Highlights
-
•
Cortical volume and cortical thickness deficits are shared between patients with schizophrenia and bipolar disorder.
-
•
The direct comparison between both disorders showed significant reductions in all measures in patients with schizophrenia.
-
•
Cortical volume decrease in schizophrenia was driven by changes in cortical thickness and surface area, whereas in bipolar disorder was exclusively explained by cortical thinning.
-
•
Reduced GI was only found in schizophrenia.
Keywords: Neuroimaging, Schizophrenia, Bipolar disorder, Surface-based morphometry, Cortical thickness, Surface area, Gyrification index
Abstract
Objectives
The profiles of cortical abnormalities in schizophrenia and bipolar disorder, and how far they resemble each other, have only been studied to a limited extent. The aim of this study was to identify and compare the changes in cortical morphology associated with these pathologies.
Methods
A total of 384 subjects, including 128 patients with schizophrenia, 128 patients with bipolar disorder and 127 sex-age-matched healthy subjects, were examined using cortical surface-based morphology. Four cortical structural measures were studied: cortical volume (CV), cortical thickness (CT), surface area (SA) and gyrification index (GI). Group comparisons for each separate cortical measure were conducted.
Results
At a threshold of P = 0.05 corrected, both patient groups showed significant widespread CV and CT reductions in similar areas compared to healthy subjects. However, the changes in schizophrenia were more pronounced. While CV decrease in bipolar disorder was exclusively explained by cortical thinning, in schizophrenia it was driven by changes in CT and partially by SA. Reduced GI was only found in schizophrenia. The direct comparison between both disorders showed significant reductions in all measures in patients with schizophrenia.
Conclusions
Cortical volume and cortical thickness deficits are shared between patients with schizophrenia and bipolar disorder, suggesting that both pathologies may be affected by similar environmental and neurodegenerative factors. However, the exclusive alteration in schizophrenia of metrics related to the geometry and curvature of the brain cortical surface (SA, GI) suggests that this group is influenced by additional neurodevelopmental and genetic factors.
1. Introduction
For over a hundred years psychotic disorders have been separated into two main varieties, schizophrenia and manic-depressive psychosis. This distinction has proved highly useful clinically and has been of heuristic value. Recent neurobiological literature supports that schizophrenia and bipolar disorder share genetic (Purcell et al., 2009; Lichtenstein et al., 2009; Craddock et al., 2006) and neurocognitive characteristics (Kim et al., 2015), supporting that the two disorders are related at the aetiological level.
An important line of research that may help to clarify the common and different neurophysiological underpinnings of both disorders is brain morphology. A number of brain studies using structural magnetic resonance imaging (sMRI) have been carried out (Bora et al., 2011; Amann et al., 2016). Among these, those based on voxel-based morphometry (VBM) and surface-based morphometry (SBM) are relevant as they allow characterizing the focal changes in grey matter (GM) tissue. In contrast to VBM, which identifies regional differences in GM volume, SBM allows measuring additional local features of the cortex, including cortical thickness (CT), cortical surface area (SA), gyrification index (GI), and cortical volume (CV), the later which is a product of CT and SA of every cortical region. Notably, recent studies have found that CT and SA seem to be heritable but genetically and phenotypically independent (Panizzon et al., 2009; Winkler et al., 2010), and they result from different ontogenic stages during corticogenesis (Pontious et al., 2008). In fact, it has been suggested that independent processes regulate CT and SA (Panizzon et al., 2009; Rimol et al., 2012): while CT may be more influenced by environmental and neurodegenerative factors (Birnbaum and Weinberger, 2017), cortical SA developmental trajectories may be predominantly influenced by early neurodevelopmental and genetic factors (Panizzon et al., 2009; Winkler et al., 2010). Thus, investigating SA and CT differences separately might lead to the identification of more specific imaging biomarkers that may provide greater insight into the neurobiology of psychiatric disorders.
Various studies have reported cortical thinning in schizophrenia compared to healthy subjects, especially in frontal and temporal regions (Rimol et al., 2012; Sugihara et al., 2017; Goldman et al., 2009; Nenadic et al., 2015; Nesvag et al., 2014), however the evidence in bipolar disorder is less conclusive (Hanford et al., 2016; Elvsashagen et al., 2013; Foland-Ross et al., 2011; Fornito et al., 2009; Lyoo et al., 2006; Maller et al., 2014). Recently, two meta-analyses by the Enhancing Neuro Imaging Genetics Through Meta-analyses (ENIGMA) Consortium have conducted the largest studies of CT and SA in both schizophrenia and bipolar disorder. The ENIGMA Schizophrenia Working Group compared 4474 patients with schizophrenia to 5098 healthy subjects and found widespread cortical thinning, with the largest effect sizes in frontal and temporal regions, as well as widespread smaller cortical SA without regional specificity (van Erp et al., 2018). On the other hand, the ENIGMA Bipolar Disorder Working Group involved 2447 bipolar patients and 4056 healthy controls. Cortical gray matter was thinner in frontal, temporal and parietal regions of both brain hemispheres, and cortical SA was reduced in isolated brain regions associated with a history of psychosis (Hibar et al., 2018). Still, no meta-analysis studies based on CT and SA have been conducted comparing patients with schizophrenia and bipolar disorder.
Cortical gyrification, represented by the local gyrification index (GI), is a morphological measure that captures a developmental window different from CT and SA. It measures the amount of cortex buried within the sulcal folds as compared with the amount of visible cortex in circular regions of interest (Schaer et al., 2012). Cortical gyrification is of great interest because is thought to be determined by factors that occur early in the maturation of the brain. Thus, alterations in GI could reflect trait vulnerability to mental disorders (Razavi et al., 2015).
Gyrification studies in schizophrenia have alternately reported both decreased and increased GI, as reviewed by White and Hilgetag (2011). However, decreased GI has been the more replicated finding in recent studies (Nesvag et al., 2014; Palaniyappan and Liddle, 2012; Matsuda and Ohi, 2018; Palaniyappan et al., 2011; Palaniyappan and Liddle, 2013). Abnormal cortical gyrification has been described in patients with a first episode of psychosis, siblings of patients, as well as high-risk and at-risk individuals, suggesting it can be an imaging biomarker for psychosis (Matsuda and Ohi, 2018; White and Gottesman, 2012). Likewise, reduced GI in patients with bipolar disorder has been found in a few studies, along with progressive changes of brain gyrification in different stages of the disorder (Cao et al., 2017; Penttila et al., 2009). As in previous studies using different brain imaging techniques, studies directly comparing gyrification patterns between schizophrenia and bipolar disorder are scarce and included small samples sizes. Decreased GI has been demonstrated in both disorders compared to healthy subjects, but it seems to be more pronounced in schizophrenia (Palaniyappan and Liddle, 2013; McIntosh et al., 2009). By contrast, higher GI has also been described in both disorders, with psychotic bipolar patients showing higher GI in cingulate cortex compared to schizophrenia and healthy subjects (Nenadic et al., 2015). Thus, further studies providing additional data are required.
The more comprehensive SBM analysis to date, in terms of CT, CV, and SA, was reported by Rimol et al. (2012). They analyzed 173 patients with schizophrenia, 139 patients with bipolar disorder and 207 healthy subjects. Patients with schizophrenia showed widespread CV reduction driven by both CT and SA reductions, compared to healthy subjects. By contrast, bipolar patients only showed CT reductions in frontal, parietal and temporal regions, but there were no significant differences in CV or SA in comparison to healthy subjects. However, this finding still needs to be replicated by taking into account the total brain volume as a confounding factor in the analysis, because unlike CT, SA and CV are highly correlated with total brain volume (Winkler et al., 2010).
