Highlights
-
•
Low CD4 nadir was associated with widespread cortical thinning in HIV+ adults, especially in the frontal and temporal regions.
-
•
Worse neurocognitive function was associated with frontal cortical thinning, even in the absence of HIV-associated neurocognitive disorders diagnosis.
-
•
Global mean cortical thickness correlates with CD4 nadir.
Keywords: Atrophy, Cortical thickness, CD4, HIV, CD4 nadir
Abstract
Background
The history of immune suppression, especially CD4 nadir, has been shown to be a strong predictor of HIV-associated neurocognitive disorders (HAND). However, the potential mechanism of this association is not well understood.
Methods
High resolution structural MRI images and neuropsychological data were obtained from fifty-nine HIV+ adults (mean age, 56.5 ± 5.8) to investigate the correlation between CD4 nadir and cortical thickness.
Results
Low CD4 nadir was associated with widespread cortical thinning, especially in the frontal and temporal regions, and global mean cortical thickness correlated with CD4 nadir. In addition, worse global neurocognitive function was associated with bilateral frontal cortical thinning, and the association largely persisted (especially in the left frontal cortex) in the subset of participants who did not meet HAND criteria.
Conclusions
These results suggest that low CD4 nadir may be associated with widespread neural injury in the brain, especially in the frontal and temporal regions. The diffuse neural injury might contribute to the prevalence and the phenotypes of HAND, as well as the difficulty treating HAND due to a broad network of brain regions affected. Low CD4 nadir related neural injury to the frontal cortex might contribute to subtle neurocognitive impairment/decline, even in the absence of HAND diagnosis.
1. Introduction
Despite largely successful viral suppression and immune recovery with modern antiretroviral treatment in the era of combination antiretroviral therapy (cART), HIV-associated neurocognitive disorders (HAND) remain highly prevalent in people with HIV (PWH) (Heaton et al., 2010; Sacktor et al., 2016). Several risk factors have been identified and proposed to contribute to HAND in the cART era, including CD4 nadir (the lowest ever lymphocyte CD4 count), which has been shown to be a strong predictor of neurocognitive impairment (Ellis et al., 2011; Heaton et al., 2010; Muñoz-Moreno et al., 2008; Robertson et al., 2007; Valcour et al., 2006). The impact of low CD4 nadir on neurocognitive function is generally referred to as an important “legacy event” effect. That is, the depth of immune suppression associated with low CD4 nadir (i.e., less than 200 cells/μl) may have caused irreversible neural injury that may persist years later. However, the neuropathogenesis of low CD4 nadir effect on brain function and neurocognitive impairment is not well understood and remains to be elucidated.
Using structural MRI techniques, several previous studies have provided evidence suggesting that low CD4 nadir might be associated with increased brain atrophy, including CSF/ventricular expansion, white matter (WM) and gray matter (GM) volume reduction, and cortical thinning (Clark et al., 2015; Cohen et al., 2010; Gongvatana et al., 2014; Guha et al., 2016; Hua et al., 2013; Jernigan et al., 2011; MacDuffie et al., 2018; Pfefferbaum et al., 2012; Sanford et al., 2017; Tate et al., 2011; Tesic et al., 2018). A recent longitudinal study further suggested that lower CD4 nadir might be associated with faster brain atrophy throughout the course of HIV disease (Nir et al., 2019). However, the findings need to be extended and additional research is necessary. These previous studies relied on neuroimaging techniques that have high sensitivity but low spatial specificity, such as total GM volume, global mean cortical thickness, and/or CSF/ventricular expansion, therefore, the spatial profile of the neural injury associated with low CD4 nadir remains largely unknown. In addition, the effect size is usually small/modest in the studies that have found an association between low CD4 nadir and brain atrophy, including marginal but non-significant findings (Chu et al., 2018; Clark et al., 2018).
In brain imaging research, the majority of MRI studies have relied on voxel-wise or similar approaches (i.e., vertex-wise approach for cortical thickness), which have the spatial specificity to relate atrophy to specific brain region(s). However, to the best of our knowledge, there is not a single published study that has found a significant association between brain atrophy and low CD4 nadir using a voxel- or vertex-wise approach. This could be due to two different scenarios: i) the brain atrophy associated with low CD4 nadir is indeed confined to local area(s) in each individual patient, but different brain areas are affected differently between individuals, i.e., low CD4 nadir is associated with atrophy in the left inferior frontal gyrus in one patient, versus the right middle temporal gyrus in another patient. The high heterogeneity in spatial locations would make it statistically impossible to detect a correlation between brain atrophy and low CD4 nadir with a voxel-/vertex-wise approach; or ii) the effect of low CD4 nadir on brain structure is diffuse and widespread, but the effect size at each voxel/vertex is small, thus it would be difficult to detect with the “standard” statistical parametric map (SPM) framework, which has limitations in detecting signal across voxels/vertices (Norman et al., 2006; Smith and Nichols, 2009). Recently, to better detect true signal across voxels (or vertices for surface-based analysis) while controlling for false positives, several new techniques have been developed, including the “threshold-free cluster enhancement” (TFCE) technique (Smith and Nichols, 2009). TFCE is a permutation-based non-parametric technique that is designed to optimize the detection of signal across voxels (even when the signal is diffuse and low in amplitude) and has been shown to have a higher sensitivity than conventional parametric approaches in detecting true signal across voxels/vertices (Smith and Nichols, 2009). One limitation of the TFCE technique is a high demand for computing power and the need for rather lengthy computational time.
In the present study, we investigated the relationship between low CD4 nadir and brain atrophy using the TFCE technique, with a focus on cortical thickness: previous studies have suggested that CD4 nadir might be more associated with cortical atrophy, whereas current viral load might be more associated with subcortical atrophy (Cohen et al., 2010; Guha et al., 2016). We predicted that if the impact of low CD4 nadir on brain atrophy is indeed confined to different local area(s) in different patients, we might not be able to obtain any significant results with the vertex-wise approach due to the high heterogeneity in spatial locations. By contrast, if low CD4 nadir is associated with diffuse and widespread atrophy that is relatively weak due to a small effect size, but the brain areas affected are relatively consistent across patients (i.e., at the same or similar anatomical locations), we predicted that the TFCE technique might be able to reveal low CD4 nadir associated cortical thinning across a broad network of brain regions.
