Abstract
Background:
Extraskeletal osteosarcoma is a rare soft-tissue sarcoma about which little is known. The objectives of this study were to describe the clinical features and natural history of extraskeletal osteosarcoma and to investigate factors affecting outcomes.
Methods:
A retrospective review of a prospectively maintained database of patients diagnosed with soft-tissue sarcoma was conducted. Patients with pathologically confirmed extraskeletal osteosarcoma from 1982 to 2012 were identified and were included in the analysis. Medical records were reviewed for clinical features, treatment, and outcomes.
Results:
Fifty-three patients were identified from the database: forty-two presented with localized disease, two presented with metastatic disease, and nine presented with recurrent (local and/or distant) disease. The median patient age at diagnosis was sixty-four years, with a median follow-up time of thirty-four months (range, one to 290 months) for survivors. Of the fifty-three patients who were identified, forty-one had lesions in the extremities, fifty-one had high-grade lesions, forty had lesions >5 cm, and forty-two had deep lesions. For patients presenting with localized disease, the median survival was 45.8 months with a three-year cumulative incidence of death due to disease of 39%. All patients with localized disease were managed with surgical resection of the primary tumor: nineteen with surgery only, ten with adjuvant radiation, five with adjuvant chemotherapy, and eight with both radiation and chemotherapy. Eighteen patients relapsed: two patients had local recurrences, ten patients had distant metastases, and six patients had local recurrences and distant metastases. In log-rank analysis, patients with superficial tumors and negative margins at resection had a higher three-year event-free survival. No significant association of disease-specific or event-free survival was found with the addition of radiation, chemotherapy, or both to surgery.
Conclusions:
For patients presenting with localized extraskeletal osteosarcoma, three-year event-free survival was higher for patients with superficial tumors and negative margins at resection. Radiation and chemotherapeutic treatment were not associated with a lower incidence of death due to disease or a longer event-free survival.
Level of Evidence:
Prognostic Level III. See Instructions for Authors for a complete description of levels of evidence.
Extraskeletal osteosarcoma is a malignant mesenchymal neoplasm that produces osteoid, bone, or chondroid material without demonstrable attachments to bone or periosteum1. The diagnosis of extraskeletal osteosarcoma is very rare and represents 2% to 5% of osteosarcomas2-6 and <1% of all soft-tissue sarcomas2,5,6. No more than an estimated 400 cases have been described in the literature2-14. Most published reports are case reports or small series, with the largest series consisting of eighty-eight cases7. Only two series have reviewed cases from prospectively gathered data6,12.
As a result, little is known regarding the clinical features or natural history of extraskeletal osteosarcoma. Compared with primary osteosarcoma of bone, patients with extraskeletal osteosarcoma are older, typically in the sixth decade of life. Outcomes have historically been poor, with high rates of metastatic disease and overall survival at five years ranging from 25% to 77%10,12.
Even less is understood about the impact of adjuvant treatments, and no published study to date has demonstrated the effectiveness of radiation or chemotherapy. The purpose of our study was to review our institution’s experience with extraskeletal osteosarcoma over a thirty-year period to better characterize the clinical course and outcomes of patients with surgically treated, localized extraskeletal osteosarcoma.
Materials and Methods
After obtaining a waiver of patient informed consent requirements from our institutional review board, we conducted a retrospective review of a prospectively maintained database of patients who received sarcoma-related care at our cancer center. We searched for all patients who were diagnosed with extraskeletal osteosarcoma from July 1982 through May 2012. Extraskeletal osteosarcoma was defined as a soft-tissue sarcoma histologically indistinguishable from primary osteosarcoma of bone, without involvement of bone or periosteum. The lack of osseous or periosteal involvement was verified intraoperatively or by pathological analysis. Cases involving visceral organs, such as breast and prostate, were excluded because epithelial components suggestive of carcinosarcoma could not be ruled out. Patients with malignant mesenchymomas with osteosarcomatous components, dedifferentiated tumors with osteogenic features, or a history of osteosarcoma of bone were also excluded. A comprehensive review of the medical records was performed. Data regarding patient characteristics, tumor characteristics, medical history, treatment, follow-up, and clinical outcome were collected.
