Abstract
Background:
Acute septic arthritis in a native joint may require more than one surgical debridement to eradicate the infection. Our objectives were to determine the prevalence of failure of a single surgical debridement for acute septic arthritis, to identify risk factors for failure of a single debridement, and to develop a prognostic probability algorithm to predict failure of a single surgical debridement for acute septic arthritis in adults.
Methods:
We collected initial laboratory and medical comorbidity data of 128 adults (132 native joints) with acute septic arthritis who underwent at least one surgical debridement at our institution between 2000 and 2011. Univariate and logistic regression analyses were used to identify potential risk factors for failure of a single surgical debridement. Stepwise variable selection was used to develop a prediction model and identify probabilities of failure of a single surgical debridement.
Results:
Of the 128 patients (132 affected joints) who underwent surgical debridement for acute septic arthritis, forty-nine (38%) of the patients (fifty joints) experienced failure of a single debridement and required at least two debridements (range, two to four debridements). Staphylococcus aureus was the most common bacterial isolate (in sixty, or 45%, of the 132 joints). Logistic regression analysis identified five independent clinical predictors for failure of a single surgical debridement: a history of inflammatory arthropathy (odds ratio [OR], 7.3; 95% confidence interval [CI], 2.4 to 22.6; p < 0.001), the involvement of a large joint (knee, shoulder, or hip) (OR, 7.0; 95% CI, 1.2 to 37.5; p = 0.02), a synovial-fluid nucleated cell count of >85.0 x 109 cells/L (OR, 4.7; 95% CI, 1.8 to 17.7; p = 0.002), S. aureus as the bacterial isolate (OR, 4.6; 95% CI, 1.8 to 11.9; p = 0.002), and a history of diabetes (OR, 2.6; 95% CI, 1.1 to 6.2; p = 0.04).
Conclusions:
Most (62%) of the septic joints were managed effectively with a single surgical debridement. Adults with a history of inflammatory arthropathy, involvement of a large joint, a synovial-fluid nucleated cell count of >85.0 x 109 cells/L, an infection with S. aureus, or a history of diabetes had a higher risk of failure of a single surgical debridement for acute septic arthritis and requiring additional surgical debridement(s).
Level of Evidence:
Prognostic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Septic arthritis is an orthopaedic emergency requiring prompt treatment. Treatment with a single surgical irrigation and debridement with antibiotics is usually effective, but more than one debridement may be necessary to eradicate the infection1.
The bacterial inoculation of a joint can develop from hematogenous seeding, direct introduction, or extension from a contiguous focus of infection2. A joint infection may damage cartilage directly by bacterial enterotoxins and indirectly from the host immune response to bacteria3. Delayed treatment of septic arthritis can result in joint degeneration, osteonecrosis, or joint instability4-6.
The clinical evaluation of a patient with suspected septic arthritis begins with a clinical examination and assessment of laboratory data7. The serologic testing consists of a white blood-cell (WBC) count, measurement of the C-reactive protein (CRP) level and of the erythrocyte sedimentation rate (ESR), and performance of aerobic and anaerobic blood cultures. Arthrocentesis is also performed to determine synovial-fluid nucleated cell count, to test for crystals, and for a Gram stain and cultures. The standard for the diagnosis of septic arthritis is a positive Gram stain or subsequent positive cultures from the arthrocentesis. A high nucleated cell count (that is, >50 × 109 cells/L) is also strongly correlated with infection2. Treatment consists of emergency surgical irrigation and debridement, followed by a course of antibiotics based on culture results8-12. Successful treatment results in the resolution of symptoms (which include pain, fever, inability to bear weight, and limited motion in the affected joint). Some patients (23% to 48%) do not improve after a single debridement and require additional debridements to treat the infection13-15.
Several studies have identified risk factors for the development of septic arthritis in adults, including diabetes mellitus16, rheumatoid arthritis17, intravenous drug use16,18, poor socioeconomic status12,19, concurrent infection, and immunosuppression17. We are aware of no published literature regarding risk factors associated with the failure of a single surgical debridement for the treatment of acute septic arthritis of a native joint. The goals of the present study were to determine the prevalence of failure of a single surgical debridement for acute septic arthritis, to identify risk factors for failure of a single surgical debridement, and to develop a prognostic probability algorithm for failure of a single surgical debridement.
