Abstract
Background:
To gain insight into how teriparatide affects various bone health parameters, we assessed the effects of teriparatide treatment with use of standard DXA (dual x-ray absorptiometry) technology and two newer technologies, high-resolution MRI (magnetic resonance imaging) and finite element analysis of quantitative CT (computed tomography) scans.
Methods:
In this phase-4, open-label study, postmenopausal women with severe osteoporosis received 20 μg/day of teriparatide. Assessments included (1) changes in areal BMD (bone mineral density) (in g/cm2) at the radius, spine, and hip on DXA, (2) changes in volumetric BMD (in mg/cm3) at the spine and hip on quantitative CT scans, (3) changes in bone microarchitecture at the radius on high-resolution MRI, (4) estimated changes in spine and hip strength according to finite element analysis of quantitative CT scans, (5) changes in bone turnover markers in serum, and (6) safety.
Results:
Thirty-five subjects were enrolled; thirty completed eighteen months and twenty-five completed an optional six-month extension. No significant changes were observed for the primary outcome, high-resolution MRI at the distal aspect of the radius. At month eighteen, the least-squares mean percentage change from baseline in total volumetric BMD at the spine was 10.05% (95% confidence interval [CI], 6.83% to 13.26%; p < 0.001), and estimated spine strength increased 17.43% (95% CI, 12.09% to 22.76%; p < 0.001). Total volumetric BMD at the hip increased 2.22% (95% CI, 0.37% to 4.06%; p = 0.021), and estimated hip strength increased 2.54% (95% CI, 0.06% to 5.01%; p = 0.045). Areal BMD increased at the lumbar spine and femoral neck, was unchanged for the total hip and at the distalmost aspect of the radius, and decreased at a point one-third of the distance between the wrist and elbow. Bone turnover markers increased at months three, six, and twenty-four (all p < 0.05). No unexpected adverse events were observed.
Conclusions:
High-resolution MRI failed to identify changes in bone microarchitecture at the distal aspect of the radius, a non-weight-bearing site that may not be suitable for assessing effects of an osteoanabolic agent. Teriparatide increased areal BMD at the spine and femoral neck and volumetric BMD at the spine and hip. Estimated vertebral and femoral strength also increased. These findings and increases in bone turnover markers through month twenty-four are consistent with the known osteoanabolic effect of teriparatide.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Osteoporosis is a systemic skeletal disorder characterized by reduced bone mineral density (BMD) and disruption in bone microarchitecture. Dual-x-ray absorptiometry (DXA) is widely used in clinical practice to measure BMD; it yields an estimate of the amount of bone (in grams) in a given area of bone (in cm2), which is referred to as the areal BMD (in g/cm2). This estimate differs from the volumetric BMD, which is the estimated bone mineral content divided by the volume of the region of interest and is expressed in mg/cm3. Although DXA is widely used, it cannot assess bone microarchitecture, an important independent risk factor for fracture in patients with osteoporosis.
Data from transiliac bone biopsies have demonstrated the important contribution of bone microarchitecture to trabecular strength1,2, suggesting that assessment of bone structure may help determine fracture risk. Histomorphometric analyses of bone biopsies have provided valuable information about bone architecture following treatment with teriparatide3-5; however, biopsies are invasive and are not amenable to serial measurements or use for routine monitoring of therapy in clinical practice.
High-resolution magnetic resonance imaging (MRI) has emerged as a noninvasive means of performing in vivo bone morphometry. This technique allows researchers to examine trabecular and cortical bone microstructure at peripheral skeletal sites, with the distal aspect of the radius being particularly amenable to this technique6,7.
Quantitative computed tomography (CT) scans are a noninvasive means of estimating bone strength and have been used frequently in clinical research to identify changes in volumetric BMD. Briefly, quantitative CT scans provide three-dimensional images to assess structural effects of drugs that act on bone. Finite element analysis based on quantitative CT data has been described in detail in the literature8-12 and allows researchers to estimate vertebral strength for a simulated compression overload and femoral strength for a simulated sideways fall. Few finite element analysis studies have been reported previously for patients treated with teriparatide13,14.
Teriparatide treatment stimulates production of new bone on trabecular and cortical bone surfaces3,4,15, resulting in increased BMD, improved bone microarchitecture and strength, and reduced fracture risk15-21. Given the osteoanabolic effect of teriparatide treatment and the importance of BMD, bone microarchitecture, and bone strength in reducing fracture risk, we designed this study to investigate several parameters of bone health with use of both conventional and novel technologies.
Subjects and Methods
Study Design
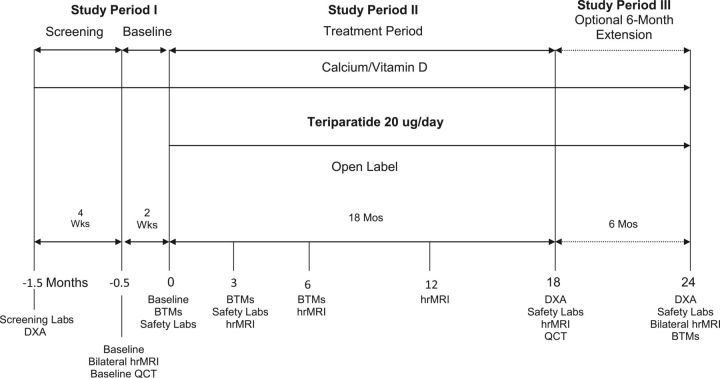
This phase-4 exploratory, open-label, single-arm study (ClinicalTrials.gov number NCT00557310) evaluated the effects of daily injection of 20 μg of teriparatide (recombinant human parathyroid hormone, 1-34) in ambulatory postmenopausal women with severe osteoporosis at five centers in the U.S. and Canada. Subjects were recruited for screening in approximately a 1:1 ratio on the basis of whether or not they had previously used oral bisphosphonates. Informed consent was obtained from each subject at study entry. There were three study periods: an initial period for screening (up to 1.5 months), an eighteen-month treatment period, and an optional six-month treatment extension (Fig. 1).
