Update
This article was updated on July 22, 2015, because the name of one of the authors, Victor W. Wong, was listed incorrectly. The name, which previously read “Victor C. Wong,” on pages 1101 and 1108, now reads “Victor W. Wong.”
An erratum has been published: J Bone Joint Surg Am. 2015 Sep 2;97(17):e59.
➤ Heterotopic ossification occurs most commonly after joint arthroplasty, spinal cord injury, traumatic brain injury, blast trauma, elbow and acetabular fractures, and thermal injury.
➤ The conversion of progenitor cells to osteogenic precursor cells as a result of cell-mediated interactions with the local tissue environment is affected by oxygen tension, pH, availability of micronutrients, and mechanical stimuli, and leads to heterotopic ossification.
➤ Radiation and certain nonsteroidal anti-inflammatory medications are important methods of prophylaxis against heterotopic ossification.
➤ Well-planned surgical excision can improve patient outcomes regardless of the joint involved or the initial cause of injury.
➤ Future therapeutic strategies are focused on targeted inhibition of local factors and signaling pathways that catalyze ectopic bone formation.
Heterotopic ossification is the formation of ectopic lamellar bone in soft tissues. It is increasingly recognized as a complication following trauma, burns, neurologic injuries, and major orthopaedic surgeries1-3. Heterotopic ossification was first described during World War I as a consequence of blast injuries, and remains a major cause of morbidity in soldiers returning from Iraq and Afghanistan1,4,5. The disability incurred as a result of heterotopic ossification is quite variable, and >20% of patients develop overt dysfunction as a result of soft-tissue loss, joint contractures, and chronic pain; rates of heterotopic ossification in the setting of civilian injury with traumatic brain injury are as high as 50%2,3,5-7.
Current research seeks to better understand the underlying cellular, biochemical, and mechanical processes to provide earlier diagnoses and develop more effective forms of treatment6,8,9 (Fig. 1). In this review, we highlight the need for improved recognition of heterotopic ossification as a common cause of morbidity after specific mechanisms of injury, and highlight factors about the epidemiology, management, and pathophysiology to improve the prevention, diagnosis, and treatment of this condition.
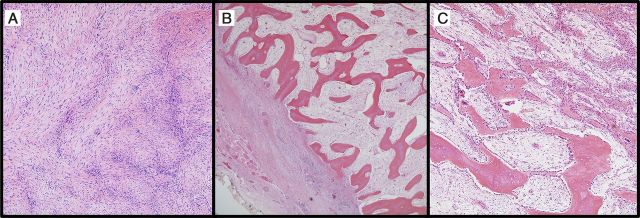
Fig. 1.
Histopathologic evidence of heterotopic ossification (hematoxylin and eosin). Fig. 1-A Early heterotopic ossification. The appearance of early heterotopic ossification may resemble reactive fibroblastic lesions. The photomicrograph shows the cellular proliferation of fibroblasts arranged in gently undulating C and S-shapes (×200). Fig. 1-B Densely mineralized osteoid interspersed with a loose and edematous fibroblastic proliferation (×40). Fig. 1-C A higher-magnification image demonstrates the tendency for maturation along the periphery of the lesion (lower left) in comparison with the more central areas (upper right) (×100). Prominent bone-lining osteoblasts are noted throughout the lesion.
Epidemiology
The prevalence of heterotopic ossification has been reported to range from 0.2% to 4% after burn injury and up to 90% after certain types of hip arthroplasty or acetabular fractures10-15. The mechanism of injury, duration of immobilization, percentage of total body surface area burned, and degree of spasticity affect the risk for heterotopic ossification12,16-19. There are also differences related to sex that affect the predisposition for heterotopic ossification, with males more commonly affected than females, perhaps as a result of differential muscle mass, variations in mechanism of injury, and distinct hormonal signaling pathways affecting osteogenesis19-21.
Clinical Presentation
Heterotopic ossification commonly presents as restriction in joint motion after an inciting trauma. Several classification schemes exist; Brooker et al. grouped heterotopic ossification at the hip into four classes to indicate the severity of heterotopic ossification11,22 (Table I). The Hastings and Graham classification system uses clinical and radiographic data to categorize heterotopic ossification at the elbow into three classes19 (Table II). These classification schemes facilitate the objective characterization of heterotopic ossification primarily for clinical research.
TABLE I.
Brooker Classification System for Heterotopic Ossification at the Hip22
| Class | Definition |
| I | Islands of bone within soft tissues of the hip |
| II | Bone spurs in the pelvis or femur but with ≥1 cm between bone surfaces |
| III | Bone spurs within the pelvis or femur with <1 cm between bone surfaces |
| IV | Ankylosis of the hip |
TABLE II.
Hastings and Graham Classification System for Heterotopic Ossification at the Elbow19
| Class | Definition |
| I | Radiographic evidence without functional deficit |
| IIA | Radiographic evidence with limitation in flexion-extension axis |
| IIB | Radiographic evidence with limitation in pronation-supination axis |
| IIIA | Ectopic bone formation and ankylosis of joint in flexion-extension axis |
| IIIB | Ectopic bone formation and ankylosis of joint in pronation-supination axis |
| IIIC | Ectopic bone formation and ankylosis of joint in pronation-supination and flexion-extension axes |
Radiography
When heterotopic ossification is suspected clinically, radiographic data identify the location and extent of disease2. Because serum calcium, phosphorus, and alkaline phosphatase levels are not routinely reliable for the diagnosis or prognostication of heterotopic ossification, radiographic imaging and clinical history are of utmost importance23.
Plain radiographs are commonly the first imaging study used to detect heterotopic ossification1. The advantages of radiographs are the low cost and relative ease of obtaining these images. The disadvantage is the difficulty of visualizing the anatomic extent of ectopic bone deposition early in the disease process. Technetium-99 bone scans are an alternative in this setting24,25. While the advantage of bone scans is the ability to detect heterotopic ossification earlier than radiographs, bone scans are expensive and of limited value in differentiating inflammation from early heterotopic ossification6,12.
Computed tomography (CT) facilitates preoperative planning by improving three-dimensional visualization of heterotopic ossification in relation to important anatomic landmarks. In some circumstances, magnetic resonance imaging may be required to more clearly define the extent of local soft-tissue or neurovascular involvement. These studies are best utilized when heterotopic ossification is adjacent to anatomic structures within a potential operative field25.
New and upcoming imaging modalities are also under review. Single-photon emission CT, or SPECT, is a potential imaging modality for early detection of heterotopic ossification with improved sensitivity16,26,27. Ultrasound has been shown to detect heterotopic ossification sooner than conventional radiography and can be used intraoperatively to visualize heterotopic ossification prior to surgical excision13,17-19,22,26,27. Raman spectroscopy is a novel imaging technology that has the potential to define the extent of heterotopic ossification earlier than currently available radiographic studies by detecting mineralized collagen within tissues24,27. Clinical trials are under way, given the recent validation in animal models.
