Highlights
-
•
Diffusion tensor imaging (DTI) and transcranial magnetic stimulation (TMS) were used to assess neurophysiological and neurostructural properties of the corticospinal tract.
-
•
Patients with a history of anterior cruciate ligament injury demonstrate asymmetry in corticospinal tract structure.
-
•
Corticospinal excitability was strongly correlated with tract volume.
Keywords: White matter, Neurophysiology, Knee, Anterior cruciate ligament, Quadriceps muscle, Cortical excitability
Abstract
Background
Underlying neural factors contribute to poor outcomes following anterior cruciate ligament reconstruction (ACLR). Neurophysiological adaptations have been identified in corticospinal tract excitability, however limited evidence exists on neurostructural changes that may influence motor recovery in ACLR patients.
Objective
To 1) quantify hemispheric differences in structural properties of the corticospinal tract in patients with a history of ACLR, and 2) assess the relationship between excitability and corticospinal tract structure.
Methods
Ten participants with ACLR (age: 22.6 ± 1.9 yrs; height: 166.3 ± 7.5 cm; mass: 65.4 ± 12.6 kg, months from surgery: 70.0 ± 23.6) volunteered for this cross-sectional study. Corticospinal tract structure (volume; fractional anisotropy [FA]; axial diffusivity [AD]; radial diffusivity [RD]; mean diffusivity [MD]) was assessed using diffusion tensor imaging, and excitability was assessed using transcranial magnetic stimulation (motor evoked potentials normalized to maximal muscle response [MEP]) for each hemisphere. Hemispheric differences were evaluated using paired samples t-tests. Correlational analyses were conducted on structural and excitability outcomes.
Results
The hemisphere of the ACLR injured limb (i.e. hemisphere contralateral to the ACLR injured limb) demonstrated lower volume, lower FA, higher MD, and smaller MEPs compared to the hemisphere of the non-injured limb, indicating disrupted white matter structure and a reduction in excitability of the corticospinal tract. Greater corticospinal tract excitability was associated with larger corticospinal tract volume.
Conclusions
ACLR patients demonstrated asymmetry in structural properties of the corticospinal tract that may influence the recovery of motor function following surgical reconstruction. More research is warranted to establish the influence of neurostructural measures on patient outcomes and response to treatment in ACLR populations.
1. Introduction
Musculoskeletal injuries, such as those to the anterior cruciate ligament (ACL), are often treated as injuries with solely musculoskeletal consequences. For instance, rupture of the ACL destabilizes the knee, creating joint laxity and functional instability. These impairments are frequently treated via surgical ACL reconstruction (ACLR) to restore static stability, and therapeutic rehabilitation to increase strength of the musculature surrounding the knee (Ingersoll et al., 2008). Despite these invasive and timely interventions, deficits in clinically meaningful measures such as quadriceps strength and patient-reported function, as well as altered mechanical loading at the knee joint, persist for years following surgery. These less than optimal outcomes have reinforced the need for researchers to study and identify the underlying physiologic mechanisms that contribute to impairments after ACLR (Ageberg, 2002).
Beyond well-appreciated musculoskeletal consequences, recent evidence has established that systemic neurological alterations also occur following ACLR (Needle et al., 2017). Specifically, alterations in afferent and reflexive neural excitability have been observed acutely following injury, due to the loss or overactivity of the mechanoreceptors, effusion, and pain originating from the joint (Lepley et al., 2015) In the later stages of injury recovery, abnormal activation and excitability of the descending corticospinal pathways has also been observed (Lepley et al., 2015; Grooms et al., 2017; Kapreli et al., 2009). These systemic changes in peripheral and central nervous system function can render traditional therapeutic approaches ineffective for patients with these impairments, potentially perpetuating poor clinical outcomes (Ingersoll et al., 2008; Needle et al., 2017; Ward et al., 2015).
The corticospinal tract is essential for motor performance. Hence, understanding if unexplored abnormalities are present in this key white matter pathway is critical for elucidating the barriers to neuromuscular recovery following ACLR. In other neurologically impaired patient populations, such as those with multiple sclerosis (MS) or stroke, structural changes in the corticospinal tract evaluated via diffusion tensor imaging (DTI) are evident (Moller et al., 2007) and linked to the recovery of corticospinal excitability and motor function (Jayaram et al., 2012). Additionally, structural white matter changes in anisotropy and diffusivity outcomes have been identified in patients with low back pain (Pijnenburg et al., 2014) and ankle instability (Terada et al., 2019), establishing a connection between musculoskeletal injury and neurostructural reorganization. While ACL injury is not a direct insult to the central nervous system, the disrupted afferent input from the lost ligament mechanoreceptors (Kennedy et al., 1982; Valeriani et al., 1999) and associated motor compensations (Paterno et al., 2007) result in altered motor cortex (Grooms et al., 2017) and corticospinal activity and excitability (Lepley et al., 2015; Lepley et al., 2019). These functional changes observed following injury, if not addressed in therapy, may result in neurostructural changes that further impede optimal voluntary motor control and potentially contribute to chronic dysfunction (Mansour et al., 2013).