Fig. 1.
Study design and treatment periods. BTMs = bone turnover markers, hrMRI = high-resolution MRI, and QCT = quantitative CT.
During the initial period, eligibility criteria were evaluated and screening procedures were performed as appropriate. Areal BMD was measured by DXA at two locations in the distal aspect of the radius (the “1/3 distal radius” and the “ultra-distal radius”), femoral neck, total hip, and lumbar spine for screening purposes and baseline measurements. The 1/3 distal radius location is a predominantly cortical site that is one-third of the distance between the wrist and elbow; the ultra-distal radius location is a site comprising both trabecular and cortical bone that is at the very distal end of the radius. The ultra-distal radius site aligned with positioning of the high-resolution MRI coil used in this study.
A subject was eligible for enrollment if she was postmenopausal, forty-five to eighty-five years of age, and had a diagnosis of osteoporosis as defined by a baseline areal BMD T-score of either (1) ≤−2.0 at the hip or spine if the subject had at least one minimal-trauma vertebral or nonvertebral fracture, or (2) ≤−3.0 at the hip or spine if the subject had no such prior fracture. Subjects were excluded if they had previously used intravenous bisphosphonates, had a cumulative total of more than five years of treatment with oral bisphosphonates over their lifetime, had a metal implant or hip replacement that would impact imaging studies, or had a medical condition (e.g., Paget disease of the bone, hyperparathyroidism, metastatic cancer, or previous radiation therapy) that would preclude use of teriparatide.
Subjects subsequently underwent baseline high-resolution MRI measurements of the right and left distal radii as well as quantitative CT of the lumbar spine and hip (Fig. 1). Blood samples were drawn for clinical laboratory assessments and measurement of systemic serum concentrations of bone turnover markers.
Subjects administered 20 μg of teriparatide daily via subcutaneous injection, and they received calcium supplements (approximately 1000 mg/day) and vitamin D (approximately 800 IU/day) for at least one month during screening and throughout the study. This study was approved by the ethics committee at each participating center and was conducted in accordance with the October 2000 revision of the Declaration of Helsinki.
High-Resolution MRI
High-resolution MRI uses a patented algorithm to transform data into a highly detailed three-dimensional model of bone microstructure as described previously22. A Signa 1.5T scanner (GE Healthcare, Waukesha, Wisconsin) and a wrist coil were used to assess the distal aspect of the right forearm. The volume acquired for trabecular measurements consisted of thirty-two slices (twenty-four of which were typically included in the region of interest) with a voxel size of 137 × 137 × 410 μm.
Objectives
The primary study objective was to determine whether teriparatide treatment results in an increase in the surface-to-curve ratio of the distal aspect of the radius from baseline to month eighteen, as determined by high-resolution MRI. The surface-to-curve ratio is a computed ratio of trabecular connectivity that provides a measurement of bone microarchitecture, involving the transition from rod-like to plate-like structures, similar to that provided by a biopsy23. Secondary objectives were to assess (1) changes from baseline to months eighteen and twenty-four in the cortical thickness, topological erosion index, and bone volume fraction of the distal aspect of the radius, as determined by high-resolution MRI (as well as the surface-to-curve ratio at month twenty-four); (2) changes from baseline to month eighteen in the volumetric BMD of the lumbar spine and hip, as determined by quantitative CT; (3) vertebral and femoral strength at month eighteen, as estimated by finite element analysis of the quantitative CT scans; and (4) changes in areal BMD from baseline to months eighteen and twenty-four as determined by DXA. The volumetric BMD calculations were performed for the trabecular and peripheral bone compartments as well as for the bone as a whole (“total”); the peripheral compartment was the outer 2 to 3 mm of bone, containing all of the cortical bone and some adjacent trabecular bone. As an exploratory objective, we also assessed whether previous oral bisphosphonate therapy affected volumetric BMD or estimated strength.
The effect of teriparatide treatment on bone remodeling was assessed by evaluating changes in two bone turnover markers from baseline to months three and six (early changes) and to month twenty-four. One of the markers, serum procollagen type 1 N-terminal propeptide (P1NP), provides a measure of bone formation; the other marker, serum carboxyterminal cross-linking telopeptide of collagen type 1 (CTX), provides a measure of bone resorption24.
Safety evaluations included physical examinations, vital signs, hematology, and clinical chemistry. Serum calcium, albumin, and creatinine were measured at least twelve hours after the last dose of teriparatide. Adverse events were assessed at the time they were originally reported and throughout the study. Specific tests for safety could be repeated in subsequent visits at the investigators’ discretion. Treatment noncompliance was considered to exist if a subject had missed >30% of the study drug doses at two consecutive study visits.
Statistical Methods
Unless otherwise specified, changes in continuous longitudinal variables between baseline and various subsequent time points were analyzed with use of the mixed-model repeated-measures method, and changes in continuous nonlongitudinal variables were analyzed with use of the analysis of variance (ANOVA) method. The actual and percentage changes in each continuous outcome (high-resolution MRI, DXA, or quantitative CT scan) were analyzed, with investigator site and prior bisphosphonate exposure in the model, for subjects who completed eighteen or twenty-four months of therapy and had both a baseline and an eighteen-month evaluation. An end-point analysis, which included subjects who discontinued the study prior to month eighteen and had a follow-up evaluation at the time they discontinued, was also performed. The nonparametric Wilcoxon signed-rank test was used to evaluate the change in strength from baseline. Significance tests were based on least-squares means and the type-III sum of squares; a p value of 0.05 (two-sided) was considered significant. Analysis was conducted on the full set of subjects who were enrolled and received at least one dose of study drug, following the intent-to-treat principle25. All analyses used SAS Drug Development software (SAS Institute, Cary, North Carolina).
