Abstract
BACKGROUND:
The safety and efficacy of intrathecal drug delivery systems (IDDSs) for the treatment of cancer-related pain have been demonstrated in randomized controlled clinical trials (RCTs). Despite positive evidence for this therapy, IDDS remains underutilized to treat cancer pain. Real-world registry data augment existing safety and effectiveness data and are presented here to broaden awareness of this therapeutic option, needed for adequate cancer-related pain treatment, and as a viable tool addressing concerns with systemic opioid use.
METHODS:
This prospective, long-term, multicenter (United States, Western Europe, and Latin America) registry started in 2003 to monitor the performance of SynchroMed Infusion Systems. Patient-reported outcomes were added in 2013. Before data acquisition, all sites obtained Ethics Committee/Institutional Review Board approval and written patient consent. The study was registered (NCT01524276 at clinicaltrials.gov) before patients were enrolled. Patients who provided informed consent were enrolled in the registry at initial IDDS implant or replacement.
RESULTS:
Through July 2017, 1403 patients with cancer pain were enrolled and implanted. The average (minimum/maximum) age of patients was 59 years (13/93 years), with 56.6% female. The most frequent cancer types were lung, breast, colon/rectal, pancreatic, and prostate. The majority of patients whose registry follow-up ended (87%; 1141/1311) were followed through death, with 4.3% (n = 57) exiting due to device explant or therapy discontinuation; the remaining 113 (8.6%) discontinued for reasons such as transfer of care, lost to follow-up, and site closure. Pain scores within the cohort of patients providing baseline and follow-up data improved significantly at 6 (P = .0007; n = 103) and 12 (P = .0026; n = 55) months compared to baseline, with EuroQol with 5 dimensions (EuroQol-5D) scores showing significant improvement at 6 months (P = .0016; n = 41). Infection requiring surgical intervention (IDDS explant, replacement, pocket revision, irrigation and debridement, etc) was reported in 3.2% of patients.
CONCLUSIONS:
Adequate and improved pain control in patients with cancer, even in advanced stages, with concurrent quality of life maintenance is attainable. Results from this large-scale, multicenter, single-group cohort supplement existing RCT data that support IDDS as a safe and effective therapeutic option with a positive benefit–risk ratio in the treatment of cancer pain.
See Editorial, p 286
KEY POINTS.
Question: Do real-world registry data support the use of intrathecal drug delivery system (IDDS) as a treatment option for cancer-related pain?
Findings: Overall device performance and safety along with positive outcomes in pain management and quality of life for the subset of patients available for analysis were demonstrated.
Meaning: Real-world data and patient outcomes presented here add to the available evidence supporting the use of IDDS for cancer-related pain.
PLAIN LANGUAGE SUMMARY
Based on a study of 1403 patients, cancer-related pain can be successfully and safely treated with medication delivery by an implantable pump directly to the spinal cord.
The World Health Organization (WHO) cancer pain treatment guidelines1 identified inadequate cancer pain management as a global health concern, with the WHO analgesic ladder developed to support stepwise progression to strong opioids as necessary to adequately control cancer-related pain. The 2018 National Comprehensive Cancer Network (NCCN) pain guidelines recommend a similar stepwise algorithm, and link survival to disease/symptom control, including pain management, and to quality of life.2 Recent studies, however, indicate that cancer pain remains undertreated in 25%3 to 77%4 of patients (lack of adherence to WHO guidelines), with undertreatment rates unchanged over the past 20 years.5 In addition, the 5-year survival rate for all cancer types has increased to 65%,6 and many survivors experience chronic pain—with the American Society of Clinical Oncology (ASCO) policy indicating their possible need for long-term use of opioids.7,8 The current US opioid epidemic has increased scrutiny of systemic opioid use,9 so finding acceptable alternative treatments for refractory cancer pain and chronic pain is pressing.
Intrathecal drug delivery systems (IDDSs) administer Food and Drug Administration (FDA)-approved preservative-free morphine sulfate or ziconotide directly to the spinal cord. IDDS has demonstrated improved cancer pain management compared to conventional medical management (CMM) and placebo10–12 and in retrospective and observational studies13,14 for patients unresponsive to escalating systemic opioid doses or experiencing intolerable side effects. For those patients, IDDS facilitates systemic medication reduction or elimination with associated risk reduction13,15 and significant cost savings.16 This single-group cohort study presents a compilation of 14 years of observational IDDS data, prospectively collected and monitored for compliance, on 1403 registry participants with cancer pain.
METHODS
This study followed appropriate guidelines for a cohort study (Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] checklist). Before data acquisition, all sites obtained Ethics Committee/Institutional Review Board approval and patient consents for all subjects. The study was registered before patients were enrolled (NCT01524276; responsible party: Medtronic; date of registration: January 2012).
Registry Description
The Implantable Systems Performance Registry (ISPR; ClinicalTrials.gov Identifier: NCT01524276), initiated in 2003, is described in detail by Konrad et al.17 Results presented here include data collected on patients implanted with the SynchroMed II Infusion System (Medtronic, Inc, Minneapolis, MN) and enrolled in the ISPR (2003–2012) as well as those implanted and enrolled in the 2013 amended registry, referred to as the “Product Surveillance Registry (PSR),” which is ongoing and collectively referred to as the “registry.” The registry platform was designed to conduct ongoing nonrandomized, active prospective postmarket surveillance under a common protocol with specific appendices for neuromodulation products/therapies, enrolling patients with eligible, commercially available products. Product performance and patient outcomes are assessed compared to baseline, but no comparison group is included. Data collection aligns with routine clinical practice and was, therefore, not limited to on-label drug administration. Registry sites contributing to these data are noted in the Acknowledgments.