2. Methods
2.1. Participants
Fifty-nine PWH from the greater Washington D.C. metropolitan area participated in the study. A telephone screening interview followed by an onsite screening visit was used to exclude participants who met at least one of the following exclusion criteria: younger than 41 or older than 70 years old; MRI contraindications such as metal implants or claustrophobia; less than eight years of education; inability to speak or understand English; illicit substance use within the previous three months (screened with urine toxicology tests); and other confounding factors such as major psychiatric disorders, stroke, and other non-HIV neurological disorders. Written informed consent approved by the Institutional Review Board at Georgetown University Medical Center was obtained prior to enrollment. Blood specimens were collected to verify viral load and current CD4 counts. Medical data and other comorbidities such as substance abuse were assessed. CD4 nadir and estimated duration of HIV infection were collected through self-report with each participant — and previous studies have shown that self-reported CD4 nadir is largely accurate and strongly correlates with actual medical records (if available) (Buisker et al., 2015; Ellis et al., 2011). Five participants could not recall their CD4 nadirs, and were excluded from the analyses involving CD4 nadir (n = 54).
2.2. Neuropsychological testing
Participants underwent a comprehensive neuropsychological assessment comprising 12 standardized tests that assessed seven neurocognitive domains, including speeded information processing, verbal fluency, learning, memory, executive function, working memory, and motor abilities. Additionally, participants completed the Lawton and Brody Activities of Daily Living questionnaire (1969) in which they self-reported any declines on everyday tasks (e.g., managing finances, managing medications, etc.). A global deficit score (GDS) was computed for each participant to determine neurocognitive impairment based on a previously published algorithm (Blackstone et al., 2012; Carey et al., 2004), with a higher GDS indicating a worse global neurocognitive function. The GDS was then used for HAND diagnosis using the standard Frascati guideline (Antinori et al., 2007). All participants were included in the GDS analysis (n = 59).
2.3. MRI data acquisition
MRI data was acquired at the Center for Functional and Molecular Imaging at Georgetown University Medical Center using a 3-Tesla Siemens Magnetom Tim Trio whole-body scanner (Erlangen, Germany) and a manufacturer supplied 12-channel phased array head coil. Structural images were acquired with a 3D T1-weighted sequence (MPRAGE, magnetization prepared rapid acquisition gradient echo) with the following parameters: TR/TE = 1900/2.52 ms, TI = 900 ms, flip angle = 9°, 160 contiguous 1 mm sagittal slices, FoV = 256 × 160 × 256 mm, 1 mm3 resolution.
2.4. MRI data preprocessing and analysis
MRI data preprocessing and analysis was performed using the SPM12 software package (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and the CAT12 toolbox release 12.6, r1434 (http://dbm.neuro.uni-jena.de/cat/) in MATLAB (release 2017b, The MathWorks, Inc., Natick, Massachusetts, United States).
Standard preprocessing procedures were used, including correction for bias-field inhomogeneities, denoising, skull-stripping, segmentation, and corrections for partial volume estimation. Segmentation output comprised GM, WM, and CSF tissue class volumes for each participant. Tissue class volumes were then used to spatially normalize all images to the template in standard Montreal Neurologic Institute (MNI) space. Cortical thickness was estimated using the projection-based thickness algorithm implemented in the CAT12 toolbox with default parameters (Dahnke et al., 2013). Spherical mapping of the cortical surface (Yotter et al., 2011) was used for inter-participant registration of cortical surfaces for each brain hemisphere, followed by resampling and 15 mm full width at half maximum (FWHM) Gaussian smoothing of vertex values before being used for statistical analyses. For GM volume-based analyses, normalized GM voxel values were modulated to preserve voxel-wise estimates of the absolute amount of tissue, then smoothed using a Gaussian kernel of 8 mm FWHM prior to statistical analysis.
In order to determine regions of cortical thickness that correlate with HIV-disease (including CD4 nadir, current CD4, and disease duration), multiple regression analyses were performed within the CAT12 software general linear model (GLM) interface, with age, education, sex, and race as four nuisance covariates, and CD4 nadir, current CD4, and disease duration as three dependent variables in a single model. The correlation between GDS and cortical thickness was also investigated, using age, education, sex, and race as nuisance covariates. Non-parametric statistical significance testing was run using 5000 resampling steps in the TFCE toolbox (Smith and Nichols, 2009), reporting results that survive an FWE vertex-level multiple comparisons correction of p < 0.05. A similar correlation was calculated using GM volume, with the addition of total intracranial volume (TIV) as a nuisance covariate.
In addition, global mean cortical thickness, total GM volume, total WM volume, total cortical and subcortical GM volume, and TIV were extracted and their relationship with CD4 nadir, current CD4, and disease duration was examined after controlling for participant age, education, sex, and race. Similar analyses were done with GM/WM volume after dividing by TIV.
3. Results
The demographical and clinical characteristics of the participants (n = 59) are shown in Table 1. Out of the 59 participants, 12 met standard Frascati criteria for asymptomatic neurocognitive impairment (ANI), two met the criteria for mild neurocognitive disorder (MND). There was no significant correlation between GDS and clinical nor demographic variables. The correlations between age, education, GDS, disease duration, current CD4, and CD4 nadir are listed in Supplementary Table 1. A scatterplot of GDS versus CD4 nadir is shown in Supplementary Figure 1.
Table 1.
