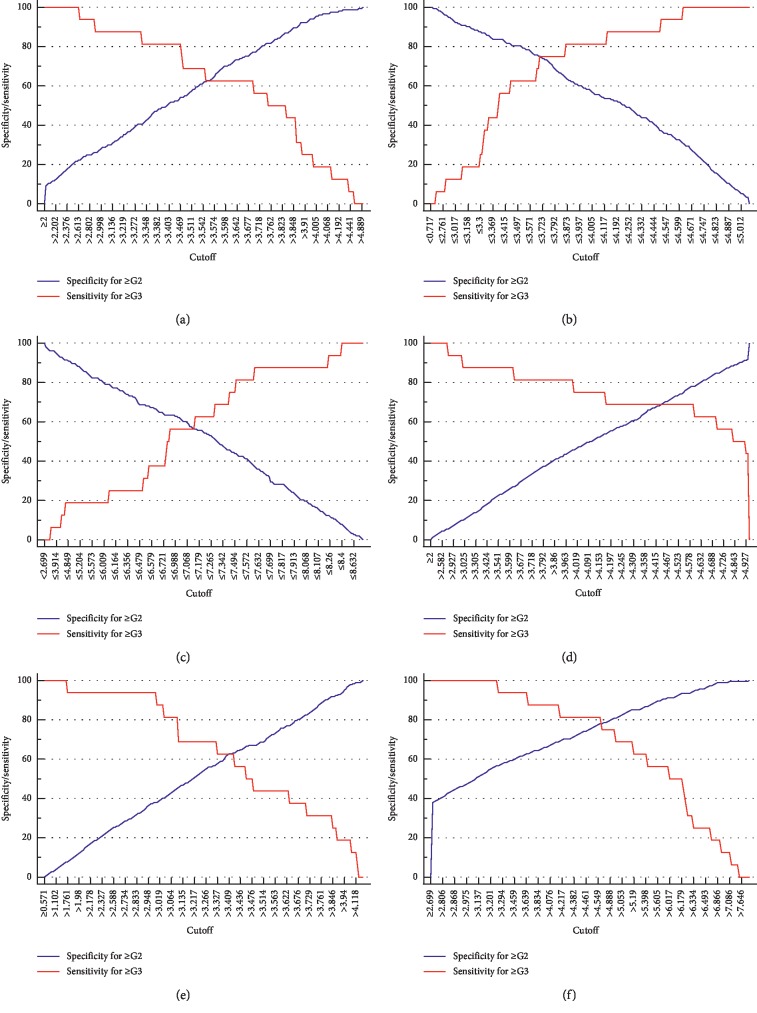
With reference to the minimum differences in specificities of predicting pathological grades ≥G2 and sensitivities of predicting pathological grades ≥G3 or in specificities of predicting pathological stages ≥S2 and sensitivities of predicting pathological stages =S4 at the same cutoffs, a single tradeoff cutoff was determined (Figure 3) [22, 23]. The corresponding diagnostic parameters based on a single tradeoff cutoff in predicting pathological grades ≥G2 and ≥G3 or pathological stages ≥S2 and =S4 of HBeAg-positive and HBeAg-negative patients were calculated (Table 4).
Figure 3.
Scatter plot of specificities of predicting pathological grades ≥G2 and of sensitivities of predicting pathological grades ≥G3 corresponding to cutoffs of anti-HBc (a, b), serum HBsAg (c, d), and HBV DNA (e, f) in HBeAg-positive (a, c, e) and HBeAg-negative (b, d, f) patients.
Of HBeAg-positive patients, with reference to the tradeoff cutoffs, the sensitivity and specificity of serial test of combination of anti-HBc and serum HBsAg for predicting pathological grades ≥G2 were 28.2% and 90.8% and of parallel test of which for predicting pathological grades ≥G3 were 81.3% and 37.9%, respectively; the sensitivity and specificity of serial test of combination of anti-HBc and serum HBsAg for predicting pathological grades ≥S2 were 21.1% and 91.6% and of parallel test of combination for predicting pathological grades ≥S4 were 87.2% and 44.2%, respectively.
