Abstract
Background:
Intrasynovial grafts are the ideal solution to replace defects in intrasynovial flexor tendons, but autologous graft sources are rarely available. The purpose of the present study was to test the hypotheses that an intrasynovial tendon prepared with repetitive freeze-thaw cycles and lyophilization (as a means of reducing immunogenicity) has increased frictional force (gliding resistance) in comparison with fresh intrasynovial tendons and that a lyophilized intrasynovial flexor tendon that is modified with carbodiimide-derivatized hyaluronic acid and gelatin has decreased frictional force in comparison with untreated lyophilized tendons.
Methods:
Thirty-six flexor digitorum profundus tendons from the second and fifth digits of canine hind paws were randomly assigned to three groups. Twelve tendons were immediately assessed both mechanically and morphologically and served as the normal tendon group. The other twenty-four tendons were prepared with repetitive freeze-thaw cycles and lyophilization and were randomly assigned to two groups, including one group in which the tendons were treated with carbodiimide-derivatized hyaluronic acid and gelatin and one group in which the tendons were not treated. The frictional force was measured during 1000 cycles of simulated flexion-extension motion in all tendons, and the mean frictional forces were compared. The tendons were then observed with use of transmitted light microscopy for residual hyaluronic acid on the tendon surface, and the smoothness of the surface was evaluated with use of scanning electron microscopy.
Results:
The frictional force after lyophilization was significantly increased by 104.9% after the first cycle and by 99.5% after 1000 cycles in comparison with the normal tendon (p < 0.05). The frictional force of the lyophilized tendons after treatment with carbodiimide-derivatized hyaluronic acid and gelatin was not significantly different from that of normal tendons. The untreated lyophilized tendon surfaces were observed on scanning electron microscopy to be rough in appearance, whereas the normal surface and the surface treated with carbodiimide-derivatized hyaluronic acid and gelatin were smooth, with residual hyaluronic acid present on the gliding surface.
Conclusions:
Lyophilization alters tendon surface morphology and increases tendon frictional force. Surface modification with carbodiimide-derivatized hyaluronic acid and gelatin can mitigate this adverse effect.
Clinical Relevance:
Tendon surface modification with carbodiimide-derivatized hyaluronic acid and gelatin can improve the gliding ability of lyophilized flexor tendons and therefore may improve the utility of lyophilized tendon allografts as a tendon graft substitute.
Injuries to the flexor tendons in the digits are common, especially in the young and working-age populations. Although immediate primary repair after injury has been generally accepted and outcomes have been improved in association with the use of new regimens of primary repair and postoperative controlled mobilization1-5, poor functional outcomes are still common, especially in zone II6-10 (i.e., the portion of the flexor tendon sheath extending from the middle part of the palm to the middle one-third of the finger). This zone contains two tendons in a fibrous sheath in which the blood supply to the tendon is at its most tenuous, being limited to one or two small vessels that are easily injured. Consequently, this is the zone in which tendon repair is most difficult technically and in which the worst results of tendon repair are recorded. In such cases, tendon autografts play an important role in reconstruction to restore hand function. However, clinical outcomes after treatment with tendon autograft are generally poor as well because of complications such as adhesion formation. Although experimental studies have shown that intrasynovial tendons have better outcomes than extrasynovial grafts do11-13, extrasynovial tendons usually are used clinically14-17 because autologous intrasynovial tendons are rarely available for use as tendon grafts. Intrasynovial tendon allografts are another possibility18-20, but fresh allografts may induce an immune reaction. To reduce immunogenicity and to facilitate storage, allografts may be subjected to repetitive freeze-thaw cycles and lyophilization18,21-25, but the effect of lyophilization on allograft function is unclear.
Previous studies also have shown that tendon frictional force (gliding resistance) is an important factor influencing the outcome of tendon repair. Higher gliding resistance results in greater adhesion formation26, and extrasynovial tendon has a higher gliding resistance than intrasynovial tendon does27. Treating the extrasynovial tendon surface with a carbodiimide-derivatized hyaluronic acid (cd-HA) and gelatin mixture has been shown to improve tendon gliding ability in vitro28-30 and to improve digital function in vivo31. Such treatment can also improve the gliding of repaired intrasynovial tendons32.