Demographic and clinical characteristics of study participants.
| HIV-positive (n = 59) | |
|---|---|
| Age [years, mean (SD)] | 56.5 (5.8) |
| Sex [n,% (male)] | 45 (76) |
| Race [n,% (African-American)]a | 46 (78) |
| Education [years, mean (SD)] | 13.9 (3.2) |
| Duration of HIV infection [years, mean (SD)] | 25.3 (8.2) |
| Current CD4 [cells/μl, median (IQR)] | 741 (503–941) |
| Nadir CD4 [cells/μl, median (IQR)]b | 190 (55–357) |
| Plasma viral load <20 copies/ml [n, (%)]c | 50 (85) |
| Global deficit score (GDS) [mean (SD)] | 0.3 (0.3) |
| HAND diagnoses [n, (%)]d | 14 (24) |
IQR, interquartile range; HAND, HIV-associated neurocognitive disorders.
Twelve participants were Caucasian and one participant was Hispanic.
Five participants were missing nadir CD4.
Four participants had plasma viral load greater than 100 copies/ml.
Twelve participants were diagnosed with asymptomatic neurocognitive impairment (ANI) and two participants were diagnosed with mild neurocognitive disorder (MND) using standard Frascati criteria.
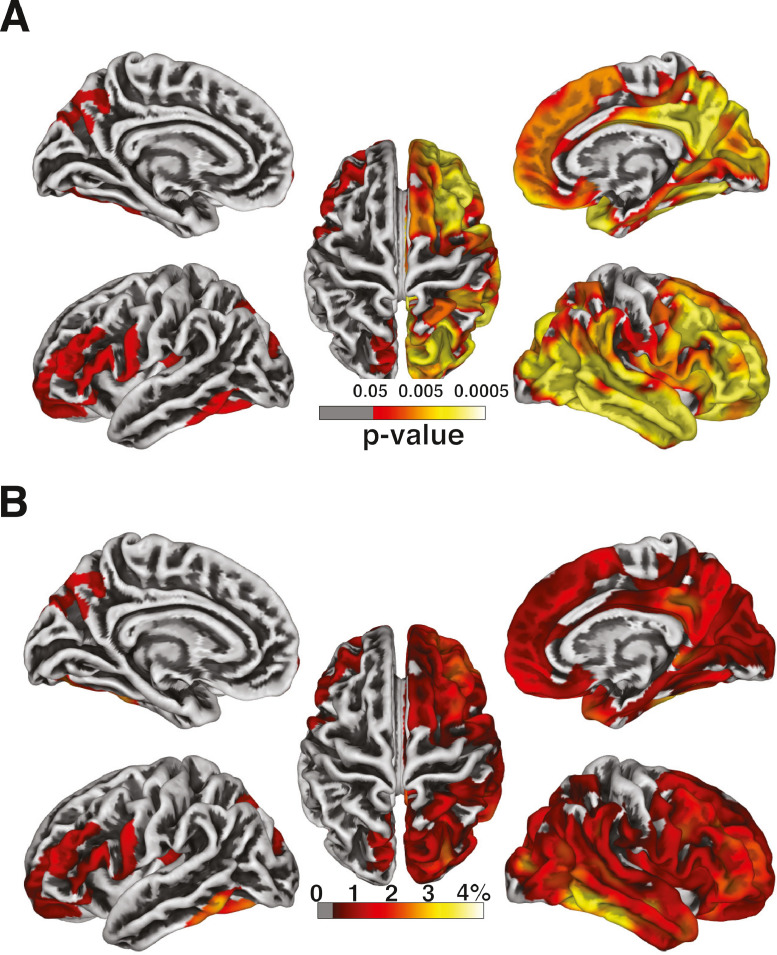
For surface-based analysis, multiple regression analysis revealed that low CD4 nadir was associated with widespread cortical thinning, especially in the right frontal and temporal regions (Fig. 1A). By contrast, no clusters survived at the threshold of p < 0.05 (FWE-corrected) for the correlation between cortical thickness and current CD4 nor disease duration. The percent (%) of cortical thinning with every 100-cell drop in nadir CD4 at each location varied between 0.41% and 4.15% (Fig. 1B). Three post-hoc analyses were conducted with each of the three clinical variables (CD4 nadir, current CD4, and disease duration) separately (Supplementary Fig. 2), and similar results were observed for CD4 nadir. We conducted an additional analysis on a subset of PWH subjects with undetectable plasma viral load (n = 50) and obtained results similar to the entire study sample (Supplementary Fig. 3).
Fig. 1.
Cortical thickness and CD4 nadir. (A) Whole-brain vertex-wise analysis of the association between cortical thickness and CD4 nadir. (B) The percent (%) of cortical thinning associated with every 100-cell drop in CD4 nadir at each location in the clusters identified in (A).