Of HBeAg-negative patients, with the standard of the tradeoff cutoffs, the sensitivity and specificity of serial test of combination of anti-HBc and serum HBV DNA for predicting pathological grades ≥G2 were 35.5% and 93.1% and of parallel test of combination for predicting pathological grades ≥G3 were 87.6% and 46.7%, respectively; the sensitivity and specificity of serial test of combination of anti-HBc and serum HBV DNA for predicting pathological grades ≥S2 were 28.8% and 87.4% and of parallel test of combination for predicting pathological grades ≥S4 were 76.7% and 37.5%, respectively.
4. Discussion
The host's immune response against HBV is the main cause leading to liver injury of HBV infection [24, 25]. The lowly efficient immune response against HBV in patients with chronic HBV infection, which lead not only to HBV persistence, but also to the disease oscillation, has not been clarified [24–26]. Some laboratory evidences have demonstrated that serum HBsAg that is overexpressed in chronic HBV infection and serum HBeAg that is not essential for HBV replication suppress host's immune responses against HBV [26–30]. Theoretically, anti-HBc levels may reflect the efficiency of host's immune responses against HBV to some extent.
Some studies have investigated the correlation of serum HBsAg and HBV DNA with liver pathological grades and stages in patients with chronic HBV infection [31–36]. The results of this study were basically consistent with those studies. However, few studies comparatively investigated the correlation of anti-HBc versus serum HBsAg and HBV DNA, and intrahepatic HBsAg and HBcAg with liver pathological grades and stages. This study showed that, in HBeAg-positive patients, anti-HBc was significantly positively correlated, but serum HBsAg and HBV DNA and intrahepatic HBcAg were all significantly negatively correlated with both pathological grades and stages; however, in HBeAg-negative patients, anti-HBc and serum HBV DNA and intrahepatic HBsAg and HBcAg were all significantly positively correlated with both pathological grades and stages, and serum HBsAg was significantly positively correlated pathological grades. The medians of anti-HBc of the same pathological grades and stages in HBeAg-positive patients were all significantly lower than those in HBeAg-negative patients.
The findings of this study demonstrated that the virological and immunological mechanisms of progression and oscillation of hepatic necroinflammation and fibrosis in HBeAg-positive patients could be different from those in HBeAg-negative patients. Serum HBsAg and HBeAg may play leading and synergistic role in immune regulation, respectively, while intrahepatic HBcAg may serve as the primary target antigen for immune responses [8, 9, 31, 32, 36]. It is postulated that, in HBeAg-positive patients, spontaneous decrease of serum HBsAg could evoke host's immune responses against HBV, which lead to decrease of serum HBsAg and HBeAg and of intrahepatic HBsAg and HBcAg, and decrease of HBV replication with hepatic aggravation of necroinflammation and progression of fibrosis, until serum HBsAg remain lower levels and HBeAg loss or convert. However, in HBeAg-negative patients, increase of intrahepatic HBcAg resulting from opportunistic increase of HBV replication could evoke again host's immune responses against HBV, which lead to decrease of serum HBsAg and of intrahepatic HBsAg and HBcAg, and decrease of HBV replication with hepatic reaggravation of necroinflammation and reprogression of fibrosis, until serum HBsAg remain again lower levels or HBsAg loss or convert.
Much progress has been made in the noninvasive assessment of hepatic fibrosis levels based on the disease-specific indicators and mathematical models [13, 14, 22, 23, 37, 38]. However, the study on serum HBV markers, especially anti-HBc, the only virological marker that can reflect the host's immune efficiency, for predicting hepatic necroinflammation intensities and fibrosis levels has special theoretical and practical importance for further elucidating and monitoring progression and oscillation during chronic HBV infection.