Improved understanding of the systemic consequences of peripheral musculoskeletal injuries, including underlying neurostructural adaptions, can aid clinician-scientists in identifying the origins of chronic impairments. This information can profoundly influence the development of more accurate prognostics and more effective rehabilitation interventions to improve motor function, such as motor skill learning and feedback/cognitive training, which have been shown to induce beneficial structural changes in white matter pathways (Hofstetter et al., 2013; Nadkarni et al., 2015; Sampaio-Baptista et al., 2013). Therefore, the purpose of this investigation was to 1) quantify hemispheric differences in structural properties of the corticospinal tract in patients with a history of ACLR, and 2) to assess the relationship between excitability and structure of the corticospinal tract.
2. Materials and methods
Participants were recruited from the Department of Orthopaedic Surgery and University population who were between the ages of 16–35 and had a history of primary, unilateral ACLR. Participants were excluded if they had a history of previous orthopedic surgery (other than ACLR), ligamentous knee injury, or sustained any lower extremity musculoskeletal injury in the last six months. Further exclusion criteria included history of a concussion or head injury in the past 6 months, previous loss of consciousness associated with a concussion, history of a stroke, cranial neurosurgery, migraines, cancer in the brain, a diagnosed neurological or psychiatric disorder, currently taking medications that alter neural activity, or imbedded intracranial metallic clips. Written, informed consent was provided prior to testing, and all procedures were approved by the University's Institutional Review Board. Participants reported to the laboratory for a single testing session in which measures of corticospinal tract structure and then excitability were assessed.
2.1. Corticospinal tract structure
All participants completed a magnetic resonance imaging (MRI) session comprised of anatomical and DTI sequences. DTI outcomes are considered to be sensitive markers of neuropathology, providing information on the microstructural architecture of white matter (Alexander et al., 2007). Lower anisotropy (fractional anisotropy [FA]), and higher diffusivity (axial diffusivity [AD]; radial diffusivity [RD]; mean diffusivity [MD]) values indicate increased water diffusion and loss of coherence of diffusion along the white matter tract (Alexander et al., 2007; Soares et al., 2013), which is interpreted as deleterious changes in white matter microstructure due to pathology, aging, disuse, or prolonged changes in neural activity or excitability (Pijnenburg et al., 2014; Mansour et al., 2013; Berghuis et al., 2019; Fujiyama et al., 2016).
MRI was performed in a 3-Tesla Siemens Prisma scanner using a 20-channel phase array receiver-only head coil (Siemens, Erlangen, Germany). DTI data were acquired using echo-planar imaging (EPI) sequences with opposite phase encoding directions (A-P, P-A) consisting of transaxial images collected in 32 gradient directions at a b-value of 1000s/mm2 with four b = 0 weighted images (TR = 5000 ms, TE = 69 ms, 40 contiguous slices, 2 × 2 × 3-mm voxel, FOV = 256 mm). Structural scans acquired included sagittal 3-D MPRAGE sequence (TR = 2300 ms, TE = 2.26 ms, 176 contiguous slices, 1 × 1 × 1-mm voxel, FOV = 220 mm, flip angle = 7°).
Anatomical scans were aligned to AC-PC, then reconstructed using FreeSurfer software suite (version 6.0) (Dale et al., 1999). Morphometric properties of the brain (e.g. cortical thickness, regional volumes) were obtained through FreeSurfer's segmentation of white matter, gray matter, and cerebrospinal fluid. DTI data were pre-processed using TORTOISE (version 3.1.1) software package for motion, eddy current, and EPI distortion corrections (Irfanoglu et al., 2015; Pierpaoli et al., 2010; Irfanoglu et al., 2018). Bilateral corticospinal tracts were reconstructed (Fig. 1) using TRACULA (in Freesurfer version 6.0), an automated method that reconstructs probabilistic distributions of major white-matter pathways using each individual's native diffusion images (Yendiki et al., 2011). TRACULA yielded diffusivity measures for each hemisphere's respective corticospinal tract, including FA, AD, RD, and MD). The hemisphere of the ACLR injured limb was defined as the hemisphere contralateral to the ACLR knee.
Fig. 1.
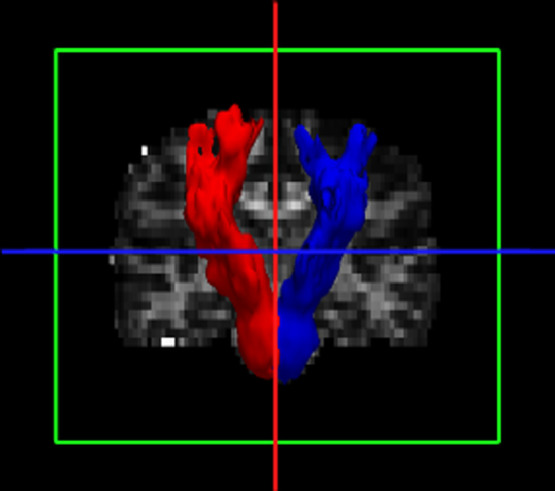
3D structural reconstruction of the right (red) and left (blue) corticospinal tracts from a selected participant (coronal view).