Clinical Risk Factors: Mechanism of Injury
Spinal Cord and Traumatic Brain Injuries
The prevalence of heterotopic ossification after central neurologic injury has been reported to range from 10% to 53%28 (Table III). Most studies combine the analysis of patients with a traumatic brain injury and those with a spinal cord injury. Although the relationship between the nervous system and the formation of bone remains incompletely understood, it is known that peripheral neurotransmitters affect osteoblast formation18,29. Risk factors for heterotopic ossification in patients with a spinal cord injury include the severity of the injury and the level of the spinal cord injury, with injuries to the thoracic and cervical spine resulting in greater severity of heterotopic ossification18. Patients with severe spasticity, impaired cognition, tracheostomy, pneumonia, and/or urinary tract infections are at a higher risk18. Thus, measures to minimize the likelihood of these contributing conditions are important to decrease the subsequent risk of heterotopic ossification. In patients after a spinal cord injury, heterotopic ossification commonly forms caudad to the level of the injury, and most commonly at the hip; heterotopic ossification at peripheral joints is rare16.
TABLE III.
| Cause of Trauma | Rate of Heterotopic Ossification (%) |
| Thermal burn | 0.2-4 |
| Hip arthroplasty | 3-90 |
| Neurologic injury | 10-53 |
| Spinal cord injury | 20 |
Patients with a traumatic brain injury share a similar set of risk factors. Unlike patients with a spinal cord injury, however, those with a traumatic brain injury may develop heterotopic ossification throughout the body, including at the hip, knee, and elbow or shoulder. In a systematic review comparing treatments used for traumatic brain injury and spinal cord injury, Aubut et al. analyzed twenty-six studies comparing pharmacologic interventions administered to these two populations30. While pharmacologic strategies in general were effective in both groups, nonsteroidal anti-inflammatory medications (NSAIDs) were more commonly used in patients after a spinal cord injury, while bisphosphonates were used in patients with a traumatic brain injury. These findings, however, must be applied with caution, given the heterogeneity evident in the dosing regimens and administration strategies among these groups31,32. When pharmacologic strategies are initiated, timing is of utmost importance as early initiation of pharmacologic therapy can limit the progression of disability over time. Compliance with therapy is also important.
Thermal Injury
The most important risk factor for heterotopic ossification among patients after a burn injury is the percentage of body surface area affected, with burns involving >20% of the body substantially increasing the likelihood of heterotopic ossification14. Additional risk factors include male sex and full-thickness injury at or near a joint14. Heterotopic ossification occurs with the highest frequency at the elbow, followed by the shoulder, and subsequently the hip (Fig. 2). Pain, erythema, swelling, and palpable bone formation are commonly reported symptoms6,12,19.
Fig. 2.
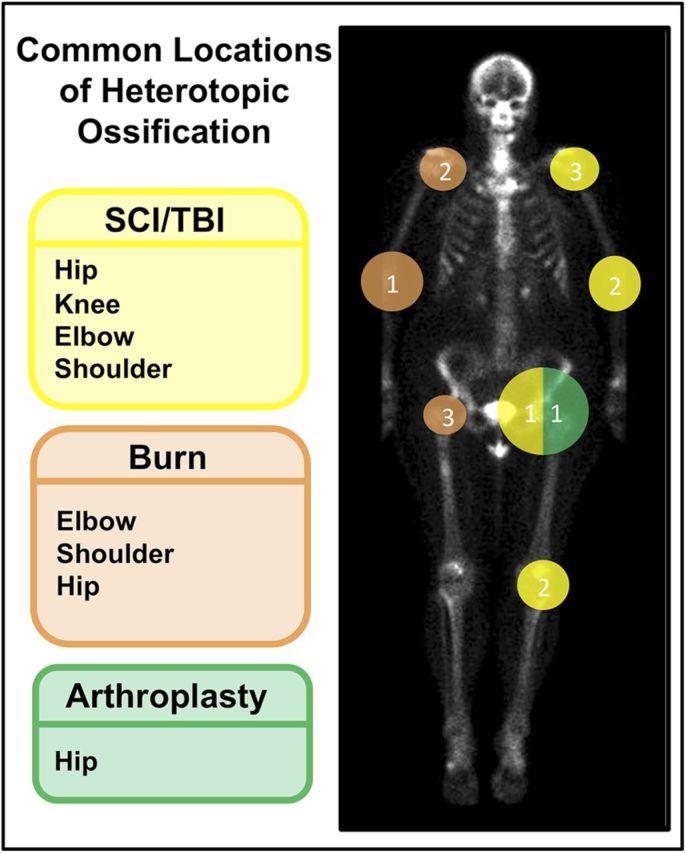
The common locations of heterotopic ossification are indicated according to the mechanism of injury. The numbering system from 1 (highest) to 3 (lowest) indicates decreasing prevalence of heterotopic ossification at the indicated joint based on the mechanism of injury. SCI = spinal cord injury, and TBI = traumatic brain injury.
Similar to patients presenting with neurologic injury, those with a burn injury often have a restriction in the range of motion at a joint as the earliest manifestation of the heterotopic ossification. It is of particular importance in the treatment of burn injuries to distinguish scar contracture near a joint from heterotopic ossification. Imaging studies and isolated joint flexibility measurements are useful in this determination. At the elbow, palpation of a locking sensation at the terminal extent of extension or flexion is more likely an indication of heterotopic ossification than of a joint contracture; no specific signs have been noted for other joints15. Few studies have analyzed the impact of pharmacologic prophylaxis or the treatment of heterotopic ossification after burn injury14,33. As a result, the treatment of established heterotopic ossification commonly involves surgical excision. Prior to surgical intervention, however, it is important to ensure adequate soft-tissue coverage of the site postoperatively, given the extensive scarring, tenuous and tight contour of skin grafts, and relative laxity of the surrounding soft tissues after thermal injury.
Hip Arthroplasty
Following hip arthroplasty, heterotopic ossification occurs at variable rates, with most studies having documented rates at approximately 40%11,13,22,34. Although many studies have isolated specific factors that increase the risk for heterotopic ossification after spinal cord injury and burn injury, it remains unclear what patient-related factors lead to heterotopic ossification after hip arthroplasty18,35. Interestingly, surgical factors, such as extended ischemia time, type of approach, and the use of cemented implants, may increase the risk of heterotopic ossification36. Both radiation and NSAID therapies are effective for prophylaxis; surgical excision is effective for treatment37,38.
Fractures
The formation of heterotopic ossification after orthopaedic trauma has been studied most extensively in the setting of acetabular fractures and elbow fractures. Heterotopic ossification occurs in approximately 40% of patients after operative fixation of an acetabular fracture39. Specific risks include the need for long-term mechanical ventilation. Injury severity score, sex, and fracture type do not affect this risk36. Importantly, the surgical approach can impact the risk for heterotopic ossification. Guo et al. recommended the use of the trochanteric flip or the posterior approach in the setting of acetabular fractures; the rates of heterotopic ossification after the trochanteric flip, anterior, or posterior approach were 33.3%, 42.1%, and 36.9%, respectively40. While an anterior approach optimizes visualization of the fracture, this approach also has a high rate of heterotopic ossification. The trochanteric flip approach preserves the vascular supply to the surrounding soft tissues, as does the posterior approach, and provides an efficient exposure by which to achieve access to the femoral head40.
The prevalence of heterotopic ossification after an elbow fracture is also approximately 40%, and it is most commonly located posteromedially41,42. More than 20% of those who develop heterotopic ossification in this setting have clinically relevant motion deficits in the form of a decreased arc of flexion-extension to <100°. Risk factors include concomitant neurologic injury, delayed internal fixation, and use of bone graft and/or bone-graft substitute. Patients with AO class-C3 fractures were more likely to require operative intervention for the management of heterotopic ossification than were those with less severe fractures41. More severe heterotopic ossification was associated with a concomitant distal humeral fracture, triad injury, Monteggia fracture-dislocation, and transolecranon fracture-dislocation. Given that a delay in intervention increases the risk for heterotopic ossification, prompt operative fixation is imperative42. Prophylaxis should be considered in patients at high risk for heterotopic ossification, and treatment is focused on surgical excision39,43,44.