The overall goal of our research program is to improve outcomes after tendon repair and reconstruction through engineering of the tendon-tendon sheath interface in order to reduce friction and, consequently, adhesion formation. The purpose of the present study was to test the hypotheses that (1) an intrasynovial tendon prepared with repetitive freeze-thaw cycles and lyophilization has increased frictional force compared with fresh intrasynovial tendon and (2) the resulting lyophilized intrasynovial flexor tendon allograft modified with cd-HA-gelatin has decreased frictional force compared with untreated lyophilized tendon.
Materials and Methods
A total of thirty-six flexor digitorum profundus tendons from the second and fifth digits were harvested from the hind paws of nine adult mongrel dogs that were killed for other studies approved by our Institutional Animal Care and Use Committee. The tendons were randomly divided into three experimental groups. Twelve tendons were immediately assessed for both mechanical and morphological evaluation after harvesting; these tendons served as the normal tendon group (Group 1). The other twenty-four tendons were treated with five freeze-thaw cycles followed by lyophilization to render them acellular21,24,33,34 and were stored at –80°C until testing. These twenty-four tendons were then randomly divided into two groups; in one group (Group 2) the tendons were treated with cd-HA-gelatin, and in the other group (Group 3) the tendons were not treated and served as the lyophilized tendon control group. The tendons were then evaluated mechanically and morphologically.
Tendon Preparation
After the dogs were killed, the tendons in Group 1 were tested as soon as possible after harvest, without any special preparation. The tendons in Groups 2 and 3 were immediately immersed into liquid nitrogen for one minute and then were thawed for five minutes in warmed saline solution at 37°C. This procedure was repeated five times, after which the tendons were lyophilized. Previously, this protocol has been shown to produce necrosis of 97% to 100% of all viable tenocytes24. After lyophilization, the tendons were stored at −80°C until testing.
Prior to mechanical testing, the tendons in Groups 2 and 3 were rehydrated in a saline solution bath for twenty-four hours at 4°C. Although our pilot data showed that the weight of the lyophilized tendons was restored to that of the prelyophilization level between five and twenty-four hours, a rehydration time of twenty-four hours was chosen on the basis of a published report in which the investigators noted that rehydration for twenty-four hours was preferable from the perspective of the restoration of mechanical properties20.
Treatment of Group-2 Tendons with cd-HA-Gelatin
For the cd-HA-gelatin treatment group, the tendons were immersed in a combined solution of 1% hyaluronic acid and 1% 1-ethyl 1-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC; Sigma Chemical, St. Louis, Missouri)35/1% N-hydroxysuccinimide (NHS; Pierce Biotechnology, Rockford, Illinois)36 with gelatin mixture for thirty seconds, after which the reaction was allowed to proceed for five minutes with the tendon wrapped in a towel moistened with phosphate-buffered saline solution. Excess reagent was washed off with phosphate-buffered saline solution. Sodium hyaluronate (Acros Organics, Pittsburgh, Pennsylvania) was dissolved in phosphate-buffered saline solution (pH 6) at 20 mg/mL. EDC/NHS activates the CO2H in the hyaluronic acid molecule and forms the intermediate O-acylisourea, which can chemically bind to exposed amino groups on the tendon surface. This chemical modification of hyaluronic acid not only increases the half-life of the hyaluronic acid but also increases the binding strength between hyaluronic acid and the tendon surface28,37-39.