2.2. Corticospinal tract excitability
Corticospinal excitability was assessed via motor evoked potentials (MEP) elicited during transcranial magnetic stimulation (TMS). MEPs represent the magnitude of excitability that is able to be transmitted through the descending corticospinal tract and alpha motor neuron (Groppa et al., 2012). Lower MEP amplitudes indicate less information is transmitted through the corticospinal tract, and ultimately will result in decreased motor output to the muscle (Needle et al., 2017).
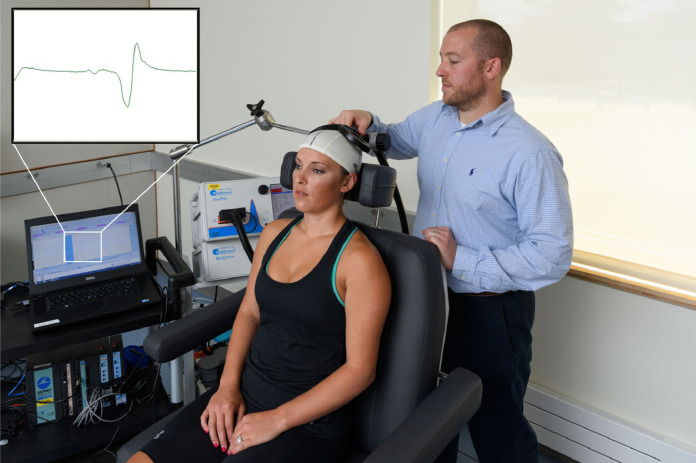
Following standard electromyography preparation, two 10 mm, pre-gelled Ag–AgCl (EL503, BIOPAC Systems Inc., Goleta, CA, USA) disk-shaped surface EMG electrodes, with an inter-electrode difference of 1.75 cm, were positioned over the distal vastus medialis muscle belly (Lepley et al., 2015). During testing, participants were seated in the testing chair (MagVenture Treatment Chair, 9016B008 MagVenture Inc., Atlanta, GA) with their knees and hips at 90° of flexion. A lycra swim cap, with a 0.5 cm grid, was placed on the participant's head to allow for identification of the motor cortex location and origin of corticospinal neurons (Lepley et al., 2015; Norte et al., 2010). A double cone angled TMS coil (D-B80, MagVenture Inc., Atlanta, GA) was positioned over the intersected grid lines and moved in increments of 0.5 cm in anterior-to posterior and medial-to-lateral directions until the optimal stimulating point was detected, which was defined as the location producing the greatest MEP amplitude in the vastus medialis (Fig. 2) (Livingston and Ingersoll, 2008). Once the optimal stimulating point was located, the coil was secured over that spot using a flexible mount (Super Flex Arm, MagVenture Inc.). Active motor thresholds (AMT) were then identified by detecting the lowest TMS intensity required to evoke a measurable (>100 μV) MEP in five out of 10 trials (Lepley et al., 2015; Pietrosimone et al., 2015). Once AMT was established, five MEPs were elicited at 120% of AMT. The five peak-to-peak MEP amplitude values were averaged and normalized to the average of three maximal muscle responses that were elicited at rest immediately following TMS testing using peripheral electrical nerve stimulation (Kapreli et al., 2009). During testing, participants generated an isometric knee extension contraction of 10% of their maximal muscle force production, which was objectively assessed and monitored by the investigator via a belt stabilized handheld dynamometer with verbal feedback provided to the participant during contraction (micro FET; Hoggann Scientific LLC, West Jordan UT) (Lepley et al., 2019).
Fig. 2.
Set-up for corticospinal excitability testing and screen shot of motor evoked potential elicited via transcranial magnetic stimulation.
2.3. Statistical analysis
Differences in corticospinal tract structural and excitability outcomes between the hemispheres of the ACLR injured and non-injured limbs were assessed using paired samples t-tests and Cohen's d effect sizes were also calculated (Cohen, 1977). Separate Pearson Product Moment correlations were conducted to investigate the relationship between outcomes of corticospinal tract structure (volume, FA, AD, RD, MD) and excitability (MEP) of the ACLR injured limb. Correlation coefficients (r) were classified as weak (0–0.4), moderate (0.4–0.7), or strong (0.7–1.0) (Cohen, 1977). All alpha levels were set a-priori at p ≤ 0.05 and all statistical analyses were performed using SPSS software version 26.0 (IBM, Armonk, NY, USA).
3. Results
A total of ten participants with a history of ACLR were included in this study. Demographic variables are reported in Table 1.
Table 1.
Demographic variables.
| N | 10 (6 female/4 male) |
|---|---|
| Age (yrs) | 22.6 ± 1.9 |
| Height (cm) | 166.3 ± 7.5 |
| Mass (kg) | 65.4 ± 12.6 |
| Months from surgery | 70.0 ± 23.6 |
| IKDC | 84.9 ± 9.9 |
| Tegner Activity level | 7.2 ± 1.4 |
Abbreviations: IKDC, International Knee Documentation Committee Self-Reported Questionnaire.