The primary tumor site was classified as upper extremity (including shoulder girdle), lower extremity (including gluteal compartment and groin), or axial (including chest wall, pelvis, perineum, and head or neck). Size was defined by the maximum diameter measured on the gross specimen. Tumors were defined as deep if they were located deep to the investing fascia of the given compartment. Margin status was defined as R0 if it was microscopically negative or R1 if it was microscopically positive at initial resection.
Statistical Analysis
Patient characteristics are summarized by frequency and percentages for categorical variables and median value for continuous variables. Event-free survival was evaluated with local recurrence, distant recurrence, and disease-specific death as events. The Kaplan-Meier method and log-rank test were used to compare event-free survival rates between groups. Competing risks analysis was used to compare the cumulative incidence of death due to disease between groups. Death due to other causes was treated as a competing event. Event times were calculated from the date of diagnosis to the first event or the latest follow-up. The variables analyzed included age (more than fifty years compared with fifty years or less), sex, primary site (extremity compared with axial), tumor grade (low compared with high), size (>5 cm compared with ≤5 cm), depth (superficial compared with deep), resection margin status (R1 compared with R0), history of radiation, and treatment type (surgery alone or with adjuvant chemotherapy and/or radiation therapy). All analysis was conducted with use of R software (version 3.0.0; R Project, Vienna, Austria), including the survival and cmprsk packages.
Source of Funding
There was no external source of funding for this study.
Results
Patient and Tumor Characteristics
Fifty-three patients were identified with pathologically confirmed extraskeletal osteosarcoma and were included in this study. The median follow-up time from diagnosis was thirty-three months (range, one to 337 months) for the overall patient cohort and thirty-four months (range, one to 290 months) for the twenty-three patients who were alive at the time of the latest follow-up. The overall cohort consisted of twenty-three male patients (43%) and thirty female patients (57%). The median patient age at diagnosis was sixty-four years (range, ten to eighty-eight years) (see Appendix).
Forty-one patients (77.4%) presented with primary sites in the lower extremity, six patients (11.3%) presented with primary sites in the upper extremity, and six patients (11.3%) presented with primary sites in an axial location. Two tumors (3.8%) were low grade. With respect to size, thirteen tumors (24.5%) were ≤5 cm, twenty tumors (37.7%) were between >5 cm and ≤10 cm, and twenty tumors (37.7%) were >10 cm. Eleven tumors (21%) were located superficially in the subcutaneous tissues (Table I).
TABLE I.
Summary of Patient Demographics and Tumor Characteristics
| Demographics and Characteristics | All Patients (N = 53) | Patients with Localized Disease (N = 42) |
| Patient age*(yr) | 64 | 64.5 |
| Patient sex† | ||
| Male | 23 (43) | 19 (45) |
| Female | 30 (57) | 23 (55) |
| Site of primary tumor† | ||
| Upper extremity | 6 (11.3) | 6 (14.3) |
| Lower extremity | 41 (77.4) | 31 (73.8) |
| Axial | 6 (11.3) | 5 (11.9) |
| Tumor grade† | ||
| Low | 2 (3.8) | 2 (4.8) |
| High | 51 (96.2) | 40 (95.2) |
| Tumor size† | ||
| ≤5 cm | 13 (24.5) | 11 (26.2) |
| >5 cm to ≤10 cm | 20 (37.7) | 14 (33.3) |
| >10 cm | 20 (37.7) | 17 (40.5) |
| Tumor depth† | ||
| Superficial | 11 (21) | 10 (24) |
| Deep | 42 (79) | 32 (76) |
The value is given as the median.
The values are given as the number of patients, with the percentage in parentheses.
Four (7.5%) of fifty-three patients had a history of radiation therapy: three for a malignant diagnosis and one for a benign condition. One patient was managed with radiation for uterine cancer, subsequently developed a sarcoma in the groin eight years later, and presented to our institution with recurrent disease. The second patient was managed with radiation for inguinal metastatic neuroendocrine carcinoma and developed a sarcoma in the groin area ten years later. The third patient had a history of breast carcinoma treated with lumpectomy and radiation and developed a sarcoma in the chest wall nine years later. The fourth patient had a history of radiation to the face for acne and developed a subcutaneous sarcoma on the side of the nose; however, the interval between radiation and development of sarcoma for this patient could not be determined from the medical record.