Previous studies have shown that serum HBsAg of HBeAg-positive patients and serum HBV DNA of HBeAg-negative patients are valuable but not very satisfactory in predicting liver inflammation intensities and fibrosis levels [31–36]. Lately, preliminary studies have demonstrated that anti-HBc may be useful in predicting liver inflammation intensities and fibrosis levels [15–20]. This study indicated that the AUC of anti-HBc of HBeAg-positive patients in predicting pathological grades ≥G2 and ≥G3 was both close to that of serum HBsAg and both significantly greater than that of serum HBV DNA, and in predicting pathological stages ≥S2 and =S4 was both close to that of serum HBsAg and, respectively, significantly greater than and close to that of serum HBV DNA. However, the AUC of anti-HBc of HBeAg-negative patients in predicting pathological grades ≥G2 and ≥G3 was both close to that of serum HBsAg and, respectively, significantly less than and close to that of serum HBV DNA, and in predicting pathological stages ≥S2 and =S4 was both close to that of serum HBsAg and both significantly less than that of serum HBV DNA. These data suggested that the validity of anti-HBc in predicting liver necroinflammation intensities and fibrosis levels of HBeAg-positive patients be close to that of serum HBsAg and superior to that of serum HBV DNA, and of HBeAg-negative patients be inferior to that of serum HBV DNA but not inferior to that of serum HBsAg.
Clear diagnosis of significant hepatic necroinflammation or fibrosis and timely diagnosis of extensive hepatic necroinflammation or cirrhosis is of great practical importance for the rational intervention of antiviral therapy [39, 40]. Therefore, this study selected single tradeoff cutoffs [22, 23], which minimize not only the misdiagnosis for predicting significant hepatic necroinflammation and fibrosis (pathological grades ≥G2 and stages ≥S2) but also the missed diagnosis for predicting extensive hepatic necroinflammation and cirrhosis (pathological grades ≥G3 and stages =S4), to evaluate the performance of anti-HBc and serum HBsAg and HBV DNA in predicting liver necroinflammation intensities and fibrosis levels.
In this study, with reference to the tradeoff cutoffs, the specificity of anti-HBc of HBeAg-positive patients for predicting significant liver necroinflammation and fibrosis and the sensitivity of which in predicting extensive liver necroinflammation and cirrhosis was all less than that of serum HBsAg and greater than that of serum HBV DNA; the specificity of anti-HBc of HBeAg-negative patients for predicting significant liver necroinflammation and fibrosis and the sensitivity of which in predicting extensive liver necroinflammation and cirrhosis was all no less than that of serum HBsAg and less than that of serum HBV DNA. It should be noted that the specificity of serum HBsAg of HBeAg-positive patients and serum HBV DNA of HBeAg-negative patients for predicting significant liver necroinflammation and fibrosis and the sensitivity of which in predicting extensive liver necroinflammation and cirrhosis was all less than 80%. However, the serial test of combination of anti-HBc and serum HBsAg of HBeAg-positive patients and of anti-HBc and serum HBV DNA of HBeAg-negative patients can all achieve satisfactory specificities for predicting significant liver necroinflammation and fibrosis, and the parallel test of combination of which can also obtain satisfactory sensitivity for predicting extensive liver necroinflammation and cirrhosis.
This study has some limitations. Firstly, synchronous investigation of other HBV markers such as serum HBcrAg and HBV RNA as well as intrahepatic HBV DNA, HBV RNA, and HBV cccDNA could be more useful for elucidating the role of anti-HBc in liver pathological grades and stages. Secondly, combined investigation with HBeAg-stopping mutant of G1896A in precore region or HBeAg-suppressing mutants of A1762T and G1764A in basal core promoter region of the HBV genome as well as HBV genotypes could be more helpful for explaining the role of anti-HBc in liver inflammation intensities and fibrosis levels. Thirdly, dynamic observation of the evolving patterns of anti-HBc versus other HBV markers could provide more valuable information for revealing the role of anti-HBc in the exacerbation and alleviation of liver necroinflammation as well as the progression and regression liver fibrosis.