Measurement of Frictional Force
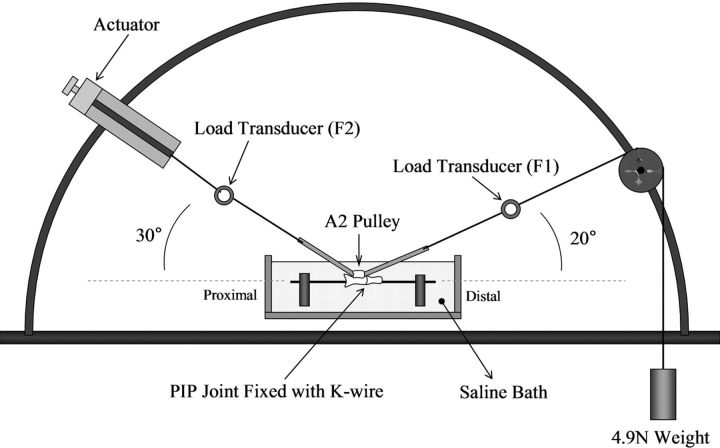
The frictional force between the flexor digitorum profundus tendon and the proximal pulley of the digit was evaluated with use of a testing device that was developed previously40-42. The experimental setup consisted of one mechanical actuator, a linear potentiometer, and a tensile load transducer, which were connected to the proximal tendon end. Another tensile load transducer, a pulley unit, and a 4.9-N weight were connected to the distal tendon end (Fig. 1). The resolution of the recorded output of the force transducers in this setup is <1 g. A normal canine proximal phalanx with an intact A2 pulley was secured on the custom-made device with the volar side upward (Figs. 2-A and 2-B) in a saline solution bath (Isotemp 202; Fisher Scientific, Houston, Texas) at 37°C. Each tendon was tested against its own phalanx and pulley. The tendon was then pulled at a rate of 2 mm/sec by the actuator against the weight with a fixed excursion of 16 mm, an estimate of normal canine tendon excursion based on previous studies43. The first two cycles of frictional testing were used to precondition the tendon and to remove any loose superficial reagent, and then data were collected during the third cycle (the first data-collection cycle). The force difference between the proximal and distal tendon ends represents the gliding resistance of the flexor digitorum profundus tendon against the A2 pulley. The gliding resistance was obtained with the equation (F2flexion − F2extension)/2, as described previously44. The motion between tendon and pulley was repeated for 1000 cycles. The number of simulated repetitive cycles (1000) was determined on the basis of the estimated total number of postoperative passive motions in a six-week in vivo canine tendon rehabilitation model31,43,45. The data on the gliding resistance were recorded after every fifty cycles up to 500 cycles and then every 100 cycles up to 1000 cycles.
Fig. 1.
Illustration depicting the testing device used to measure frictional force (gliding resistance). The actuator was positioned at an angle of 30° and the distal load-transducer was positioned at an angle of 20°. F1 = force 1, F2 = force 2, PIP = proximal interphalangeal, and K-wire = Kirschner wire.
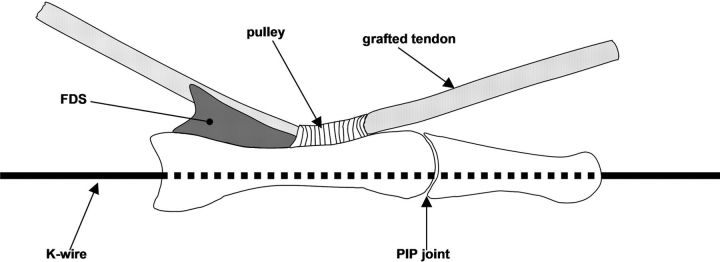
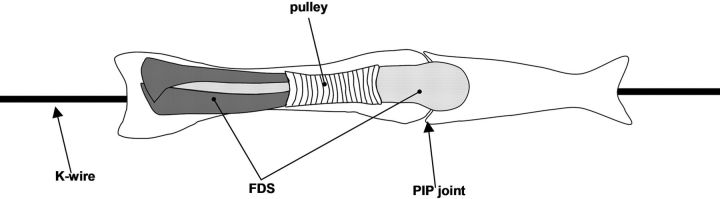
Fig. 2-A Fig. 2-B.
Figs. 2-A and 2-B Illustrations depicting the lateral view (Fig. 2-A) and top view (Fig. 2-B) of the pulley unit. The proximal phalanx with an intact A2 pulley was mounted in the device and was fixed in full proximal interphalangeal (PIP) joint extension with a longitudinal Kirschner wire (K-wire). FDS = flexor digitorum superficialis.