Materials and Methods
Patients
At our institution, the orthopaedic service treats all patients diagnosed with septic arthritis with surgical debridement. Therefore, we queried our billing database to identify all patients at least eighteen years of age who were treated for septic arthritis between 2000 and 2011. We included patients for whom orthopaedic consultation was obtained; we did not include those who may have been treated nonoperatively with serial aspirations by other services because of high surgical risk or because the patient declined to undergo surgery. Exclusion criteria included a history of septic arthritis (nine patients), osteomyelitis of a bone contiguous with the affected joint (five patients), a history of fracture surgery with an arthrotomy of the joint/joint capsule (twenty patients), previous arthroplasty (seventy-three patients), a joint with an implanted foreign body (such as suture material) from a previous procedure (six patients), or a treatment plan at the time of the initial debridement that included conducting a subsequent second debridement (nineteen patients). The records of the remaining 128 patients (132 joints) were reviewed to obtain the results of serologic and joint-fluid analyses. Institutional review board approval for this study was obtained.
The diagnosis of septic arthritis was made when the patient had a clinical presentation consistent with acute septic arthritis (acute onset of pain, the inability to bear weight or move the affected joint, fevers, and joint effusion) and when any of the following occurred: (1) a positive Gram stain (that is, for bacteria) from joint aspiration; (2) aerobic or anaerobic cultures that grew bacteria; (3) aspirated fluid with a nucleated cell count of >50.0 × 109 cells/L. To minimize information bias, patients with an incomplete work-up and follow-up history were excluded.
Data obtained for all of the patients included age, sex, race, date of presentation, duration of prodromal symptoms, history of fever (a temperature of >38.5°C), which joint was affected, history of any surgical procedures involving the affected joint, presence of concurrent infection, method of surgical debridement, and history of the following comorbidities: cancer, diabetes, insulin use, immunosuppression, HIV (human immunodeficiency virus), inflammatory arthropathy (such as rheumatoid arthritis or spondyloarthropathy), sickle cell disease, coronary artery disease, tobacco use, or obesity (a body mass index [BMI] of >30 kg/m2).
Hospital Course
Surgery
All patients had either an open or an arthroscopic irrigation and debridement of the affected joint for the index procedure, as determined by the attending surgeon. Open debridement was the preferred initial treatment (68% of the affected joints). In general, the affected joints were debrided and then irrigated with sterile saline solution. With the exception of a few patients, in whom antibiotics were initiated prior to orthopaedic consultation, broad-spectrum antibiotics were routinely withheld preoperatively until deep intraoperative specimens for culture were obtained. Cultures were monitored, and antibiotic selection was later adjusted as necessary based on these results.
Postoperative Period
Patients were monitored in the hospital for at least forty-eight hours postoperatively to assess the success of the initial surgical debridement. For all patients, an infectious disease consultant determined the appropriate antibiotic regimen.
During the postoperative period, any of the following signs or symptoms were considered a recurrence of infection and failure of a single surgical debridement: persistent purulent discharge from a drain or incisional site(s), increasing pain, decreasing range of motion, persistent fevers, or persistent elevation of serologic inflammatory markers. Patients showing steady clinical improvement after forty-eight hours were discharged from our institution with continued antibiotic therapy. Serologic markers (ESR, CRP, and CBC [complete blood count]) were routinely monitored during antibiotic therapy in an outpatient setting to assess the patient’s response to the treatment.
Outpatient Follow-up
Outpatient infectious disease and orthopaedic follow-up continued for the length of the antibiotic therapy (three to twelve weeks of oral or intravenous therapy, determined by infectious disease consultants on the basis of clinical examination and return of serologic markers to near-baseline values). Any increase in inflammatory markers prompted clinical reevaluation. Any patient with worsening symptoms was evaluated in the clinic and/or our emergency room. Repeat aspiration and serologic studies were performed. Those having recurrence of the infection were readmitted to the hospital, and an additional debridement was performed.