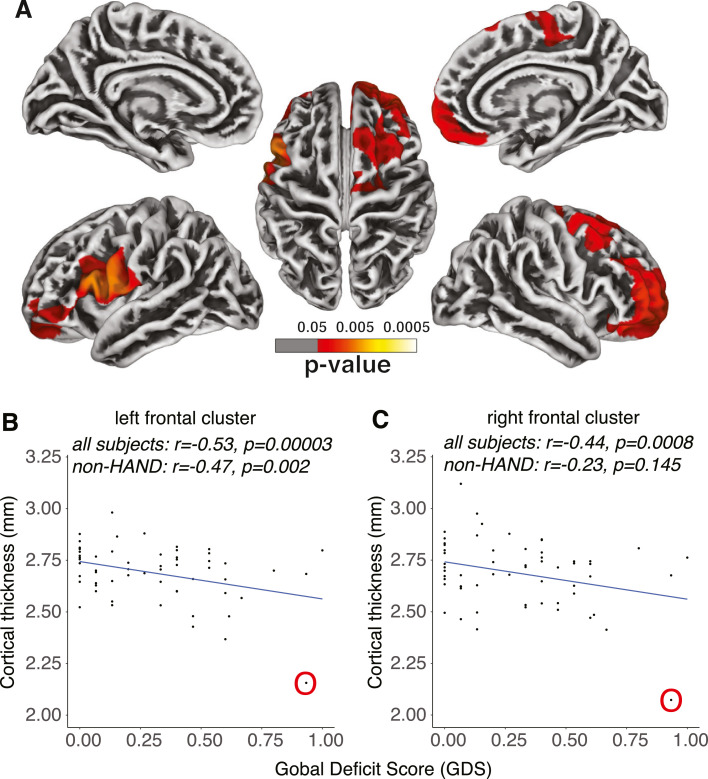
Higher GDS score was primarily associated with cortical thinning in the left and right prefrontal cortex, as well as part of the left premotor area (Fig. 2A). The brain areas negatively associated with GDS in cortical thickness overlapped well with those positively associated with CD4 nadir, especially in the prefrontal cortex. To further investigate the correlation between cortical thickness and GDS, we extracted the mean cortical thickness of the two clusters identified in Fig. 2A, and investigated the association between the mean cortical thickness of each cluster and GDS using a partial correlation after controlling for the linear effects of age, education, sex, and race. As expected with how the ROIs were defined, there was a strong negative correlation between GDS and mean cortical thickness for both clusters (Fig. 2B and C). Similar results were observed when the outlier subject (circled in O) was excluded (left frontal cluster, r = −0.419, p = 0.0016; right frontal cluster, r = −0.301, p = 0.027). More importantly, in the subset of participants (n = 45) who did not meet HAND diagnostic criteria yet, the negative correlation between GDS and mean cortical thickness was still significant in the left frontal cluster (p = 0.0020) (Fig. 2B), although not in the right frontal cluster (p = 0.14) (Fig. 2C), suggesting that frontal atrophy might contribute to early and mild global neurocognitive decline in PWH who do not meet HAND diagnostic criteria.
Fig. 2.
Cortical thickness and GDS. (A) Whole-brain vertex-wise analysis of the association between cortical thickness and GDS. The scatter plots of the mean cortical thickness from the clusters in (B) the left and (C) the right hemisphere identified in (A). The correlations between mean cortical thickness and GDS were examined both across all participants and within the subset of non-HAND participants who did not meet HAND diagnostic criteria yet (i.e., GDS less than 0.5). One outlier subject (with severe current immunosuppression, see main text) is identified and circled in O. GDS, global deficit score.
No clusters survived at the threshold of p < 0.05 (FWE-corrected) for the correlation analyses between HIV disease duration and cortical thickness, nor between current plasma viral load and cortical thickness.
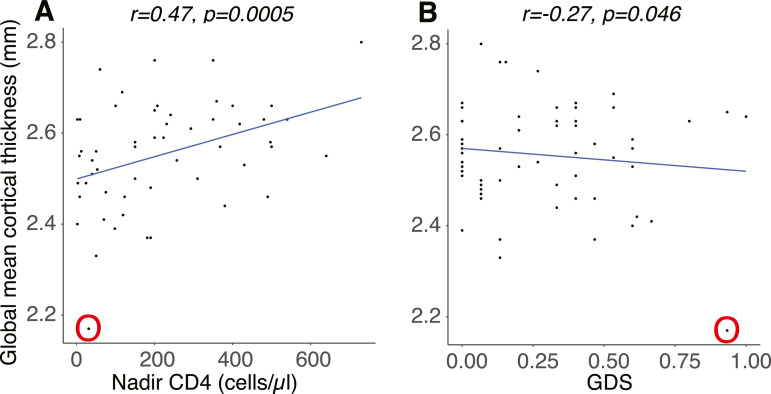
The global mean cortical thickness strongly correlated with CD4 nadir (r = 0.513, p = 0.00005) (Fig. 3A), but not disease duration (r = 0.269, p = 0.058), nor current CD4 (r = 0.029, p = 0.843). In the subset of participants with undetectable viral load (n = 50, Table 1), the positive correlation between global mean cortical thickness and CD4 nadir was also significant (r = 0.415, p = 0.0069). In addition, there was a marginal negative correlation between global mean cortical thickness and GDS (r = −0.27, p = 0.046) (Fig. 3B), however, the correlation was no longer significant when the outlier subject (circled in O) was excluded (p = 0.380).
Fig. 3.
Global mean cortical thickness. The correlations between global mean cortical thickness and (A) nadir CD4 and (B) GDS. The same outlier subject (with severe current immunosuppression) is circled in O. GDS, global deficit score. .
For volumetric analyses, we did not observe any significant correlations between GM volume and CD4 nadir, either using a voxel-based morphometry approach (no cluster survived at the threshold p < 0.05, FWE-corrected), or simply using global measurements such as total GM volume (p = 0.107), total cortical GM volume (p = 0.096), or total subcortical GM volume (p = 0.683), suggesting that a surface-based approach might be more sensitive to detect low CD4 nadir induced brain atrophy than a voxel-based approach. No significant correlations between GM volume and current CD4 nor disease duration were observed.
4. Discussion
In the present study, using a non-parametric statistical approach with cortical thickness we provide evidence suggesting that low CD4 nadir was associated with widespread cortical atrophy (specifically, cortical thinning), especially in the prefrontal and temporal regions. Lower global neurocognitive function (i.e., higher GDS) was primarily associated with cortical thinning in bilateral prefrontal areas, and the association persisted even when only examining a subset of participants who did not meet HAND diagnostic criteria, especially in the left frontal cortex.