Sample Size
To our knowledge, no published data on high-resolution MRI analysis of trabecular architecture in teriparatide-treated subjects were available when this study was designed. Results from a study of hypogonadal men treated with testosterone26 and other unpublished data were therefore considered in determining the sample size and power. To optimize the chances of meeting statistical power requirements, the study was designed to enroll approximately thirty-three subjects. It was assumed that 30% of subjects would discontinue before completing the eighteen-month measurements. The study was predicted to have ≥80% power to detect a change in the surface-to-curve ratio of 0.57 (equivalent to 9%) with a standard deviation of 0.90 at a two-sided significance level of 0.05 with use of a paired t test.
Source of Funding
Lilly USA, LLC (Indianapolis, Indiana) sponsored this study and was the employer of some of the authors. Eli Lilly and Company provided compensation to inVentiv Health Clinical for provision of technical writing services provided by E. Gallagher. The clinical investigators who conducted the study did not receive compensation related to authorship of this manuscript.
Results
Baseline Demographics and Characteristics
Of the eighty-two subjects screened, thirty-five were assigned to treatment, thirty completed eighteen months, and twenty-five completed twenty-four months of teriparatide treatment (Fig. 2). The most common reason for early discontinuation was an adverse event. Two subjects who experienced adverse events during the period between providing consent and starting the study drug were considered screening failures and discontinued. Baseline demographics and characteristics according to prior bisphosphonate use are summarized in Table I. The one subject inadvertently enrolled in the study who did not meet the eligibility criteria (representing a protocol violation) was included in the safety analyses but not in the efficacy analyses. Therefore, the full-set analysis involved thirty-four subjects, 97% of whom were compliant with the study drug regimen.
Fig. 2.
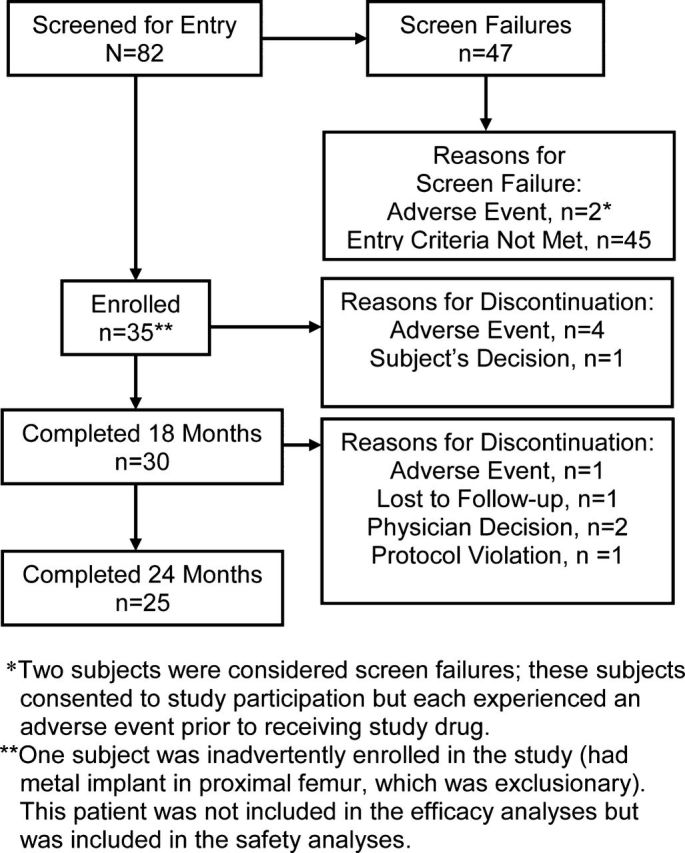
Flow of subjects through the study.
TABLE I.