Statistical Analysis
Univariate analysis, using a two-tailed Student t test for continuous variables and Fisher exact test for binary variables, was performed. Variables with a p value of <0.20 were included as initial candidates in the stepwise variable selection. For all continuous laboratory variables (WBC, ESR, CRP, and synovial-fluid nucleated cell count), thresholds were created and optimized to detect a difference, thus allowing for variable inclusion in our model and assisting with risk stratification. Stepwise variable selection using logistic regression was performed to identify a subset of significant independent clinical variables present in individuals with failure of a single surgery.
A prognostic model for failure of a single surgery was created using the identified risk factors. The Hosmer-Lemeshow goodness-of-fit test was performed to detect any departure from a good model fit. A receiver operating characteristic (ROC) curve was constructed to determine the diagnostic performance for identifying patients who may experience failure of a single surgery. A two-sided p value of <0.05 was considered significant for all tests.
Source of Funding
Statistical analysis was supported by the University of Rochester CTSA (Clinical and Translational Science Awards) number UL1 TR000042 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Results
Univariate Analysis
No difference in the effectiveness of a single surgical debridement was seen with regard to sex, race, or age (p > 0.05). We were unable to demonstrate any difference in the effectiveness of a single surgical debridement between open and arthroscopic debridement (p > 0.05). The forty-nine patients (fifty joints) with a failed single debridement differed from the seventy-nine patients (eighty-two joints) with a successful single debridement (using p < 0.20) by duration of prodromal symptoms (5.3 ± 4.5 compared with 6.2 ± 5.4 days, respectively), positive Gram stain from arthrocentesis (56% compared with 38%, respectively), history of diabetes (65% compared with 46%, respectively), history of immunosuppression (41% compared with 23%, respectively), history of inflammatory arthropathy (39% compared with 17%, respectively), coronary artery disease (31% compared with 15%, respectively), Staphylococcus aureus infection (66% compared with 33%, respectively), methicillin-resistant S. aureus (MRSA) infection (48% compared with 20%, respectively), positive blood culture (23% compared with 9%, respectively), large joint involvement (94% compared with 81%, respectively), an initial CRP level of >180 mg/L (51% compared with 66%, respectively), a WBC of >11.5 × 109/L (35% compared with 61%, respectively), and a synovial-fluid nucleated cell count of >85.0 × 109 cells/L (35% compared with 14%, respectively) (Table I).
TABLE I.
Univariate Analysis of Demographic Variables for Successful Versus Failed Single Surgical Debridement
| Variable | Successful Single Surgical Debridement | Failed Single Surgical Debridement | P Value |
| Male* | 47 (59.5%) | 35 (71.4%) | 0.28 |
| Age* (yr) | 53.8 ± 19.0 | 57.1 ± 18.6 | 0.12 |
| Race* | |||
| Caucasian | 52 (65.8%) | 36 (73.4%) | 0.32 |
| African-American | 19 (24.1%) | 12 (24.5%) | 1.00 |
| Hispanic | 8 (10.1%) | 1 (2.0%) | 0.15 |