Management
Prophylaxis
NSAIDs
NSAIDs prevent heterotopic ossification by inhibiting the osteogenic differentiation of progenitor cells45,46. Prostaglandin E2 is a major contributor to heterotopic ossification formation, fracture-healing, and bone regeneration47-59. Numerous recommendations exist with regard to dosing; indomethacin, a nonselective cyclooxygenase (COX)-1 and COX-2 inhibitor, is commonly administered at an oral dose of 75 mg twice per day or 25 mg three times per day for three to six weeks postoperatively15,60,61. The optimal timing, dose, and duration of treatment have yet to be determined (Table IV). Selective COX-2 inhibitors are also an option to consider, given the risk of gastrointestinal distress associated with nonselective NSAIDs47,62,63. Vasileiadis et al. reviewed five studies that demonstrated that selective COX-2 inhibitors are equally as effective as NSAIDs in the prevention of heterotopic ossification in patients undergoing total hip arthroplasty64,65.
TABLE IV.
Prophylactic Options for Postoperative Heterotopic Ossification*
| Treatment Used in Study | Clinically Important Heterotopic Ossification† | Level of Evidence |
| Saudan et al.55 (2007) | I | |
| Celecoxib | 6/117 (5.1%) | |
| Ibuprofen | 16/123 (13.0%) | |
| Grohs et al.57 (2007) | I | |
| Rofecoxib | 3/46 (6.5%) | |
| Indomethacin | 0/50 (0.0%) | |
| Karunakar et al.50 (2006) | I | |
| Placebo | 13/62 (20.9%) | |
| Indomethacin | 9/59 (15.2%) | |
| Fransen et al.104 (2006) | I | |
| Placebo | 26/407 (6.4%) | |
| Ibuprofen | 11/391 (2.8%) | |
| Burd et al.66 (2001) | I | |
| Radiation therapy | 3/78 (3.8%) | |
| NSAIDs | 8/72 (11.1%) | |
| Kölbl et al.105 (1997) | I | |
| Radiation therapy | 1/188 (0.5%) | |
| NSAIDs | 2/113 (1.8%) | |
| Kölbl et al.37 (1997) | I | |
| Radiation therapy | 0/100 (0.0%) | |
| NSAIDs | 6/183 (3.3%) | |
| Beckmann et al.63 (2014) | III | |
| No treatment | 23/92 (25.0%) | |
| Naproxen | 11/196 (5.6%) | |
| Brunnekreef et al.54 (2013) | III | |
| Etoricoxib | 0/42 (0.0%) | |
| Le Duff et al.60 (2011) | III | |
| Indomethacin and 1000 mL saline solution jet lavage | 6/111 (5.4%) | |
| Indomethacin, radiation therapy, and 1000 mL saline solution jet lavage | 23/332 (6.9%) | |
| Indomethacin, radiation therapy, and 2000 mL saline solution jet lavage | 5/247 (2.0%) | |
| Indomethacin, radiation therapy, 2000 mL saline solution jet lavage, and debris drape | 5/294 (1.7%) | |
| Nunley et al.59 (2011) | III | |
| Aspirin | 1/151 (0.7%) | |
| Warfarin | 4/46 (8.7%) | |
| van der Heide et al.56 (2007) | III | |
| Placebo | 2/99 (2.0%) | |
| Indomethacin | 49/170 (28.9%) | |
| Rofecoxib | 0/42 (0.0%) |
Clinically important heterotopic ossification was determined to be Brooker score of III or IV by radiographic imaging. Drug names ending in -coxib are selective COX-2 inhibitors.
The values are given as the number of patients who developed heterotopic ossification divided by the total number in the treatment group.
While NSAIDs have demonstrated prophylactic efficacy against heterotopic ossification, it is also important to consider the impact of these medications on fracture union; heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion39,66. Given these risks, NSAIDs must be used with caution after orthopaedic injuries because of the potential risk of nonunion50,67.
Bisphosphonates
Bisphosphonates are antiresorptive agents that induce osteoclast apoptosis and inhibit calcification. While some studies have demonstrated that bisphosphonates are effective for prophylaxis against heterotopic ossification28, others have found this therapy to be ineffective and do not recommend routine use of these medications specifically after orthopaedic interventions33,64,68. Studies have examined the effect of first-generation bisphosphonates on heterotopic ossification as this generation affects osteoclasts and osteoblasts; subsequent generations of bisphosphonates are less likely to be of use against heterotopic ossification as they affect only osteoclasts. While one systematic review found bisphosphonates to be ineffective for preventing heterotopic ossification, others have found that they are indeed effective in patients with burn injuries and spinal cord injuries specifically28,69.
Radiation
Radiation is effective for prophylaxis against heterotopic ossification at the hip. While the rates of heterotopic ossification after hip arthroplasty have been reported to range from approximately 5% to 90%, the prevalence after radiation therapy decreased to 25%70,71. Even low-dose radiation minimizes heterotopic ossification after hip arthroplasty72. The utility of radiation as prophylaxis at joints other than the hip, however, has not been adequately studied73,74. Radiation may be given at a dose of 700 to 800 cGy in a single fraction administered from twenty-four hours preoperatively until forty-eight to seventy-two hours postoperatively70. Both preoperative radiation and postoperative radiation were found to be equally effective at the hip, and no specific differences in complications were stated75. Although no cases of malignancy after prophylactic radiation have been reported to date, this is a theoretical complication to consider. Additional side effects include progressive soft-tissue contracture, delayed wound-healing, nonunion, or inhibited ingrowth of press-fit hip implants7,74.
Because radiation and NSAID therapy are both effective methods of prophylaxis, the question remains as to which is better8,76,77. Studies have demonstrated no difference between NSAIDs and radiation in preventing heterotopic ossification38,53,78. A study performed by Moore et al. corroborated these results in the setting of acetabular fractures79. The decision to use radiation or NSAID therapy for prophylaxis can be tailored to individual patient conditions. For example, because side effects of soft-tissue contracture are particularly detrimental to patients with burns, NSAID therapy may be preferable to radiation in the absence of gastrointestinal contraindications. On the other hand, patients undergoing hip arthroplasty may be candidates for either prophylactic modality. Additional factors, including long-term medication compliance and cost, must be considered.
Treatment
Physical Therapy
There are a number of different opinions on the value of physical therapy in the treatment of heterotopic ossification, as no clear evidence exists as to the ultimate effect of joint motion on the progression of overlying heterotopic ossification80. While some believe that too much motion immediately following the injury exacerbates heterotopic ossification, others believe that heterotopic ossification progresses because of lack of motion9,18,80. Although there is no consensus, physical therapy may be helpful for those experiencing worsening range of motion that limits daily functionality.
Pharmaceutical Intervention
Despite the large number of prophylactic strategies available, no current pharmaceutical treatments exist to address heterotopic ossification once present. A stage-II U.S. Food and Drug Administration trial is under way, however, to evaluate the effect of retinoic acid receptor (RAR) agonists on patients with fibrodysplasia ossificans progressiva81. Palovarotene is an RAR agonist that has mitigated heterotopic ossification in mouse models of fibrodysplasia ossificans progressiva. It is thought that targeting RAR gamma, a receptor expressed on chondrogenic cells and chondrocytes that acts as a transcriptional repressor, will inhibit osteogenic activity in chondrocytes prior to endochondral ossification81. Although palovarotene has demonstrated efficacy in mouse models of fibrodysplasia ossificans progressiva, it remains unclear whether this strategy will be effective for other acquired forms of heterotopic ossification in human subjects.