At presentation to our institution, forty-two patients had localized disease and two had metastatic disease and no prior treatment. Nine patients presented with recurrent disease after undergoing surgical resection of the primary site at another facility. Of these nine patients, three presented with a local recurrence, two presented with a distant recurrence, and four presented with both local and distant recurrence.
Patient and tumor characteristics for the forty-two patients presenting with localized disease are summarized in Table I and are similar to the overall patient group. The median age was 64.5 years (range, ten to eighty-eight years) and there was a slight female preponderance. The lower extremity was the most common site and the tumors were mostly high grade, >5 cm, and deep.
Treatment of Localized Disease
All forty-two patients underwent surgical resection of the primary tumor. Prior to presentation at our institution, fourteen patients had had no prior treatment, eighteen patients had undergone biopsy only, and ten patients had had resection of the primary tumor. Of these ten patients, eight underwent re-resection of the primary site. Re-resection was performed for incomplete resections in four cases and for margins that could not be verified at our institution in the remaining four cases. No residual tumor was identified in the four specimens obtained from the re-resection procedures conducted for ambiguous margins at the initial resection.
Of the thirty-two patients with initial resection of the primary site at our institution, seven (22%) had microscopically positive margins. Two of these cases were re-resected with no residual tumor identified in the re-resected specimens. A summary of treatment modalities for the study cohort is presented in Figure 1.
Fig. 1.
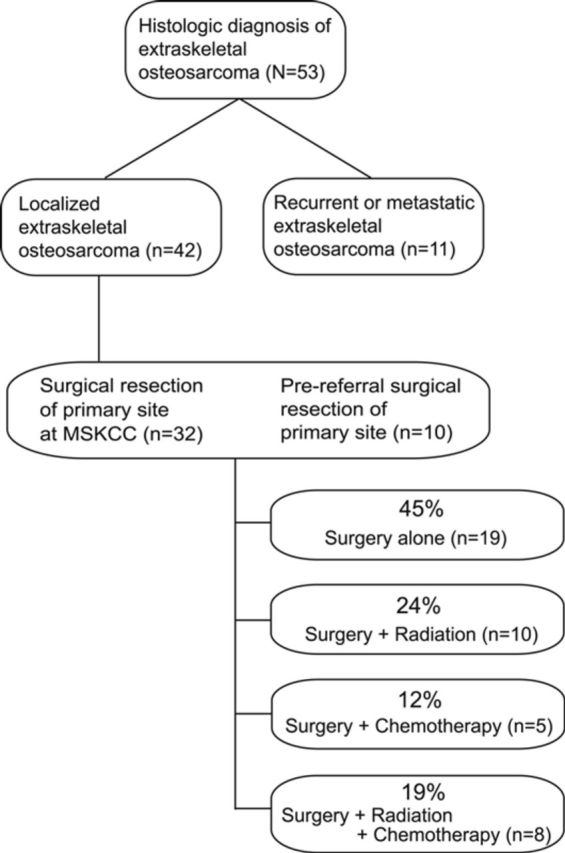
Flowchart showing diagnoses and treatment modalities in patients with extraskeletal osteosarcoma. MSKCC = Memorial Sloan-Kettering Cancer Center.
Nineteen patients with localized disease were treated with surgery alone. Ten patients received adjuvant radiation therapy and no chemotherapy: two patients received brachytherapy and eight patients were managed with postoperative radiation therapy. None received preoperative radiation therapy.
Five patients received adjuvant chemotherapy without radiation. All but one received preoperative chemotherapy. Chemotherapy was primarily doxorubicin-based but varied widely.
Eight patients received both adjuvant chemotherapy and radiation. Four patients received brachytherapy, one received preoperative radiation, and three received postoperative radiation therapy. Chemotherapy was administered preoperatively to two patients and postoperatively to six patients.
Overall Outcome
At the latest follow-up, twenty-one patients had died of disease, all of whom developed pulmonary metastases at some point during follow-up. Three patients died of disease-unrelated causes without developing a recurrence and six patients died of unknown causes, one with local recurrent disease and one with distant recurrent disease. One patient was alive with disease at the latest follow-up and twenty-two were alive with no evidence of disease.