Baseline Demographics and Characteristics (FSA Population)*
| Prior Bisphosphonate Treatment |
|||
| Variable | All Subjects, N = 34 | Yes, N = 21 | No, N = 13 |
| Age† (yr) | 62 ± 8 | 61 ± 8 | 63 ± 9 |
| Origin‡ | |||
| White | 31 (91) | 19 (90) | 12 (92) |
| Hispanic or West Asian | 3 (9) | 2 (10) | 1 (8) |
| Time since menopause† (yr) | 17 ± 12 | 16 ± 9 | 18 ± 16 |
| Prevalent fracture‡ | |||
| Any vertebral fracture | 4 (12) | 1 (5) | 3 (23) |
| Any nonvertebral fracture | 17 (50) | 11 (52) | 6 (46) |
| Areal BMD T-score† | |||
| Lumbar spine | −2.9 ± 1.0 (n = 30) | −2.8 ± 1.2 (n = 18) | −3.1 ± 0.8 (n = 12) |
| Femoral neck | −2.4 ± 0.7 (n = 34) | −2.5 ± 0.7 (n = 21) | −2.2 ± 0.7 (n = 13) |
| Total hip | −1.8 ± 0.8 (n = 34) | −1.8 ± 0.9 (n = 21) | −1.7 ± 0.7 (n = 13) |
| Areal BMD† (g/cm2) | |||
| 1/3 distal radius | 0.6 ± 0.9 (n = 34) | 0.6 ± 0.1 (n = 21) | 0.6 ± 0.1 (n = 13) |
| Ultra-distal radius | 0.3 ± 0.1 (n = 34) | 0.3 ± 0.1 (n = 21) | 0.3 ± 0.1 (n = 13) |
| Volumetric BMD† (mg/cm3) | |||
| Spine | 145.9 ± 19.55 (n = 30) | 149.2 ± 21.17 (n = 19) | 140.3 ± 15.7 (n = 11) |
| Hip | 207.6 ± 27.91 (n = 27) | 208.7 ± 27.36 (n = 17) | 205.9 ± 30.24 (n = 10) |
| Total lumbar spine strength† (N) | 4236 ± 888.09 (n = 30) | 4428.47 ± 1001.87 (n = 19) | 3904.73 ± 537.98 (n = 11) |
| Total hip strength† (N) | 2786.48 ± 520.46 (n = 27) | 2728.24 ± 550.96 (n = 17) | 2885.50 ± 474.96 (n = 10) |
| P1NP† (μg/L) | 42.8 ± 20.3 (n = 33) | 38.2 ± 18.7 (n = 21) | 50.8 ± 21.1 (n = 12) |
| CTX† (ng/mL) | 0.6 ± 0.3 (n = 33) | 0.6 ± 0.3 (n = 21) | 0.7 ± 0.2 (n = 12) |
FSA = full-set analysis, CTX = serum carboxyterminal cross-linking telopeptide of collagen type 1, and P1NP = serum procollagen type 1 N-terminal propeptide.
The values are given as the mean and standard deviation, with the number of subjects with data in parentheses.
The values are given as the number of subjects, with the percentage in parentheses.
High-Resolution MRI Findings
There were no significant changes from baseline (p > 0.05) in the estimated surface-to-curve ratio of the distal aspect of the radius in subjects who completed eighteen months of treatment with teriparatide (the primary outcome), in subjects who completed twenty-four months of treatment (a secondary outcome), or in those subjects who dropped out prior to month eighteen and had a follow-up high-resolution MRI at the time they discontinued (Table II). There also were no significant differences in other secondary MRI parameters that were assessed at month eighteen or twenty-four or at the end point for all subjects with follow-up data (all p > 0.05).
TABLE II.
Percentage Change in High-Resolution MRI Assessments (FSA Population)*
| Parameter | Month-18 Completers, N = 25† | P Value | Month-24 Completers, N = 21† | P Value | End Point, N = 32†‡ | P Value |
| Surface-to-curve ratio§ | 2.37 (−9.44 to 14.18) | 0.679 | −2.38 (−19.54 to 14.66) | 0.770 | 0.31 (−13.29 to 13.91) | 0.963 |
| Cortical thickness# | −0.59 (−6.27 to 5.09) | 0.830 | −0.69 (−7.08 to 5.70) | 0.821 | −0.97 (−5.99 to 4.06) | 0.695 |
| Bone volume fraction** | 0.31 (−3.45 to 4.07) | 0.865 | −3.39 (−8.42 to 1.64) | 0.172 | −2.49 (−6.32 to 1.34) | 0.193 |
| Topological erosion index†† | 3.46 (−3.41 to 10.33) | 0.305 | 8.82 (−1.28 to 18.92) | 0.082 | 5.72 (−2.71 to 14.15) | 0.175 |
FSA = full-set analysis.
The values are given as the least-squares mean percentage change from baseline, with the 95% CI in parentheses.
Includes data for those subjects who dropped out prior to month 18 and had a follow-up MRI scan at the time they discontinued.
A measure of the ratio of plates to rods in the analysis volume.
A surface-based measurement.
A measure of mechanical competence of bone.
The ratio of the sum of topological parameters expected to increase with bone erosion compared with the sum of those expected to decrease33.
Volumetric BMD and Strength Estimates
Vertebral Outcomes
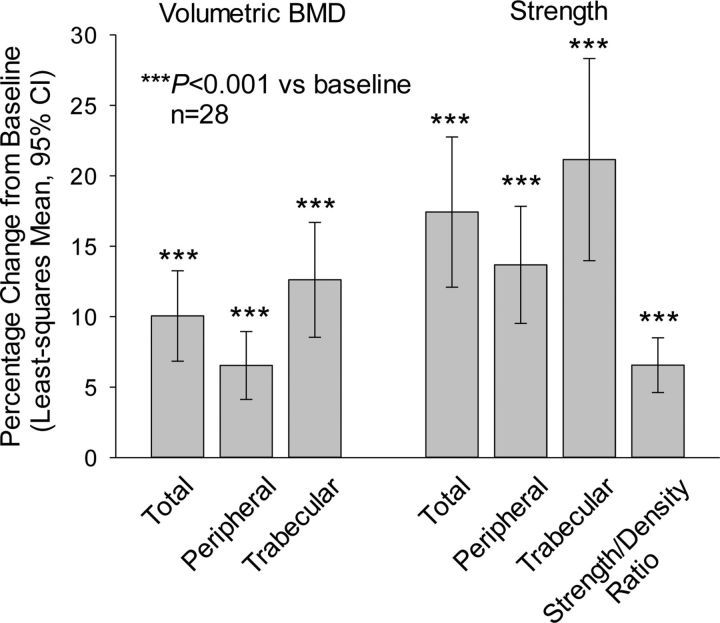
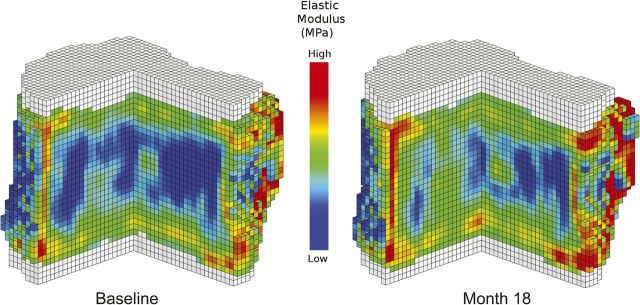
Teriparatide treatment significantly increased volumetric BMD at the lumbar spine at month eighteen (Fig. 3) and at the end point for all subjects with follow-up data; significant increases were also seen overall and in both of the bone compartments assessed (all p < 0.001) (see Appendix). Additionally, estimated lumbar spine bone strength, overall and in both bone components, was significantly increased from baseline to both month eighteen (Fig. 3) and the end point for all subjects with follow-up data (all p < 0.001). As shown in Figure 4, treatment-induced improvement in bone material properties is evident in the quantitative CT-based finite element models of the L3 vertebra from a representative subject before and after treatment with teriparatide.