Patients
Potential registry patients are identified from the practices of participating physicians as meeting specific indications (eg, Chronic Intractable Malignant Pain) for the SynchroMed II Infusion System and are enrolled at initial implant or at the time of replacement for a previously implanted pump. The patient or legally authorized representative provides written authorization and/or consent per institution and geographical requirements before data collection. Patients inaccessible for follow-up, excluded per local law, or currently enrolled in or planning to enroll in any concurrent drug and/or device study that may confound results are ineligible. Data are only included for patients who consent to enroll. After enrollment, patients are followed per standard of care, with status updates obtained every 6 months through therapy discontinuation or registry exit.
Data Collection
Registry evolution has resulted in data collection changes over time. A numerical pain rating scale (NPRS) assessment was initiated in 2010, with a single pain score (0: no pain; 10: worst pain, assessed as “current pain”) collected during scheduled study visits. The EuroQol with 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and 5 possible responses (having no problems, having slight problems, having moderate problems, having severe problems, and being unable to do/having extreme problems) (EQ-5D-5L) and EQ Health Visual Analog Scale (EQ Health-VAS) assessments of quality of life were added to the registry in 2013. The United Kingdom value set18 was used to calculate the EQ-5D Index value, which ranges from 1 (best health state) to −0.285 (worst health state). A positive mean index value change indicates an improvement (ie, increase) in health. The EuroQol Visual Analog Scale (EQ-VAS) assesses patient-reported health on that day, ranging from 0 (worst health) to 100 (best health), and a positive mean visual analog scale (VAS) change indicates an improvement in patient-reported health. Neither of these patient-reported outcomes allowed for retrospective collection of data. Thus, limited baseline pain and EQ-5D scores were available for older registry entries.
The American Society of Anesthesiology (ASA) physical status scores were collected as standard practice at the time of IDDS implant consideration at a single center on a subset (n = 649) of enrolled subjects.
Safety data currently collected in the registry include all events that appear or worsen after enrollment and are a result of implanted or external components of the IDDS, the implant procedure, or the infusion therapy. Adverse events (AEs) were categorized using Medical Dictionary for Regulatory Activities (MedDRA) criteria and terminology.
Analytic Methods
Data included in this analysis were collected through July 31, 2017, from patients in the registry who were treated with IDDS for cancer pain. Summary statistics are presented either as percentages for categorical variables or mean (standard deviation [SD]; or minimum/maximum) or median (interquartile range [IQR]) for continuous values.
Patient survival was defined as freedom from all-cause mortality and estimated using Kaplan–Meier survival analysis methods. Survival time was defined as months from the patient’s first implant recorded in the registry to death. Patients who remained alive were censored at their last follow-up in the registry.
Pain and EQ-5D patient-reported outcomes were analyzed for therapy-naive patients who were enrolled in the registry with their first pump implant and provided pain/EQ-5D baseline data before pump implant. Analysis is provided on 2 cohorts of patients: those with 6-month data collected and those with 12-month data collected; all patients with required data for analysis were included in each analysis set. Paired t tests were used to test within-patient change from baseline to follow-up for paired data. The Hochberg method19 was used to adjust the significance level for the multiple statistical tests that were performed (change in pain, EQ-5D, and EQ-VAS from baseline to 6 and 12 months).
Events related to product performance, requiring surgical intervention (including infections requiring surgical intervention), and reports of patient death have been collected consistently since registry inception. Summaries of these events include all patients. AE reporting was expanded in 2010 to include serious AEs (SAEs) of any etiology related to the device. The subset of active and newly enrolled patients after this protocol change is included in the analysis of AEs. Safety summaries are presented as the percentage of patients who experienced ≥1 event, with 95% Wilson score confidence intervals (CIs).
RESULTS
Patient Demographics and Implant Details
A total of 7867 IDDS patients were enrolled in the registry at 64 sites across the United States, Europe, and Latin America, with 1403 from 37 sites being treated for cancer pain (Supplemental Digital Content, Figure 1, http://links.lww.com/AA/C934). The majority of patients were enrolled at 1 registry site (n = 1136, 81.0%, Phoenix, AZ), with an additional 7 sites contributing 12.7% of patients (n = 178) and the remaining 29 sites each contributing ≤10 patients.
The average (minimum/maximum) age of the patients was 59 years (13/93 years), with 56.6% female (Table 1). The SynchroMed II 40-mL pump was the prevalent pump implanted (1267/1505 pumps, 84.2%), and the InDura Model 8709 catheter the most common catheter (980/1535 catheters, 63.8%), with Ascenda Models 8780 and 8781 (53 and 282 catheters, respectively; total 335 of 535 catheters, 21.8%) also implanted. Medical history data indicated median (IQR) duration from cancer diagnosis to implant of 28.9 months (14.9–66.0 months), with median (IQR) duration from implant to death/last visit of 3.2 months (1.2–9.4 months).
Table 1.