The profiles of cortical abnormalities in schizophrenia and bipolar disorder, and how far they resemble each other, have only been studied to a limited extent. The aim of this study is to investigate and compare the brain cortical alterations that underlie schizophrenia and bipolar disorder. To circumvent the limitations of previous studies related to examining a single or a few brain features and the lack of explicit inter-group comparisons between patients with both pathologies, in this work three groups of subjects are studied using multiple SBM features. Specifically, 128 patients with schizophrenia, 128 patients with bipolar disorder, and 127 healthy subjects are compared based on four local cortical measures: (1) cortical volume, (2) cortical thickness, (3) surface area, and (4) gyrification index. Given that the brain abnormalities occurring in these two disorders may affect multiple and distinct brain morphological features, the analysis of different metrics may be more specific and sensitive in discriminating the similarities and differences between disorders. We hypothesize that if schizophrenia and bipolar disorder share a common underlying pathophysiology, then these similarities must be also reflected on their brain structural changes.
2. Method
2.1. Participants
The patient sample (n = 256) consisted of 128 patients with schizophrenia and 128 patients with bipolar disorder recruited from two hospitals (Hospital Benito Menni, Sant Boi de Llobregat; and Hospital Clínic in Barcelona). All patients were diagnosed using DSM-IV criteria. Patients were excluded if: (a) they were aged <18 years or >65 years, (b) had a history of brain trauma or neurological disease, and (c) had shown alcohol/substance abuse within 12 months prior to participation. Clinical evaluation also included the Positive and Negative Syndrome Scale (PANSS) (Kay SR and Opler, 1987), and in the bipolar disorder group symptoms were also scored using the Young Mania Rating Scale (YMRS) (Young et al., 1978) and Hamilton Depression Rating Scale HDRS-21 (Hamilton, 1960) and the Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979). Overall severity of illness was rated using the Global Assessment of Functioning scale (GAF) (Endicott et al., 1976). Pre-morbid IQ was estimated using the Word Accentuation Test (Test de Acentuación de Palabras, TAP; (Del Ser et al., 1997, Gomar et al., 2011), a word reading test requiring pronunciation of Spanish words whose accents have been removed. This test is conceptually similar to the UK National Adult Reading Test (Nelson HE, 1991) and the American Wide-Range Achievement Test (Wilkinson, 1993). Patients were also required to have a current IQ in the normal range (i.e.,>70), as measured using four subtests of the Wechsler Adult Intelligence Scale III (WAIS-III: vocabulary, similarities, block design, and matrix reasoning).
Patients with schizophrenia were on treatment with antipsychotics (second generation AP n = 81, first generation AP n = 9; combination n = 31) with a chlorpromazine equivalent doses of 585 mg/d (SD± 449) (Data missing n = 7). Most of the bipolar patients were on treatment with mood stabilizers (n = 100) (lithium alone or in combination n = 78, valproate alone or in combination n = 14, other mood stabilizers n = 8) and with antidepressants (n = 30) (Data missing for n = 13); of them 81 were taking antipsychotics (second generation AP n = 67, first generation AP n = 7; combination n = 7) with a chlorpromazine equivalent doses of 308 mg/d (SD ± 263) (Data missing for n = 10).
A subsample of the subjects included in this study was previously included in another study by our group. Specifically, 37 patients with schizophrenia, 38 patients with bipolar disorder, and 38 healthy controls were analyzed using VBM 6.
The control sample consisted of 127 healthy individuals selected to be age-, sex- and estimated premorbid IQ-matched to the patients and met the same exclusion criteria. All controls were recruited from non-medical staff working in the hospital, their relatives and acquaintances, plus independent sources in the community. They were questioned and excluded if they reported a history of mental illness and/or treatment with psychotropic medication. All individuals were right-handed.
All participants gave written informed consent and the study was approved by the hospital research ethics committee.
2.2. MRI data acquisition
All subjects were scanned in the same 1.5 Tesla GE Signa scanner (General Electric Medical Systems, Milwaukee, WI, USA) located at the Sant Joan de Déu Hospital in Barcelona (Spain). High-resolution structural-T1 MRI data were acquired with the following acquisition parameters: matrix size 512 × 512; 180 contiguous axial slices; voxel resolution 0.47 ×0.47 × 1 mm3; echo (TE), repetition (TR) and inversion (TI) times (TE/TR/TI) = 3.93 ms/2000 ms/710 ms, respectively; flip angle 15°.
2.3. Surface-based morphometry
Structural MRI data were analyzed with the FreeSurfer image analysis suite, (http://surfer.nmr.mgh.harvard.edu/). Briefly, the pre-processing included motion correction, removal of non-brain tissue, automated Talairach transformation, tessellation of the grey- and white-matter boundaries and surface deformation (Fischl et al., 2004). A number of deformation procedures were performed in the data analysis pipeline, including surface inflation and registration to a spherical atlas. This method uses both intensity and continuity information from the entire three-dimensional images in the segmentation and deformation procedures to produce vertex-wise representations of CT, SA, CV, and GI. The resultant maps are sensitive to sub-millimeter differences between groups and have been validated against histological data (Rosas et al., 2002).
All subjects included in this study passed the standardized quality-control protocols from the ENIGMA consortium (http://enigma.ini.usc.edu/protocols/imaging-protocols) that have previously been applied in large-scale multi-centre studies of major depression and bipolar disorder (add ref: https://www.nature.com/articles/mp201773).
Prior to the statistical analyses, the individual CT, SA, CV, and GI maps were smoothed using a 2D Gaussian filter on the cortex with a full width at half maximum of 20 mm (Chung et al., 2003). Finally, general linear models were applied to the individual maps and the intracranial volume was included as a covariate in the models (Palaniyappan and Liddle, 2012). As all groups are matched by age and gender, these factors were not included in the statistical model. However, we repeated the analyses and verified that the results did not change after controlling for these factors. Statistical inference was carried out with FreeSurfer tools based on non-parametric permutation testing, using a cluster-wise correction method for multiple comparisons with initial cluster-forming threshold of sig = 2.6 (i.e., p < 0.0025, two tails) and 5000 iterations applied. In these analyses, only those clusters with a corrected value of p < 0.05 were considered significant. Anatomical locations of the significant regions were determined with reference to the surface atlas included in FreeSurfer.
3. Results
Demographic, clinical and neuropsychological data for the patients and controls are shown in Table 1. Differences in demographic characteristics among the groups were examined using chi-square tests for categorical variables and ANOVA tests for continuous variables. The groups were matched for age, sex and estimated premorbid IQ (TAP score).
Table 1.