Operative Intervention
Surgical excision is effective for the treatment of heterotopic ossification and should be considered as an option for patients with functional deficits as a result of the disorder. Given the inherent fragility of the soft tissues, however, it is important to counsel patients on the risks regarding delayed wound-healing, infection, nerve injury, and recurrent contracture10,82. Timing of operative intervention is an important consideration. The etiology of heterotopic ossification and the degree of bone maturation should guide the timing of intervention; traumatic heterotopic ossification can generally be resected at six to nine months, spinal cord injury-related heterotopic ossification at twelve months, and traumatic brain injury-related heterotopic ossification at eighteen months83. Although the definition of complete bone maturation remains inconsistent within the literature, operative intervention should be considered when there is a lack of functional improvement with nonoperative forms of treatment.
After orthopaedic intervention specifically, early excision is recommended by some because of the relative preservation of tissue planes that are important for differentiating ectopic bone from normal callus and scar at the site of a recent intervention84. While outcomes are often satisfactory after excision, severe complications can also occur. For acetabular fractures, complications have been reported to occur at a rate of 33.3% and have included intraoperative femoral neck fractures, sciatic nerve injury, femoral head osteonecrosis, and recurrence of heterotopic ossification32. In summary, when there is lack of clinical improvement with nonoperative interventions or progressive disability in the setting of stable imaging findings, it is prudent to consider operative intervention85,86.
Current Research
Much of our understanding of heterotopic ossification stems from research on fibrodysplasia ossificans progressiva87, which is a congenital condition resulting in spontaneous heterotopic ossification throughout the body. Overactivation of the bone morphogenetic protein (BMP) cascade through constitutive activation of the activin type-1 receptor (ACVR1) leads to abnormal skeletogenesis in the form of endochondral ossification. Identification of the cells that contribute to the fibrodysplasia ossificans progressiva phenotype in humans, however, has remained elusive. In animal models, global expression of the ca-ALK2 (constitutively active activin-like kinase 2) mutation, Q207D, results in uniform in utero lethality and elevated levels of Smad 1/5/8 suggestive of a pro-osteogenic phenotype88. Although a number of common cellular mechanisms have been described between fibrodysplasia ossificans progressiva and acquired forms of heterotopic ossification, no single unifying mechanism currently exists.
Osteogenic Precursors
Tissues prone to heterotopic ossification demonstrate an abnormally heightened or prolonged inflammatory response to injury9. Heterotopic ossification occurs as a result of the pathologic recruitment of local and distant circulating cellular precursors. Recent advances have highlighted a number of critical cell populations involved in this process (Table V). Mesenchymal stem cells are thought to be the major cell population involved in the formation of heterotopic ossification. In the setting of trauma-induced heterotopic ossification, muscle-derived mesenchymal stem cells demonstrate increased osteogenic potential through increased BMP-4 expression. This leads to increased vascular proliferation and osteogenesis89. Given the numerous signaling pathways that are important for this process, however, it is most likely that a combination of different cell types is involved89-92.
TABLE V.
Cells Types Contributing to Heterotopic Ossification
| Study | Cell Types* | Model† | Findings† |
| Wu et al.106 (2013) | Muscle satellite cells | Rat (HO) | Both burn and burn serum increase osteogenic potential |
| Medici et al.99 (2010) | Vascular endothelial cells | Mouse (HO) | Convert to multipotent stem-like cells increasing local osteogenesis |
| Lounev et al.95 (2009) | MyoD-expressing skeletal muscle, SMMHC-expressing vascular smooth muscle, and Tie2-expressing endothelial cells | Mouse (HO) | <5% contribution to fibroproliferative stage; <1% to chondrogenic stage; not detected in heterotopic ossification; 40% to 50% contribution at each stage of heterotopic ossification‡ |
| Suda et al.107 (2009) | Bone marrow-derived circulating osteogenic precursors | Mouse (HO) | Cells present in fibroproliferative stage lesions |
| Kan et al.108 (2009) | Macrophages and/or monocytes | Mouse (HO) | Macrophages help mediate the injury response triggering HO |
| Kalajzic et al.109 (2008) | Alpha-smooth muscle actin-expressing cells (pericyte and/or myofibroblast phenotype) | Mouse | Increased osteogenic potential in osteoblast ablation model |
| Kaplan et al.110 (2007) | Hematopoietic stem cells via bone marrow transplant | Human (FOP) | Stem cell replacement not sufficient to prevent ectopic skeletogenesis |
| Hematopoietic lineage cells | Mouse (HO) | Not represented during any stage of the heterotopic skeletal anlagen |
MyoD = myogenic differentiation 1, and SMMHC = smooth muscle myosin heavy chain.
HO = heterotopic ossification, and FOP = fibrodysplasia ossificans progressiva.
Stages of the heterotopic skeletal anlagen can be divided as follows: fibroproliferative, chondrogenic, and osteogenic.
Recent experiments utilizing lineage tracing have also identified a role for nonosteogenic cell populations in heterotopic ossification93. Tie2-expressing cells that affect angiogenesis contribute to 50% of the bone-forming cells in heterotopic lesions. A recent study by Wosczyna et al. showed that heterotopic ossification lesions in their model were positive for Tie2 but negative for VE (vascular endothelial)-cadherin, indicating a nonendothelial origin94. These cells are known to respond to inflammation by undergoing endochondral differentiation, and they form heterotopic bone in response to BMP stimulation95. Fibrocytes also differentiate into osteoblasts and chondrocytes and contribute to the formation of heterotopic ossification93.
Local Microenvironment
Local factors play a role in the development of ectopic bone. BMPs are central to tissue homeostasis and osteogenesis; as part of the transforming growth factor (TGF)-beta superfamily, BMPs induce the formation of receptor complexes with inherent serine-threonine kinase activity. Although more than twenty different types of BMPs have been discovered, the BMP-2/4 subfamily is especially relevant for its osteoinductive properties. Activation of the BMP-2 receptor is one of the major pathways leading to heterotopic ossification formation96-98. The BMP-2 receptor is activated by a ligand, which leads to downstream phosphorylation of Smad 1/5/8. As a result, gene transcription, cell differentiation, and cell proliferation are affected, and this results in increased production of osteogenic factors. Numerous studies have demonstrated upregulation of this pathway locally after burn injuries98. In addition to local BMP-mediated mechanisms, retinoid receptors are also important in chondrogenesis. Exogenous retinoids inhibit chondrogenesis and are sufficient to inhibit heterotopic ossification in traumatic and congenital mouse models92.