Demographics and Baseline Data of Patients With Cancer Pain Enrolled in the Product Surveillance Registry
| Age at enrollment, y (minimum/maximum) | n = 1403a | 59 (13/93) |
| Female, % | n = 794 | 56.6 |
| Male, % | n = 609 | 43.4 |
| Type of cancer, n (%) | n = 592a | |
| Lung | 89 (15.0) | |
| Breast | 65 (11.0) | |
| Colon/rectal | 64 (10.8) | |
| Pancreatic | 49 (8.3) | |
| Prostate | 35 (5.9) | |
| Bladder | 23 (3.9) | |
| Other/unknown | 267 (45.1) | |
| Months from diagnosis to implant | n = 491a | |
| Median (IQR range) | 28.9 (14.9–66.0) | |
| Months from implant to death/last visit | n = 491a | |
| Median (IQR range) | 3.2 (1.2–9.4) | |
| Baseline pain score | n = 283a | |
| Mean (SD) | 6.8 (2.4) | |
| Median | 7.0 | |
| Baseline EQ-5D Index score | n = 139a | |
| Mean (SD) | 0.372 (0.269) | |
| Median | 0.379 | |
| ASA physical status at enrollmentb | n = 649 | |
| Mean | 3.12 |
Abbreviations: ASA, American Society of Anesthesiologists; IQR, interquartile range; SD, standard deviation.
A total of 1403 represents total enrollment between August 2003 and July 2017—protocol modifications (ie, addition of cancer type and patient-reported outcomes) result in smaller n values in these categories. All available data are included in each analysis set.
ASA status was collected as standard of care at one center. Data are presented for this single-center cohort of enrolled patients.
ASA physical status for 91.1% (n = 591) of the analyzed subgroup was III or IV, with 8.9% (n =
58) ASA II; with an existing cancer diagnosis, there were no ASA I subjects. The average ASA score within this cohort subset was 3.12.
Patient Survival
Patient postimplant survival was 39%, 24%, 16%, 11%, and 5% at 0.5, 1, 2, 3, and 10 years, respectively (Figure 1). As of July 31, 2017, 92 IDDS patients remained actively enrolled in the registry and 1311 had exited the registry. Complete follow-up (not lost to follow-up or withdrawal) was high, with the majority of patients (1141/1311; 87%) followed from implant through death, and only 4.3% exiting the study due to device explant or therapy discontinuation; the remaining 113 patients (8.6%) discontinued for reasons such as transfer of care, lost to follow-up, and site closure. Among patients exiting the registry due to death, >90% (1052/1141) expired due to disease-related (neoplasm) causes. Only 2 deaths were possibly associated with therapy: one was reported as infection with death secondary to postoperative pneumonia after device implantation, and one was due to pulmonary embolus secondary to drug withdrawal as a result of missed pump refill. Of the 87 remaining patient deaths, none was associated with product performance, therapy, or surgical implantation. The duration from implant to last patient follow-up ranged from <1 month to >14 years.
Figure 1.
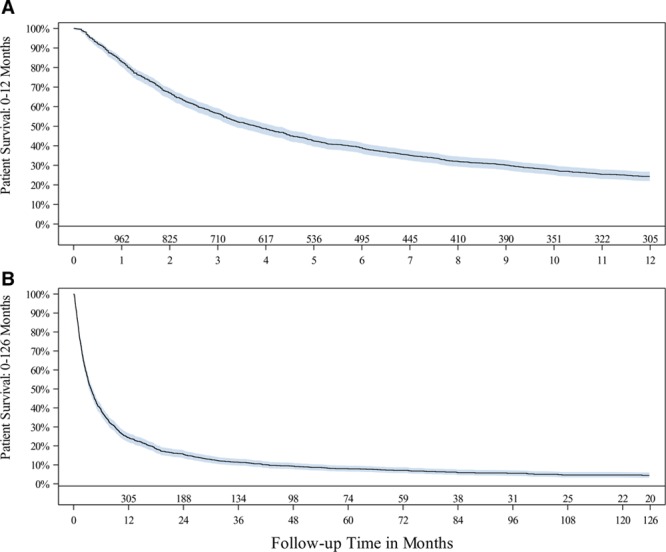
Patient survival from all-cause death through (A) 12 mo and (B) 126 mo. Shaded area represents the 95% confidence interval at that time point. Number of patients at risk are shown at select months of follow-up. Data are shown when there are ≥20 patients in each 3-mo interval.
Pain
Data were analyzed on the subcohort of 283 patients for whom baseline pain scores were available; these patients reported an average baseline pain score of 6.8 (SD, 2.4). A subset of these 283 patients had both baseline and follow-up (6 and/or 12 months) pain scores. Under an adjusted significance level of .017, these patients demonstrated statistically significant improvement in average pain from baseline (6.6; SD, 2.4) to 6 months (5.5; SD, 2.6, n = 103), with an average change of −1.1 (95% CI, −0.5 to −1.7; P = .0007), and from baseline (6.9; SD, 2.3) to 12 months (5.4; SD, 2.5; n = 55), with an average change of −1.4 (95% CI, −0.5 to −2.3; P = .0026) (Figure 2).
Figure 2.