Means and standard deviations of structural and excitability measures of the corticospinal tract are displayed in Table 2. The hemisphere of the ACLR injured limb demonstrated lower volume, lower FA, and higher MD compared to the hemisphere of the non-injured limb (p < 0.05), indicating altered white matter structure with increased general diffusion and loss of coherence on diffusion along the corticospinal tract direction (Soares et al., 2013). These differences also demonstrated strong effect sizes (Table 2). No differences were detected for AD or RD measures (p > 0.05). Additionally, MEPs were smaller for the hemisphere of the ACLR injured limb, signifying a reduction in excitability through the corticospinal tract to the quadriceps muscle (p < 0.05) (Groppa et al., 2012).
Table 2.
Means and standard deviations for structural and excitability measures.
| Hemisphere of ACLR injured limb | Hemisphere of non-injured limb | T | p | ES (95% CI) | ||
|---|---|---|---|---|---|---|
| CST structure | ||||||
| Volume (voxels - 2 mm) | 567.1 ± 75.3 | 659.7 ± 64.3 | −3.64 | 0.005* | −1.27 (−2.23; −0.31) | |
| FA | 0.49 ± 0.01 | 0.53 ± 0.02 | −2.72 | 0.02* | −2.42 (−3.58; −1.27) | |
| AD | 1.17×10−3 ± 0.37×10−3 | 1.17×10−3 ± 0.02×10−3 | 1.01 | 0.34 | 0.00 (−0.88; 0.88) | |
| RD | 4.97×10−4 ± 0.16×10−4 | 5.00×10−4 ± 0.15×10−4 | −0.74 | 0.47 | −0.19 (−1.06; 0.69) | |
| MD | 7.58×10−4 ± 0.35×10−4 | 7.23×10−4 ± 0.10×10−4 | 3.14 | 0.01* | 1.30 (0.34; 2.27) | |
| CST excitability | ||||||
| MEP | 0.013 ± 0.007 | 0.028 ± 0.010 | −2.26 | 0.04* | −1.66 (−2.68; −0.65) | |
Abbreviations: CST, corticospinal tract; FA, fractional anisotropy; AD, axial diffusivity; RD, radial diffusivity; MD, mean diffusivity; MEP, motor evoked potential; ES, effect size; CI, confidence interval.
Significant difference between hemispheres (p < 0.05).
Correlation analyses (Table 3) revealed a strong, positive correlation between corticospinal tract volume and MEP of the ACLR injured limb, indicating that smaller corticospinal tract volume is related to lower corticospinal excitability. No other significant correlations were identified.
Table 3.
Results of correlation analyses for the ACLR injured limb.
| MEP | |
|---|---|
| Volume | r = 0.890; p = 0.001* |
| FA | r = −0.08, p = 0.81 |
| AD | r = 0.09, p = 0.79 |
| RD | r = 0.13, p = 0.72 |
| MD | r = 0.11, p = 0.75 |
Abbreviations: FA, fractional anisotropy; AD, axial diffusivity; RD, radial diffusivity; MD, mean diffusivity; MEP, motor evoked potential.
Significant correlation between volume and MEP at p = 0.001.
4. Discussion
This investigation sought to assess hemispheric differences in structural properties of the corticospinal tract in patients with a history of ACLR, while also establishing the relationship between structure and excitability of the corticospinal tract. Our results demonstrate that the hemisphere of the ACLR limb displayed smaller corticospinal tract volume, lower FA, and higher MD values relative to the hemisphere of the non-injured limb. Smaller corticospinal tract volume was also strongly correlated with lesser excitability. These findings suggest that ACLR patients demonstrate asymmetry in structural properties of the corticospinal tract that may influence the recovery of motor function following injury and surgical reconstruction.
Neurological impairments after musculoskeletal injury are commonly evaluated in the literature by changes in the excitability of the corticospinal tract via TMS (Needle et al., 2017). However, a major limitation in this methodology is that it is unable to identify the underlying factors responsible for the reduction in corticospinal drive. Excitability of the corticospinal tract (i.e. the ability of the corticospinal tract to transmit descending motor signals) is undoubtedly influenced by the structural architecture of the white matter axons that are transmitting that signal (Groppa et al., 2012), which was corroborated by the significant relationship between excitability and volume in the current study (Table 3). Evaluating corticospinal excitability in combination with non-invasive DTI allows for the evaluation of the structural make-up of white matter neural tracts and its influence on corticospinal drive to the muscle. This combined methodology represents a promising technique to help elucidate the underlying neurophysiological and neuroanatomical mechanisms that impede voluntary motor control in these patients, as well as identify patients that are experiencing CNS dysfunction during recovery.