Evaluation of Residual Hyaluronic Acid Binding on the Tendon Surface
Following mechanical testing, a 5-mm longitudinal segment of the tendon portion that glided against the pulley was excised and was stained with biotinylated hyaluronic acid binding protein (HABP; EMD Biosciences, San Diego, California) to determine residual binding of hyaluronic acid. Tendons were embedded in Tissue-Tek (Sakura Finetek USA, Torrance, California), were sectioned transversely at 8 μm with use of a cryostat (Leica, Heidelberg, Germany), and were collected on supercharged glass slides. After washing in a 0.1-M phosphate-buffered saline solution with 3% Triton X-100 for five minutes, the sections were incubated in a blocking solution consisting of 1% bovine serum albumin in 0.1-M phosphate-buffered saline solution plus 0.5% Triton X-100 for twenty minutes. The sections were then washed with 0.1-M phosphate-buffered saline solution three times and were incubated in 0.2% HABP in 1% bovine serum albumin/0.1-M phosphate-buffered saline solution for two hours at room temperature. Negative control sections were incubated with a 1% bovine serum albumin/0.1-M phosphate-buffered saline solution instead of the biotinylated hyaluronic acid binding protein. The slides were washed with 0.1-M phosphate-buffered saline solution three times for five minutes and were incubated in avidin-biotin peroxidase (VECTASTAIN ABC kit; Vector Laboratories, Burlingame, California) for thirty minutes at room temperature, followed by three washes in 0.1-M phosphate-buffered saline solution for five minutes. The sections were stained with the DAB Peroxidase Substrate Kit (Vector Laboratories) for 3.5 minutes, and then the slides were washed by repeated dipping in double-distilled water. The sections were dehydrated by means of serial washes in 80%, 95%, and 100% alcohol solutions, followed by two xylene washes. Each slide was examined with use of transmitted light microscopy (Nikon Eclipse E400; Nikon, Melville, New York) to evaluate for residual hyaluronic acid binding on the tendon surface.
Scanning Electron Microscopy
Three tendons in each group were prepared for scanning electron microscopy. The selected tendons were washed in phosphate-buffered saline solution and were fixed in a solution of buffered glutaraldehyde and osmium tetroxide. After dehydration in graded acetone, the specimens were coated with gold-palladium alloy and were examined with scanning electron microscopy (FE-SEM S-4700; Hitachi, Hitachi, Japan) in secondary electron mode at 3 kV. The surface of the tendon was qualitatively assessed for the smoothness of the tendon surface.
Statistical Analysis
The mean frictional force (and standard deviation) was determined for each treatment group. One-way analysis of variance was used for the comparison among the three treatment groups (the control group of normal flexor digitorum profundus tendons, the group in which lyophilized tendons were treated with cd-HA-gelatin, and the group in which lyophilized tendons were not treated). A Tukey-Kramer post hoc test for each pairwise comparison was used if there was a significant difference among the three groups. A p value of <0.05 was considered to indicate significance in all cases.