| Duration of prodromal symptoms* (d) | 6.2 ± 5.4 | 5.3 ± 4.5 | 0.14 |
| Large joint involvement* | 64 (81.0%) | 46 (93.9%) | 0.08 |
| Recent arthroscopy* | 15 (19.0%) | 13 (26.5%) | 0.46 |
| Concurrent infection* | 30 (38.0%) | 24 (49.0%) | 0.33 |
| History of cancer* | 17 (21.5%) | 11 (22.5%) | 1.00 |
| History of diabetes* | 36 (45.6%) | 32 (65.3%) | 0.05 |
| Current insulin use* | 10 (12.7%) | 11 (22.5%) | 0.24 |
| History of immunosuppression* | 18 (22.8%) | 20 (40.8%) | 0.054 |
| HIV positive* | 5 (6.3%) | 2 (4.1%) | 0.87 |
| Inflammatory arthropathy* | 13 (16.5%) | 19 (38.8%) | 0.01 |
| Sickle cell disease* | 2 (2.5%) | 2 (4.1%) | 1.00 |
| Coronary artery disease* | 12 (15.2%) | 15 (30.6%) | 0.07 |
| Tobacco use* | 15 (19.0%) | 12 (24.5%) | 0.63 |
| BMI >30 kg/m2* | 21 (26.6%) | 14 (28.6%) | 1.00 |
| Fever >38.5°C* | 12 (15.2%) | 9 (18.4%) | 0.85 |
| Cell count >85.0 × 109 cells/L* | 11 (13.9%) | 17 (34.7%) | 0.011 |
| CRP >180 mg/L* | 52 (65.8%) | 25 (51.0%) | 0.14 |
| WBC >11.5 × 109/L* | 48 (60.8%) | 17 (34.7%) | 0.007 |
| Positive blood culture* | 7 (8.9%) | 11 (22.5%) | 0.06 |
| Presence of crystals in synovial fluid† | 10 (12.2%) | 10 (20.0%) | 0.47 |
| Positive Gram stain† | 31 (37.8%) | 28 (56.0%) | 0.05 |
| S. aureus infection† | 27 (32.9%) | 33 (66.0%) | <0.001 |
| Methicillin-resistant S. aureus infection† | 16 (19.5%) | 24 (48.0%) | <0.001 |
Values are given as the mean number of patients and standard deviation for continuous variables and as the number and percentage of patients for categorical variables. N = 79 patients in the successful group, and N = 49 patients in the failed group.
Values are given as the number and percentage of affected joints. N = 82 joints in the successful group, and N = 50 joints in the failed group.
When expressed as continuous variables, the mean nucleated cell count (70.6 ± 58.3 compared with 89.4 ± 68.8 × 109 cells/L; p = 0.02) and WBC (12.3 ± 5.1 compared with 14.0 ± 4.7 × 109/L; p = 0.004) differed significantly between the groups, with both markers trending higher in patients who failed a single debridement. The CRP level tended to be higher in the patients who failed a single surgical debridement, but this difference between the groups did not reach significance (171.2 ± 113.9 compared with 195.9 ± 104.9 mg/L; p = 0.07) (Table II).
TABLE II.
Univariate Analysis of Serologic and Arthrocentesis Variables for Successful Versus Failed Single Surgical Debridement
| Variable | Successful, N = 79* | Failed, N = 49* | P Value |
| WBC (× 109/L) | 12.3 ± 5.1 | 14.0 ± 4.7 | 0.004 |
| Platelets (× 109/L) | 284.2 ± 142.5 | 275.9 ± 157.7 | 0.62 |
| ESR (mm/hr) | 75.0 ± 36.7 | 77.0 ± 34.0 | 0.66 |
| CRP (mg/L) | 171.2 ± 113.9 | 195.9 ± 104.9 | 0.07 |
| Cell count (× 109 cells/L) | 70.6 ± 58.3 | 89.4 ± 68.8 | 0.02 |
Values are given as the mean and standard deviation for continuous variables.
Arthrocentesis was performed on all 128 patients (132 affected joints). For forty-seven joint aspirations (36%), bacteria did not grow on aerobic/anaerobic culture. A sterile or negative culture was more common in patients successfully treated with a single debridement (thirty-seven of seventy-nine [47%] compared with nine of forty-nine [18%]; p = 0.001).