Outcome of Localized Disease
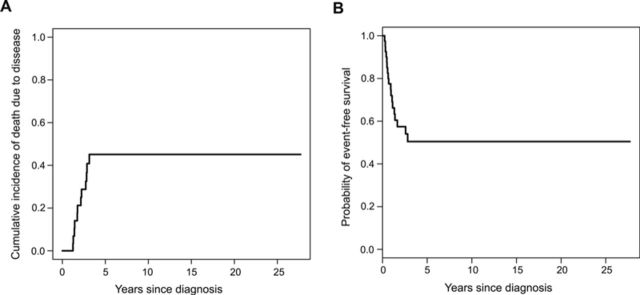
Among the forty-two patients presenting with localized disease, fourteen had died of disease, two had died of causes unrelated to disease, and five had died of unknown causes at the time of the latest follow-up. No patients were alive with disease at the time of the latest follow-up and twenty-one patients were alive with no evidence of disease. The median follow-up time of survivors was thirty-four months. The cumulative incidence of death due to disease was a three-year incidence of 39% (Fig. 2-A). A significantly lower incidence of death due to disease (p = 0.01) was found when patients with superficial tumors (0%) were compared with those with deep tumors (52%). No significant difference between subgroups for other variables was found.
Fig. 2.
Kaplan-Meier curves showing cumulative incidence of death due to disease (Fig. 2-A) and event-free survival (Fig. 2-B) for forty-two patients with localized extraskeletal osteosarcoma. The three-year cumulative incidence of death due to disease was 39%, and the median event-free survival was 45.8 months.
Eighteen patients experienced a relapse: two had a local recurrence, ten had a distant recurrence (lung), and six recurred at both local and distant sites. Of these last six patients, one had both local and distant recurrences identified at the same time, three recurred locally first, and two were found to have metastatic disease at first relapse. Overall, eight patients (19%) had local recurrence and sixteen patients (38%) developed metastases, all to the lung. No relapses were observed after thirty-five months. The Kaplan-Meier curve for three-year event-free survival was 50%; the median survival was 45.8 months (Fig. 2-B). Event-free survival was better in patients with superficial tumors compared with those with deep tumors and in patients with R0 margin status compared with those with R1 margin status. Because of the small sample size, the effect of margin status on local or distant recurrence could not be distinguished. Adjuvant treatment was not associated with decreased incidence of death due to disease or event-free survival. The results of competing risks analysis comparing the cumulative incidence of death due to disease are summarized in Table II and the results of the log-rank test comparing event-free survival rates are summarized in Table III.
TABLE II.
Competing Risks Analysis Comparing the Cumulative Incidence of Death Due to Disease in Patients with Localized Extraskeletal Osteosarcoma
| Variable | No. of Patients | No. of Events | Three-Year Cumulative Incidence of Death Due to Disease* | P Value |
| All patients | 42 | 14 | 0.39 (0.22 to 0.56) | — |
| Patient age | 0.12 | |||
| Fifty years or less | 11 | 1 | 0.14 (0.00 to 0.42) | |
| More than fifty years | 31 | 13 | 0.45 (0.25 to 0.65) | |
| Patient sex | 0.53 | |||
| Female | 23 | 8 | 0.47 (0.21 to 0.72) | |
| Male | 19 | 6 | 0.30 (0.07 to 0.53) | |
| Site of primary tumor | 0.73 | |||
| Axial | 5 | 1 | 0.38 (0.00 to 1.00) | |
| Extremity | 37 | 13 | 0.39 (0.21 to 0.57) | |
| Tumor grade | 0.25 | |||
| Low | 2 | 0 | 0.00 (0.00 to 0.00) | |
| High | 40 | 14 | 0.41 (0.23 to 0.59) | |
| Tumor size | 0.20 | |||
| ≤5 cm | 11 | 2 | 0.21 (0.00 to 0.49) | |
| >5 cm | 31 | 12 | 0.45 (0.24 to 0.65) | |
| Tumor depth | 0.01 | |||
| Superficial | 10 | 0 | 0.00 (0.00 to 0.00) | |
| Deep | 32 | 14 | 0.52 (0.31 to 0.73) | |
| Resection margin status | 0.99 | |||
| R0 | 35 | 12 | 0.39 (0.21 to 0.58) | |
| R1 | 7 | 2 | 0.38 (0.00 to 0.85) | |
| Patient history of radiation therapy | 0.20 | |||
| No | 39 | 14 | 0.42 (0.24 to 0.61) | |
| Yes | 3 | 0 | 0.00 (0.00 to 0.00) | |
| Treatment type | 0.30 | |||
| Surgery alone | 19 | 5 | 0.36 (0.09 to 0.62) | |
| Surgery and chemotherapy | 5 | 0 | 0.00 (0.00 to 0.00) | |
| Surgery, radiation therapy, and chemotherapy | 8 | 3 | 0.40 (0.01 to 0.79) | |
| Surgery and radiation therapy | 10 | 6 | 0.55 (0.19 to 0.91) |
The values are given as the three-year cumulative incidence of death due to disease, with the 95% confidence interval shown in parentheses.