5. Conclusions
This study revealed that, following aggravation of liver necroinflammation and progression of liver fibrosis, anti-HBc increases gradually in HBeAg-positive patients and further increases gradually in HBeAg-negative patients. Anti-HBc is a valuable but unsatisfactory indicator in predicting liver necroinflammatory intensities and fibrosis levels in both HBeAg-positive and HBeAg-negative patients; however, anti-HBc can improve the performance of serum HBsAg of HBeAg-positive patients and serum HBV DNA of HBeAg-negative patients in predicting liver necroinflammatory intensities and fibrosis levels.
Acknowledgments
The quantitative anti-HBc kits were kindly provided by Innodx Biotech Co. Ltd. (Xiamen, China). This work was supported by the “13th Five-year” National Science and Technology Major Project of China (2017ZX10203202) and Shanghai Municipal Hospital of Joint Research Projects in Emerging Cutting-Edge Technology (SHDC12016237).
Abbreviations
- ALT:
Alanine transferase
- Anti-HBc:
Hepatitis B core antigen antibody
- AST:
Aspartate transferase
- AUC:
Areas under ROC curves
- ChE:
Cholinesterase
- CI:
Confidence interval
- CMIA:
Chemiluminescence microparticle immunoassay
- GGT:
Gamma-glutamyl transpeptidase
- HBcAg:
Hepatitis B core antigen
- HBcrAg:
Hepatitis B core-related antigen
- HBeAg:
Hepatitis B e antigen
- HBsAg:
Hepatitis B surface antigen
- HBV:
Serum hepatitis B virus
- IQR:
Interquartile range
- PLT:
Platelet
- ROC:
Receiver-operating characteristic
- SE:
Standard error.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
This study was approved by the independent ethics committees of Shanghai Public Health Clinical Center, Fudan University.
Disclosure
The fund supporters and reagent provider had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Zhang-qing Zhang conceived and designed the study, analyzed the data, and drafted the manuscript. Bi-sheng Shi, Wei Lu, and Dan-ping Liu collated the data. Bi-sheng Shi and Dan-ping Liu performed the experiments. Wei Lu, Dan Huang, Bi-sheng Shi, and Zhan-qing Zhang collaboratively collected the data. Yan-ling Feng performed pathological diagnoses and immunohistochemistry. Zhan-qing Zhang, Bi-sheng Shi, Wei Lu, and Yan-ling Feng critically revised the manuscript.
References
- 1.European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. Journal of Hepatology. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Jaroszewicz J., Serrano B. C., Wursthorn K., et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. Journal of Hepatology. 2010;52(4):514–522. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen T., Thompson A. J. V., Bowden S., et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. Journal of Hepatology. 2010;52(4):508–513. doi: 10.1016/j.jhep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y. J., Cho H. C., Choi M. S., et al. The change of the quantitative HBsAg level during the natural course of chronic hepatitis B. Liver International. 2011;31(6):817–823. doi: 10.1111/j.1478-3231.2011.02516.x. [DOI] [PubMed] [Google Scholar]
- 5.Seto W.-K., Wong D. K.-H., Fung J., et al. Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B. Clinical Microbiology and Infection. 2014;20(11):1173–1180. doi: 10.1111/1469-0691.12739. [DOI] [PubMed] [Google Scholar]
- 6.Maasoumy B., Wiegand S. B., Jaroszewicz J., et al. Hepatitis B core-related antigen (HBcrAg) levels in the natural history of hepatitis B virus infection in a large European cohort predominantly infected with genotypes A and D. Clinical Microbiology and Infection. 2015;21:601.e1–606.e10. doi: 10.1016/j.cmi.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z. Q., Zhang X. N., Lu W., et al. Distinct patterns of serum hepatitis B core-related antigen during the natural history of chronic hepatitis B. BMC Gastroenterol. 2017;17:p. 140. doi: 10.1186/s12876-017-0703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia W., Song L.-W., Fang Y.