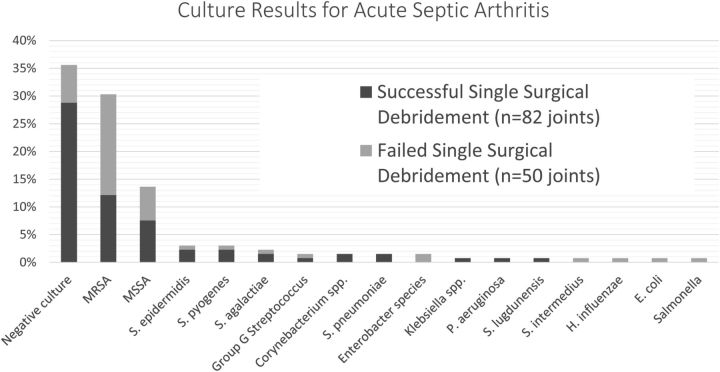
S. aureus was the most common isolate, found in sixty (45%) of the 132 joints and fifty-eight (68%) of the eighty-five joints with a positive culture from aspiration. S. aureus was identified in twenty-seven (33%) of the eighty-two joints treated successfully with a single debridement compared with thirty-three (66%) of the fifty joints failing initial debridement (p < 0.001). Among the sixty joints for which S. aureus grew on culture, forty (67%) were identified as having MRSA. MRSA was also identified significantly more often in the joints experiencing a failed single surgical debridement (48% compared with 20%; p <0.001). No other significant differences were observed between the two groups on analysis of culture data (p > 0.05). Culture results are summarized in Figure 1.
Fig. 1.
The final culture results for adults with acute septic arthritis. Negative or sterile culture results were the most common. Staphylococcus aureus was the most common bacterial isolate identified (45%) and more common in joints that failed a single surgical debridement (66% compared with 33%; p < 0.001). No other significant differences were observed between the two groups on analysis of culture data (p > 0.05). MRSA = methicillin-resistant S. aureus, MSSA = methicillin-sensitive S. aureus, S. epidermidis = Staphylococcus epidermidis, S. pyogenes = Streptococcus pyogenes, S. agalactiae = Streptococcus agalactiae, S. pneumoniae = Streptococcus pneumoniae, P. aeruginosa = Pseudomonas aeruginosa, S. lugdunensis = Staphylococcus lugdunensis, S. intermedius = Streptococcus intermedius, H. influenzae = Haemophilus influenzae, and E. coli = Escherichia coli.
The most commonly affected joint was the knee (ninety-four, or 71%, of the 132 joints). Other affected joints were the shoulder (seventeen, or 13%), the ankle (eight, or 6%), the elbow (eight, or 6%), the hip (three, or 2%), and the wrist (two, or 2%). Larger joints (knee, shoulder, and hip) were more likely to fail a single surgical debridement compared with smaller joints (elbow, ankle, and wrist) (p = 0.02). Open surgical debridement was performed as the initial surgery for fifty-four (68%) of the seventy-nine patients in the successful single surgical debridement group and thirty-six (73%) of the forty-nine in the failed initial debridement group (p = 0.56).
Forty-nine (38%) of the patients (fifty joints) had a failed single surgical debridement. Thirty-three (67%) of these patients required two procedures, nine (18%) required three procedures, and seven (14%) had four procedures. Twenty-seven (55%) of the forty-nine patients had been discharged and required a hospital readmission for unplanned debridement. The second debridement occurred at a mean of 25.5 days after the initial debridement (range, two to 204 days). Five patients had a recurrence of septic arthritis more than 100 days after the initial debridement. All five recurrences occurred in the same joint and grew the same strain of bacteria identified at the initial debridement and, therefore, are considered a failure of a single debridement. There were four cases of polyarticular septic arthritis; one of these patients failed the initial debridement and three were successfully treated with a single surgery. All four cases involved one knee and one shoulder. The mean duration of follow-up was nine months (range, 1.5 to 69.5 months) for the successful single-surgery group and 4.9 months (range, 1.5 to fourteen months) for the failed single-surgery group.
Multivariate Analysis
We identified five independent predictors that were associated with failure of a single debridement: a history of inflammatory arthropathy (odds ratio [OR], 7.3; 95% confidence interval [CI], 2.4 to 22.6; p < 0.001), the involvement of a large joint (knee, shoulder, or hip) (OR, 7.0; 95% CI, 1.2 to 37.5; p = 0.02), a synovial-fluid nucleated cell count of >85.0 × 109 cells/L (OR, 4.7; 95% CI, 1.8 to 17.7; p = 0.002), S. aureus as the bacterial isolate (OR, 4.6; 95% CI, 1.8 to 11.9; p = 0.002), and a history of diabetes (OR, 2.6; 95% CI, 1.1 to 6.2; p = 0.04) (Table III).