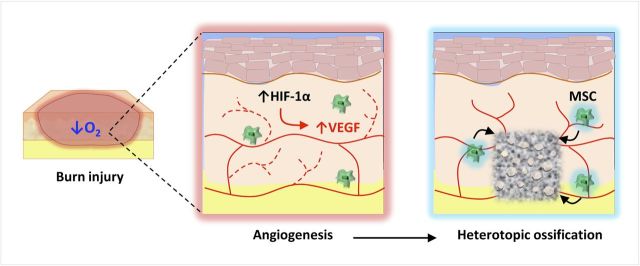
Oxygen tension, pH, micronutrients, and mechanical stimuli also impact bone formation54. Hypoxia-inducible factor 1-alpha (HIF-1α) is a key transcriptional regulator of the cellular response to ischemia through stimulation of vascular endothelial cell precursors99,100 (Fig. 3). Under hypoxic conditions, HIF-1α translocates to the nucleus where it dimerizes with HIF-1β to bind to a hypoxia response element present on multiple hypoxia-related genes for angiogenic cytokines; these cytokines including VEGF (vascular endothelial growth factor), bFGF (basic fibroblast growth factor), PDGF (platelet-derived growth factor), and angiopoietin-2 are essential for endothelial cell motility, recruitment, and proliferation. HIF-1α signaling also sustains differentiation of hypoxic prechondrogenic cells during skeletogenesis by regulating sex-determining region Y-box 9, which is required as a cartilage precursor to heterotopic ossification101. Although it is known that HIF-1α upregulates BMP-2 signaling, the use of HIF-1α as a therapeutic target has not been exploited92,99-101.
Fig. 3.
Burn injury and the resulting hypoxic environment increase HIF-1α production, which leads to angiogenesis as a result of increased levels of VEGF. Angiogenesis is an important step in the process of bone formation. MSC = mesenchymal stem cell.
Future Directions
Prompt recognition, accurate diagnosis, and initiation of appropriate treatment and prophylactic management strategies in the setting of heterotopic ossification can mitigate the disability attributable to this debilitating disease process. Current guidelines indicate that radiation and NSAIDs are effective forms of prophylaxis, and surgical intervention is an effective treatment option. Given the extent of disability caused by heterotopic ossification, we must improve our mechanistic understanding of this process. MicroRNA constructs are targeted therapies that can potentially improve current modalities of treatment and prophylaxis against heterotopic ossification through local HIF-1α inhibition102. NFκB (nuclear factor kappa B) may be another potential target to inhibit chondrogenesis, osteogenesis, and angiogenesis103. Additional local therapies include toll-like receptor and RAR modulation81. In addition to improved pharmacologic agents, early detection of heterotopic ossification is also important. Both Raman spectroscopy and near-infrared imaging are under review in the form of human and animal-based studies. By improving our ability to treat and detect heterotopic ossification, we can reduce the severe personal and societal costs associated with the progression of this disease.
Footnotes
Investigation performed at the Department of Surgery, University of Michigan Health Systems, Ann Arbor, Michigan
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. None of the authors, or their institution(s), have had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, no author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
Disclaimer: The opinions or assertions contained in this paper are the private views of the authors and are not to be construed as reflecting the views, policy or positions of the Department of the Navy, Department of Defense, nor the U.S. Government.
References
- 1.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009. May;91(5):1084-91. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. J Am Acad Orthop Surg. 2004. Mar-Apr;12(2):116-25. [DOI] [PubMed] [Google Scholar]
- 3.Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005. May;37(3):129-36. [DOI] [PubMed] [Google Scholar]
- 4.Potter BK, Forsberg JA, Davis TA, Evans KN, Hawksworth JS, Tadaki D, Brown TS, Crane NJ, Burns TC, O’Brien FP, Elster EA. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010. December;92(Suppl 2):74-89. [DOI] [PubMed] [Google Scholar]
- 5.Forsberg JA, Davis TA, Elster EA, Gimble JM. Burned to the bone. Sci Transl Med. 2014. September 24;6(255):55fs37. [DOI] [PubMed] [Google Scholar]
- 6.Kung TA, Jebson PJ, Cederna PS. An individualized approach to severe elbow burn contractures. Plast Reconstr Surg. 2012. April;129(4):663e-73e. [DOI] [PubMed] [Google Scholar]
- 7.Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys. 2006. August 1;65(5):1289-99. [DOI] [PubMed] [Google Scholar]
- 8.Baird EO, Kang QK. Prophylaxis of heterotopic ossification - an updated review. J Orthop Surg. 2009;4:12 Epub 2009 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coons D, Godleski M. Range of motion exercises in the setting of burn-associated heterotopic ossification at the elbow: case series and discussion. Burns. 2013. June;39(4):e34-8. Epub 2012 Nov 16. [DOI] [PubMed] [Google Scholar]
- 10.Maender C, Sahajpal D, Wright TW. Treatment of heterotopic ossification of the elbow following burn injury: recommendations for surgical excision and perioperative prophylaxis using radiation therapy. J Shoulder Elbow Surg. 2010. December;19(8):1269-75. Epub 2010 Sep 18. [DOI] [PubMed] [Google Scholar]
- 11.Bedi A, Zbeda RM, Bueno VF, Downie B, Dolan M, Kelly BT. The incidence of heterotopic ossification after hip arthroscopy. Am J Sports Med. 2012. April;40(4):854-63. Epub 2012 Jan 20. [DOI] [PubMed] [Google Scholar]
- 12.Cipriano CA, Pill SG, Keenan MA. Heterotopic ossification following traumatic brain injury and spinal cord injury. J Am Acad Orthop Surg. 2009. November;17(11):689-97. [DOI] [PubMed] [Google Scholar]
- 13.Rath E, Sherman H, Sampson TG, Ben Tov T, Maman E, Amar E. The incidence of heterotopic ossification in hip arthroscopy. Arthroscopy. 2013. March;29(3):427-33. Epub 2013 Jan 23. [DOI] [PubMed] [Google Scholar]
- 14.Medina A, Shankowsky H, Savaryn B, Shukalak B, Tredget EE. Characterization of heterotopic ossification in burn patients. J Burn Care Res. 2014. May-Jun;35(3):251-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen HC, Yang JY, Chuang SS, Huang CY, Yang SY. Heterotopic ossification in burns: our experience and literature reviews. Burns. 2009. September;35(6):857-62. Epub 2009 May 29. [DOI] [PubMed] [Google Scholar]
- 16.Kluger G, Kochs A, Holthausen H. Heterotopic ossification in childhood and adolescence. J Child Neurol. 2000. June;15(6):406-13. [DOI] [PubMed] [Google Scholar]
- 17.Hunt JL, Arnoldo BD, Kowalske K, Helm P, Purdue GF. Heterotopic ossification revisited: a 21-year surgical experience. J Burn Care Res. 2006. Jul-Aug;27(4):535-40. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MP, Torres SJ, Mehta S, Ahn J. Heterotopic ossification after central nervous system trauma: a current review. Bone Joint Res. 2013. March;2(3):51-7. Epub 2013 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastings H, 2nd, Graham TJ. The classification and treatment of heterotopic ossification about the elbow and forearm. Hand Clin. 1994. August;10(3):417-37. [PubMed] [Google Scholar]
- 20.Abrams GD, Bellino MJ, Cheung EV. Risk factors for development of heterotopic ossification of the elbow after fracture fixation. J Shoulder Elbow Surg. 2012. November;21(11):1550-4. Epub 2012 Sep 2. [DOI] [PubMed] [Google Scholar]