Fig. 3.
Vertebral outcomes for month-eighteen completers (full-set analysis). The whiskers indicate the 95% CI.
Fig. 4.
Quantitative CT-based finite element models of the L3 vertebra from a representative study subject before and after treatment with teriparatide. Treatment resulted in an improvement in material properties (e.g., the elastic modulus shown), which resulted in a significant increase in the overall estimate of vertebral compressive strength.
Hip Outcomes
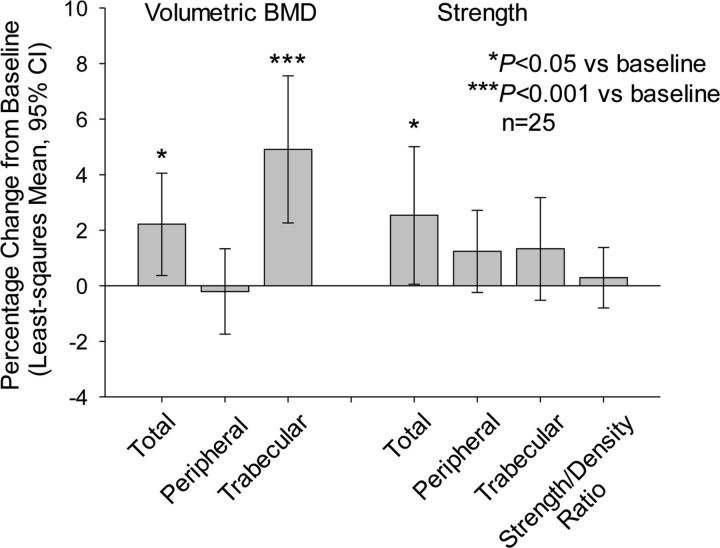
The volumetric BMD for the total hip was significantly higher than baseline after eighteen months of teriparatide treatment (Fig. 5) and at the end point for all subjects with follow-up data (p < 0.05) (see Appendix). The volumetric BMD in the trabecular area of the hip was significantly increased from baseline to both month eighteen and the end point for all subjects with follow-up data (p < 0.001), but the peripheral volumetric BMD was not significantly different from baseline at month eighteen or at the end point (p > 0.05). Total hip strength was also significantly improved at month eighteen (p < 0.05) (Fig. 5). Bone strength estimates in the individual hip compartments at month eighteen were not significantly different from baseline (p > 0.05).
Fig. 5.
Hip outcomes for month-eighteen completers (full-set analysis). The whiskers indicate the 95% CI.
Effect of Oral Bisphosphonates
In an exploratory analysis, prior oral bisphosphonate use did not significantly influence the effect of teriparatide treatment on volumetric BMD or estimated bone strength at the lumbar spine or hip (all p > 0.05) (see Appendix).
Areal BMD
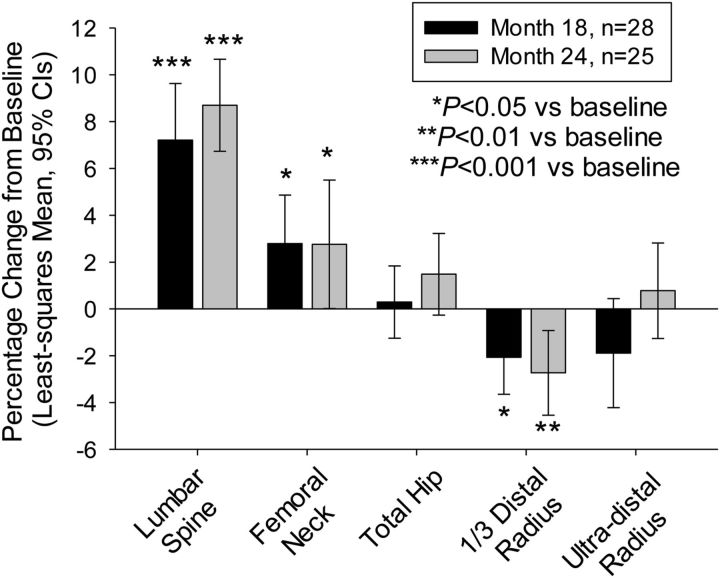
The lumbar spine and femoral neck skeletal sites displayed significant increases in areal BMD, as assessed with DXA, at months eighteen and twenty-four and at the end point for all subjects with follow-up data (p < 0.001 for the lumbar spine, p < 0.05 for the femoral neck) (Fig. 6; see Appendix). There were no significant changes in areal BMD for the total hip (p > 0.05). Assessments of areal BMD for the 1/3 distal radius site were significantly lower than baseline (p ≤ 0.013). The only assessment of areal BMD for the ultra-distal radius that differed significantly from baseline was the actual change at month eighteen (p = 0.049).