TABLE III.
Log-Rank Test Comparing Event-Free Survival Rates in Patients with Localized Extraskeletal Osteosarcoma
| Variable | No. of Patients | No. of Events | Three-Year Event-Free Survival Rate* | P Value |
| All patients | 42 | 18 | 0.50 (0.36 to 0.70) | — |
| Patient age | 0.09 | |||
| Fifty years or less | 11 | 2 | 0.75 (0.50 to 1.00) | |
| More than fifty years | 31 | 16 | 0.42 (0.27 to 0.67) | |
| Patient sex | 0.73 | |||
| Female | 23 | 10 | 0.49 (0.30 to 0.78) | |
| Male | 19 | 8 | 0.53 (0.34 to 0.83) | |
| Site of primary tumor | 0.12 | |||
| Axial | 5 | 3 | NA† | |
| Extremity | 37 | 15 | 0.54 (0.39 to 0.75) | |
| Tumor grade | 0.93 | |||
| Low | 2 | 1 | 0.50 (0.13 to 1.00) | |
| High | 40 | 17 | 0.50 (0.36 to 0.71) | |
| Tumor size | 0.26 | |||
| ≤5 cm | 11 | 3 | 0.70 (0.47 to 1.00) | |
| >5 cm | 31 | 15 | 0.43 (0.27 to 0.68) | |
| Tumor depth | 0.03 | |||
| Superficial | 10 | 1 | 0.89 (0.71 to 1.00) | |
| Deep | 32 | 17 | 0.38 (0.23 to 0.63) | |
| Resection margin status | ||||
| R0 | 35 | 13 | 0.57 (0.41 to 0.78) | 0.03 |
| R1 | 7 | 5 | 0.17 (0.03 to 1.00) | |
| Patient history of radiation therapy | 0.08 | |||
| No | 39 | 16 | 0.53 (0.38 to 0.73) | |
| Yes | 3 | 2 | NA† | |
| Treatment type | 0.83 | |||
| Surgery alone | 19 | 7 | 0.55 (0.34 to 0.88) | |
| Surgery and chemotherapy | 5 | 1 | 0.80 (0.52 to 1.00) | |
| Surgery, radiation therapy, and chemotherapy | 8 | 4 | 0.50 (0.25 to 1.00) | |
| Surgery and radiation therapy | 10 | 6 | 0.36 (0.15 to 0.87) |
The values are given as the three-year event-free survival rate, with the 95% confidence interval in parentheses.
NA = not available. These values are not estimable for axial sites and those with prior radiation therapy because the last event occurred before year 3.
Compared with the outcomes in the overall localized disease cohort, the survival rates among the thirty-two patients who underwent primary surgery at our institution were similar, with a three-year cumulative incidence of death due to disease of 41% and an event-free survival of 44%. Patients with superficial tumors had a significantly lower incidence of death due to disease at three years and patients with R0 margin status tended toward higher event-free survival (see Appendix).
Discussion
Extraskeletal osteosarcoma is a rare soft-tissue sarcoma and constitutes 0.6% of all soft-tissue sarcomas followed prospectively at our institution. To our knowledge, this study represents the largest series of patients managed at a single institution analyzed to date (see Appendix). The patient and tumor characteristics of our cohort are similar to those previously reported. The median age in our series was sixty-four years, consistent with the reported range of fifty to sixty-seven years10,13. Extraskeletal osteosarcoma is reportedly uncommon under the age of thirty years5,7,8 and only four patients in our series were in this age group. We found a slight female predominance in our series; however, sex distributions vary5,9. As in other series, the most common site was the lower extremity (77.4%) and the majority of primary tumors were deep to the superficial compartmental fascia (79%).