In the cART era, low CD4 nadir has been associated with increased risk of HAND (Ellis et al., 2011; Heaton et al., 2010; Muñoz-Moreno et al., 2008; Robertson et al., 2007; Valcour et al., 2006), but the potential mechanism of this association is not well understood. While previous studies have provided evidence suggesting that low CD4 nadir might contribute to brain atrophy in PWH, these studies have been using approaches that have high sensitivity but low spatial specificity (i.e., total GM volume or global mean cortical thickness). Therefore, the spatial profile of the association between low CD4 nadir and brain atrophy remains to be investigated. There are three possible scenarios: the impact of low CD4 nadir on brain atrophy is confined to local areas, but with different brain areas affected in different patients; the impact is global and widespread (but with a small effect size at each voxel/vertex), with no brain regions that are preferentially affected; or the impact is widespread, but some brain regions (i.e., frontal cortex) are more vulnerable than other regions. These scenarios have different implications for the neuropathological process of low CD4 nadir on brain structure/function, and likewise may require different strategies/approaches in developing interventional therapies to prevent/treat HAND that is partially due to the history of immune suppression (as measured by CD4 nadir). These scenarios, however, cannot be differentiated using global measurements such as CSF/ventricular expansion or total GM volume, which do not have the spatial specificity. In the present study, we confirmed that global mean cortical thickness was associated with CD4 nadir (Fig. 3A) in line with previous reports (Chu et al., 2018; Guha et al., 2016). More importantly, using the TFCE technique that has a high sensitivity while maintaining spatial specificity (i.e., a vertex-wise approach to search the entire cortex) and controlling for false positives (p < 0.05, FWE-corrected), we provided evidence suggesting that the impact of low CD4 nadir on cortical atrophy is diffuse and widespread, with the frontal and temporal regions particularly vulnerable. The finding of an association between low CD4 nadir and widespread cortical atrophy is novel and has important clinical implications, i.e., the history of immune suppression may have a broad impact on the entire brain structure and function, which in turn might contribute to the high prevalence of HAND in the cART era and make it difficult to effectively treat HAND due to a broad network of brain regions affected. These results further bolster the importance of early initiation of cART during acute infection to facilitate immune recovery (Sharma et al., 2019) and to reduce the risk of HAND (Crum-Cianflone et al., 2013). In addition, the vulnerability of frontal and temporal regions to low CD4 nadir is interesting, as it might contribute to the phenotypes of neurocognitive impairment in the cART era, including the prevalence of mild symptoms and the affected neurocognitive domains (Heaton et al., 2011, 2010).
The frontal region has long been considered as the essential hub of brain networks involved in executive function (Stuss and Alexander, 2000), and frontal atrophy has been frequently detected in PWH (Becker et al., 2011; Clifford et al., 2017; Jernigan et al., 2005; Li et al., 2014; Sanford et al., 2018; Spies et al., 2016; Towgood et al., 2011) and has been associated with neurocognitive impairment (Nichols et al., 2019). Recently, using a novel meta-analysis technique, we have provided quantitative evidence suggesting that the frontal cortex may be the most consistently affected brain region in PWH in the cART era, especially in cognitively “normal” PWH (Israel et al., 2019). Based on the meta-analysis results and previous studies, we have proposed a neural model of HAND severity (Israel et al., 2019), in which we hypothesize that the high prevalence of frontal atrophy may directly contribute to the highly prevalent executive dysfunction in the cART era (Heaton et al., 2011) and may underlie early and more subtle neurocognitive impairment in PWH (Kamat et al., 2016; Prakash et al., 2017). In the present study, we found that higher GDS was associated with cortical thinning in the bilateral frontal regions, and the association between GDS and left frontal cortex thickness remained significant in the subset of PWH who did not meet HAND diagnostic criteria. This finding provides further evidence supporting a critical role of frontal atrophy in mild forms of neurocognitive impairment in PWH, including mild neurocognitive decline prior to the onset of HAND diagnosis. The overlap between the brain areas associated with GDS and low CD4 nadir in the frontal region suggests that the history of immune suppression might contribute to the frontal atrophy that underlies the high prevalence of executive dysfunction and mild neurocognitive impairment in PWH in the cART era. Although the high prevalence of executive deficits in PWH in cART era supports a critical role of prefrontal atrophy in global cognitive decline (Israel et al., 2019), and previous studies have suggested a central role of prefrontal cortex in cognitive aging, future studies are needed to thoroughly investigate the association between neurocognitive performance of each individual neurocognitive domain and cortical thickness (or brain atrophy in general).
Previous studies have suggested low current CD4 counts might be associated with cortical thinning (Thompson et al., 2005). In the present study, the correlation between current CD4 and cortical thickness was significant across many brain areas only when including one outlier participant whose current CD4 (31 cells/μl) was his new CD4 nadir (Supplementary Fig. 1B). In line with previous reports (Thompson et al., 2005), this suggests that significant current immune suppression (i.e., with current CD4 counts less than 100 cells/μl) may lead to potentially widespread cortical thinning. Taken together, these results suggest that significant current immune dysfunction might be detrimental to brain structure, leading to cortical thinning (especially in the prefrontal cortex), which might recover (at least partially) along with the recovery of immune function (Pfefferbaum et al., 2014). This again supports the importance of early initiation of cART after seroconversion. The association between cortical thinning and low CD4 nadir might be a direct or indirect consequence of the impact of low CD4 on cortical thickness prior to cART initiation.
In the present study, we did not observe a significant correlation between GM volume and CD4 nadir using a voxel-based morphometry approach, suggesting that cortical thickness might be more sensitive to capture brain atrophy due to low CD4 nadir. Using a global measurement approach focusing on total GM volume, we found a weak but non-significant correlation between CD4 nadir and total cortical GM volume (p = 0.096), but not total subcortical GM volume (p = 0.683), which—while not significant—is in line with previous reports that suggest low CD4 nadir might be more associated with cortical than subcortical atrophy (Cohen et al., 2010; Guha et al., 2016).
There are some limitations of the present study. First, there was a significant correlation between disease duration and age in this cohort of PWH participants, which might limit our capability to detect the probable impact of disease duration on brain structure (Cohen et al., 2010; Gongvatana et al., 2014; Tesic et al., 2018). Second, unlike previous studies (Cohen et al., 2010; Guha et al., 2016), we did not observe a correlation between current viral load and subcortical atrophy; this might be due to the fact that the majority of participants (50 out of 59) in the present study had undetectable viral load, and only three had a plasma viral load higher than 150 copies/μl. Third, while several previous studies have suggested an impact of low CD4 nadir on WM volume (Gongvatana et al., 2014; Hua et al., 2013; Jernigan et al., 2011; Sanford et al., 2017; Tate et al., 2011), WM hyperintensities (WMH) (Moulignier et al., 2018), and WM integrity (Bell et al., 2018; Cordero et al., 2017), we did not find a significant impact of CD4 nadir on total WM volume in the present study (p = 0.713), and we could not assess WMH nor WM integrity as this study focused on T1-weighted structural images. To fully understand the neuropathogenesis of low CD4 nadir on brain structure/function, future multimodal MRI studies are needed to assess and integrate the impact of low CD4 nadir on brain atrophy, WM injury/abnormalities, shift in neurochemistry, and altered neuronal activity.