Demographic and clinical features of the patients and controls
| Patients with schizophrenia (N = 128) | Patients with bipolar disorder (N = 128) | Controls (N = 127) | P value | |
|---|---|---|---|---|
| Age (years) | 41 ± 10 | 41 ± 10 | 39 ± 10 | 0.334 |
| Sex (male/female) | 74/54 | 74/54 | 73/54 | 0.998 |
| Pre-morbid IQ (TAP) | 22 ± 4 (a) | 22 ± 4 (b) | 22 ± 4 (i) | 0.288 |
| Age of onset (years) | 22 ± 6 (b) | 26 ± 9 (e) | ||
| Duration of illness (years) | 18 ± 11 (b) | 14 ± 10 (e) | ||
| Positive syndrome | 13 ± 5 (c) | 7 ± 4 (f) | ||
| Negative syndrome | 17 ± 6 (c) | 9 ± 5 (f) | ||
| Disorganized syndrome | 9 ± 3 (c) | 5 ± 2 (f) | ||
| GAF score | 46 ± 14 (d) | 63 ± 19 (f) | ||
| HDRS-21 score | 7 ± 9 (g) | |||
| MADRS score | 8 ± 12 (h) | |||
| YMRS score | 5 ± 9 (e) |
TAP: Word Accentuation Test (Test de Acentuación de Palabras). GAF: Global Assessment of Functioning. HDRS-21: Hamilton Depression Rating Scale, 21 items version. MADRS: Montgomery-Asberg Depression Scale. YMRS: Young Mania Rating Scale. Values are given as mean ± standard deviation.
(a) Missing data for 10 patients. (b) Missing data for 8 patients. (c) Missing data for 23 patients. (d) Missing data for 27 patients. (e) Missing data for 7 patients. (f) Missing data for 25 patients. (g) Missing data for 11 patients. (h) Missing data for 15 patients. (i) Missing data for 13 patients.
3.1. SBM differences between patients with schizophrenia and healthy controls
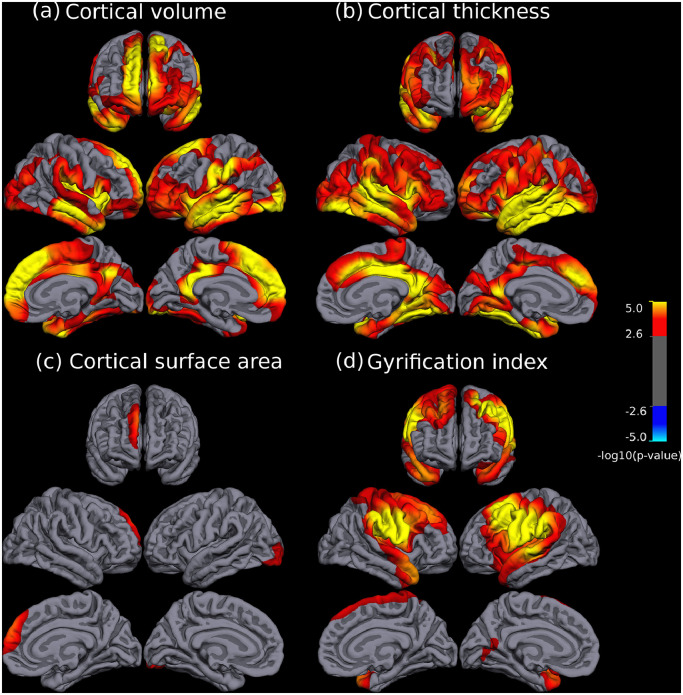
There were significant differences in CV, CT, SA and GI between both groups, which always involved reductions in the patient group (see Fig. 1 and Table 2). Patients with schizophrenia showed a widespread and bilateral pattern of reduction in CV (Fig. 1a) including medial and orbitofrontal regions, both insula and surrounding cortex, the temporal cortices and the posterior cingulate, among other regions (see Table 2 for a complete list of affected regions). CT reductions followed a broadly similar pattern to that observed in CV (Fig. 1b) but less extended in fronto medial regions and affecting larger areas of the temporal, parietal and occipital lobes, with enlarged abnormality in ventrolateral prefrontal and premotor cortices (see list on Table 2). In contrast, patient related reductions in SA were much less abundant involving only the right superior frontal cortex and a small area of the left lateral occipital cortex (see Fig. 1c). Finally, the GI had a differential pattern of bilateral abnormalities including reductions in temporal poles, superior temporal gyrus, insula, parietal areas (mainly supramarginal and postcentral gyri) and frontal caudal regions (see Fig. 1d and Table 2).
Fig. 1.
Brain regions showing significant statistical differences between patients with schizophrenia and healthy subjects at p = 0.05, corrected for multiple comparisons across space. Panels depict cortical volume (a), cortical thickness (b), cortical surface area (c), and gyrification index (d), respectively. The right side of the image represents the left side of the brain. The statistical maps show uncorrected values (i.e., sig = −log10(p)) masked by the significant clusters. The statistical results were only significant in the direction of reduced values in patients.
Table 2.
Results from the SBM differences between patients with schizophrenia and healthy controls analyses. Report of significant regions where patients showed reduced cortical volume, thickness, surface area and gyrification index compared to healthy controls, at p < 0.05, cluster-based corrected for multiple comparisons. Structures are labeled using the Deskian-Killiany atlas included in FreeSurfer and they are ordered by overlapping percentage. The overlapping percentage of each brain region is calculated as 100% x NumVcluster/NumVregion, where NumVcluster is the number of vertices of the cluster within a region and NumVregion is the total number of vertices of that region.
| Metrics | Main peak | Cluster | |||
|---|---|---|---|---|---|
| MNI Coordinates (x,y,z) | p-value | Number of vertices | Cluster p-value | Main involved structures (ordered by overlapping percentage) | |
| Cortical volume | |||||
| Right Brain | |||||
| Superior frontal cortex | (7, 54, 31) | <1e−8 | 85575 | 2e−4 | Insula(100), Isthmus Cingulate (100), Posterior Cingulate (100), Parsorbitalis (100), Transverse Temporal(100), Temporal Pole (100), Frontal Pole(100), Entorhinal (100), Parsopercularis (99),Fusiform (98), Superior Frontal(92), Superior Temporal (86), Paracentral (81) |
| Left Brain | |||||
| Middle temporal cortex | (−54, −14, −20) | <1e−8 | 92976 | 2e−4 | Lateral Orbitofrontal (100), Isthmus Cingulate (100), Parsorbitalis (100), Parstriangularis (100),Transverse Temporal (100), Frontal Pole (100), Insula (96), Superior Frontal (95), Middle Temporal (91), Superior Temporal (89), Lateral Occipital (84), Bankssts (84), Posterior Cingulate (83) |
| Cortical Thickness | |||||
| Right Brain | |||||
| Insula | (36, −8, 7) | <1e−9 | 110037 | 2e−4 | Insula (100), Isthmus Cingulate (100), Posterior Cingulate (100), Supramarginal (100), Parsopercularis (100), Parstriangularis (100), Superior Temporal (100), Parahippocampal (100), Middle Temporal (100), Transverse Temporal (100), Temporal Pole (100), Entorhinal (100), Bankssts (100) |
| Left Brain | |||||
| Insula | (−35, −6, 11) | 1e−10 | 109936 | 2e−4 | Superior Temporal (100), Fusiform (100), Middle Temporal (100), Temporal Pole (100), Transverse Temporal (100), Bankssts (100), Entorhinal (100), Inferior Temporal (100), Insula (99), Lingual (95), Parsopercularis (93), Parsorbitalis (91), Parstriangularis (91) |
| Cortical Surface Area | |||||
| Right Brain | |||||
| Superior frontal cortex | (8, 55, 31) | <1e−3 | 2149 | 3e−3 | Superior Frontal (18), Frontal Pole (5) |
| Left Brain | |||||
| Lateral occipital cortex | (−30, −90, −15) | <1e−3 | 1970 | <8e−4 | Lateral occipital (31) |
| Gyrification index | |||||
| Right Brain | |||||
| Supramarginal | (56, −21, 31) | <1e−6 | 55951 | 2e−4 | Postcentral (100), Transverse Temporal (100), Precentral (98), Temporal Pole (98), Supramarginal (94), Caudal Middle Frontal (91), Superior Temporal (78), Parsopercularis (57), Superior Frontal, (48), Insula (46), Rostral Middle Frontal (37) |
| Left Brain | |||||