- 21.Ranganathan K, Peterson JR, Agarwal S, Oluwatobi E, Loder S, Forsberg JA, Davis TA, Wang SC, Levi B. Role of gender in burn-induced heterotopic ossification and mesenchymal cell osteogenic differentiation. Plast Reconstr Surg. [In press]. [DOI] [PMC free article] [PubMed]
- 22.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973. December;55(8):1629-32. [PubMed] [Google Scholar]
- 23.Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002. March;43(3):346-53. [PubMed] [Google Scholar]
- 24.Peterson JR, Okagbare PI, De La Rosa S, Cilwa KE, Perosky JE, Eboda ON, Donneys A, Su GL, Buchman SR, Cederna PS, Wang SC, Kozloff KM, Morris MD, Levi B. Early detection of burn induced heterotopic ossification using transcutaneous Raman spectroscopy. Bone. 2013. May;54(1):28-34. Epub 2013 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perosky JE, Peterson JR, Eboda ON, Morris MD, Wang SC, Levi B, Kozloff KM. Early detection of heterotopic ossification using near-infrared optical imaging reveals dynamic turnover and progression of mineralization following Achilles tenotomy and burn injury. J Orthop Res. 2014. November;32(11):1416-23. Epub 2014 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crane NJ, Elster EA. Vibrational spectroscopy: a tool being developed for the noninvasive monitoring of wound healing. J Biomed Opt. 2012. January;17(1):010902. [DOI] [PubMed] [Google Scholar]
- 27.Crane NJ, Polfer E, Elster EA, Potter BK, Forsberg JA. Raman spectroscopic analysis of combat-related heterotopic ossification development. Bone. 2013. December;57(2):335-42. Epub 2013 Sep 5. [DOI] [PubMed] [Google Scholar]
- 28.Teasell RW, Mehta S, Aubut JL, Ashe MC, Sequeira K, Macaluso S, Tu L; SCIRE Research Team. A systematic review of the therapeutic interventions for heterotopic ossification after spinal cord injury. Spinal Cord. 2010. July;48(7):512-21. Epub 2010 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichel LM, Salisbury E, Moustoukas MJ, Davis AR, Olmsted-Davis E. Molecular mechanisms of heterotopic ossification. J Hand Surg Am. 2014. March;39(3):563-6. Epub 2013 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aubut JA, Mehta S, Cullen N, Teasell RW. ERABI Group; Scire Research Team. A comparison of heterotopic ossification treatment within the traumatic brain and spinal cord injured population: an evidence based systematic review. NeuroRehabilitation. 2011;28(2):151-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAuliffe JA, Wolfson AH. Early excision of heterotopic ossification about the elbow followed by radiation therapy. J Bone Joint Surg Am. 1997. May;79(5):749-55. [DOI] [PubMed] [Google Scholar]
- 32.Wu XB, Yang MH, Zhu SW, Cao QY, Wu HH, Wang MY, Cuellar DO, 3rd, Mauffrey C. Surgical resection of severe heterotopic ossification after open reduction and internal fixation of acetabular fractures: a case series of 18 patients. Injury. 2014. October;45(10):1604-10. Epub 2014 May 27. [DOI] [PubMed] [Google Scholar]
- 33.Shafer DM, Bay C, Caruso DM, Foster KN. The use of eidronate disodium in the prevention of heterotopic ossification in burn patients. Burns. 2008. May;34(3):355-60. Epub 2007 Sep 14. [DOI] [PubMed] [Google Scholar]
- 34.Spinarelli A, Patella V, Petrera M, Abate A, Pesce V, Patella S. Heterotopic ossification after total hip arthroplasty: our experience. Musculoskelet Surg. 2011. April;95(1):1-5. Epub 2011 Jan 6. [DOI] [PubMed] [Google Scholar]
- 35.Regis D, Sandri A, Sambugaro E. Incidence of heterotopic ossification after surface and conventional total hip arthroplasty: a comparative study using anterolateral approach and indomethacin prophylaxis. Biomed Res Int. 2013;2013:293528 Epub 2013 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Firoozabadi R, O’Mara TJ, Swenson A, Agel J, Beck JD, Routt M. Risk factors for the development of heterotopic ossification after acetabular fracture fixation. Clin Orthop Relat Res. 2014. November;472(11):3383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kölbl O, Flentje M, Eulert J, Barthel T, Knelles D, Kraus U. [Prospective study on the prevention of heterotopic ossification after total hip replacement. Non-steroidal anti-inflammatory agents versus radiation therapy]. Strahlenther Onkol. 1997. December;173(12):677-82. German. [DOI] [PubMed] [Google Scholar]
- 38.Pakos EE, Ioannidis JP. Radiotherapy vs. nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2004. November 1;60(3):888-95. [DOI] [PubMed] [Google Scholar]
- 39.Sagi HC, Jordan CJ, Barei DP, Serrano-Riera R, Steverson B. Indomethacin prophylaxis for heterotopic ossification after acetabular fracture surgery increases the risk for nonunion of the posterior wall. J Orthop Trauma. 2014. July;28(7):377-83. [DOI] [PubMed] [Google Scholar]
- 40.Guo JJ, Tang N, Yang HL, Qin L, Leung KS. Impact of surgical approach on postoperative heterotopic ossification and avascular necrosis in femoral head fractures: a systematic review. Int Orthop. 2010. March;34(3):319-22. Epub 2009 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foruria AM, Augustin S, Morrey BF, Sánchez-Sotelo J. Heterotopic ossification after surgery for fractures and fracture-dislocations involving the proximal aspect of the radius or ulna. J Bone Joint Surg Am. 2013. May 15;95(10):e66. [DOI] [PubMed] [Google Scholar]
- 42.Foruria AM, Lawrence TM, Augustin S, Morrey BF, Sanchez-Sotelo J. Heterotopic ossification after surgery for distal humeral fractures. Bone Joint J. 2014. December;96-B(12):1681-7. [DOI] [PubMed] [Google Scholar]
- 43.Matta JM, Siebenrock KA. Does indomethacin reduce heterotopic bone formation after operations for acetabular fractures? A prospective randomised study. J Bone Joint Surg Br. 1997. November;79(6):959-63. [DOI] [PubMed] [Google Scholar]
- 44.Veltman ES, Lindenhovius AL, Kloen P. Improvements in elbow motion after resection of heterotopic bone: a systematic review. Strateg Trauma Limb Reconstr. 2014. August;9(2):65-71. Epub 2014 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang JK, Li CJ, Liao HJ, Wang CK, Wang GJ, Ho ML. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology. 2009. April 28;258(2-3):148-56. Epub 2009 Jan 22. [DOI] [PubMed] [Google Scholar]
- 46.Chang JK, Li CJ, Wu SC, Yeh CH, Chen CH, Fu YC, Wang GJ, Ho ML. Effects of anti-inflammatory drugs on proliferation, cytotoxicity and osteogenesis in bone marrow mesenchymal stem cells. Biochem Pharmacol. 2007. November 1;74(9):1371-82. Epub 2007 Jul 7. [DOI] [PubMed] [Google Scholar]
- 47.Barbato M, D’Angelo E, Di Loreto G, Menna A, Di Francesco A, Salini V, Zoppi U, Cavasinni L, La Floresta P, Romanò CL. Adherence to routine use of pharmacological prophylaxis of heterotopic ossification after total hip arthroplasty: results from an Italian multicenter, prospective, observational survey. J Orthop Traumatol. 2012. June;13(2):63-7. Epub 2012 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010. May;21(5):294-301. Epub 2010 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vuolteenaho K, Moilanen T, Moilanen E. Non-steroidal anti-inflammatory drugs, cyclooxygenase-2 and the bone healing process. Basic Clin Pharmacol Toxicol. 2008. January;102(1):10-4. Epub 2007 Oct 31. [DOI] [PubMed] [Google Scholar]