In summary, using a vertex-wise approach along with the TFCE technique and a conservative threshold, the present study investigated the impact of low CD4 nadir on the entire cortex. The results support the hypothesis that low CD4 nadir is associated with widespread cortical atrophy in PWH, especially in the frontal and temporal regions, which might contribute to the phenotypes and prevalence of HAND in the cART era and partially explain the difficulty treating HAND. These findings support the importance of early cART initiation to prevent significant immune suppression, which is linked to widespread cortical thinning. In addition, the significant correlation between GDS and frontal cortex thickness (including in PWH without HAND) suggests that frontal atrophy may contribute to the high prevalence of neurocognitive impairment and decline in the cART era, even in those who do not meet HAND diagnostic criteria.
Financial support
This work is supported by the National Institute of Mental Health [grant number 1R01MH108466 to X.J.].
Conference presentation
None.
CRediT authorship contribution statement
Shiva Hassanzadeh-Behbahani: Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Kyle F. Shattuck: Formal analysis, Writing - original draft. Margarita Bronshteyn: Data curation. Matthew Dawson: Data curation. Monica Diaz: Data curation. Princy Kumar: Data curation. David J. Moore: Writing - review & editing. Ronald J. Ellis: Writing - review & editing. Xiong Jiang: Conceptualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
None.
Acknowledgments
We wish to thank all participants for their time and participation, and the assistance for patient care from the Georgetown University Clinical Research Unit (GU-CRU), which has been funded in whole or in part with Federal funds (Grant # UL1TR000101 previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise.”
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102155.
Appendix. Supplementary materials
References
- Antinori A., Arendt G., Becker J.T., Brew B.J., Byrd D.A., Cherner M., Clifford D.B., Cinque P., Epstein L.G., Goodkin K., Gisslen M., Grant I., Heaton R.K., Joseph J., Marder K., Marra C.M., McArthur J.C., Nunn M., Price R.W., Pulliam L., Robertson K.R., Sacktor N., Valcour V., Wojna V.E. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.T., Maruca V., Kingsley L.A., Sanders J.M., Alger J.R., Barker P.B., Goodkin K., Martin E., Miller E.N., Ragin A., Sacktor N., Selnes O., Study M.A.C. Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology. 2011;54:113–121. doi: 10.1007/s00234-011-0854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R.P., Barnes L.L., Towe S.L., Chen N.-.K., Song A.W., Meade C.S. Structural connectome differences in HIV infection: brain network segregation associated with nadir CD4 cell count. J. Neurovirol. 2018;24:454–463. doi: 10.1007/s13365-018-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K., Moore D.J., Franklin D.R., Clifford D.B., Collier A.C., Marra C.M., Gelman B.B., McArthur J.C., Morgello S., Simpson D.M., Ellis R.J., Atkinson J.H., Grant I., Heaton R.K. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin. Neuropsychol. 2012;26:894–908. doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisker T.R., Dufour M.-S.K., Myers J.J. Recall of nadir CD4 cell count and most recent HIV viral load among HIV-Infected, socially marginalized adults. AIDS Behav. 2015;19:2108–2116. doi: 10.1007/s10461-015-1018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey C.L., Woods S.P., Gonzalez R., Conover E., Marcotte T.D., Grant I., Heaton R.K., Group H.N.R.C. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J. Clin. Exp. Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Chu K., Tran T., Wei K., Lammering J.C., Sondergaard A., Mogadam E., Shriner K., King K.S. Distinguishing brain impact of aging and HIV severity in chronic HIV using multiparametric mr imaging and MR spectroscopy. Open Forum Infect. Dis. 2018;5 doi: 10.1093/ofid/ofy243. ofy243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark U.S., Arce Rentería M., Hegde R.R., Morgello S. Early life stress-related elevations in reaction time variability are associated with brain volume reductions in HIV+ adults. Front. Behav. Neurosci. 2018;12:6. doi: 10.3389/fnbeh.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark U.S., Walker K.A., Cohen R.A., Devlin K.N., Folkers A.M., Pina M.J., Tashima K.T. Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia. 2015;70:263–271. doi: 10.1016/j.neuropsychologia.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford K.M., Samboju V., Cobigo Y., Milanini B., Marx G.A., Hellmuth J.M., Rosen H.J., Kramer J.H., Allen I.E., Valcour V.G. Progressive brain atrophy despite persistent viral suppression in HIV patients older than 60 years. J. Acquir. Immune Defic. Syndr. 2017;76:289–297. doi: 10.1097/QAI.0000000000001489. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.A., Harezlak J., Schifitto G., Hana G., Clark U., Gongvatana A., Paul R., Taylor M., Thompson P., Alger J., Brown M., Zhong J., Campbell T., Singer E., Daar E., McMahon D., Tso Y., Yiannoutsos C.T., Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J. Neurovirol. 2010;16:25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero D.M., Towe S.L., Chen N.-.K., Robertson K.R., Madden D.J., Huettel S.A., Meade C.S. Cocaine dependence does not contribute substantially to white matter abnormalities in HIV infection. J. Neurovirol. 2017;23:441–450. doi: 10.1007/s13365-017-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum-Cianflone N.F., Moore D.J., Letendre S., Poehlman Roediger M., Eberly L., Weintrob A., Ganesan A., Johnson E., Del Rosario R., Agan B.K., Hale B.R. Low prevalence of neurocognitive impairment in early diagnosed and managed HIV-infected persons. Neurology. 2013;80:371–379. doi: 10.1212/WNL.0b013e31827f0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahnke R., Yotter R.A., Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336–348. doi: 10.1016/j.neuroimage.2012.09.050. [DOI] [PubMed] [Google Scholar]