| Caudal middle frontal cortex | (−35, 11, 54) | <1e−7 | 57680 | 2e−4 | Superior Temporal (100), Caudal Middle Frontal (100), Parsopercularis (100), Insula (100), Transverse Temporal (100), Supramarginal (99), Temporal Pole (91), Precentral (72), Postcentral (72), Bankssts (53) |
| Precuneus | (−19, −59,15) | 1e−4 | 2311 | 0.017 | Pericalcarine(27),Precuneus (17), Lingual (11) |
3.2. SBM differences between patients with bipolar disorder and healthy controls
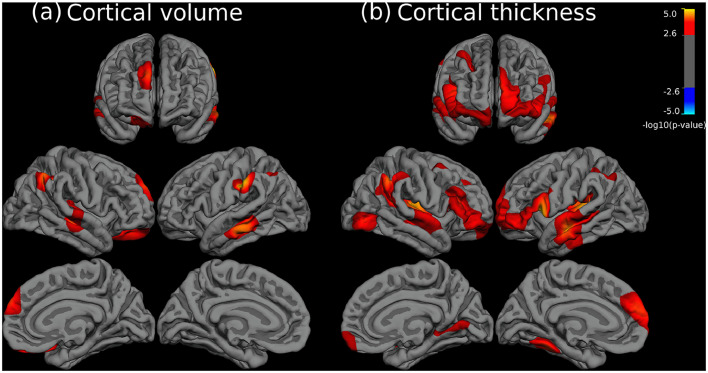
As shown in Fig. 2 there were significant differences in CV and CT between patients with bipolar disorder and healthy controls. As in the previous analyses between schizophrenia patients and controls, these only involved reductions in patients and were frequently placed in the same areas. However, such reductions were clearly less marked in extension than those observed in patients with schizophrenia. Specifically, alteration of CV occurred in moderately sized clusters located in the right frontal cortex (in superior and orbitofrontal areas), temporal lobes and bilateral parietal cortex (supramarginal gyrus and adjacent areas) (see full description in Table 3). Cortical thinning abnormalities were slightly more prominent than those in CV, including clusters of larger extension (Fig. 2b). Both left and right hemispheres had frontal reductions in CT, mainly including parts of the medial and lateral prefrontal cortices, temporal lobes, insulae and supramarignal gyri, while the occipital cortex was only affected in two sites of the right hemisphere (see Table 3). Finally, in contrast to CV and CT results, patients with bipolar disorder did not show any significant area of SA or GI abnormalities when compared to healthy subjects.
Fig. 2.
Brain regions showing significant statistical differences between patients with bipolar disorder and healthy subjects at p = 0.05, corrected for multiple comparisons across space. Left and right panels depict cortical volume (a) and cortical thickness (b), respectively. The right side of the image represents the left side of the brain. The statistical maps show uncorrected values (i.e., sig = −log10(p)) masked by the significant clusters. The statistical results were only significant in the direction of reduced values in patients.
Table 3.
Results from the SBM differences between patients with bipolar disorder and healthy controls analyses. Report of significant regions where patients showed reduced cortical volume and thickness compared to healthy controls, at p < 0.05, cluster-based corrected for multiple comparisons. Structures are labeled using the Deskian-Killiany atlas included in FreeSurfer and they are ordered by overlapping percentage. The overlapping percentage of each brain region is calculated as 100% x NumVcluster/NumVregion, where NumVcluster is the number of vertices of the cluster within a region and NumVregion is the total number of vertices of that region.
| Metrics | Main peak | Cluster | |||
|---|---|---|---|---|---|
| MNI Coordinates (x,y,z) | p-value | Number of vertices | Cluster p-value | Main involved structures (ordered by overlapping percentage) | |
| Cortical volume | |||||
| Right Brain | |||||
| Inferior parietal cortex | (36, −46, 36) | <1e−8 | 3994 | 8e−4 | Inferior parietal (29), Superior parietal (9), Supramarginal (3) |
| Middle temporal cortex | (48, −27, −11) | <1e−4 | 4127 | 6e−4 | Transverse temporal (90), Superior temporal (26), Middle temporal (22), Insula (10) |
| Superior frontal cortex | (9, 56, 30) | <1e−3 | 1244 | 0.04 | Superior frontal (10) |
| Lateral orbitofrontal cortex | (17, 41, −18) | <1e−3 | 3219 | 8e−4 | Lateral orbitofrontal (64), Medial orbitofrontal (9), Frontal pole (5) |
| Left Brain | |||||
| Supramarginal gyrus | (−55, −30,35) | <1e−5 | 2554 | 0.01 | Supramarginal (24), Postcentral (5) |
| Middle temporal cortex | (−64, −26, −16) | <1e−4 | 3381 | 4e−4 | Middle temporal (55), Bankssts (14), Superior temporal (6), Inferior temporal (5) |
| Inferior parietal cortex | (−32, −58, 37) | <1e−4 | 2576 | 0.013 | Inferior parietal (17), Superior parietal (9), Supramarginal (3) |
| Cortical Thickness | |||||
| Right Brain | |||||
| Transverse temporal cortex | (41, −26, 3) | <1e−4 | 16257 | 2e−4 | Transverse temporal (100), Frontal pole (75), Superior temporal (63), Parsorbitalis 63), Insula (60), Parstriangularis (51) |
| Inferior parietal cortex | (48, −47, 38) | <1e−4 | 7193 | 2e−4 | Supramarginal (39), Inferior parietal (32), Bankssts (26) |
| Lateral occipital cortex | (42, −77, −5) | <1e−3 | 1426 | 0.02 | Lateral occipital (22), Inferior temporal (3) |
| Caudal middle frontal cortex | (30, 14, 47) | <1e−3 | 2478 | 5e−3 | Caudal middle frontal (43), Superior frontal (5) |
| Lingual gyrus | (14, −61, 2) | <1e−3 | 1639 | 0.036 | Lingual (32), Pericalcarine (11), Parahippocampal (7) |
| Left Brain | |||||
| Parsopercularis | (−42, 10, 8) | <1e−5 | 21876 | 2e−4 | Transverse temporal (100), Parsopercularis (88), Parsorbitalis (65), Parstriangularis (63), Superior temporal (54), Middle temporal (49), Frontal pole (45), Bankssts (38), Insula (37) |
| Fusiform gyrus | (−32, −45, −20) | <1e−3 | 1724 | 0.02 | Fusiform (35) |
| Supramarginal gyrus | (−40, −51, 35) | <1e−3 | 3350 | 5e−3 | Inferior parietal (13), Superior parietal (12), Supramarginal (12) |
3.3. SBM differences between patients with schizophrenia and bipolar disorder
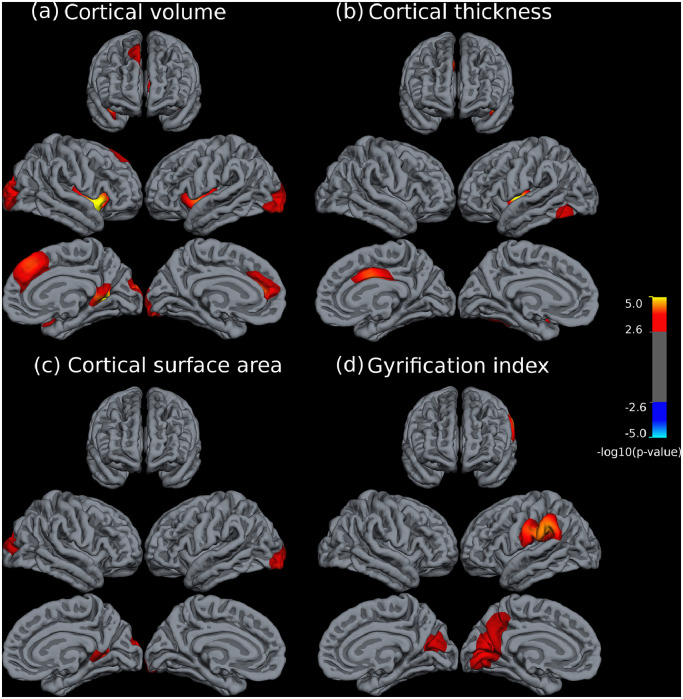
The comparison between the two groups of patients revealed that patients with schizophrenia were significantly more affected in all cortical measures (i.e. all significant results in CV, CT, SA or GI involved reductions in schizophrenia) (see Fig. 3). Specifically, there were seven clusters with significant CV differences that were primarily located in a bilateral fashion in the dorsomedial prefrontal and anterior cingulate cortex, in caudal occipital areas, both lateral sulci, right ventral posterior cingulate and right temporal pole (see Table 4). All clusters were placed within areas that had shown CV reductions in the healthy control vs. schizophrenia comparison (see both Figs. 1a and 3a). Similarly, albeit less abundantly, differences in CT between both disorders were also located in areas of previous schizophrenia vs. healthy subject reductions (Fig. 3b). These mainly included abnormalities in the right dorsal cingulate, left lateral sulcus and left inferior temporal gyrus, posteriorly (see Table 4).