Compared with primary osteosarcoma of bone, several characteristics of extraskeletal osteosarcoma are markedly different. Primary osteosarcoma of bone occurs in much younger patients, with a peak incidence in the second decade of life compared with the seventh decade seen in our series of patients with extraskeletal osteosarcoma. Additionally, the male predominance seen in primary osteosarcoma is not found among patients with extraskeletal osteosarcoma. Finally, primary osteosarcoma of bone is the most common malignant bone tumor, in sharp contrast to the rarity of extraskeletal osteosarcoma among soft-tissue sarcomas.
Extraskeletal osteosarcoma is generally considered a high-grade lesion9,10. To date, less than ten cases of low-grade extraskeletal osteosarcoma have been reported15,16. High rates of survival have been reported for low-grade osteosarcoma of bone17; however, an improved prognosis in this small subset of patients in extraskeletal osteosarcoma has not been demonstrated. Some have speculated that complete surgical resection alone can lead to prolonged remissions15. Our series included two patients with low-grade tumors. One underwent complete surgical resection without adjuvant treatment and remained disease-free for more than twenty years after initial diagnosis. The second patient developed extraskeletal osteosarcoma after radiation for a benign condition. The primary tumor was initially misdiagnosed and was incompletely excised. Disease recurred locally twice within two years and the patient underwent repeat surgical resections, as well as chemotherapy after his first recurrence. The recurrent lesions had no features of dedifferentiation. This patient died of unknown causes but without evidence of disease four years after initial diagnosis. These cases highlight the importance of complete surgical resection.
Radiation therapy is a known predisposing factor for the development of bone and soft-tissue sarcomas, including extraskeletal osteosarcoma18. A history of radiation therapy has been reported in 5% to 10% of cases of extraskeletal osteosarcoma, with the development of secondary sarcoma after an average of fifteen years5,9. In our series, four (7.5%) of fifty-three patients had received radiation therapy, on average, nine years prior to presentation. Although the poor prognosis of radiation-induced soft-tissue sarcomas has been established19,20, we were unable to demonstrate any significant difference in outcome in these patients, likely because of the small number of patients with secondary sarcomas.
Extraskeletal osteosarcoma has high rates of local recurrence and distant metastases; the reported rates were 69% for local recurrence and 80% for distant metastases in a previously published series5. We had a local recurrence rate of 19% and a distant recurrence rate of 38% among patients presenting with localized disease. These figures are comparable with those found in a similar group of patients with localized disease reported by Ahmad et al. in 2002, who found a local recurrence rate of 20% and distant recurrence rate of 37%11. Overall survival has also been reportedly poor, ranging from 25% to 76% at five years5,12. Our incidence of death due to disease was 39% at three years.
In addition to patient age, the prognostic significance of tumor grade, size, and location in localized soft-tissue sarcoma of the extremities has been demonstrated21. We were unable to demonstrate any association of these factors with outcomes, likely because of the small sample size. Positive margin status was associated with a higher risk of local and systemic relapse but was not associated with lower incidence of death due to disease. Larger series of soft-tissue sarcomas have also demonstrated a lack of association between positive margin and outcome22,23. With respect to extraskeletal osteosarcoma, previous studies have only shown large tumor size and positive margins to be significant prognostic factors8,11.
At our institution, the current approach to the treatment of extraskeletal osteosarcoma is similar to that used for other soft-tissue sarcomas. Patients with localized disease are treated with wide surgical resection of the primary site. Adjuvant radiation and chemotherapy are considered for high-risk lesions (high grade, large) and for incomplete resections. This treatment strategy is reflected in this series of localized extraskeletal osteosarcoma in that patients with low-grade lesions were initially managed with surgery alone and 50% of those lesions initially treated with surgery alone were ≤5 cm. In contrast, only 8% of lesions in patients receiving adjuvant therapy were ≤5 cm. This bias in selection likely influenced our ability to demonstrate associations with benefit of adjuvant therapy (or lack thereof) in this series.
Adjuvant radiation therapy decreases local recurrence in soft-tissue sarcomas. However, most studies regarding radiation and soft-tissue sarcoma do not include cases of extraskeletal osteosarcoma, if specified at all24. In our series, eighteen (43%) of forty-two patients with localized disease had radiation as part of their treatment, but no difference in death due to disease or event-free survival was found.