-Q., et al. Antibody to hepatitis B core antigen levels in the natural history of chronic hepatitis B: a prospective observational study. Medicine. 2014;93(29):p. e322. doi: 10.1097/md.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song L.-W., Liu P.-G., Liu C.-J., et al. Quantitative hepatitis B core antibody levels in the natural history of hepatitis B virus infection. Clinical Microbiology and Infection. 2015;21(2):197–203. doi: 10.1016/j.cmi.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Yu Y., Li G., et al. Natural history of serum HBV-RNA in chronic HBV infection. Journal of Viral Hepatitis. 2018;25(25):1038–1047. doi: 10.1111/jvh.12908. [DOI] [PubMed] [Google Scholar]
- 11.Terrault N. A., Lok A. S. F., McMahon B. J., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarin S. K., Kumar M., Lau G. K., et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatology International. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou J., Wang G., Wang F., et al. Guideline of prevention and treatment for chronic hepatitis B (2015 update) Journal of Clinical and Translational Hepatology. 2017;5(4):297–318. doi: 10.14218/jcth.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 15.Yuan Q., Song L. W., Cavallone D., et al. Total hepatitis B core antigen antibody, a quantitative non-invasive marker of hepatitis B virus induced liver disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130209.e130209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M. R., Zheng H. W., Lu J. H., et al. Serum hepatitis B core antibody titer use in screening for significant fibrosis in treatment-naive patients with chronic hepatitis B. Oncotarget. 2017;8:11063–11070. doi: 10.18632/oncotarget.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J., Song L., Zhao H., et al. Serum hepatitis B core antibody as a biomarker of hepatic inflammation in chronic hepatitis B patients with normal alanine aminotransferase. Scientific Reports. 2017;7(1):p. 2747. doi: 10.1038/s41598-017-03102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M.-R., Lu J.-H., Ye L.-H., et al. Quantitative hepatitis B core antibody level is associated with inflammatory activity in treatment-naïve chronic hepatitis B patients. Medicine. 2016;95(34):p. e4422. doi: 10.1097/md.0000000000004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J., Mao R.-C., Li X.-L., et al. A novel noninvasive index for the prediction of moderate to severe fibrosis in chronic hepatitis B patients. Digestive and Liver Disease. 2018;50(5):482–489. doi: 10.1016/j.dld.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 20.Li J., Zhang T.-Y., Song L.-W., et al. Role of quantitative hepatitis B core antibody levels in predicting significant liver inflammation in chronic hepatitis B patients with normal or near-normal alanine aminotransferase levels. Hepatology Research. 2018;48(3):E133–E145. doi: 10.1111/hepr.12937. [DOI] [PubMed] [Google Scholar]
- 21.Brunt E. M. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31(1):241–246. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 22.Liu D. P., Lu W., Zhang Z. Q., et al. Comparative evaluation of GPR versus APRI and FIB-4 in predicting different levels of liver fibrosis of chronic hepatitis B. Journal of Viral Hepatitis. 2018;25(5):581–589. doi: 10.1111/jvh.12842. [DOI] [PubMed] [Google Scholar]
- 23.Liu D., Li J., Lu W., et al. Gamma-glutamyl transpeptidase to cholinesterase and platelet ratio in predicting significant liver fibrosis and cirrhosis of chronic hepatitis B. Clinical Microbiology and Infection. 2019;25(4):514.e1–514.e8. doi: 10.1016/j.cmi.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Peeridogaheh H., Meshkat Z., Habibzadeh S., et al. Current concepts on immunopathogenesis of hepatitis B virus infection. Virus Research. 2018;245:29–43. doi: 10.1016/j.virusres.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Busch K., Thimme R. Natural history of chronic hepatitis B virus infection. Medical Microbiology and Immunology. 2015;204(1):5–10. doi: 10.1007/s00430-014-0369-7. [DOI] [PubMed] [Google Scholar]