Fig. 3.
Brain regions showing significant statistical differences between patients with schizophrenia and patients with bipolar disorder at p = 0.05, corrected for multiple comparisons across space. Panels depict cortical volume (a), cortical thickness (b), cortical surface area (c), and gyrification index (d), respectively. The right side of the image represents the left side of the brain. The statistical maps show uncorrected values (i.e., sig = −log10(p)) masked by the significant clusters. The statistical results were only significant in the direction of reduced values in patients with schizophrenia.
Table 4.
Results from the SBM differences between patients with schizophrenia and bipolar disorder analyses. Report of significant regions where patients showed reduced cortical volume, thickness, surface area and gyrification index compared to healthy controls, at p < 0.05, cluster-based corrected for multiple comparisons. Structures are labeled using the Deskian-Killiany atlas included in FreeSurfer and they are ordered by overlapping percentage. The overlapping percentage of each brain region is calculated as 100% x NumVcluster/NumVregion, where NumVcluster is the number of vertices of the cluster within a region and NumVregion is the total number of vertices of that region.
| Metrics | Main peak | Cluster | |||
|---|---|---|---|---|---|
| MNI Coordinates (x,y,z) | p-value | Number of vertices | Cluster p-value | Main involved structures (ordered by overlapping percentage) | |
| Cortical volume | |||||
| Right Brain | |||||
| Insula | (36, 7, −11) | <1e−5 | 5931 | 2e−4 | Insula (75), Parsopercularis (26), Temporal pole (15) |
| Lingual gyrus | (26, −57, 7) | <1e−5 | 3839 | 8e−4 | Isthmus cingulate (50), Lingual (24), Precuneus (18), Pericalcarine (14) |
| Superior frontal cortex | (78, 34, 45) | <1e−4 | 3632 | 2e−4 | Superior frontal (31) |
| Lateral occipital cortex | (17, −94, 15) | <1e−4 | 3072 | 2e−4 | Lateral occipital (44), Cuneus (5) |
| Left Brain | |||||
| Insula | (−36, −1, −6) | 4398 | 8e−4 | Insula (65), Parsopercularis (22), Parstriangularis (4) | |
| Superior frontal cortex | (−15, 42, 14) | 2041 | 0.0054 | Rostral anterior cingulate(24), Superior frontal (14) | |
| Lateral occipital cortex | (−25, −94, −16) | 3186 | 2e−4 | Lateral occipital (50) | |
| Cortical Thickness | |||||
| Right Brain | |||||
| Posterior cingulate cortex | (4, −4, 30) | <1e−4 | 2922 | 0.015 | Posterior cingulate (55), Caudal anterior cingulate (54) |
| Left Brain | |||||
| Insula | (−36, −9, −2) | 1e−5 | 3900 | 0.00240 | Insula (51), Superior temporal (17) |
| Fusiform gyrus | (−41, −46, −15) | <1e−3 | 2616 | 0.0028 | Inferior temporal (30), Fusiform (24), Lateral occipital (2) |
| Cortical Surface Area | |||||
| Right Brain | |||||
| Lingual gyrus | (24, −55, 7) | <1e−3 | 2183 | 0.026 | Isthmus cingulate (37), Lingual (21), Precuneus (6) |
| Lateral occipital cortex | (18, −94, 16) | <1e−3 | 1862 | <5e−3 | Lateral occipital (27), Cuneus (8) |
| Left Brain | |||||
| Lateral occipital cortex | (−28, −95, −14) | <1e−3 | 1592 | < 5e−3 | Lateral occipital (25) |
| Gyrification index | |||||
| Right Brain | |||||
| Cuneus | (9, −70, 19) | 1e−3 | 3407 | 8e−4 | Cuneus (67), Pericalcarine (53), Precuneus (15), Lingual (4) |
| Left Brain | |||||
| Supramarginal gyrus | (−56, −37, 32) | <1e−4 | 9049 | 2e−4 | Supramarginal (68), Postcentral (26), Superior temporal (6) |
| Cuneus | (−13, −69, 15) | <1e−3 | 9587 | 2e−4 | Pericalcarine (79), Precuneus (67), Cuneus (64), Lingual (45), Isthmus cingulate (11) |
In contrast, SA results not always had such anatomical matching with areas of SA reduction found in the schizophrenia vs. healthy control comparison. However, all reported differences in SA between both disorders included areas that had shown CV differences in the same cross-disorder comparison (see Fig. 3a and c). These comprised both posterior occipital regions and the right ventral posterior cingulate (see Table 4). Finally, inter-disorder differences in GI included a cluster in the left supramarginal gyrus (which had previously shown extensive reductions in the schizophrenia vs. control comparison) but also included bilateral abnormalities in the cuneus that spread over the left precuneus and left primary visual areas and which, apart from a small fraction of the left side cluster, were not present at all in the schizophrenia vs. control comparison (see Figs. 1d and 3d).
4. Discussion
We found that patients with schizophrenia showed widespread CV and CT reductions compared to healthy subjects. CV reductions in medial brain structures, frontotemporal regions including the insula, and lateral occipital cortex are partially consistent with previous meta-analyses of VBM studies (Bora et al., 2011; Honea et al., 2005). Similarly, CT reductions have also been reported in schizophrenia in several studies (Rimol et al., 2012; Sugihara et al., 2017; Goldman et al., 2009).