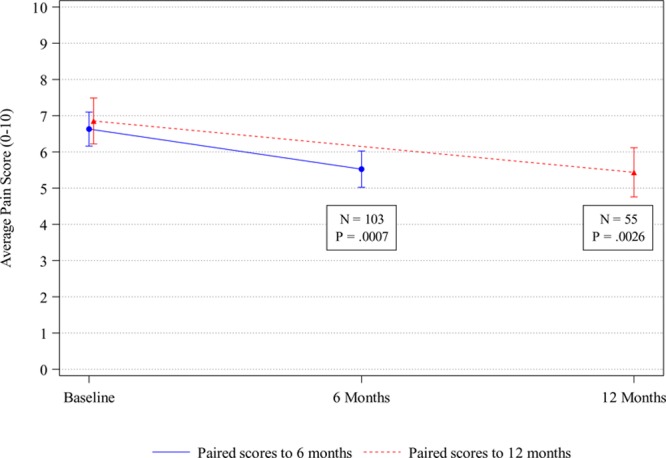
Patient-reported outcomes: pain. Average pain scores and 95% confidence intervals for patients with paired baseline and 6- or 12-mo assessment data. Pain scores range from 0 (no pain) to 10 (worst pain).
Quality of Life
Patient-reported quality of life, as indicated by the EQ-5D Index value and the EQ-5D Health-VAS, demonstrated statistically significant improvement compared to baseline at 6 months (n = 41) under an adjusted significance level of .017. The average EQ-5D Index value improved from 0.386 (SD, 0.252) to 0.556 (SD, 0.252) with an average change of +0.171 (95% CI, 0.069–0.273; P = .0016). The average EQ-5D Health-VAS score improved from 45.2 (SD, 21.6) to 58.2 (SD, 20.7) with an average change of +13.0 (95% CI, 4.5–21.5; P = .0036). Change in quality of life from baseline to 12 months, however, was not statistically significant (P = .13, adjusted significance level of .025, for EQ-5D Index value; and P = .22, adjusted significance level of .05, for VAS) (Figure 3).
Figure 3.
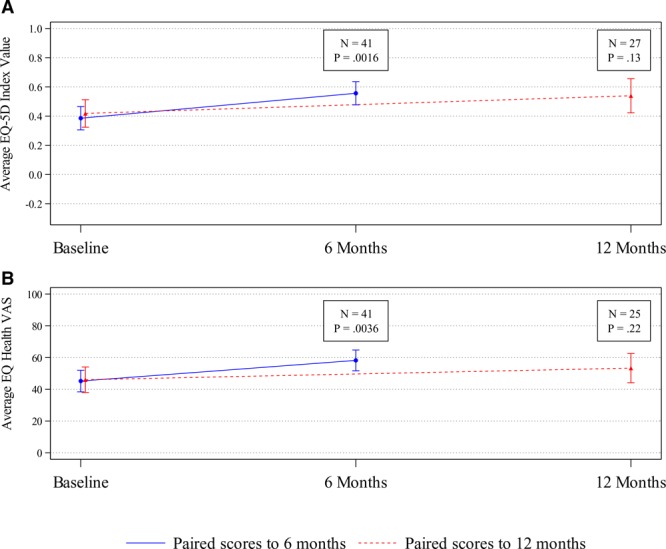
Patient-reported outcomes: quality of life. A, Average EQ-5D Index scores and 95% confidence intervals for patients with paired baseline and 6- or 12-mo assessment data. EQ-5D Index scores range from −0.285 (worst health state) to 1 (best health state). B, Average EQ-5D Health-VAS and 95% confidence intervals for patients with paired baseline and 6- or 12-mo assessment data. EQ-5D Health-VAS scores range from 0 (worst health) to 100 (best health). EQ-5D indicates EuroQol with 5 dimensions; EQ-5D Health-VAS, EuroQol Health Visual Analog Scale.
Safety
Within the full cancer pain cohort (n = 1403), infection requiring surgical intervention (IDDS explant, replacement, pocket revision, irrigation and debridement, etc) was reported in 3.2% (95% CI, 2.4–4.3) of patients. Pneumonia was the only reported infection resulting in death (n = 1), and possibly attributed to the device or a therapy-related surgical procedure. Events defined as product performance related (events with an etiology associated with pump, catheter, or patient programmer performance) and occurring in >1% of patients were catheter dislodgement (3.8%; 95% CI, 2.9–4.9), pump motor stall (1.8%; 95% CI, 1.2–2.6), catheter occlusion (1.5%; 95% CI, 0.98–2.3), catheter kink (1.5%; 95% CI, 0.98–2.3), and catheter break/cut (1.2%; 95% CI, 0.8–1.9). Magnetic resonance imaging (MRI) exposure, a potential cause of motor stall, has been actively tracked within the registry since 2013. A total of 73 MRIs was reported for 51 patients, with all but 1 reported as event-free. All the MRI-induced motor stalls recovered within the expected 24-hour period.
Of the 706 patients who were followed after the 2010 expanded AE data collection, 40% (279/706; 95% CI, 36–43) experienced ≥1 AE that was related to the device components, implant procedure, or delivery of therapy. The most frequently occurring AEs were adverse drug reaction (24.5%; 95% CI, 21.5–27.8) and medical device site pain (10.1%; 95% CI, 8.1–12.5). Sixty-eight SAEs were reported in 54 patients (7.65%) (Table 2).
Table 2.