- 26.Tsai K.-N., Kuo C.-F., Ou J.-H. J. Mechanisms of hepatitis B virus persistence. Trends in Microbiology. 2018;26(1):33–42. doi: 10.1016/j.tim.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi B., Ren G., Hu Y., et al. HBsAg inhibits IFN-α production in plasmacytoid dendritic cells through TNF-α and IL-10 induction in monocytes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044900.e44900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y., Hu Y., Shi B., et al. HBsAg inhibits TLR9-mediated activation and IFN-α production in plasmacytoid dendritic cells. Molecular Immunology. 2009;46(13):2640–2646. doi: 10.1016/j.molimm.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Wang S., Chen Z., Hu C., et al. Hepatitis B virus surface antigen selectively inhibits TLR2 ligand-induced IL-12 production in monocytes/macrophages by interfering with JNK activation. The Journal of Immunology. 2013;190(10):5142–5151. doi: 10.4049/jimmunol.1201625. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J., Imanishi H., Morisaki H., et al. Recombinant HBsAg inhibits LPS-induced COX-2 expression and IL-18 production by interfering with the NFκB pathway in a human monocytic cell line, THP-1. Journal of Hepatology. 2005;43(3):465–471. doi: 10.1016/j.jhep.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Martinot-Peignoux M., Carvalho-Filho R., Lapalus M., et al. Hepatitis B surface antigen serum level is associated with fibrosis severity in treatment-naïve, e antigen-positive patients. Journal of Hepatology. 2013;58(6):1089–1095. doi: 10.1016/j.jhep.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Marcellin P., Martinot-Peignoux M., Asselah T., et al. Serum levels of hepatitis B surface antigen predict severity of fibrosis in patients with e antigen-positive chronic hepatitis B. Clinical Gastroenterology and Hepatology. 2015;13(8):1532–1539. doi: 10.1016/j.cgh.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Goyal S. K., Jain A. K., Dixit V. K., et al. HBsAg level as predictor of liver fibrosis in HBeAg positive patients with chronic hepatitis B virus infection. Journal of Clinical and Experimental Hepatology. 2015;5(3):213–220. doi: 10.1016/j.jceh.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balkan A., Namiduru M., Balkan Y., Mete A., Karaoglan I., Bosnak V. Are serum quantitative hepatitis B surface antigen levels, liver histopathology and viral loads related in chronic hepatitis B-infected patients? Saudi Journal of Gastroenterology. 2016;22(3):208–214. doi: 10.4103/1319-3767.182454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P., Du H., Tong G., et al. Serum hepatitis B surface antigen correlates with fibrosis and necroinflammation: a multicentre perspective in China. Journal of Viral Hepatitis. 2018;25(9):1017–1025. doi: 10.1111/jvh.12903. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z.-Q., Lu W., Wang Y.-B., et al. Measurement of the hepatitis B core-related antigen is valuable for predicting the pathological status of liver tissues in chronic hepatitis B patients. Journal of Virological Methods. 2016;235:92–98. doi: 10.1016/j.jviromet.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 37.European Association for the Study of the Liver. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. Journal of Hepatology. 2015;63(1):237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Lemoine M., Shimakawa Y., Nayagam S., et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65(8):1369–1376. doi: 10.1136/gutjnl-2015-309260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desmet V. J., Roskams T. Cirrhosis reversal: a duel between dogma and myth. Journal of Hepatology. 2004;40(5):860–867. doi: 10.1016/j.jhep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Jung Y. K., Yim H. J. Reversal of liver cirrhosis: current evidence and expectations. The Korean Journal of Internal Medicine. 2017;32(2):213–228. doi: 10.3904/kjim.2016.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.