Fig. 6.
Change in areal BMD. The whiskers indicate the 95% CI.
Biochemical Markers of Bone Turnover
Teriparatide-treated subjects had significantly greater levels of serum P1NP (p < 0.001 compared with baseline) at all time points measured, and CTX levels were also significantly greater (p < 0.05 compared with baseline) except at the end point for all subjects with follow-up data (see Appendix). The increase in P1NP was greater than that in CTX at all time points, consistent with an osteoanabolic action of teriparatide treatment that results initially in bone modeling followed by an increase in bone remodeling27. P1NP concentrations remained elevated after twenty-four months of teriparatide treatment, suggesting that teriparatide continued to stimulate new bone formation at this time point. Changes in serum P1NP at month three were correlated with the increase in estimated vertebral strength at month eighteen (Pearson correlation coefficient = 0.401, Pearson p = 0.04) (see Appendix).
Safety
Thirty-two (91%) of the thirty-five subjects reported at least one adverse event during the study. Five subjects experienced serious adverse events, and one of these subjects experienced two serious adverse events (a fall and visual impairment). The most common adverse events were arthralgia (four, 11%); muscle spasms (four, 11%); and diarrhea, insomnia, headache, a fall, and decreased weight (three, 9%, each). In five (14%) of the subjects, an adverse event resulted in discontinuation; the event was vertigo, chest pain, malaise, dizziness, and neurilemmoma (one of the serious adverse events) in one subject each. Vertigo and dizziness were noted as being related to the study drug in the opinion of the investigators. The neurilemmoma, which was identified during MRI to assess a subject’s hearing loss, was later diagnosed as a benign acoustic neuroma, which the investigator considered unrelated to the study drug.
Teriparatide treatment can transiently increase serum calcium levels28. Hypercalcemia was identified in five subjects. One subject had hypercalcemia at baseline and discontinued the study after approximately three months because of an adverse event (vertigo) that emerged during treatment. No subject discontinued because of hypercalcemia. One subject had an elevated alkaline phosphatase level at baseline that remained elevated, but stable, throughout the study.
Discussion
Researchers are challenged to find technologies for performing noninvasive assessments of bone health. In the current trial, high-resolution MRI failed to detect changes in bone microarchitecture at the distal aspect of the radius after treatment with teriparatide, a bone anabolic agent approved for the treatment of osteoporosis. There are several potential explanations for this failure to detect an effect.
First, areal BMD at the ultra-distal radius site (approximately the same region as that assessed in the high-resolution MRI) was not significantly different at months eighteen and twenty-four compared with baseline. Furthermore, the 1/3 distal radius site, which consists predominantly of cortical bone, had a significant loss of areal BMD at months eighteen and twenty-four compared with baseline. It is possible that teriparatide treatment increases cortical remodeling, resulting in transient “cortical porosity” and an apparent loss of areal BMD as measured by DXA.
Second, the anatomical site (distal aspect of the radius) that was used for the high-resolution MRI measurements is not weight-bearing. Data from animal models show that it is important to have some level of mechanical loading of the bone to achieve a substantial osteoanabolic response with teriparatide treatment29. The distal aspect of the radius is minimally loaded compared with lower-extremity sites, such as the tibia. This effect of mechanical loading has been suggested in a clinical study of idiopathic osteoporosis in premenopausal women treated with teriparatide30. In that study, substantial increases in bone parameters were observed in the distal aspect of the tibia but not the distal aspect of the radius as measured by high-resolution CT scanning.
Third, most published information on high-resolution MRI is from trials conducted by a single investigator at a single research-dedicated MRI facility at an academic center. In the present multicenter trial, the high-resolution MRI units were also used for routine clinical care. Furthermore, there were technical issues at some sites in the present trial, although there were no obvious outliers when the MRI data were analyzed according to investigator site.
Finally, the lack of a detectable response in the high-resolution MRI data may be due to the absence of biologic effect of teriparatide. However, this is unlikely since the secondary outcomes—areal BMD, volumetric BMD, finite element analysis estimates of vertebral and hip strength, and bone turnover markers—were consistent with the known osteoanabolic effect of teriparatide.
Teriparatide treatment has consistently been associated with increased vertebral strength estimates. However, assessment of changes in femoral strength is more complex because of the impact of cortical remodeling on the peripheral compartment. During cortical remodeling, new cortical bone replaces existing cortical bone, a process that may eventually improve the material properties of the cortical bone10,14,31. In the present study, quantitative CT analysis revealed no improvement in volumetric BMD of the peripheral compartment at the hip; indeed, it revealed a possible decline, which may be due to cortical remodeling. Biomechanically, however, reductions in peripheral volumetric BMD at the hip do not necessarily imply reductions in overall femoral strength.
In the Fracture Prevention Trial17, which evaluated the effect of teriparatide on fractures in postmenopausal women with osteoporosis, 20 μg/day of teriparatide for a mean of nineteen months reduced new nonvertebral fragility fractures by 53%. The major “nonvertebral” sites included the hip, wrist, humerus, pelvis, and ankle. The Fracture Prevention Trial did not have enough hip or wrist fracture events to assess the impact of teriparatide at those specific sites. However, those findings suggest that any transient cortical remodeling with teriparatide treatment does not result in an increase in nonvertebral fractures in the short term and that the nonvertebral fracture risk reduction is maintained during longer-term treatment, which is often followed by antiresorptive therapy. Further, it is reassuring that femoral strength improved significantly after eighteen months of teriparatide treatment in the present trial.