In our study, SA reductions in patients with schizophrenia were also found, indicating that although the reduction in CV is largely explained by cortical thinning, it is also affected by SA changes. It is worth mentioning that a previous study by our group (Landin-Romero et al., 2017) found that schizoaffective patients showed widespread CV reduction and a region of decreased SA in the left fusiform gyrus. Interestingly, this area is close to the left lateral occipital cortex, where we have reported SA reduction in patients with schizophrenia. Additionally, our results are partially consistent with a recent ENIGMA meta-analysis, which found that patients with schizophrenia had widespread thinner cortex, with the largest effect sizes in frontal and temporal lobe regions (van Erp et al., 2018). However, opposite to our focal SA reduction findings, they found smaller but widespread SA reduction, which may be explained by a higher sensitivity to detect small changes due to larger sample size (van Erp et al., 2018). Another finding of this study is reduced GI in patients with schizophrenia compared to healthy subjects. Abnormalities were found in frontal, temporal and parietal regions. Decreased GI in the left insula, superior/middle/inferior frontal cortex, supramarginal cortex and Broca's area has been also reported in previous studies (Palaniyappan and Liddle, 2012; Palaniyappan et al., 2011; Spalthoff et al., 2018). Also in agreement with our results, another study based on two large independent samples of Swedish and Norwegian patients with schizophrenia showed reduced GI in the left pericentral region, comparared to healthy subjects (Nesvag et al., 2014).
Patients with bipolar disorder showed reduced CV in right frontal, temporal and parietal regions compared to healthy subjects. Interestingly, abnormalities in the grey matter of the right prefrontal cortex and temporal lobe were described as the most robust in a meta-analysis of VBM studies in bipolar disorder (Selvaraj et al., 2012). Cortical thinning in bipolar disorder were found in similar but more extensive areas than CV. Similar areas with reduced CT have already been reported by other authors, including the left superior and middle temporal cortex (Elvsashagen et al., 2013; Maller et al., 2014), superior frontal cortex (Elvsashagen et al., 2013; Foland-Ross et al., 2011; Maller et al., 2014), orbitofrontal cortex (Foland-Ross et al., 2011; Lyoo et al., 2006), and right parahippocampal gyrus (Hulshoff Pol et al., 2012). More recently, the largest ENIGMA meta-analysis of CT and SA in bipolar disorder found evidence of widespread bilateral cortical thinning in frontal, temporal and parietal regions (Hibar et al., 2018). Longer duration of illness and medication were associated with reduced CT in these regions. Interestingly, there is a substantial overlap between some of the reported regions with the strongest effect sizes (i.e., left frontal operculum and left fusiform gyrus) and our results. In addition, opposite to our negative results in relation to SA abnormalities in bipolar patients, they found localized SA reduction in the insula. Although, it was only found in a subgroup of young adults and it was associated with a history of psychosis and medication (Hibar et al., 2018). Furthermore, no region of significant gyrification abnormalities between patients with bipolar disorder and healthy subjects were found in our study. Hence, our results point towards a reduction in the cortical volume of patients with bipolar disorder, which is exclusively explained by cortical thinning but not by changes in the surface area or gyrification pattern. Finally, it is important to mention that patients with bipolar disorder showed significant thinning of the right caudal middle frontal cortex (DLPFC). Reduced functional activation of this area has been reported in a number of functional MRI studies, e.g. (Pomarol-Clotet et al., 2015).
One of the main novelties of the present study concerns the direct comparison between patients with schizophrenia and bipolar disorder. This analysis is important to evaluate to what extent the underlying cortical morphological alterations differ between these two disorders. To date, the limited number of studies that have compared both disorders usually found more grey matter deficits in schizophrenia. However, the cortical regions implicated by these deficits were rather heterogeneous across studies (Arnone et al., 2009; Brown et al., 2011; Ivleva et al., 2017; Shahab et al., 2018). In agreement with these studies, we found that CV reductions are more pronounced in patients with schizophrenia. Our results point to significant abnormalities affecting frontal, temporal and occipital regions. Recently, the European Network on Psychosis, Affective disorders, and Cognitive Trajectory analyzed a big sample of patients with schizophrenia and bipolar disorder combining ROI and VBM analyses. In line with our results, both disorders exhibited shared fronto-temporo-occipital grey matter deficits, and schizophrenia was associated with more severe abnormalities in a number of regions including the insula (Maggioni et al., 2017). In addition to widespread CV reduction, in our study patients with schizophrenia showed reduced CT in relation to patients with bipolar disorder in the right anterior/posterior cingulate cortex and left temporal regions, including the insula. Only a few studies have directly compared cortical thickness between patients with schizophrenia and bipolar disorder. A recent review identified a similar pattern of cortical thinning in both disorders in frontal and temporal regions, including the parahippocampal and fusiform gyrus, suggesting some common neuropathology (Hanford et al., 2016). Likewise, a similar bilateral cortical thinning in both groups, mainly affecting the operculum and the anterior/posterior cingulate cortex was reported in Knochel et al. (2016). Similar to us, these studies found a more pronounced cortical thinning in schizophrenia than in bipolar disorder, involving similar but also different regions. In this sense, our study adds more evidence to the hypothesis that thinning of the frontal cortex may represent a biological feature shared by both disorders. As was found by Hulshoff Pol et al. (2012), common areas of thinner cortex could be associated with higher genetic liabilities for both disorders.
In terms of SA, patients with schizophrenia showed smaller SA than patients with bipolar disorder in posterior brain regions, including the lingual gyrus, precuneus, cuneus and occipital cortex. It is worth noting that only one previous study attempted to compare abnormalities in CV, CT, and SA among schizophrenia, bipolar disorder, and healthy controls (Rimol et al., 2012). Our study adds two novelties to this previous study. First, in addition to these cortical metrics, we also compared the gyrification patterns. Second, we took into account the total brain volume as a confounding factor in the analyses. Our findings are partially in line with this previous study. One the one hand, in both studies patients with schizophrenia showed CV, CT and SA reductions compared with healthy subjects. Moreover, SA reductions were detected in patients with schizophrenia, but not in bipolar patients. On the other hand, however, while the SA reductions found by Rimol et al. (2012) in schizophrenia were widespread, only focal SA changes were identified in our study. The biggest discrepancy between both studies is that we found CV reductions in bipolar patients, while Rimol et al. (2012) did not. Despite CT reductions were found in patients with bipolar disorder in both studies, in Rimol et al. (2012) a non-significant SA increase was detected in patients which counteracted the cortical thinning. This discrepancy may be explained by differences in the statistical models used in both studies. In our study, the intra-cranial volume was included as a covariate because it is highly correlated with SA, and thus with CV (Winkler et al., 2010). If this factor is not taken into account, local morphological differences may be confounded with differences in total brain volume. The comparison between patients groups carried out in Rimol et al. (2012) found that practically all differences in CV were driven by changes in SA, while in our in study significant changes in both SA and CT were found.
In line with previous studies, suggesting that CT may be more influenced by additional environmental and neurodegenerative factors (Birnbaum and Weinberger, 2017), while cortical SA developmental trajectories in psychosis may be predominantly influenced by early neurodevelopmental and genetic factors (Panizzon et al., 2009; Winkler et al., 2010; Jalbrzikowski et al., 2019), our results support the hypothesis for a strongest neurodevelopmental disturbance in schizophrenia than in bipolar disorder, as SA reductions were only found in patients with schizophrenia.