TABLE III.
Multivariate Logistic Regression of Identified Independent Risk Factors for Failure of a Single Surgery in Adults with Acute Septic Arthritis
| Variable | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
| Inflammatory arthropathy | 7.3 | 2.4-22.6 | <0.001 |
| Large joint involvement | 7.0 | 1.2-37.5 | 0.02 |
| Synovial-fluid nucleated cell count >85.0 × 109 cells/L | 4.7 | 1.8-17.7 | 0.002 |
| S. aureus | 4.6 | 1.8-11.9 | 0.002 |
| Diabetes | 2.6 | 1.1-6.2 | 0.04 |
Prognostic Model
We constructed a prognostic model with a scale of six points (range, zero to five points); one point was assigned for each risk factor noted. The predictive probabilities of failure of a single surgery based on this model and the analysis related to our study population are presented in the tables in the Appendix. The area under the ROC curve was calculated to be 0.79 for this model, demonstrating good-to-excellent prognostic capabilities (see Appendix). The Hosmer-Lemeshow goodness-of-fit test did not indicate any departure from a good model fit (p = 0.90).
Discussion
Septic arthritis often can be treated by a single surgical debridement with appropriate antibiotic therapy. Sometimes, however, a patient’s clinical picture worsens and a second debridement becomes necessary. To our knowledge, risk factors for failure of a single surgical debridement have not previously been identified. The purpose of this study was to determine the prevalence of failure of a single surgical debridement, to identify risk factors associated with a failed single surgical debridement for the treatment of acute septic arthritis of a native joint, and to develop a prognostic probability algorithm.
In this study, 62% (eighty-two of 132) of the joint infections were successfully managed with a single debridement. Open debridement was the preferred initial treatment in 68% of affected joints, although we were unable to demonstrate any clinical differences between open and arthroscopic debridement (p > 0.05). This is in agreement with other studies that have demonstrated equal treatment efficacy between open and arthroscopic surgical debridement of the wrist, knee, shoulder, and hip11,15,20-23.
In this study, 36% of joint aspirations did not yield a positive culture result despite a clinical scenario suggestive of septic arthritis. This is comparable with what has been published in other studies, in which the prevalence of no growth on culture has ranged from 0% to 40%8,18. The most common bacterial isolate was S. aureus, accounting for 45% (sixty of 132) of all samples. This is similar to reports from other countries (37% to 56%)12,24,25. When patients with no growth on culture (that is, sterile or negative culture) were excluded from analysis, S. aureus accounted for 68% (fifty-eight of eighty-five) of all positive cultures and was identified as a risk factor for failure in our study. MRSA represented 30% of all cultures, 47% of all positive cultures, and 67% of all S. aureus cultures, but was not an independent risk factor for failure of a single surgery by multivariate analysis. This prevalence of MRSA is substantially higher than previously reported in other studies18,26 and raises concern that the prevalence of MRSA as a cause of septic arthritis continues to rise.
A synovial-fluid nucleated cell count of >50 × 109 cells/L from aspiration is commonly cited as a threshold for diagnosing acute septic arthritis, even in the presence of a sterile Gram stain result15,27. Rashkoff et al. reported a variability from 20 to 150 × 109 cells/L in confirmed cases of septic arthritis28. Our study had a range of 2.0 to 294 × 109 cells/L for patients treated for septic arthritis. A threshold of 50 × 109 may not be as reliable as previously believed.