Limited data exist regarding adjuvant chemotherapy for extraskeletal osteosarcoma. Two reports from the same institution have suggested that the response to doxorubicin-based chemotherapy was low and cisplatin-based chemotherapy had limited therapeutic benefit11,25. A third study suggested that the response rate to chemotherapy in extraskeletal osteosarcoma was at least as favorable as that in soft-tissue sarcoma in general; however, the chemotherapeutic regimens were heterogeneous13. In contrast, a higher five-year survival rate of 76% was seen in a series of seventeen patients managed with a chemotherapeutic regimen designed for primary osteosarcoma of bone12. These patients were, on average, younger, with a median age of forty-four years. These results parallel the improved median survival rate of patients older than forty years with primary osteosarcoma of bone treated with both surgery and chemotherapy26. Only 36% of patients in our series received adjuvant chemotherapy, and no difference in overall or event-free survival was found.
These studies highlight the uncertainty in the treatment of extraskeletal osteosarcoma. In most published series, its treatment has been similar to that of other soft-tissue sarcomas. Because extraskeletal osteosarcoma is histologically indistinguishable from primary osteosarcoma of bone, it is tempting to attribute a similar pathophysiology to both diseases. This remains to be established, but there may be a rationale to use bone osteogenic sarcoma chemotherapy if systemic therapy is needed. Further study of the role of adjuvant therapy in extraskeletal osteosarcoma is certainly warranted.
Appendix
A figure showing a bar graph of the age distribution of patients diagnosed with extraskeletal osteosarcoma and tables showing (1) a competing risks analysis comparing the cumulative incidence of death due to disease in patients with localized extraskeletal osteosarcoma and primary surgical treatment at our institution, (2) a log-rank test comparing event-free survival rates in patients with localized extraskeletal osteosarcoma and primary surgical treatment at our institution, and (3) a summary of published series on extraskeletal osteosarcoma are available with the online version of this article as a data supplement at jbjs.org.
Investigation performed at the Department of Surgery and the Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY
Peer Review: This article was reviewed by the Editor-in-Chief and one Deputy Editor, and it underwent blinded review by two or more outside experts. The Deputy Editor reviewed each revision of the article, and it underwent a final review by the Editor-in-Chief prior to publication. Final corrections and clarifications occurred during one or more exchanges between the author(s) and copyeditors.
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. One or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Enzinger FM Weiss SW. Soft tissue tumors. St. Louis: CV Mosby; 1983:733-41. [Google Scholar]
- 2.Allan CJ Soule EH. Osteogenic sarcoma of the somatic soft tissues. Clinicopathologic study of 26 cases and review of literature. Cancer. 1971 May;27(5):1121-33. [DOI] [PubMed] [Google Scholar]
- 3.Lorentzon R Larsson SE Boquist L. Extra-osseous osteosarcoma: a clinical and histopathological study of four cases. J Bone Joint Surg Br. 1979 May;61(2):205-8. [DOI] [PubMed] [Google Scholar]
- 4.Rao U Cheng A Didolkar MS. Extraosseous osteogenic sarcoma: clinicopathological study of eight cases and review of literature. Cancer. 1978 Apr;41(4):1488-96. [DOI] [PubMed] [Google Scholar]
- 5.Sordillo PP Hajdu SI Magill GB Golbey RB. Extraosseous osteogenic sarcoma. A review of 48 patients. Cancer. 1983 Feb 15;51(4):727-34. [DOI] [PubMed] [Google Scholar]
- 6.McCarter MD Lewis JJ Antonescu CR Brennan MF. Extraskeletal osteosarcoma: analysis of outcome of a rare neoplasm. Sarcoma. 2000;4(3):119-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung EB Enzinger FM. Extraskeletal osteosarcoma. Cancer. 1987 Sep 1;60(5):1132-42. [DOI] [PubMed] [Google Scholar]