Another main contribution of this study was the gyrification abnormalities found in patients with schizophrenia compared to bipolar disorder. In particular, we found a decreased GI in patients with schizophrenia in the left parietal and temporal cortex, as well as in both sides of the dorsal medial part of the occipital cortex. To date, only a few studies have directly compared GI between both disorders, but including small sample of patients. For instance, a previous study found that patients with schizophrenia and bipolar disorder have a similar pattern of reduced prefrontal gyrification, which was associated with cognitive impairments (McIntosh et al., 2009). Another study reported an overlapping reduction in gyrification (i.e., 25%) in the lateral prefrontal cortex in both patient groups (Palaniyappan and Liddle, 2013). Finally, decreased GI has been observed in adolescents with psychotic bipolar disorder and schizophrenia, suggesting it may represent a shared endophenotype for psychosis (Janssen et al., 2014).
This study has some limitations. In common with other neuroimaging studies of psychosis, patients were medicated. Furthermore, our samples were made up of right-handed subjects, which may induce a sampling bias. It is important to note that left-handedness has been found to be over-represented in schizophrenia. Moreover, our samples were recruited to be matched by age, gender and premorbid IQ and this may cause selection bias. For example, the proportion of men with schizophrenia was higher than that of women (74/54) and it caused a selection bias of the bipolar patients and healthy subjects. On the other hand, a limitation related to matching by age is that this may cause a selection bias in terms of years of evolution. Unfortunately, it is not possible to match these two variables at the same time, so we preferred to recruit age-matched participants. Finally, it is important to note that we matched by premorbid IQ and patients were also required to have a current IQ in the normal range (i.e.>70). We hope that this procedure contributed to reducing any IQ-related sampling bias. Finally, subjects with alcohol or drug abuse were excluded. Data on substance abuse in the past were not systematically collected and might have differed between the three groups. Therefore, our results should not be generalized to the whole population of patients and healthy controls but to subpopulations satisfying these clinical and demographic characteristics. Future studies should investigate the value of cortical morphology for distinguishing major psychiatric disorders, for instance, by using machine learning algorithms as a diagnostic prediction tool in psychosis (Salvador et al., 2017). Moreover, new studies should be conducted to investigate the alterations in white matter tissue using multiple features derived from diffusion MRI data (Canales-Rodriguez et al., 2014) allowing the individual characterization of microstructural parameters (Daducci et al., 2015) and their relation to white matter genes (Gas et al., 2019). Multi-modal studies (Pomarol-Clotet et al., 2010) may help to provide a better understanding of the underlying neurobiology of schizophrenia and bipolar disorder, as well as a more robust biomedical background to the aetiology of either condition, which might ultimately lead to improvements in diagnosis and treatment.
5. Conclusions
Cortical volume and cortical thickness deficits are shared between patients with schizophrenia and bipolar disorder, suggesting that both pathologies may be affected by similar environmental and neurodegenerative factors. However, in bipolar disorder, these abnormalities are less pronounced. These results are in line with previous studies using different imaging techniques, as well as with genetics and neuropsychological studies, which point towards a shared neurobiological underpinning between both disorders. However, in schizophrenia, these abnormalities are more pronounced and may be related to psychotic symptoms. The exclusive alteration in schizophrenia of metrics related to the local geometry and curvature of the brain cortical surface suggests that this group is influenced by additional neurodevelopmental and genetic factors. Taken together, these data point towards a shared neurobiological underpinning between both pathologies, but with additional components affecting schizophrenia.
Financial disclosures
None.
Declaration of Competing of Interest
All authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the Generalitat de Catalunya (2017SGR01271 to EP-C and 2017SGR1265 to PF-C from AGAUR and PERIS SLT006/17/232 to MM from Departament de Salut) and several grants funded by Instituto de Salud Carlos III co-funded by the European Regional Development Fund/European Social Fund “Investing in your future”: Miguel Servet Research Contract (CPII16/00018 to EP-C), Sara Borrell Research Contract (CD1800029 to EJC-R), and Research Projects (PI14/01148 to EP-C, PI14/01151 to RS, PI15/00277 to EJC-R, PI18/00810 to EP-C, PI18/00877 to RS, PI1801535 to MM and PI18/00880 to PJM). Also by grants from Ministerio de Ciencia, Innovación y Universidades: Juan de la Cierva-Formación contract (FJCI-2015-25278 to PF-C). EV, CB and JG thank the support of the Spanish Ministry of Science, Innovation and Universities (PI15/00283) integrated into the Plan Nacional de I+D+I y cofinanciado por el ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER); CIBERSAM; and the Comissionat per a Universitats i Recerca del DIUE de la Generalitat de Catalunya to the Bipolar Disorders Group (2017 SGR 1365) and the project SLT006/17/00357, from PERIS 2016–2020 (Departament de Salut). CERCA Programme/Generalitat de Catalunya. The funding organizations played no role in the study design, data collection and analysis, or manuscript approval.
Footnotes
All authors did the research and wrote this manuscript. They have made a direct and substantial contribution to the study reported and participated to a sufficient degree. S. Alonso-Lana, A. Guerrero, N. Moro, C. Bosque, J. Gomar, JM. Goikolea, C Bonnin, .E. Vieta and S. Sarró participated in the data collection. E Pomarol-Clotet, E .Canales-Rodriguez, P. McKenna and M. Madre conceived and designed the study. E .Canales-Rodriguez, T. Maristany, P. Salgado-Pineda, P. Fuentes-Claramote and R. Salvador analyzed and interpreted the data and did the statistical analyses. All authors participated in writing the article and they provided a critical discussion and conclusions.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Mercè Madre, Email: mmadre.hbmenni@hospitalarias.es.
Erick J. Canales-Rodríguez, Email: ejcanalesr@gmail.com.