- Ellis R.J., Badiee J., Vaida F., Letendre S., Heaton R.K., Clifford D., Collier A.C., Gelman B., McArthur J., Morgello S., McCutchan J.A., Grant I., CHARTER Group CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS Lond. Engl. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongvatana A., Correia S., Dunsiger S., Gauthier L., Devlin K.N., Ross S., Navia B., Tashima K.T., DeLaMonte S., Cohen R.A. Plasma cytokine levels are related to brain volumes in HIV-infected individuals. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2014;9:740–750. doi: 10.1007/s11481-014-9567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A., Brier M.R., Ortega M., Westerhaus E., Nelson B., Ances B.M. Topographies of cortical and subcortical volume loss in HIV and aging in the cART Era. J. Acquir. Immune Defic. Syndr. 2016;73:374–383. doi: 10.1097/QAI.0000000000001111. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Clifford D.B., Franklin D.R., Woods S.P., Ake C., Vaida F., Ellis R.J., Letendre S.L., Marcotte T.D., Atkinson J.H., Rivera-Mindt M., Vigil O.R., Taylor M.J., Collier A.C., Marra C.M., Gelman B.B., McArthur J.C., Morgello S., Simpson D.M., McCutchan J.A., Abramson I., Gamst A., Fennema-Notestine C., Jernigan T.L., Wong J., Grant I., CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: Charter study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Franklin D.R., Ellis R.J., McCutchan J.A., Letendre S.L., Leblanc S., Corkran S.H., Duarte N.A., Clifford D.B., Woods S.P., Collier A.C., Marra C.M., Morgello S., Mindt M.R., Taylor M.J., Marcotte T.D., Atkinson J.H., Wolfson T., Gelman B.B., McArthur J.C., Simpson D.M., Abramson I., Gamst A., Fennema-Notestine C., Jernigan T.L., Wong J., Grant I., CHARTER Group, HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Boyle C.P., Harezlak J., Tate D.F., Yiannoutsos C.T., Cohen R., Schifitto G., Gongvatana A., Zhong J., Zhu T., Taylor M.J., Campbell T.B., Daar E.S., Alger J.R., Singer E., Buchthal S., Toga A.W., Navia B., Thompson P.M., HIV Neuroimaging Consortium Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatment. NeuroImage Clin. 2013;3:132–142. doi: 10.1016/j.nicl.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel S.M., Hassanzadeh-Behbahani S., Turkeltaub P.E., Moore D.J., Ellis R.J., Jiang X. Different roles of frontal versus striatal atrophy in HIV-associated neurocognitive disorders. Hum. Brain Mapp. 2019 doi: 10.1002/hbm.24577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T.L., Archibald S.L., Fennema-Notestine C., Taylor M.J., Theilmann R.J., Julaton M.D., Notestine R.J., Wolfson T., Letendre S.L., Ellis R.J., Heaton R.K., Gamst A.C., Franklin D.R., Jr., Clifford D.B., Collier A.C., Gelman B.B., Marra C., McArthur J.C., McCutchan J.A., Morgello S., Simpson D.M., Grant I., Franklin D.R.J., Clifford D.B., Collier A.C., Gelman B.B., Marra C., McArthur J.C., McCutchan J.A., Morgello S., Simpson D.M., Grant I., Franklin D.R., Jr., Clifford D.B., Collier A.C., Gelman B.B., Marra C., McArthur J.C., McCutchan J.A., Morgello S., Simpson D.M., Grant I., Franklin D.R.J., Clifford D.B., Collier A.C., Gelman B.B., Marra C., McArthur J.C., McCutchan J.A., Morgello S., Simpson D.M., Grant I., Franklin D.R., Jr., Clifford D.B., Collier A.C., Gelman B.B., Marra C., McArthur J.C., McCutchan J.A., Morgello S., Simpson D.M., Grant I., Franklin D.R.J. Clinical factors related to brain structure in HIV: the Charter study. J. Neurovirol. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T.L., Gamst A.C., Archibald S.L., Fennema-Notestine C., Mindt M.R., Marcotte T.D., Marcotte T.L., Heaton R.K., Ellis R.J., Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am. J. Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kamat R., Doyle K.L., Iudicello J.E., Morgan E.E., Morris S., Smith D.M., Little S.J., Grant I., Woods S.P., Translational Methamphetamine AIDS Research Center (TMARC) Group Neurobehavioral disturbances during acute and early HIV infection. Cognit. Behav. Neurol. Off. J. Soc. Behav. Cognit. Neurol. 2016;29:1–10. doi: 10.1097/WNN.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li H., Gao Q., Yuan D., Zhao J. Structural gray matter change early in male patients with HIV. Int. J. Clin. Exp. Med. 2014;7:3362–3369. [PMC free article] [PubMed] [Google Scholar]
- MacDuffie K.E., Brown G.G., McKenna B.S., Liu T.T., Meloy M.J., Tawa B., Archibald S., Fennema-Notestine C., Atkinson J.H., Ellis R.J., Letendre S.L., Hesselink J.R., Cherner M., Grant I., TMARC Group Effects of HIV infection, methamphetamine dependence and age on cortical thickness, area and volume. NeuroImage Clin. 2018;20:1044–1052. doi: 10.1016/j.nicl.2018.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulignier A., Savatovsky J., Assoumou L., Lescure F.-.X., Lamirel C., Godin O., Valin N., Tubiana R., Canestri A., Roux P., Sadik J.-.C., Salomon L., Abrivard M., Katlama C., Yazdanpanah Y., Pialoux G., Girard P.-.M., Costagliola D., Microvascular Brain Retina and Kidney (MicroBREAK) Study Group Silent cerebral small-vessel disease is twice as prevalent in middle-aged individuals with well-controlled, combination antiretroviral therapy-treated human immunodeficiency virus (HIV) than in HIV-Uninfected individuals. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018;66:1762–1769. doi: 10.1093/cid/cix1075. [DOI] [PubMed] [Google Scholar]