Our study had several limitations. It was retrospective in design and may have inherent bias from incomplete documentation in the medical record. The surgical procedures were performed by different surgeons, and some variability in patients’ hospital course and treatment may have occurred during the perioperative period. Also, because a portion of the patients had negative cultures, it is possible that some of those treated surgically actually had an inflammatory arthropathy rather than septic arthritis. However, the generally accepted criteria for diagnosis of septic arthritis were used in this study. Although all patients were treated initially with broad-spectrum antibiotics, individual patient medication allergies and operative culture sensitivities meant that the selected antibiotic regimen could not be standardized, and it is beyond the scope of this project to be able to comment on the effectiveness of different antibiotic regimens. It is also possible that patients having one surgical debridement at our institution may have undergone an additional procedure elsewhere; however, this was never documented in their follow-up record.
Our small sample size limits our ability to perform internal validation and may have resulted in an over-fitted algorithm. Furthermore, although our data provide prognostic information on the risk that a patient will fail a single surgery, we do not have any data to support the concept that a planned second surgery, based on risk factors alone, will result in an improved clinical course or lead to a quicker resolution of the infection. Additional prospective studies should be performed to confirm our identified risk factors and prognostic algorithm for the risk of failure of a single surgical debridement in treating acute septic arthritis.
In summary, we found open and arthroscopic debridements similarly effective for the initial treatment of septic arthritis. S. aureus was the most common bacterial isolate (in 45%), and there was a high prevalence of MRSA (30% of all cultures). Adults with a history of inflammatory arthropathy, involvement of a larger joint, a synovial-fluid nucleated cell count of >85.0 × 109 cells/L, infection with S. aureus, or a history of diabetes had a higher risk of failure of a single surgical debridement for acute septic arthritis in a native joint. We believe that the model we developed has prognostic value and may be useful to physicians in counseling their adult patients with acute septic arthritis, especially in the early treatment course.
Appendix
The ROC curve of our prognostic model and tables showing the predicted probabilities of failure of a single surgical debridement and the analysis related to our study population are available with the online version of this article as a data supplement at jbjs.org.
Acknowledgments
Note: The authors acknowledge Katie Evans, PhD, and Susan Messing, PhD, for their assistance with statistical analysis preparation.
Footnotes
Investigation performed at the University of Rochester Medical Center, Rochester, New York
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. None of the authors, or their institution(s), have had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, no author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Chen CM, Lin HH, Hung SC, Huang TF, Chen WM, Liu CL, Chen TH. Surgical treatment for septic arthritis of the knee joint in elderly patients: a 10-year retrospective clinical study. Orthopedics. 2013. April;36(4):e434-43. [DOI] [PubMed] [Google Scholar]
- 2.Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002. October;15(4):527-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riegels-Nielson P, Frimodt-Möller N, Jensen JS. Rabbit model of septic arthritis. Acta Orthop Scand. 1987. February;58(1):14-9. [DOI] [PubMed] [Google Scholar]
- 4.Choi IH, Pizzutillo PD, Bowen JR, Dragann R, Malhis T. Sequelae and reconstruction after septic arthritis of the hip in infants. J Bone Joint Surg Am. 1990. September;72(8):1150-65. [PubMed] [Google Scholar]
- 5.Wada A, Fujii T, Takamura K, Yanagida H, Urano N, Surijamorn P. Operative reconstruction of the severe sequelae of infantile septic arthritis of the hip. J Pediatr Orthop. 2007. December;27(8):910-4. [DOI] [PubMed] [Google Scholar]