- 50.Karunakar MA, Sen A, Bosse MJ, Sims SH, Goulet JA, Kellam JF. Indometacin as prophylaxis for heterotopic ossification after the operative treatment of fractures of the acetabulum. J Bone Joint Surg Br. 2006. December;88(12):1613-7. [DOI] [PubMed] [Google Scholar]
- 51.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009. April;50(Suppl):S29-34. Epub 2008 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt SA, Kjaersgaard-Andersen P, Pedersen NW, Kristensen SS, Pedersen P, Nielsen JB. The use of indomethacin to prevent the formation of heterotopic bone after total hip replacement. A randomized, double-blind clinical trial. J Bone Joint Surg Am. 1988. July;70(6):834-8. [PubMed] [Google Scholar]
- 53.Vavken P, Castellani L, Sculco TP. Prophylaxis of heterotopic ossification of the hip: systematic review and meta-analysis. Clin Orthop Relat Res. 2009. December;467(12):3283-9. Epub 2009 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunnekreef JJ, Hoogervorst P, Ploegmakers MJ, Rijnen WH, Schreurs BW. Is etoricoxib effective in preventing heterotopic ossification after primary total hip arthroplasty? Int Orthop. 2013. April;37(4):583-7. Epub 2013 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saudan M, Saudan P, Perneger T, Riand N, Keller A, Hoffmeyer P. Celecoxib versus ibuprofen in the prevention of heterotopic ossification following total hip replacement: a prospective randomised trial. J Bone Joint Surg Br. 2007. February;89(2):155-9. [DOI] [PubMed] [Google Scholar]
- 56.van der Heide HJ, Koorevaar RC, Lemmens JA, van Kampen A, Schreurs BW. Rofecoxib inhibits heterotopic ossification after total hip arthroplasty. Arch Orthop Trauma Surg. 2007. September;127(7):557-61. Epub 2006 Nov 16. [DOI] [PubMed] [Google Scholar]
- 57.Grohs JG, Schmidt M, Wanivenhaus A. Selective COX-2 inhibitor versus indomethacin for the prevention of heterotopic ossification after hip replacement: a double-blind randomized trial of 100 patients with 1-year follow-up. Acta Orthop. 2007. February;78(1):95-8. [DOI] [PubMed] [Google Scholar]
- 58.Cullen N, Perera J. Heterotopic ossification: pharmacologic options. J Head Trauma Rehabil. 2009. Jan-Feb;24(1):69-71. [DOI] [PubMed] [Google Scholar]
- 59.Nunley RM, Zhu J, Clohisy JC, Barrack RL. Aspirin decreases heterotopic ossification after hip resurfacing. Clin Orthop Relat Res. 2011. June;469(6):1614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Duff MJ, Takamura KB, Amstutz HC. Incidence of heterotopic ossification and effects of various prophylactic methods after hip resurfacing. Bull NYU Hosp Jt Dis. 2011;69(Suppl 1):S36-41. [PubMed] [Google Scholar]
- 61.Richards AM, Klaassen MF. Heterotopic ossification after severe burns: a report of three cases and review of the literature. Burns. 1997. February;23(1):64-8. [DOI] [PubMed] [Google Scholar]
- 62.Xue D, Zheng Q, Li H, Qian S, Zhang B, Pan Z. Selective COX-2 inhibitor versus nonselective COX-1 and COX-2 inhibitor in the prevention of heterotopic ossification after total hip arthroplasty: a meta-analysis of randomised trials. Int Orthop. 2011. January;35(1):3-8. Epub 2009 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014. March 24;42(6):1359-64. Epub 2014 Mar 24. [DOI] [PubMed] [Google Scholar]
- 64.Vasileiadis GI, Sakellariou VI, Kelekis A, Galanos A, Soucacos PN, Papagelopoulos PJ, Babis GC. Prevention of heterotopic ossification in cases of hypertrophic osteoarthritis submitted to total hip arthroplasty. Etidronate or indomethacin? J Musculoskelet Neuronal Interact. 2010. June;10(2):159-65. [PubMed] [Google Scholar]
- 65.Vasileiadis GI, Sioutis IC, Mavrogenis AF, Vlasis K, Babis GC, Papagelopoulos PJ. COX-2 inhibitors for the prevention of heterotopic ossification after THA. Orthopedics. 2011. June;34(6):467. [DOI] [PubMed] [Google Scholar]
- 66.Burd TA, Lowry KJ, Anglen JO. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J Bone Joint Surg Am. 2001. December;83(12):1783-8. [DOI] [PubMed] [Google Scholar]
- 67.Pountos I, Georgouli T, Calori GM, Giannoudis PV. Do nonsteroidal anti-inflammatory drugs affect bone healing? A critical analysis. ScientificWorldJournal. 2012;2012:606404 Epub 2012 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaman SR. Heterotopic ossification of the elbows in a major petrol burn. BMJ Case Rep. 2012. Epub 2012 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haran M, Bhuta T, Lee B. Pharmacological interventions for treating acute heterotopic ossification. Cochrane Database Syst Rev. 2004;(4):CD003321 Epub 2004 Oct 18. [DOI] [PubMed] [Google Scholar]
- 70.Popovic M, Agarwal A, Zhang L, Yip C, Kreder HJ, Nousiainen MT, Jenkinson R, Tsao M, Lam H, Milakovic M, Wong E, Chow E. Radiotherapy for the prophylaxis of heterotopic ossification: a systematic review and meta-analysis of published data. Radiother Oncol. 2014. October;113(1):10-7. Epub 2014 Sep 11. [DOI] [PubMed] [Google Scholar]
- 71.Back DL, Smith JD, Dalziel RE, Young DA, Shimmin A. Incidence of heterotopic ossification after hip resurfacing. ANZ J Surg. 2007. August;77(8):642-7. [DOI] [PubMed] [Google Scholar]
- 72.Bosse MJ, Poka A, Reinert CM, Ellwanger F, Slawson R, McDevitt ER. Heterotopic ossification as a complication of acetabular fracture. Prophylaxis with low-dose irradiation. J Bone Joint Surg Am. 1988. September;70(8):1231-7. [PubMed] [Google Scholar]
- 73.Ploumis A, Belbasis L, Ntzani E, Tsekeris P, Xenakis T. Radiotherapy for prevention of heterotopic ossification of the elbow: a systematic review of the literature. J Shoulder Elbow Surg. 2013. November;22(11):1580-8. [DOI] [PubMed] [Google Scholar]
- 74.Hamid N, Ashraf N, Bosse MJ, Connor PM, Kellam JF, Sims SH, Stull DE, Jeray KJ, Hymes RA, Lowe TJ. Radiation therapy for heterotopic ossification prophylaxis acutely after elbow trauma: a prospective randomized study. J Bone Joint Surg Am. 2010. September 1;92(11):2032-8. [DOI] [PubMed] [Google Scholar]
- 75.Seegenschmiedt MH, Martus P, Goldmann AR, Wölfel R, Keilholz L, Sauer R. Preoperative versus postoperative radiotherapy for prevention of heterotopic ossification (HO): first results of a randomized trial in high-risk patients. Int J Radiat Oncol Biol Phys. 1994. August 30;30(1):63-73. [DOI] [PubMed] [Google Scholar]
- 76.Davoodi P, Fernandez JMOSJ. O SJ. Postburn sequelae in the pediatric patient: clinical presentations and treatment options. J Craniofac Surg. 2008. July;19(4):1047-52. [DOI] [PubMed] [Google Scholar]
- 77.Gaur A, Sinclair M, Caruso E, Peretti G, Zaleske D. Heterotopic ossification around the elbow following burns in children: results after excision. J Bone Joint Surg Am. 2003. August;85(8):1538-43. [DOI] [PubMed] [Google Scholar]