There is limited cadaveric evidence that the corticospinal tract of the left hemisphere is larger than that of the right (Rademacher et al., 2001). However, the balance of in vivo data suggests that healthy individuals display symmetrical anatomical and structural properties of the corticospinal tract, even when accounting for handedness or laterality (Reich et al., 2006; White et al., 1997; Lee et al., 2016; Dalamagkas et al., 2019; Seizeur et al., 2014). Therefore, assessment of white matter asymmetries is often of clinical value, commonly used as a marker of traditional neurological disease (Reich et al., 2006). The participants with ACLR in the current study demonstrated reduced corticospinal tract volume of the hemisphere of the ACLR injured limb compared to the non-injured side, indicating potential atrophy of the corticospinal tract serving the injured limb. This volumetric asymmetry likely developed over time due to reductions in afferent signaling, reduced motor output, and motor inhibition that is present in these patients (Lepley et al., 2019; Luc-Harkey et al., 2017). Theoretical models have suggested that the human body will limit activation of, or even specifically inhibit, motor tracts to prevent unwanted movement of an injured joint (Ingersoll et al., 2008; Needle et al., 2017; Ward et al., 2015; Pietrosimone et al., 2012). This is hypothesized to be a protective mechanism to avoid painful and unnecessary limb movement. It is plausible that these neurostructural changes occur as an adaptation to the reduced signaling coming from the motor cortex, which helps to explain the strong correlation detected between corticospinal tract structure and excitability in this data set (Table 3). Unfortunately, due to the cross-sectional nature of this study, we are unable to determine cause and effect, or when these changes occur during the recovery process. It is important to note that the participants in the current study were on average 70.0 ± 23.6 months post-surgery (range: 66.6 – 96.5 months), which is nearly six years removed from surgery. In agreement with the excitability literature, corticospinal deficits appear to be progressive over time (Needle et al., 2017; Lepley et al., 2015), with larger deficits the further a patient is removed from the injury. This may help to explain our findings, that almost six years of reduced signaling from the motor cortex post-ACLR would allow for negative structural adaptations in the corticospinal tract. However, larger scale longitudinal investigations are needed to confirm this finding.
The interpretation of DTI outcomes can be complex (Jones et al., 2013). DTI measurements assess water diffusion through a tissue, which is highly sensitive to differences in the microstructural architecture of cellular membranes. The sensitivity of these outcomes creates an effective method for detecting microscopic tissue properties, but results must be interpreted carefully. For instance, FA is a highly sensitive outcome for assessing overall structural changes in axonal tissue, but is a relatively non-specific biomarker of neuropathology, whereas MD is specific to the degree of organization and myelination in the neural tracts (Alexander et al., 2007; Soares et al., 2013). One strength of the current study is the reporting of multiple DTI outcomes, including FA, AD, RD and MD, which help to better characterize the collective microstructural differences of the tissue. In the current study, we detected smaller FA in the hemisphere of the injured limb of patients with ACLR (Table 2). This corroborates recent work by Terada et al. (2019) who also discovered structural white matter differences in patients following peripheral musculoskeletal injury, with smaller FA values of the superior cerebellar peduncle in patients with a history of ankle sprains compared to controls. Another study by Lewis et al. (2018) reported reduced FA of the midbrain and corpus callosum in patients with knee osteoarthritis compared to controls. Similarly, others have also observed reduced FA values in a wide range of pathologies that negatively impair the nervous system and motor function, including ischemic stroke and demyelination (Reich et al., 2006). Interestingly, Stinear et al. (2007) demonstrated that corticospinal tract structure and FA values were related to motor recovery following stroke, as FA values predicted the potential for clinical improvement in MEPs and clinical functional scores (Fugl-Meyer upper limb score). In a similar manner, future work should explore the potential for neurostructural properties to predict intervention response in those with musculoskeletal trauma.
In addition to FA abnormalities, we also detected higher MD values in the hemisphere of the injured limb in patients with ACLR (Table 2), which indicates potential differences in myelination of corticospinal axons (Soares et al., 2013; Swaiman's pediatric neurology, 2018). Higher MD has also been detected in a variety of motor diseases, including chronic stroke patients, MS, neurodegenerative diseases, and inflammation and edema (Reich et al., 2006). Notably, in MS patients, MD of the corticospinal tract has been correlated with disability scores (pyramidal function system score) that are related to motor function (Lin et al., 2005), and others have found a significant relationship between corticospinal tract structure and hip and ankle strength in MS patients (Reich et al., 2008). Although stroke and other neurological disorders present a direct insult to the brain, and ACL injuries influence neuroplasticity through disruption of afferent feedback and motor compensations\disuse, these collective studies indicate that motor impairments are connected to changes observed in corticospinal white matter. Though function was not explicitly assessed in our cohort, there is a documented link between corticospinal excitability and restoration of clinical function (quadriceps muscle strength, self-reported outcomes) in patients with ACLR (Pietrosimone et al., 2013; Lepley et al., 2014; Norte et al., 2018). The published data from other neurologically impaired populations help to establish a relationship between clinical outcomes and DTI derived values of corticospinal tract structure in pathological populations, work that will need to be continued and verified in ACLR cohorts.
Identifying these systemic changes in nervous system function can help clinicians understand the complex effects that these injuries have on both the neurological and musculoskeletal systems. Importantly, this new information can help researchers and clinicians to identify the origins of clinical impairments for the purpose of developing novel evidence-based therapeutic approaches to improve motor function. DTI assessments represent a strong technique to identify indicators of disease and monitor the effectiveness of treatments in neuropathologies (Alexander et al., 2007). Therefore, more research is warranted to establish the effectiveness of these techniques to predict recovery of function and response to treatment in ACLR and other musculoskeletal patients. More specifically, future research should focus on the extent to which these neuroanatomical and neurophysiological alterations contribute to functional outcomes, including sensorimotor function and balance, muscle strength, lower extremity biomechanics, and self-reported function. To that end, previous work has shown that neurostructural outcomes can be improved through specific types of interventions. Rather than traditional strength or endurance training, motor skill learning, feedback/cognitive training, and coordination interventions have all shown to induce beneficial structural changes in task-relevant white matter pathways (Hofstetter et al., 2013; Nadkarni et al., 2015; Sampaio-Baptista et al., 2013). Future work in ACLR populations can help establish the effectiveness of these interventions on improving clinical, neurophysiological, and neurostructural outcomes.