- 6.Smith RL, Kajiyama G, Schurman DJ. Staphylococcal septic arthritis: antibiotic and nonsteroidal anti-inflammatory drug treatment in a rabbit model. J Orthop Res. 1997. November;15(6):919-26. [DOI] [PubMed] [Google Scholar]
- 7.Kallio MJ, Unkila-Kallio L, Aalto K, Peltola H. Serum C-reactive protein, erythrocyte sedimentation rate and white blood cell count in septic arthritis of children. Pediatr Infect Dis J. 1997. April;16(4):411-3. [DOI] [PubMed] [Google Scholar]
- 8.Thiery JA. Arthroscopic drainage in septic arthritides of the knee: a multicenter study. Arthroscopy. 1989;5(1):65-9. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Choi NH, Ko SH, Linton JA, Park HW. Arthroscopic treatment of septic arthritis of the hip. Clin Orthop Relat Res. 2003. February;407:211-4. [DOI] [PubMed] [Google Scholar]
- 10.Ivey M, Clark R. Arthroscopic debridement of the knee for septic arthritis. Clin Orthop Relat Res. 1985. October;199:201-6. [PubMed] [Google Scholar]
- 11.Wirtz DC, Marth M, Miltner O, Schneider U, Zilkens KW. Septic arthritis of the knee in adults: treatment by arthroscopy or arthrotomy. Int Orthop. 2001;25(4):239-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996. December;117(3):423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balabaud L, Gaudias J, Boeri C, Jenny JY, Kehr P. Results of treatment of septic knee arthritis: a retrospective series of 40 cases. Knee Surg Sports Traumatol Arthrosc. 2007. April;15(4):387-92. Epub 2006 Dec 6. [DOI] [PubMed] [Google Scholar]
- 14.Abdel MP, Perry KI, Morrey ME, Steinmann SP, Sperling JW, Cass JR. Arthroscopic management of native shoulder septic arthritis. J Shoulder Elbow Surg. 2013. March;22(3):418-21. Epub 2012 Jun 27. [DOI] [PubMed] [Google Scholar]
- 15.Sammer DM, Shin AY. Comparison of arthroscopic and open treatment of septic arthritis of the wrist. J Bone Joint Surg Am. 2009. June;91(6):1387-93. [DOI] [PubMed] [Google Scholar]
- 16.Kaandorp CJ, Van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheum. 1995. December;38(12):1819-25. [DOI] [PubMed] [Google Scholar]
- 17.Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001. January;40(1):24-30. [DOI] [PubMed] [Google Scholar]
- 18.Mehta P, Schnall SB, Zalavras CG. Septic arthritis of the shoulder, elbow, and wrist. Clin Orthop Relat Res. 2006. October;451:42-5. [DOI] [PubMed] [Google Scholar]
- 19.Gupta MN, Sturrock RD, Field M. Prospective comparative study of patients with culture proven and high suspicion of adult onset septic arthritis. Ann Rheum Dis. 2003. April;62(4):327-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarrett MP, Grossman L, Sadler AH, Grayzel AI. The role of arthroscopy in the treatment of septic arthritis. Arthritis Rheum. 1981. May;24(5):737-9. [DOI] [PubMed] [Google Scholar]
- 21.Jeon IH, Choi CH, Seo JS, Seo KJ, Ko SH, Park JY. Arthroscopic management of septic arthritis of the shoulder joint. J Bone Joint Surg Am. 2006. August;88(8):1802-6. [DOI] [PubMed] [Google Scholar]
- 22.Nusem I, Jabur MK, Playford EG. Arthroscopic treatment of septic arthritis of the hip. Arthroscopy. 2006. August;22(8):902.e1-3. [DOI] [PubMed] [Google Scholar]
- 23.Sammer DM, Shin AY. Comparison of arthroscopic and open treatment of septic arthritis of the wrist. Surgical technique. J Bone Joint Surg Am. 2010. March;92(Suppl 1 Pt 1):107-13. [DOI] [PubMed] [Google Scholar]
- 24.Kaandorp CJ, Krijnen P, Moens HJ, Habbema JD, van Schaardenburg D. The outcome of bacterial arthritis: a prospective community-based study. Arthritis Rheum. 1997. May;40(5):884-92. [DOI] [PubMed] [Google Scholar]
- 25.Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997. March;36(3):370-3. [DOI] [PubMed] [Google Scholar]
- 26.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005. April 7;352(14):1436-44. [DOI] [PubMed] [Google Scholar]
- 27.Shmerling RH, Delbanco TL, Tosteson AN, Trentham DE. Synovial fluid tests. What should be ordered? JAMA. 1990. August 22-29;264(8):1009-14. [PubMed] [Google Scholar]
- 28.Rashkoff ES, Burkhalter WE, Mann RJ. Septic arthritis of the wrist. J Bone Joint Surg Am. 1983. July;65(6):824-8. [PubMed] [Google Scholar]