Bone turnover marker results from the current trial were consistent with earlier studies5,27. It is worth noting that P1NP levels remained elevated (118.9% above baseline) at month twenty-four in the present trial, suggesting ongoing stimulation of osteoblasts at twenty-four months. An exploratory objective of this study was to determine whether prior oral bisphosphonate therapy would impact volumetric BMD or strength estimates of the spine and hip. Although no significant differences between subjects with and without prior bisphosphonate exposure were observed, it is possible that a larger sample size could have revealed differences in some parameters between these two groups. No unexpected adverse events were seen in this study involving elderly patients with severe osteoporosis. A total of five adverse events led to study discontinuation, and two (vertigo and dizziness) were considered by the investigators to be related to the study drug. Hypercalcemia was noted in five subjects but did not lead to any study discontinuations.
Limitations of the study include the small sample size, open-label design with no placebo or active comparator group, and use of high-resolution MRI at a non-weight-bearing site. Although estimates of strength by finite element analysis are predictive of incident vertebral fractures in elderly men32, to our knowledge no study has linked changes in strength associated with osteoporosis treatments to changes in fracture incidence. The effect of enhancing the spatial resolution of the MRI at the expense of signal-to-noise ratio is also unknown. Strengths of the study include the use of multiple validated imaging parameters and bone turnover markers to provide evidence supporting the osteoanabolic effect of teriparatide treatment.
In summary, high-resolution MRI failed to detect significant changes in bone microarchitecture at the non-weight-bearing site studied, the distal aspect of the radius, after eighteen or twenty-four months of treatment with teriparatide. This finding is consistent with the observed reductions in areal BMD (on DXA) at the distal aspect of the radius. Additional studies will be needed to determine whether high-resolution MRI can be used to monitor osteoporosis treatment results at a weight-bearing site, such as the tibia. In contrast, analysis of the secondary outcomes of the present study revealed increases in areal BMD in the lumbar spine and femoral neck (but not the total hip), volumetric BMD, bone turnover markers, and estimates of vertebral and femoral strength based on finite element analysis of quantitative CT data. These results are consistent with the known osteoanabolic effect of teriparatide at conventional weight-bearing skeletal sites. The increases in estimated vertebral and femoral strength are consistent with data from other finite element analysis studies and with the clinical trials of fracture outcomes that have shown teriparatide to be highly effective at treating bone fragility associated with osteoporosis and at reducing the numbers of both vertebral and nonvertebral fractures.
Appendix
Tables showing changes in volumetric BMD and estimated strength at the spine and hip (at months eighteen and twenty-four) as assessed by quantitative CT, areal BMD (at months eighteen and twenty-four), and bone turnover markers (at months three, six, and twenty-four), as well as figures showing outcomes according to prior bisphosphonate use, changes in bone turnover markers, and the correlation between changes in P1NP at month three and estimated vertebral strength at month eighteen, are available with the online version of this article as a data supplement at jbjs.org.
Acknowledgments
Note: The authors acknowledge Peiqi Chen, PhD, of Eli Lilly and Company, for assistance with study design and statistical analysis; Kathleen A. Taylor, PhD, of Lilly USA, for assistance with study design; and Eileen R. Gallagher, of inVentiv Health, for technical writing assistance.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, one or more of the authors has had another relationship, or has engaged in another activity, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Marcus R, Majumder S. The nature of osteoporosis. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. 2nd ed San Diego: Academic Press; 2001. p 3-17. [Google Scholar]
- 2.Parfitt AM. Implications of architecture for the pathogenesis and prevention of vertebral fracture. Bone. 1992;13(Suppl 2):S41-7. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003. November;18(11):1932-41. [DOI] [PubMed] [Google Scholar]
- 4.Ma YL, Zeng Q, Donley DW, Ste-Marie LG, Gallagher JC, Dalsky GP, Marcus R, Eriksen EF. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006. June;21(6):855-64. [DOI] [PubMed] [Google Scholar]
- 5.Dempster DW, Zhou H, Recker RR, Brown JP, Bolognese MA, Recknor CP, Kendler DL, Lewiecki EM, Hanley DA, Rao DS, Miller PD, Woodson GC, 3rd, Lindsay R, Binkley N, Wan X, Ruff VA, Janos B, Taylor KA. Skeletal histomorphometry in subjects on teriparatide or zoledronic acid therapy (SHOTZ) study: a randomized controlled trial. J Clin Endocrinol Metab. 2012. August;97(8):2799-808. Epub 2012 Jun 14. [DOI] [PubMed] [Google Scholar]
- 6.Gomberg BR, Saha PK, Wehrli FW. Topology-based orientation analysis of trabecular bone networks. Med Phys. 2003. February;30(2):158-68. [DOI] [PubMed] [Google Scholar]
- 7.Wehrli FW, Gomberg BR, Saha PK, Song HK, Hwang SN, Snyder PJ. Digital topological analysis of in vivo magnetic resonance microimages of trabecular bone reveals structural implications of osteoporosis. J Bone Miner Res. 2001. August;16(8):1520-31. [DOI] [PubMed] [Google Scholar]
- 8.Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003. October;33(4):744-50. [DOI] [PubMed] [Google Scholar]