Therapy-Related Serious Adverse Events
| MedDRA SOCa |
MedDRA Preferred Term | No. of Serious Events | No. (%) of Patients n = 706b |
95% Confidence Interval |
|---|---|---|---|---|
| Infections and infestations | Subtotal | 17 | 17 (2.41) | 1.51–3.82 |
| Implant site infection | 10 | 10 (1.42) | 0.77–2.59 | |
| Medical device site infection | 3 | 3 (0.42) | 0.14–1.24 | |
| Wound infection | 2 | 2 (0.28) | 0.08–1.03 | |
| Incision site infection | 1 | 1 (0.14) | 0.03–0.80 | |
| Meningitis | 1 | 1 (0.14) | 0.03–0.80 | |
| Psychiatric disorders | Subtotal | 5 | 4 (0.57) | 0.22–1.45 |
| Withdrawal syndrome | 5 | 4 (0.57) | 0.22–1.45 | |
| Nervous system disorders | Subtotal | 9 | 9 (1.27) | 0.67–2.40 |
| Cerebrospinal fluid leakage | 5 | 5 (0.71) | 0.30–1.65 | |
| Headache | 2 | 2 (0.28) | 0.08–1.03 | |
| Hypoesthesia | 1 | 1 (0.14) | 0.03–0.80 | |
| Spinal cord hematoma | 1 | 1 (0.14) | 0.03–0.80 | |
| Respiratory, thoracic, and mediastinal disorders | Subtotal | 2 | 2 (0.28) | 0.08–1.03 |
| Acute respiratory failure | 1 | 1 (0.14) | 0.03–0.80 | |
| Respiratory failure | 1 | 1 (0.14) | 0.03–0.80 | |
| General disorders and administration site conditions | Subtotal | 29 | 23 (3.26) | 2.18–4.84 |
| Adverse drug reaction | 12 | 10 (1.42) | 0.77–2.59 | |
| Pain | 9 | 8 (1.13) | 0.58–2.22 | |
| Drug withdrawal syndrome | 7 | 5 (0.71) | 0.30–1.65 | |
| Medical device site hematoma | 1 | 1 (0.14) | 0.03–0.80 | |
| Injury, poisoning and procedural complications | Subtotal | 6 | 6 (0.85) | 0.39–1.84 |
| Overdose | 3 | 3 (0.42) | 0.14–1.24 | |
| Postlumbar puncture syndrome | 1 | 1 (0.14) | 0.03–0.80 | |
| Procedural pain | 1 | 1 (0.14) | 0.03–0.80 | |
| Wound dehiscence | 1 | 1 (0.14) | 0.03–0.80 | |
| Totalc | 68 | 54 (7.65) | 5.91–9.85 |
Abbreviations: AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; SOC, System Organ Class.
AE seriousness was added to the registry in April 2010. This table includes AEs categorized as serious in the active patients (n = 706) from April 2010 to July 31, 2017.
The total number of patients with events may not represent the sum of all rows because a patient may have experienced >1 type of event.
DISCUSSION
Data from this large-scale multicenter registry supplement existing randomized controlled trial (RCT) data demonstrating the safety and effectiveness of IDDS as a therapeutic option for the treatment of cancer pain. Both pain and quality of life scores demonstrated significant improvement from baseline at 6 months after IDDS implantation, with significant improvement from baseline in pain scores at 12 months. Only 4.3% of discontinued patients exited the study due to device explant or therapy discontinuation. Infection requiring surgical intervention occurred in 3.2% of patients.
The WHO1 and NCCN2 guidelines sought to address undertreatment of pain by optimizing medication selection and escalating dose to maintain adequate pain control with disease progression. Systemic opioids represented the standard of care in both guidelines. The NCCN guidelines were expanded to include palliative and interventional referrals but do not address the lack of effectiveness data for long-term opioid use and inherent risks. Despite accepted use, there are no existing RCT data demonstrating long-term effectiveness of systemic opioids, and they are also associated with tolerance, hyperalgesia, addiction, sleep disorders, breathing/brain deterioration with early dementia, hypothalamic–pituitary deregulation, fractures, depression, and side effects including lethargy, sedation, nausea with vomiting, mental cloudiness, and constipation that significantly impact quality of life.20 Treatment options for cancer pain should also consider potential patient survival by addressing both immediate and longer-term pain management needs. Two prospective multicenter studies (IDDS + CMM versus CMM10 and active treatment versus placebo12) provide RCT support for IDDS effectiveness in pain relief, toxicity reduction, and quality of life improvement. Compared to CMM, IDDS-treated patients have also had lower utilization and total medical costs in the first year after implantation, driven by the savings in hospitalization and emergency department visits. A recent retrospective claims analysis found savings of $15,142 (P = .0097) at 2 months and $63,498 (P = .03) at 12 months compared to CMM after starting IDDS.16
Perceived IDDS risks associated with implantation and management have been identified as a limiting factor in therapy acceptance, and a possible reason for the delayed referral to pain physicians for treatment after cancer diagnosis. IDDS risk data presented here, however, compare favorably to that for intravenous ports placed for medication delivery.21,22 ASA scores collected on a subset of subjects additionally indicate the health status of a large proportion of patients included in this analysis were predisposed to increased surgical risk. Surgical-related SAEs included infection (2.41%), postdural puncture headaches/cerebral spinal fluid leaks (1.27%), and pump pocket hematoma (0.28%). IDDS implantation, as a minimally invasive surgery, resulted in clinically significant improvements to pain, function, and quality of life.