References
- Amann BL, Canales-Rodriguez EJ, Madre M. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr. Scand. 2016;133(1):23–33. doi: 10.1111/acps.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br. J. Psychiatry. 2009;195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017;18:727. doi: 10.1038/nrn.2017.125. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr. Res. 2011;127(1-3):46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Brown GG, Lee JS, Strigo IA, Caligiuri MP, Meloy MJ, Lohr J. Voxel-based morphometry of patients with schizophrenia or bipolar I disorder: a matched control study. Psychiatry Res. 2011;194(2):149–156. doi: 10.1016/j.pscychresns.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales-Rodriguez EJ, Pomarol-Clotet E, Radua J. Structural abnormalities in bipolar euthymia: a multicontrast molecular diffusion imaging study. Biol. Psychiatry. 2014;76(3):239–248. doi: 10.1016/j.biopsych.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Cao B, Passos IC, Wu MJ, Zunta-Soares GB, Mwangi B, Soares JC. Brain gyrification and neuroprogression in bipolar disorder. Acta Psychiatr. Scand. 2017;135(6):612–613. doi: 10.1111/acps.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Robbins S. Deformation-based surface morphometry applied to gray matter deformation. Neuroimage. 2003;18(2):198–213. doi: 10.1016/s1053-8119(02)00017-4. [DOI] [PubMed] [Google Scholar]
- Craddock N, O'Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr. Bull. 2006;32(1):9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daducci A, Canales-Rodriguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran JP. Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. Neuroimage. 2015;105:32–44. doi: 10.1016/j.neuroimage.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Del Ser T, Gonzalez-Montalvo JI, Martinez-Espinosa S, Delgado-Villapalos C, Bermejo F. Estimation of premorbid intelligence in Spanish people with the Word Accentuation Test and its application to the diagnosis of dementia. Brain Cogn. 1997;33(3):343–356. doi: 10.1006/brcg.1997.0877. [DOI] [PubMed] [Google Scholar]
- Elvsashagen T, Westlye LT, Boen E. Bipolar II disorder is associated with thinning of prefrontal and temporal cortices involved in affect regulation. Bipolar Disord. 2013;15(8):855–864. doi: 10.1111/bdi.12117. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch. Gen. Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Thompson PM, Sugar CA. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. Am. J. Psychiatry. 2011;168(5):530–539. doi: 10.1176/appi.ajp.2010.10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Wood SJ. Anterior cingulate cortex abnormalities associated with a first psychotic episode in bipolar disorder. Br. J. Psychiatry. 2009;194(5):426–433. doi: 10.1192/bjp.bp.107.049205. [DOI] [PubMed] [Google Scholar]
- Gas C, Canales-Rodriguez EJ, Radua J. Discoidin domain receptor 1 gene variants are associated with decreased white matter fractional anisotropy and decreased processing speed in schizophrenia. J. Psychiatr. Res. 2019;110:74–82. doi: 10.1016/j.jpsychires.2018.12.021. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch. Gen. Psychiatry. 2009;66(5):467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomar JJ, Ortiz-Gil J, McKenna PJ. Validation of the Word Accentuation Test (TAP) as a means of estimating premorbid IQ in Spanish speakers. Schizophr. Res. 2011;128(1-3):175–176. doi: 10.1016/j.schres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanford LC, Nazarov A, Hall GB, Sassi RB. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 2016;18(1):4–18. doi: 10.1111/bdi.12362. [DOI] [PubMed] [Google Scholar]
- Hibar DP, Westlye LT, Doan NT. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol. Psychiatry. 2018;23(4):932–942. doi: 10.1038/mp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, van Baal GC, Schnack HG. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch. Gen. Psychiatry. 2012;69(4):349–359. doi: 10.1001/archgenpsychiatry.2011.1615. [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Clementz BA, Dutcher AM. Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biol. Psychiatry. 2017;82(1):26–39. doi: 10.1016/j.biopsych.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Freedman D, Hegarty CE. Structural brain alterations in youth with psychosis and bipolar spectrum symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2019 doi: 10.1016/j.jaac.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen J, Aleman-Gomez Y, Schnack H. Cortical morphology of adolescents with bipolar disorder and with schizophrenia. Schizophr. Re.s. 2014;158(1-3):91–99. doi: 10.1016/j.schres.2014.06.040. [DOI] [PubMed] [Google Scholar]
- Kay SR FA, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;108:104–113. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kim MD, Seo HJ, Yun HJ. The relationship between cognitive decline and psychopathology in patients with schizophrenia and bipolar disorder. Clin. Psychopharm. Neurosci. 2015;13(1):103–108. doi: 10.9758/cpn.2015.13.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochel C, Reuter J, Reinke B. Cortical thinning in bipolar disorder and schizophrenia. Schizophr. Res. 2016;172(1-3):78–85. doi: 10.1016/j.schres.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Landin-Romero R, Canales-Rodriguez EJ, Kumfor F. Surface-based brain morphometry and diffusion tensor imaging in schizoaffective disorder. Austr. N. Z. J. Psychiatry. 2017;51(1):42–54. doi: 10.1177/0004867416631827. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Sung YH, Dager SR. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8(1):65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Maggioni E, Crespo-Facorro B, Nenadic I. Common and distinct structural features of schizophrenia and bipolar disorder: The European Network on Psychosis, Affective disorders and Cognitive Trajectory (ENPACT) study. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Thaveenthiran P, Thomson RH, McQueen S, Fitzgerald PB. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. J. Affect. Disord. 2014;169:118–127. doi: 10.1016/j.jad.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Ohi K. Cortical gyrification in schizophrenia: current perspectives. Neuropsychiatr. Dis. Treat. 2018;14:1861–1869. doi: 10.2147/NDT.S145273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Moorhead TW, McKirdy J. Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiatr. Scand. 2009;119(3):192–198. doi: 10.1111/j.1600-0447.2008.01286.x. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nelson HE WJ. NFER-Nelson; Windsor, Berkshire: 1991. The Revised National Adult Reading Test. [Google Scholar]
- Nenadic I, Maitra R, Dietzek M. Prefrontal gyrification in psychotic bipolar I disorder vs. schizophrenia. J. Affect. Disord. 2015;185:104–107. doi: 10.1016/j.jad.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Nesvag R, Schaer M, Haukvik UK. Reduced brain cortical folding in schizophrenia revealed in two independent samples. Schizophr. Res. 2014;152(2-3):333–338. doi: 10.1016/j.schres.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J. Psychiatry Neurosci. 2012;37(6):399–406. doi: 10.1503/jpn.110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr. Bull. 2013;40(3):675–684. doi: 10.1093/schbul/sbt050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Folding of the prefrontal cortex in schizophrenia: regional differences in gyrification. Biol. Psychiatry. 2011;69(10):974–979. doi: 10.1016/j.biopsych.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttila J, Paillere-Martinot ML, Martinot JL. Cortical folding in patients with bipolar disorder or unipolar depression. J. Psychiatry Neurosci. 2009;34(2):127–135. [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Alonso-Lana S, Moro N. Brain functional changes across the different phases of bipolar disorder. Br. J. Psychiatry. 2015;206(2):136–144. doi: 10.1192/bjp.bp.114.152033. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Canales-Rodriguez EJ, Salvador R. Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol. Psychiatry. 2010;15(8):823–830. doi: 10.1038/mp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev. Neurosci. 2008;30(1-3):24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi MJ, Zhang T, Liu T, Wang X. Cortical Folding Pattern and its Consistency Induced by Biological Growth. Sci. Rep. 2015;5:14477. doi: 10.1038/srep14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Nesvag R, Hagler DJ., Jr. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol. Psychiatry. 2012;71(6):552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salvador R, Radua J, Canales-Rodriguez EJ. Evaluation of machine learning algorithms and structural features for optimal MRI-based diagnostic prediction in psychosis. PLoS ONE. 2017;12(4) doi: 10.1371/journal.pone.0175683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Schmansky N, Fischl B, Thiran JP, Eliez S. How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. J. Vis. Exp. 2012;59:e3417. doi: 10.3791/3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Arnone D, Job D. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14(2):135–145. doi: 10.1111/j.1399-5618.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- Shahab S, Mulsant BH, Levesque ML. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology. 2018 doi: 10.1038/s41386-018-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalthoff R, Gaser C, Nenadic I. Altered gyrification in schizophrenia and its relation to other morphometric markers. Schizophr. Res. 2018;202:195–202. doi: 10.1016/j.schres.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Sugihara G, Oishi N, Son S, Kubota M, Takahashi H, Murai T. Distinct patterns of cerebral cortical thinning in schizophrenia: a neuroimaging data-driven approach. Schizophr. Bull. 2017;43(4):900–906. doi: 10.1093/schbul/sbw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TGM, Walton E, Hibar DP. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol. Psychiatry. 2018 doi: 10.1016/j.biopsych.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Gottesman I. Brain connectivity and gyrification as endophenotypes for schizophrenia: weight of the evidence. Curr. Top. Med. Chem. 2012;12(21):2393–2403. doi: 10.2174/156802612805289953. [DOI] [PubMed] [Google Scholar]
- White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev. Psychopathol. 2011;23(1):339–352. doi: 10.1017/S0954579410000842. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Vol. 3. Jastak Association; Wilmington, DE: 1993. (Wide Range Achievement Test–Revision). : [Google Scholar]
- Winkler AM, Kochunov P, Blangero J. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53(3):1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]