4.1. Limitations
Although this study detected differences in corticospinal tract structure and excitability in a cohort of participants with ACLR, the cross-sectional nature of this study makes it difficult to determine how these adaptations occur overtime throughout the injury and recovery processes. Future longitudinal, prospective investigations are warranted to understand the evolution of neural changes in this population and how they ultimately influence clinical outcomes. Our results were also discovered in a general population of participants with ACLR, which included mixed sexes, graft types, concomitant meniscal injury, and varying times from surgery. Future studies should control for these confounding variables to understand the effect of specific injury characteristics on neural consequences of ACL injury.
Lastly, this study did not include a matched healthy control group; therefore, we are unable to determine what effect ACL injury had on the neural function and structure of the non-injured limb. There is evidence (Lepley et al., 2015; Pietrosimone et al., 2015) of bilateral neural deficits following ACL injury, in which the non-injured limb demonstrates alterations in corticospinal excitability, and clinical deficits in muscle strength compared to healthy control groups. It is likely that bilateral decreases in corticospinal excitability could initiate bilateral changes in corticospinal tract structure, or help to identify differences in mechanisms contributing to corticospinal excitability deficits between limbs, such as intracortical inhibition (Luc-Harkey et al., 2017). Future work should look to incorporate an appropriately matched healthy control group, or utilize longitudinal study designs as mentioned above, to help better understand the negative consequences joint injury has on non-injured limb function.
5. Conclusions/Implications
Participants with a history of ACLR displayed smaller corticospinal tract volume, lower FA, and higher MD values in the hemisphere of their ACLR limb compared to their contralateral side. Smaller corticospinal tract volume was also strongly correlated with lesser excitability. These findings suggest that ACLR patients demonstrate asymmetry in structural properties of the corticospinal tract that may influence the recovery of motor function following surgical reconstruction.
Funding
This research was supported by a Faculty Seed Grant from the University of Connecticut's Brain Imaging Research Center (BIRC).
CRediT authorship contribution statement
Adam S. Lepley: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Monica T. Ly: Data curation, Formal analysis, Methodology, Software, Visualization, Writing - original draft, Writing - review & editing. Dustin R. Grooms: Conceptualization, Data curation, Investigation, Methodology, Writing - review & editing. Jeffery M. Kinsella-Shaw: Conceptualization, Funding acquisition, Investigation, Methodology, Writing - review & editing. Lindsey K. Lepley: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Writing - review & editing.
Declaration of Competing Interest
The Authors declare that there is no conflict of interest.
Acknowledgements
The authors would like to acknowledge Elisa Medeiros (MRI services manager, University of Connecticut's Brain Imaging Research Center) for their assistance and support in MRI data collection and design.
References
- Ingersoll C.D., Grindstaff T.L., Pietrosimone B.G., Hart J.M. Neuromuscular consequences of anterior cruciate ligament injury. Clin. Sports Med. 2008;27(3):383–404. doi: 10.1016/j.csm.2008.03.004. vii. [DOI] [PubMed] [Google Scholar]
- Ageberg E. Consequences of a ligament injury on neuromuscular function and relevance to rehabilitation - using the anterior cruciate ligament-injured knee as model. J Electromyogr. Kinesiol. 2002;12(3):205–212. doi: 10.1016/s1050-6411(02)00022-6. [DOI] [PubMed] [Google Scholar]
- Needle A.R., Lepley A.S., Grooms D.R. Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med. 2017;47(7):1271–1288. doi: 10.1007/s40279-016-0666-y. [DOI] [PubMed] [Google Scholar]
- Lepley A.S., Gribble P.A., Thomas A.C., Tevald M.A., Sohn D.H., Pietrosimone B.G. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand. J. Med. Sci. Sports. 2015;25(6):828–839. doi: 10.1111/sms.12435. PMID: 25693627. [DOI] [PubMed] [Google Scholar]
- Grooms D.R., Page S.J., Nichols-Larsen D.S., Chaudhari A.M., White S.E., Onate J.A. Neuroplasticity associated with anterior cruciate ligament reconstruction. J. Orthop Sports Phys. Ther. 2017;47(3):180–189. doi: 10.2519/jospt.2017.7003. [DOI] [PubMed] [Google Scholar]
- Kapreli E., Athanasopoulos S., Gliatis J. Anterior cruciate ligament deficiency causes brain plasticity: a functional MRI study. Am. J. Sports Med. 2009;37(12):2419–2426. doi: 10.1177/0363546509343201. [DOI] [PubMed] [Google Scholar]
- Ward S., Pearce A.J., Pietrosimone B., Bennell K., Clark R., Bryant A.L. Neuromuscular deficits after peripheral joint injury: a neurophysiological hypothesis. Muscle Nerve. 2015;51(3):327–332. doi: 10.1002/mus.24463. [DOI] [PubMed] [Google Scholar]