Therapy-related SAEs included respiratory failure (0.14%), adverse drug reactions (1.42%), pain (1.13%), and overdose (0.42%), none of which resulted in death. Most of these events were likely secondary to opioid rotation, drug delivery escalation, or return of underlying symptoms. Drug withdrawal SAEs (0.71%) were related to missed pump refill appointments, catheter complications, dosing changes, or pump motor stall associated with off-label medication usage. SynchroMed II motor stall risks were addressed by a redesign in collaboration with the FDA addressing gear corrosion. The SynchroMed II pump motor may stall during MRI, but the pump should resume normal function after MRI exposure. No permanent motor stalls after MRI were reported in this cohort of patients; temporary motor stalls were reported but resolved after MRI, with no reports of post-MRI drug withdrawal or sequelae.
Analysis of pain and quality of life measurements within the subset of patients providing both baseline and follow-up data offer additional, albeit noncomparative, evidence that IDDS is an effective treatment option for patients experiencing significant cancer pain. Compared with baseline, patients followed through 6 months had improved pain and functional status, with those followed up to 1 year maintaining improved pain management. Patient survival data indicate that a subset of patients have survived through 10 years of active treatment, which necessitates ≥1 pump replacement. Given the increasing survival and incidence of chronic cancer pain in long-term survivors, IDDS therapy offers a long-term treatment option that avoids the toxicities associated with oral medications.
Uncontrollable pain is often cited as the most feared aspect associated with a cancer diagnosis, with severity of pain associated with decreased treatment compliance,23 reduced survival,2 and increased rates of suicidal ideation and suicide.24 In this cohort of patients, baseline pain was classified as severe, with a baseline median pain score in the presence of standard pain control measures of 7/10. Although not directly assessed in this registry, earlier intervention with IDDS with improved pain control and reduction in side effects associated with oral medications may positively impact overall survival. Patients here had a median time between cancer diagnosis and IDDS therapy of >2 years. Although this may represent a delay in onset of severe pain in some of these patients, it likely represents a prolonged duration of poorly controlled pain in many, emphasizing the importance of a pain management plan, established at cancer diagnosis in coordination with pain specialists, addressing timely advancement from conservative therapies to advanced interventions when appropriate. Inclusion of these specific pain management plans as part of palliative care in Survivorship Care Plans,25 with buy-in from the diverse providers seen by cancer patients, would serve well in achieving the goals established by the WHO Guidelines >20 years ago.
Opioids remain the mainstay of treatment for cancer pain in the United States, but their identified risks and the opioid epidemic have placed undue burden on the prescribing provider and may result in risk of undermanagement of pain. The safety and outcome data presented here, as well as reduced risk of drug diversion or medication surplus after patient demise, strongly support a more widespread acceptance of IDDS for cancer pain. Although IDDS is not free from all medication-related risks, consensus statements regarding the safety, side effects, and best clinical practices for IDDS,26–29 published regularly since 2000 for nonmalignant pain, offer additional support for wider acceptance of this needed therapy.
Limitations
The registry data were collected using protocols that allowed clinicians to maintain their standard clinical practice. Patient-reported outcomes were only added in the past few years, so data were presented for the more recent, limited subset of the registry cohort. AE collection expanded in 2010, but surgical interventions and reasons for discontinuation have been captured consistently since registry inception. Although large registries are becoming more widely accepted in the assessment of safety and patient outcomes, the registry is limited by not having a direct comparator. Only implanted patients continue and provide data in the registry; no concurrent nonimplanted group is available for comparison to those patients receiving IDDS therapy. In addition, most patients presented here were treated at a single center in the United States. This center demonstrates a higher referral pattern from oncology with more extensive experience with aggressive treatment of cancer-related pain. Comparison of patient outcomes from this center to that of all other centers indicated no difference in the rate of SAEs but did demonstrate a difference in AE rates (higher AE rate, statistically significant) as well as a difference in EuroQol with 5 dimensions Visual Analog Scale (EQ-5D-VAS) scores at 6 months (greater improvement, statistically significant) for this center. These data likely reflect more aggressive dosing and titration patterns of IDDS therapy. Predictably, the most frequently occurring AEs were medication related, as well as medical device site pain. Finally, the registry represents a heterogeneous patient population. Although all had cancer pain as the primary indication for IDDS, essentially all types of cancer were included, as were many different pain etiologies (ie, pain due to tumors or to cancer treatments), and patients were enrolled at various stages of disease.
CONCLUSIONS
This registry remains unique in terms of enrollment numbers and duration of follow-up for patients with cancer pain. Adequate and improved pain control in patients with cancer, even in advanced stages, with concurrent quality of life maintenance are attainable. Results from this large-scale, multicenter registry supplement existing RCT data that support IDDS as a safe and effective therapeutic option with a positive benefit–risk ratio in the treatment of cancer pain.