- 8.Bane BL Evans HL Ro JY Carrasco CH Grignon DJ Benjamin RS Ayala AG. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990 Jun 15;65(12):2762-70. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS Fetsch JF Wasdhal DA Lee BP Pritchard DJ Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995 Dec 1;76(11):2253-9. [DOI] [PubMed] [Google Scholar]
- 10.Lidang Jensen M Schumacher B Myhre Jensen O Steen Nielsen O Keller J. Extraskeletal osteosarcomas: a clinicopathologic study of 25 cases. Am J Surg Pathol. 1998 May;22(5):588-94. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad SA Patel SR Ballo MT Baker TP Yasko AW Wang X Feig BW Hunt KK Lin PP Weber KL Chen LL Zagars GK Pollock RE Benjamin RS Pisters PW. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002 Jan 15;20(2):521-7. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein-Jackson SY Gosheger G Delling G Berdel WE Exner GU Jundt G Machatschek JN Zoubek A Jürgens H Bielack SS; Cooperative Osteosarcoma Study Group COSS. Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. J Cancer Res Clin Oncol. 2005 Aug;131(8):520-6. Epub 2005 May 26. [DOI] [PubMed] [Google Scholar]
- 13.Torigoe T Yazawa Y Takagi T Terakado A Kurosawa H. Extraskeletal osteosarcoma in Japan: multiinstitutional study of 20 patients from the Japanese Musculoskeletal Oncology Group. J Orthop Sci. 2007 Sep;12(5):424-9. Epub 2007 Sep 28. [DOI] [PubMed] [Google Scholar]
- 14.Lee S Lee MR Lee SJ Ahn HK Yi J Yi SY Seo SW Sung KS Park JO Lee J. Extraosseous osteosarcoma: single institutional experience in Korea. Asia Pac J Clin Oncol. 2010 Jun;6(2):126-9. [DOI] [PubMed] [Google Scholar]
- 15.Sabatier R Bouvier C de Pinieux G Sarran A Brenot-Rossi I Pedeutour F Chetaille B Viens P Weiller PJ Bertucci F. Low-grade extraskeletal osteosarcoma of the chest wall: case report and review of literature. BMC Cancer. 2010;10:645 Epub 2010 Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyriazoglou AI Vieira J Dimitriadis E Arnogiannaki N Teixeira MR Pandis N. 12q amplification defines a subtype of extraskeletal osteosarcoma with good prognosis that is the soft tissue homologue of parosteal osteosarcoma. Cancer Genet. 2012 Jun;205(6):332-6. [DOI] [PubMed] [Google Scholar]
- 17.Schwab JH Antonescu CR Athanasian EA Boland PJ Healey JH Morris CD. A comparison of intramedullary and juxtacortical low-grade osteogenic sarcoma. Clin Orthop Relat Res. 2008 Jun;466(6):1318-22. Epub 2008 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiklund TA Blomqvist CP Räty J Elomaa I Rissanen P Miettinen M. Postirradiation sarcoma. Analysis of a nationwide cancer registry material. Cancer. 1991 Aug 1;68(3):524-31. [DOI] [PubMed] [Google Scholar]
- 19.Cha C Antonescu CR Quan ML Maru S Brennan MF. Long-term results with resection of radiation-induced soft tissue sarcomas. Ann Surg. 2004 Jun;239(6):903-9; discussion 909-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladdy RA Qin LX Moraco N Edgar MA Antonescu CR Alektiar KM Brennan MF Singer S. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol. 2010 Apr 20;28(12):2064-9. Epub 2010 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisters PW Leung DH Woodruff J Shi W Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996 May;14(5):1679-89. [DOI] [PubMed] [Google Scholar]
- 22.Novais EN Demiralp B Alderete J Larson MC Rose PS Sim FH. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res. 2010 Nov;468(11):3003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stojadinovic A Leung DH Hoos A Jaques DP Lewis JJ Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002 Mar;235(3):424-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang JC Chang AE Baker AR Sindelar WF Danforth DN Topalian SL DeLaney T Glatstein E Steinberg SM Merino MJ Rosenberg SA. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998 Jan;16(1):197-203. [DOI] [PubMed] [Google Scholar]
- 25.Patel SR Benjamin RS. Primary extraskeletal osteosarcoma—experience with chemotherapy. J Natl Cancer Inst. 1995 Sep 6;87(17):1331-3. [DOI] [PubMed] [Google Scholar]
- 26.Manoso MW Healey JH Boland PJ Athanasian EA Maki RG Huvos AG Morris CD. De novo osteogenic sarcoma in patients older than forty: benefit of multimodality therapy. Clin Orthop Relat Res. 2005 Sep;438:110-5. [DOI] [PubMed] [Google Scholar]