- Moller M., Frandsen J., Andersen G., Gjedde A., Vestergaard-Poulsen P., Ostergaard L. Dynamic changes in corticospinal tracts after stroke detected by fibretracking. J Neurol. Neurosurg. Psychiatry. 2007;78(6):587–592. doi: 10.1136/jnnp.2006.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram G., Stagg C.J., Esser P., Kischka U., Stinear J., Johansen-Berg H. Relationships between functional and structural corticospinal tract integrity and walking post stroke. Clin. Neurophysiol. 2012;123(12):2422–2428. doi: 10.1016/j.clinph.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenburg M., Caeyenberghs K., Janssens L. Microstructural integrity of the superior cerebellar peduncle is associated with an impaired proprioceptive weighting capacity in individuals with non-specific low back pain. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M., Johnson N., Kosik K., Gribble P. Quantifying brain white matter microstructure of people with lateral ankle sprain. Med. Sci. Sports Exerc. 2019;51(4):640–646. doi: 10.1249/MSS.0000000000001848. [DOI] [PubMed] [Google Scholar]
- Kennedy J.C., Alexander I.J., Hayes K.C. Nerve supply of the human knee and its functional importance. Am. J. Sports Med. 1982;10(6):329–335. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- Valeriani M., Restuccia D., Di Lazzaro V., Franceschi F., Fabbriciani C., Tonali P. Clinical and neurophysiological abnormalities before and after reconstruction of the anterior cruciate ligament of the knee. Acta Neurol. Scand. 1999;99(5):303–307. doi: 10.1111/j.1600-0404.1999.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Paterno M.V., Ford K.R., Myer G.D., Heyl R., Hewett T.E. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin. J. Sport Med. 2007;17(4):258–262. doi: 10.1097/JSM.0b013e31804c77ea. [DOI] [PubMed] [Google Scholar]
- Lepley A.S., Grooms D.R., Burland J.P., Davi S.M., Kinsella-Shaw J.M., Lepley L.K. Quadriceps muscle function following anterior cruciate ligament reconstruction: systemic differences in neural and morphological characteristics. Exp. Brain Res. 2019;237(5):1267–1278. doi: 10.1007/s00221-019-05499-x. [DOI] [PubMed] [Google Scholar]
- Mansour A.R., Baliki M.N., Huang L. Brain white matter structural properties predict transition to chronic pain. Pain. 2013;154(10):2160–2168. doi: 10.1016/j.pain.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter S., Tavor I., Tzur Moryosef S., Assaf Y. Short-term learning induces white matter plasticity in the fornix. J. Neurosci. 2013;33(31):12844–12850. doi: 10.1523/JNEUROSCI.4520-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni N.K., Perera S., Studenski S.A., Rosano C., Aizenstein H.J., VanSwearingen J.M. Callosal hyperintensities and gait speed gain from two types of mobility interventions in older adults. Arch. Phys. Med. Rehabil. 2015;96(6):1154–1157. doi: 10.1016/j.apmr.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C., Khrapitchev A.A., Foxley S. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 2013;33(50):19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J.M., Marques P., Alves V., Sousa N. A Hitchhiker's guide to diffusion tensor imaging. Front. Neurosci. 2013;7:31. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis K.M.M., Fagioli S., Maurits N.M. Age-related changes in brain deactivation but not in activation after motor learning. NeuroImage. 2019;186:358–368. doi: 10.1016/j.neuroimage.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Fujiyama H., Van Soom J., Rens G. Age-Related changes in frontal network structural and functional connectivity in relation to bimanual movement control. J. Neurosci. 2016;36(6):1808–1822. doi: 10.1523/JNEUROSCI.3355-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Irfanoglu M.O., Modi P., Nayak A., Hutchinson E.B., Sarlls J., Pierpaoli C. DR-BUDDI (Diffeomorphic registration for blip-up blip-down diffusion imaging) method for correcting echo planar imaging distortions. NeuroImage. 2015;106:284–299. doi: 10.1016/j.neuroimage.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C., Walker L., Irfanoglu M.O. TORTOISE: an integrated software package for processing of diffusion MRI data. Paper presented at: ISMRM 18th Annual Meeting; Stockholm, Sweden; 2010. [Google Scholar]
- Irfanoglu M.O., Nayak A., Jenkins J., Pierpaoli C. TORTOISEv3: improvements and new features of the NIH diffusion MRI processing pipeline. Paper presented at: ISMRM 25th Annual Meeting; Honolulu, HI; 2018. [Google Scholar]
- Yendiki A., Panneck P., Srinivasan P. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinform. 2011;5:23. doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S., Oliviero A., Eisen A. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2012;123(5):858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepley A.S., Bahhur N.O., Murray A.M., Pietrosimone B.G. Quadriceps corticomotor excitability following an experimental knee joint effusion. Knee Surg. Sports Traumatol. Arthrosc. 2015;23(4):1010–1017. doi: 10.1007/s00167-013-2816-1. [DOI] [PubMed] [Google Scholar]