- 9.Orwoll ES, Marshall LM, Nielson CM, Cummings SR, Lapidus J, Cauley JA, Ensrud K, Lane N, Hoffmann PR, Kopperdahl DL, Keaveny TM; Osteoporotic Fractures in Men Study Group. Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res. 2009. March;24(3):475-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keaveny TM. Biomechanical computed tomography-noninvasive bone strength analysis using clinical computed tomography scans. Ann N Y Acad Sci. 2010. March;1192:57-65. [DOI] [PubMed] [Google Scholar]
- 11.Kopperdahl DL, Hoffmann P, Sigurdsson S, Aspelund T, Siggeirsdottir K, Eiriksdottir G, Harris T, Gudnason VF, Keaveny TM. Enhancement of hip fracture prediction using finite element analysis of CT scans [Abstract]. J Bone Miner Res. 2010;25(Suppl S1):Z114 http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=918e83ed-ab2f-4503-adfb-e0cde8677656. Accessed 2014 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin S, Kopperdhal DL, Melton LJ, 3rd, Achenbach SJ, Therneau TM, Riggs BL, Keaveny TM, Khosla S. Association of hip strength estimates by finite-element analysis with fractures in women and men. J Bone Miner Res. 2011. July;26(7):1593-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res. 2007. January;22(1):149-57. [DOI] [PubMed] [Google Scholar]
- 14.Keaveny TM, McClung MR, Wan X, Kopperdahl DL, Mitlak BH, Krohn K. Femoral strength in osteoporotic women treated with teriparatide or alendronate. Bone. 2012. January;50(1):165-70. Epub 2011 Oct 17. [DOI] [PubMed] [Google Scholar]
- 15.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetić K, Müller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001. October;16(10):1846-53. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997. August 23;350(9077):550-5. [DOI] [PubMed] [Google Scholar]
- 17.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001. May 10;344(19):1434-41. [DOI] [PubMed] [Google Scholar]
- 18.Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, Edouard C, Klenerman L, Neer RM, Renier JC, Slovik D, Vismans FJ, Potts JT., Jr Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Br Med J. 1980. June 7;280(6228):1340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003. January;18(1):9-17. [DOI] [PubMed] [Google Scholar]
- 20.Misof BM, Roschger P, Cosman F, Kurland ES, Tesch W, Messmer P, Dempster DW, Nieves J, Shane E, Fratzl P, Klaushofer K, Bilezikian J, Lindsay R. Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab. 2003. March;88(3):1150-6. [DOI] [PubMed] [Google Scholar]
- 21.Reeve J, Hesp R, Williams D, Hulme P, Klenerman L, Zanelli JM, Darby AJ, Tregear GW, Parsons JA. Anabolic effect of low doses of a fragment of human parathyroid hormone on the skeleton in postmenopausal osteoporosis. Lancet. 1976. May 15;1(7968):1035-8. [DOI] [PubMed] [Google Scholar]
- 22.Greenspan SL, Perera S, Recker R, Wagner JM, Greeley P, Gomberg BR, Seaman P, Kleerekoper M. Changes in trabecular microarchitecture in postmenopausal women on bisphosphonate therapy. Bone. 2010. April;46(4):1006-10. Epub 2010 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehrli FW, Ladinsky GA, Jones C, Benito M, Magland J, Vasilic B, Popescu AM, Zemel B, Cucchiara AJ, Wright AC, Song HK, Saha PK, Peachey H, Snyder PJ. In vivo magnetic resonance detects rapid remodeling changes in the topology of the trabecular bone network after menopause and the protective effect of estradiol. J Bone Miner Res. 2008. May;23(5):730-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J; Committee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int. 2000;11(Suppl 6):S2-17. [DOI] [PubMed] [Google Scholar]
- 25.Gillings D, Koch G. The application of the principle of intention-to-treat to the analysis of clinical trials. Drug Inf J. 1991;25:411-24. [Google Scholar]
- 26.Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, Wright AC, Zemel B, Cucchiara A, Snyder PJ. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res. 2005. October;20(10):1785-91. Epub 2005 Jun 20. [DOI] [PubMed] [Google Scholar]
- 27.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005. August 8-22;165(15):1762-8. [DOI] [PubMed] [Google Scholar]
- 28.FORTEO® (teriparatide [rDNA origin] injection) for subcutaneous use prescribing information. Eli Lilly and Company. 2012. http://pi.lilly.com/us/forteo-pi.pdf. Accessed 2013 Sep 18. [Google Scholar]
- 29.Turner RT, Lotinun S, Hefferan TE, Morey-Holton E. Disuse in adult male rats attenuates the bone anabolic response to a therapeutic dose of parathyroid hormone. J Appl Physiol (1985). 2006. September;101(3):881-6. Epub 2006 May 4. [DOI] [PubMed] [Google Scholar]
- 30.Cohen A, Boutroy S, Stein E, Liu X, Lappe J, Recker R, Dempster D, Zhou H, McMahon D, Zhang C, Young P, Zhou B, Wang J, Guo XE, Shane E. High resolution peripheral quantitative computed tomography (HRpQCT) detects improved trabecular volumetric BMD and microarchitecture in premenopausal women with idiopathic osteoporosis treated with teriparatide. J Bone Miner Res 2011;26(Suppl 1). http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=6a58a567-7a2b-4b2c-8663-1aea3e53dd86. Accessed 2012 Oct 29. [Google Scholar]
- 31.Keaveny TM, Hoffmann PF, Singh M, Palermo L, Bilezikian JP, Greenspan SL, Black DM. Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res. 2008. December;23(12):1974-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Sanyal A, Cawthon PM, Palermo L, Jekir M, Christensen J, Ensrud KE, Cummings SR, Orwoll E, Black DM, Keaveny TM; Osteoporotic Fractures in Men (MrOS) Research Group. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res. 2012. April;27(4):808-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomberg BR, Wehrli FW, Vasilić B, Weening RH, Saha PK, Song HK, Wright AC. Reproducibility and error sources of micro-MRI-based trabecular bone structural parameters of the distal radius and tibia. Bone. 2004. July;35(1):266-76. [DOI] [PubMed] [Google Scholar]