- Muñoz-Moreno J.A., Fumaz C.R., Ferrer M.J., Prats A., Negredo E., Garolera M., Pérez-Alvarez N., Moltó J., Gómez G., Clotet B. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res. Hum. Retroviruses. 2008;24:1301–1307. doi: 10.1089/aid.2007.0310. [DOI] [PubMed] [Google Scholar]
- Nichols M.J., Gates T.M., Soares J.R., Moffat K.J., Rae C.D., Brew B.J., Cysique L.A. Atrophic brain signatures of mild forms of neurocognitive impairment in virally suppressed HIV infection. AIDS Lond. Engl. 2019;33:55–66. doi: 10.1097/QAD.0000000000002042. [DOI] [PubMed] [Google Scholar]
- Nir T.M., Jahanshad N., Ching C.R.K., Cohen R.A., Harezlak J., Schifitto G., Lam H.Y., Hua X., Zhong J., Zhu T., Taylor M.J., Campbell T.B., Daar E.S., Singer E.J., Alger J.R., Thompson P.M., Navia B.A., HIV Neuroimaging Consortium Progressive brain atrophy in chronically infected and treated HIV+ individuals. J. Neurovirol. 2019 doi: 10.1007/s13365-019-00723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman K.A., Polyn S.M., Detre G.J., Haxby J.V. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cognit. Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rogosa D.A., Rosenbloom M.J., Chu W., Sassoon S.A., Kemper C.A., Deresinski S., Rohlfing T., Zahr N.M., Sullivan E.V. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol. Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Rosenbloom M.J., Sassoon S.A., Kemper C.A., Deresinski S., Rohlfing T., Sullivan E.V. Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol. Psychiatry. 2012;72:361–370. doi: 10.1016/j.biopsych.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A., Hou J., Liu L., Gao Y., Kettering C., Ragin A.B. Cognitive function in early HIV infection. J. Neurovirol. 2017;23:273–282. doi: 10.1007/s13365-016-0498-4. [DOI] [PubMed] [Google Scholar]
- Robertson K.R., Smurzynski M., Parsons T.D., Wu K., Bosch R.J., Wu J., McArthur J.C., Collier A.C., Evans S.R., Ellis R.J. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS Lond. Engl. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Sacktor N., Skolasky R.L., Seaberg E., Munro C., Becker J.T., Martin E., Ragin A., Levine A., Miller E. Prevalence of HIV-associated neurocognitive disorders in the Multicenter Aids Cohort Study. Neurology. 2016;86:334–340. doi: 10.1212/WNL.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R., Fellows L.K., Ances B.M., Collins D.L. Association of brain structure changes and cognitive function with combination antiretroviral therapy in HIV-Positive individuals. JAMA Neurol. 2018;75:72–79. doi: 10.1001/jamaneurol.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R., Fernandez Cruz A.L., Scott S.C., Mayo N.E., Fellows L.K., Ances B.M., Collins D.L. Regionally specific brain volumetric and cortical thickness changes in HIV-Infected patients in the HAART Era. J. Acquir. Immune Defic. Syndr. 2017;74:563–570. doi: 10.1097/QAI.0000000000001294. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Schlusser K.E., de la Torre P., Tambussi G., Draenert R., Pinto A.N., Metcalf J.A., Neaton J.D., Laeyendecker O., INSIGHT START Study Group The benefit of immediate compared with deferred antiretroviral therapy on CD4+ cell count recovery in early HIV infection. AIDS Lond. Engl. 2019;33:1335–1344. doi: 10.1097/QAD.0000000000002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Spies G., Ahmed-Leitao F., Fennema-Notestine C., Cherner M., Seedat S. Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. J. Neurovirol. 2016;22:149–158. doi: 10.1007/s13365-015-0379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T., Alexander M.P. Executive functions and the frontal lobes: a conceptual view. Psychol. Res. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Tate D.F., Delong A., McCaffrey D.E., Kertesz K., Paul R.H., Conley J., Russell T., Coop K., Gillani F., Flanigan T., Tashima K., Hogan J.W. Recent clinical history and cognitive dysfunction for attention and executive function among human immunodeficiency virus-infected patients. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2011;26:614–623. doi: 10.1093/arclin/acr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesic T., Boban J., Bjelan M., Todorovic A., Kozic D., Brkic S. Basal ganglia shrinkage without remarkable hippocampal atrophy in chronic aviremic HIV-positive patients. J. Neurovirol. 2018;24:478–487. doi: 10.1007/s13365-018-0635-3. [DOI] [PubMed] [Google Scholar]
- Thompson P.M., Dutton R.A., Hayashi K.M., Toga A.W., Lopez O.L., Aizenstein H.J., Becker J.T. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc. Natl. Acad. Sci. USA. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towgood K.J., Pitkanen M., Kulasegaram R., Fradera A., Kumar A., Soni S., Sibtain N.A., Reed L., Bradbeer C., Barker G.J., Kopelman M.D. Mapping the brain in younger and older asymptomatic HIV-1 men: frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex J. Devoted Study Nerv. Syst. Behav. 2011;48:230–241. doi: 10.1016/j.cortex.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Valcour V., Yee P., Williams A.E., Shiramizu B., Watters M., Selnes O., Paul R., Shikuma C., Sacktor N. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection – the Hawaii Aging with HIV cohort. J. Neurovirol. 2006;12:387–391. doi: 10.1080/13550280600915339. [DOI] [PubMed] [Google Scholar]
- Yotter R.A., Thompson P.M., Gaser C. Algorithms to improve the reparameterization of spherical mappings of brain surface meshes. J. Neuroimaging Off. J. Am. Soc. Neuroimaging. 2011;21:e134–e147. doi: 10.1111/j.1552-6569.2010.00484.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.