- 78.Ayers DC, Evarts CM, Parkinson JR. The prevention of heterotopic ossification in high-risk patients by low-dose radiation therapy after total hip arthroplasty. J Bone Joint Surg Am. 1986. December;68(9):1423-30. [PubMed] [Google Scholar]
- 79.Moore KD, Goss K, Anglen JO. Indomethacin versus radiation therapy for prophylaxis against heterotopic ossification in acetabular fractures: a randomised, prospective study. J Bone Joint Surg Br. 1998. March;80(2):259-63. [DOI] [PubMed] [Google Scholar]
- 80.Holavanahalli RK, Helm PA, Parry IS, Dolezal CA, Greenhalgh DG. Select practices in management and rehabilitation of burns: a survey report. J Burn Care Res. 2011. Mar-Apr;32(2):210-23. [DOI] [PubMed] [Google Scholar]
- 81.Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, Chandraratna RA, Mishina Y, Enomoto-Iwamoto M, Pacifici M, Iwamoto M. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat Med. 2011. April;17(4):454-60. Epub 2011 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. Results after early excision. J Bone Joint Surg Br. 2004. April;86(3):396-403. [DOI] [PubMed] [Google Scholar]
- 83.Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop Relat Res. 1991. February;263:13-29. [PubMed] [Google Scholar]
- 84.Genet F, Marmorat JL, Lautridou C, Schnitzler A, Mailhan L, Denormandie P. Impact of late surgical intervention on heterotopic ossification of the hip after traumatic neurological injury. J Bone Joint Surg Br. 2009. November;91(11):1493-8. [DOI] [PubMed] [Google Scholar]
- 85.Zou D, Han Y, Han W, Sulan You J, Wang L, Huang Y. Heterotrophic ossification on a skin graft in a postburn scar of the chin. J Craniofac Surg. 2011. November;22(6):2369-72. [DOI] [PubMed] [Google Scholar]
- 86.Lee EK, Namdari S, Hosalkar HS, Keenan MA, Baldwin KD. Clinical results of the excision of heterotopic bone around the elbow: a systematic review. J Shoulder Elbow Surg. 2013. May;22(5):716-22. Epub 2013 Feb 4. [DOI] [PubMed] [Google Scholar]
- 87.Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011;6:80 Epub 2011 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J, ten Dijke P. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2010. June;25(6):1208-15. [DOI] [PubMed] [Google Scholar]
- 89.Kluk MW, Ji Y, Shin EH, Amrani O, Onodera J, Jackson WM, Nesti LJ. Fibroregulation of mesenchymal progenitor cells by BMP-4 after traumatic muscle injury. J Orthop Trauma. 2012. December;26(12):693-8. [DOI] [PubMed] [Google Scholar]
- 90.Kan L, Peng CY, McGuire TL, Kessler JA. Glast-expressing progenitor cells contribute to heterotopic ossification. Bone. 2013. March;53(1):194-203. Epub 2012 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kan L, Kessler JA. Evaluation of the cellular origins of heterotopic ossification. Orthopedics. 2014. May;37(5):329-40. [DOI] [PubMed] [Google Scholar]
- 92.Medici D, Olsen BR. The role of endothelial-mesenchymal transition in heterotopic ossification. J Bone Miner Res. 2012. August;27(8):1619-22. Epub 2012 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis TA, O’Brien FP, Anam K, Grijalva S, Potter BK, Elster EA. Heterotopic ossification in complex orthopaedic combat wounds: quantification and characterization of osteogenic precursor cell activity in traumatized muscle. J Bone Joint Surg Am. 2011. June 15;93(12):1122-31. [DOI] [PubMed] [Google Scholar]
- 94.Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012. May;27(5):1004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009. March 1;91(3):652-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramirez DM, Ramirez MR, Reginato AM, Medici D. Molecular and cellular mechanisms of heterotopic ossification. Histol Histopathol. 2014. October;29(10):1281-5. Epub 2014 May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted-Davis EA, Davis AR. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011. October;112(10):2748-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peterson JR, De La Rosa S, Sun H, Eboda O, Cilwa KE, Donneys A, Morris M, Buchman SR, Cederna PS, Krebsbach PH, Wang SC, Levi B. Burn injury enhances bone formation in heterotopic ossification model. Ann Surg. 2014. May;259(5):993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010. December;16(12):1400-6. Epub 2010 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dilling CF, Wada AM, Lazard ZW, Salisbury EA, Gannon FH, Vadakkan TJ, Gao L, Hirschi K, Dickinson ME, Davis AR, Olmsted-Davis EA. Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification. J Bone Miner Res. 2010. May;25(5):1147-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun X, Wei Y. The role of hypoxia-inducible factor in osteogenesis and chondrogenesis. Cytotherapy. 2009;11(3):261-7. [DOI] [PubMed] [Google Scholar]
- 102.Lin L, Shen Q, Leng H, Duan X, Fu X, Yu C. Synergistic inhibition of endochondral bone formation by silencing Hif1α and Runx2 in trauma-induced heterotopic ossification. Mol Ther. 2011. August;19(8):1426-32. Epub 2011 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kosaka T, Imakiire A, Mizuno F, Yamamoto K. Activation of nuclear factor kappaB at the onset of ossification of the spinal ligaments. J Orthop Sci. 2000;5(6):572-8. [DOI] [PubMed] [Google Scholar]
- 104.Fransen M, Anderson C, Douglas J, MacMahon S, Neal B, Norton R, Woodward M, Cameron ID, Crawford R, Lo SK, Tregonning G, Windolf M; HIPAID Collaborative Group. Safety and efficacy of routine postoperative ibuprofen for pain and disability related to ectopic bone formation after hip replacement surgery (HIPAID): randomised controlled trial. BMJ. 2006. September 9;333(7567):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kölbl O, Knelles D, Barthel T, Kraus U, Flentje M, Eulert J. Randomized trial comparing early postoperative irradiation vs. the use of nonsteroidal antiinflammatory drugs for prevention of heterotopic ossification following prosthetic total hip replacement. Int J Radiat Oncol Biol Phys. 1997. December 1;39(5):961-6. [DOI] [PubMed] [Google Scholar]
- 106.Wu X, Walters TJ, Rathbone CR. Skeletal muscle satellite cell activation following cutaneous burn in rats. Burns. 2013. June;39(4):736-44. [DOI] [PubMed] [Google Scholar]
- 107.Suda RK, Billings PC, Egan KP, Kim JH, McCarrick-Walmsley R, Glaser DL, Porter DL, Shore EM, Pignolo RJ. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009. September;27(9):2209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kan L, Liu Y, McGuire TL, Berger DM, Awatramani RB, Dymecki SM, Kessler JA. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009. January;27(1):150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalajzic Z, Li H, Wang LP, Jiang X, Lamothe K, Adams DJ, Aguila HL, Rowe DW, Kalajzic I. Use of an alpha-smooth muscle actin GFP reporter to identify an osteoprogenitor population. Bone. 2008. September;43(3):501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, Senitzer D, Forman SJ, Emerson SG. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007. February;89(2):347-57. [DOI] [PubMed] [Google Scholar]