- Norte G.E., Pietrosimone B.G., Hart J.M., Hertel J., Ingersoll C.D. Relationship between transcranial magnetic stimulation and percutaneous electrical stimulation in determining the quadriceps central activation ratio. Am. J. Phys. Med. Rehabil. 2010 doi: 10.1097/PHM.0b013e3181f1c00e. [DOI] [PubMed] [Google Scholar]
- Livingston S.C., Ingersoll C.D. Intra-rater reliability of a transcranial magnetic stimulation technique to obtain motor evoked potentials. Int. J. Neurosci. 2008;118(2):239–256. doi: 10.1080/00207450701668020. [DOI] [PubMed] [Google Scholar]
- Pietrosimone B.G., Lepley A.S., Ericksen H.M., Clements A., Sohn D.H., Gribble P.A. Neural excitability alterations after anterior cruciate ligament reconstruction. J. Athl. Train. 2015;50(6):665–674. doi: 10.4085/1062-6050-50.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Academic Press; New York: 1977. Statistical Power Analysis For Behavioral Sciences. [Google Scholar]
- Rademacher J., Burgel U., Geyer S. Variability and asymmetry in the human precentral motor system: a cytoarchitectonic and myeloarchitectonic brain mapping study. Brain. 2001;124(Pt 11):2232–2258. doi: 10.1093/brain/124.11.2232. [DOI] [PubMed] [Google Scholar]
- Reich D.S., Smith S.A., Jones C.K. Quantitative characterization of the corticospinal tract at 3T. AJNR Am. J. Neuroradiol. 2006;27(10):2168–2178. [PMC free article] [PubMed] [Google Scholar]
- White L.E., Andrews T.J., Hulette C. Structure of the human sensorimotor system. II: Lateral symmetry. Cereb. Cortex. 1997;7(1):31–47. doi: 10.1093/cercor/7.1.31. [DOI] [PubMed] [Google Scholar]
- Lee D.H., Lee D.W., Han B.S. Symmetrical location characteristics of corticospinal tract associated with hand movement in the human brain: a probabilistic diffusion tensor tractography. Medicine (Baltimore) 2016;95(15):e3317. doi: 10.1097/MD.0000000000003317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalamagkas K., Tsintou M., Rathi Y. Individual variations of the human corticospinal tract and its hand-related motor fibers using diffusion MRI tractography. Brain Imaging Behav. 2019 doi: 10.1007/s11682-018-0006-y. PMID: 30617788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizeur R., Magro E., Prima S., Wiest-Daessle N., Maumet C., Morandi X. Corticospinal tract asymmetry and handedness in right- and left-handers by diffusion tensor tractography. Surg. Radiol. Anat. 2014;36(2):111–124. doi: 10.1007/s00276-013-1156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luc-Harkey B.A., Harkey M.S., Pamukoff D.N. Greater intracortical inhibition associates with lower quadriceps voluntary activation in individuals with ACL reconstruction. Exp. Brain Res. 2017;235(4):1129–1137. doi: 10.1007/s00221-017-4877-8. [DOI] [PubMed] [Google Scholar]
- Pietrosimone B.G., McLeod M.M., Lepley A.S. A theoretical framework for understanding neuromuscular response to lower extremity joint injury. Sports Health. 2012;4(1):31–35. doi: 10.1177/1941738111428251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Knosche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Lewis G.N., Parker R.S., Sharma S., Rice D.A., McNair P.J. Structural brain alterations before and after total knee arthroplasty: a longitudinal assessment. Pain Med. 2018;19(11):2166–2176. doi: 10.1093/pm/pny108. [DOI] [PubMed] [Google Scholar]
- Stinear C.M., Barber P.A., Smale P.R., Coxon J.P., Fleming M.K., Byblow W.D. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Swaiman's Pediatric Neurology . 6th ed. Elsevier; New York: 2018. Principles and Practice. [Google Scholar]
- Lin X., Tench C.R., Morgan P.S., Niepel G., Constantinescu C.S. 'Importance sampling' in MS: use of diffusion tensor tractography to quantify pathology related to specific impairment. J. Neurol. Sci. 2005;237(1–2):13–19. doi: 10.1016/j.jns.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Reich D.S., Zackowski K.M., Gordon-Lipkin E.M. Corticospinal tract abnormalities are associated with weakness in multiple sclerosis. AJNR Am. J. Neuroradiol. 2008;29(2):333–339. doi: 10.3174/ajnr.A0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrosimone B.G., Lepley A.S., Ericksen H.M., Gribble P.A., Levine J. Quadriceps strength and corticospinal excitability as predictors of disability after anterior cruciate ligament reconstruction. J. Sport Rehabil. 2013;22(1):1–6. doi: 10.1123/jsr.22.1.1. [DOI] [PubMed] [Google Scholar]
- Lepley A.S., Ericksen H.M., Sohn D.H., Pietrosimone B.G. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. Knee. 2014;21(3):736–742. doi: 10.1016/j.knee.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Norte G.E., Hertel J.N., Saliba S.A., Diduch D.R., Hart J.M. Quadriceps and patient-reported function in ACL-Reconstructed patients: a principal component analysis. J. Sport Rehabil. 2018:1–9. doi: 10.1123/jsr.2017-0080. [DOI] [PubMed] [Google Scholar]