ACKNOWLEDGMENTS
The authors thank the investigators and their sites for contributing data used in this study to the Product Surveillance Registry: Lisa Stearns, MD, Phoenix, AZ; Alaa Abd-Elsayed, MD, PhD, Madison, WI; Christophe Perruchoud, MD, Morges, Switzerland; Behzad Aalaei, MD, Highland, IN; Shakil Ahmed, MD, New York, NY; Charles Edward Anderson, MD, Palm Springs, CA; Mark Barhorst, MD, Houston, TX; Aaron Calodney, MD, Tyler, TX; Orlando Charry, MD, Minneapolis, MN; Neal Coleman, MD, Muncie, IN; Shrif Costandi, MD, Cleveland, OH; Sam Eldabe, MD, Middlesbrough, United Kingdom; Eric Grigsby, MD, Napa, CA; Vajira Gunawardane, MD, Westminster, MD; John Hatheway, MD, Spokane, WA; Jon Hillyer, MD, Bremerton, WA; John Huffman, MD, Silver Spring, MD; Eduardo Ibarra, MD, Cayey, Puerto Rico; Frank Jordan, MD, Knoxville, TN; Brian Kahan, MD, Annapolis, MD; Peter Konrad, MD, Nashville, TN; Alon Mogilner, MD, Manhasset, NY; Rosa Navarro, MD, South Bend, IN; Eric Pearson, MD, Meridian, MS; Robert Plunkett, MD, Buffalo, NY; Stephen Pyles, MD, Ocala, FL; Richard Rauck, MD, Winston Salem, NC; Mark Romanoff, MD, Charlotte, NC; Mahendra Sanapati, MD, Evansville, IN; David Schultz, MD, Edina, MN; Thomas Silvestrini, MD, Duluth, MN; Konstantin Slavin, MD, Chicago, IL; Kevin Smith, MD, San Diego, CA; Ashish Udeshi, MD, Merritt Island, FL; Angela Vallejo, MD, Bloomington, IL; Kenneth Willis, MD, Huntsville, AL; and Carlos Jaime Yepes, MD, Medellin, Colombia. Sarah Staples, MA, ELS, formatted and uploaded content after approval from all authors and was paid by Medtronic.
DISCLOSURES
Name: Lisa M. Stearns, MD.
Contribution: This author helped conceive, design, analyze, and interpret the data; draft the manuscript; and critically revise the manuscript for important intellectual content.
Conflicts of Interest: L. M. Stearns is a paid consultant for Medtronic, Flowonix, Nevro, and Boston Scientific and receives research support from Medallion Therapeutics, Inc, Medtronic, Boston Scientific, and Piramal for services unrelated to the current research.
Name: Alaa Abd-Elsayed, MD, MPH.
Contribution: This author helped conceive, design, analyze, and interpret the data; draft the manuscript; and critically revise the manuscript for important intellectual content.
Conflicts of Interest: A. Abd-Elsayed is a paid consultant for Medtronic, Avanos, StimWave, and Sollis.
Name: Christophe Perruchoud, MD.
Contribution: This author helped analyze and interpret the data; draft the manuscript; and critically revise the manuscript for important intellectual content.
Conflicts of Interest: C. Perruchoud is a paid consultant for Medtronic.
Name: Robert Spencer, MS, MBA.
Contribution: This author helped conceive, design, analyze, and interpret the data; draft the manuscript; and critically revise the manuscript for important intellectual content.
Conflicts of Interest: R. Spencer is a Medtronic employee.
Name: Krisstin Hammond, BS.
Contribution: This author helped design, analyze, and interpret the data; draft the manuscript; and critically revise the manuscript for important intellectual content.
Conflicts of Interest: None.
Name: Katherine Stromberg, MS.
Contribution: This author helped conceive, design, analyze, and interpret the data; draft the manuscript; and critically revise the manuscript for important intellectual content.
Conflicts of Interest: K. Stromberg is a Medtronic employee.
Name: Todd Weaver, PhD, MPH.
Contribution: This author helped design the study; conceive, design, analyze, and interpret the data; draft the manuscript; and critically revise the manuscript for important intellectual content.
Conflicts of Interest: T. Weaver is a Medtronic employee.
This manuscript was handled by: Honorio T. Benzon, MD.
Supplementary Material
FOOTNOTES
GLOSSARY
- 5D =
- 5 dimension
- 5L =
- 5 level
- AE =
- adverse event
- ASA =
- American Society of Anesthesiology
- ASCO =
- American Society of Clinical Oncology
- CI =
- confidence interval
- CMM =
- conventional medical management
- EQ-5D-5L =
- EuroQol with 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and 5 possible responses (having no problems, having slight problems, having moderate problems, having severe problems, and being unable to do/having extreme problems)
- EQ-5D-VAS =
- EuroQol with 5 dimensions Visual Analog Scale
- EQ Health-VAS =
- EuroQol Health Visual Analog Scale (100: best; 0: worst)
- EQ-VAS =
- EuroQol Visual Analog Scale
- FDA =
- Food and Drug Administration
- IDDS =
- intrathecal drug delivery system
- IQR =
- interquartile range
- ISPR =
- Implantable Systems Performance Registry
- MedDRA =
- Medical Dictionary for Regulatory Activities
- MRI =
- magnetic resonance imaging
- n =
- number (usually of subjects or occurrences)
- NPRS =
- numerical pain rating score (0: no pain; 10: worst pain)
- NCCN =
- National Comprehensive Cancer Network
- PSR =
- Product Surveillance Registry
- RCT =
- randomized controlled trial
- SAE =
- serious adverse event
- SD =
- standard deviation
- STROBE =
- Strengthening the Reporting of Observational Studies in Epidemiology
- VAS =
- visual analog scale
- WHO =
- World Health Organization
Published ahead of print 18 September 2019.
C. Perruchoud is currently affiliated with the Clinique de la douleur, La Tour Hospital, Geneva, Switzerland.
Funding: Medtronic underwrote preparation of this manuscript and maintains the systems performance registry that was the basis for this analysis.
Conflicts of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Presented in part as posters at the World Congress on Regional Anesthesia & Pain Medicine, New York, NY, April 2018; the World Congress of the World Institute of Pain, Dublin, Ireland, May 2018; American Society of Regional Anesthesia Annual Pain Medicine Meeting, San Antonio, TX, November 2018; and the North American Neuromodulation Society (NANS), Las Vegas, NV, January 2019.
Clinical Trial Number: ClinicalTrials.gov Identifier: NCT01524276 at https://clinicaltrials.gov/ct2/show/NCT01524276.
Listen to this Article of the Month podcast and more from OpenAnesthesia.org® by visiting http://journals.lww.com/anesthesia-analgesia/pages/default.aspx.
Reprints will not be available from the authors.
REFERENCES
- 1.World Health Organization. Cancer Pain Relief with a Guide to Opioid Availability. 1996. 2nd ed Geneva: World Health Organization; Available at: http://apps.who.int/iris/bitstream/10665/37896/1/9241544821.pdf. Accessed April 4, 2018. [Google Scholar]
- 2.National Comprehensive Cancer Network (NCCN). Available at: https://www.nccn.org/store/login/login.aspx?eturnURL=https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed March 7, 2018.
- 3.Reis-Pina P, Lawlor PG, Barbosa A. Adequacy of cancer-related pain management and predictors of undertreatment at referral to a pain clinic. J Pain Res. 2017;10:2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh H, Banipal RPS, Singh B. Assessment of adequacy of pain management and analgesic use in patients with advanced cancer using the brief pain inventory and pain management index calculation. J Glob Oncol. 2017;3:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pergolizzi JV, Gharibo C, Ho KY. Treatment considerations for cancer pain: a global perspective. Pain Pract. 2015;15:778–792. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. [DOI] [PubMed] [Google Scholar]
- 7.Ferris FD, Bruera E, Cherny N, et al. Palliative cancer care a decade later: accomplishments, the need, next steps – from the American Society of Clinical Oncology. J Clin Oncol. 2009;27:3052–3058. [DOI] [PubMed] [Google Scholar]
- 8.Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34:3325–3345. [DOI] [PubMed] [Google Scholar]
- 9.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. Jama. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith TJ, Staats PS, Deer T, et al. ; Implantable Drug Delivery Systems Study Group. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040–4049. [DOI] [PubMed] [Google Scholar]
- 11.Smith TJ, Coyne PJ, Staats PS, et al. An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM). Ann Oncol. 2005;16:825–833. [DOI] [PubMed] [Google Scholar]
- 12.Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63–70. [DOI] [PubMed] [Google Scholar]
- 13.Brogan SE, Winter NB, Okifuji A. Prospective observational study of patient-controlled intrathecal analgesia: impact on cancer-associated symptoms, breakthrough pain control, and patient satisfaction. Reg Anesth Pain Med. 2015;40:369–375. [DOI] [PubMed] [Google Scholar]
- 14.Sayed D, Monroe F, Orr WN, et al. Retrospective analysis of intrathecal drug delivery: outcomes, efficacy, and risk for cancer-related pain at a high volume academic medical center. Neuromodulation. 2018;21:660–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caraway D, Walker V, Becker L, Hinnenthal J. Successful discontinuation of systemic opioids after implantation of an intrathecal drug delivery system. Neuromodulation. 2015;18:508–515. [DOI] [PubMed] [Google Scholar]
- 16.Stearns LJ, Narang S, Albright RE, Jr, et al. Assessment of health care utilization and cost of targeted drug delivery and conventional medical management vs conventional medical management alone for patients with cancer-related pain. JAMA Netw Open. 2019;2:e191549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konrad PE, Huffman JM, Stearns LM, et al. Intrathecal Drug Delivery Systems (IDDS): the Implantable Systems Performance Registry (ISPR). Neuromodulation. 2016;19:848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27:7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 20.Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705–713. [DOI] [PubMed] [Google Scholar]
- 21.Ruppen W, Derry S, McQuay HJ, Moore RA. Infection rates associated with epidural indwelling catheters for seven days or longer: systematic review and meta-analysis. BMC Palliat Care. 2007;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jong PC, Kansen PJ. A comparison of epidural catheters with or without subcutaneous injection ports for treatment of cancer pain. Anesth Analg. 1994;78:94–100. [PubMed] [Google Scholar]
- 23.Bajpai J, Puri A, Shah K, et al. Chemotherapy compliance in patients with osteosarcoma. Pediatr Blood Cancer. 2013;60:41–44. [DOI] [PubMed] [Google Scholar]
- 24.Aboumrad M, Shiner B, Riblet N, Mills PD, Watts BV. Factors contributing to cancer-related suicide: a study of root-cause analysis reports. Psycho-oncology. 2018;27:2237–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCanney J, Winckworth-Prejsnar K, Schatz AA, et al. Addressing survivorship in cancer care. J Natl Compr Canc Netw. 2018;16:801–806. [DOI] [PubMed] [Google Scholar]
- 26.Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference–2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436–464. [DOI] [PubMed] [Google Scholar]
- 27.Deer TR, Levy R, Prager J, et al. Polyanalgesic Consensus Conference–2012: recommendations to reduce morbidity and mortality in intrathecal drug delivery in the treatment of chronic pain. Neuromodulation. 2012;15:467–482. [DOI] [PubMed] [Google Scholar]
- 28.Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20:96–132. [DOI] [PubMed] [Google Scholar]
- 29.Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): recommendations for intrathecal drug delivery: guidance for improving safety and mitigating risks. Neuromodulation. 2017;20:155–176. [DOI] [PubMed] [Google Scholar]