Source of Funding
This study was supported by a grant from the Musculoskeletal Transplant Foundation (MTF), which was used for salaries, materials, and supplies.
Results
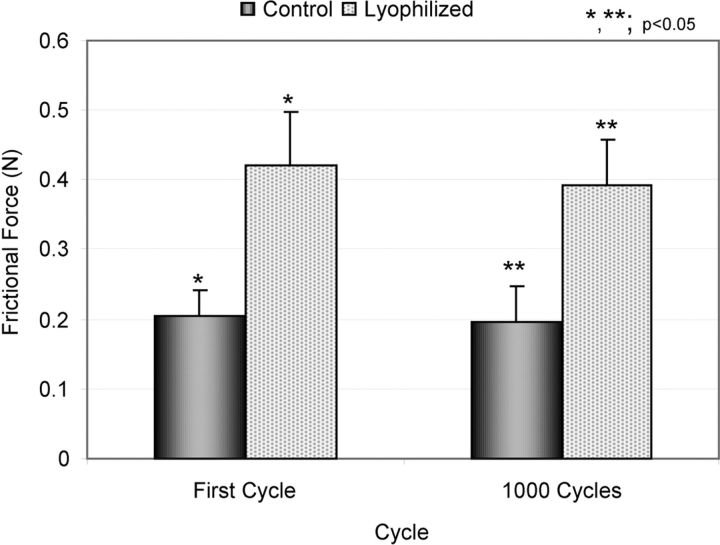
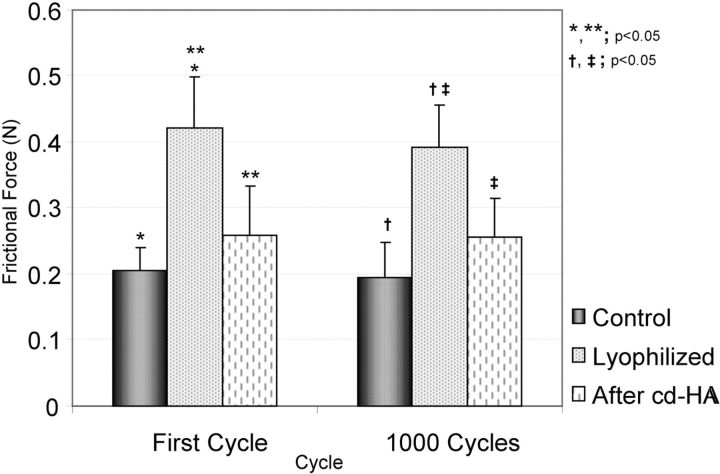
The weight of the lyophilized tendon rehydrated in a saline solution bath at 4°C recovered to normal levels within three to four hours. The mean tendon weight (and standard deviation) was 0.87 ± 0.10 g before lyophilization, 0.27 ± 0.04 g after lyophilization, and 0.85 ± 0.10 g after three hours of rehydration. In the normal flexor digitorum profundus tendon group, the frictional force did not change significantly, even after 1000 cycles. The mean frictional force of normal flexor digitorum profundus tendon was 0.206 ± 0.035 N at the first cycle and 0.196 ± 0.052 N at 1000 cycles. The frictional force of the flexor digitorum profundus tendon immediately following lyophilization was significantly increased in comparison with that of the normal flexor digitorum profundus tendon (p < 0.05) (0.422 ± 0.076 N at the first cycle and 0.391 ± 0.066 N at 1000 cycles) (Fig. 3). The frictional force of the lyophilized flexor digitorum profundus tendon after surface modification with cd-HA-gelatin was significantly decreased in comparison with the untreated lyophilized tendon (p < 0.05) (Fig. 4). The frictional force of the lyophilized flexor digitorum profundus tendon treated with cd-HA-gelatin was 0.259 ± 0.074 N at the first cycle and 0.255 ± 0.061 N at 1000 cycles. There was no significant difference between normal flexor digitorum profundus tendon and lyophilized flexor digitorum profundus tendon treated with cd-HA-gelatin (Fig. 4). After 1000 cycles, the statistical relationship among the three groups remained the same. Transmitted light microscopy showed that the lyophilized tendon surface treated with cd-HA-gelatin was well covered with a thin layer of cd-HA-gelatin even after 1000 cycles of tendon excursion (Fig. 5). Scanning electron microscopy showed that the surface of untreated lyophilized tendons appeared to be rough but that the surface of the normal tendon and the tendon treated with cd-HA-gelatin appeared to be smooth, even after 1000 cycles of tendon excursion (Fig. 6).
Fig. 3.
Bar graph illustrating the frictional force (gliding resistance) for the groups of normal and untreated lyophilized tendons. The values are expressed as the mean and the standard deviation.
Fig. 4.
Bar graph illustrating the frictional force (gliding resistance) for the groups of normal tendons, untreated lyophilized tendons, and lyophilized tendons treated with cd-HA-gelatin. The values are expressed as the mean and the standard deviation.
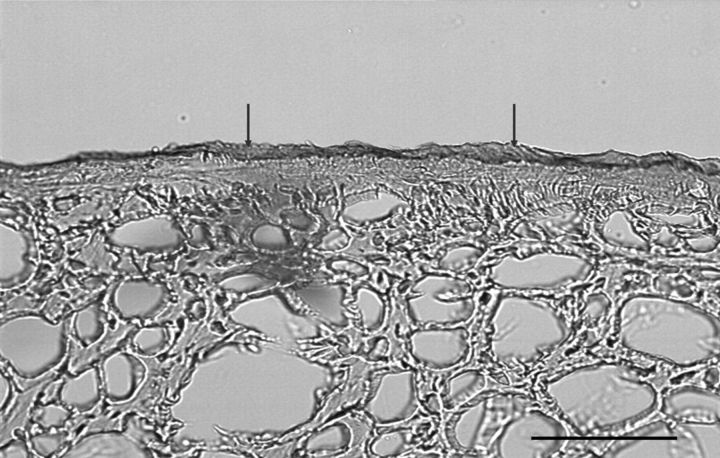
Fig. 5.
Transmitted light microscopy image showing the appearance of residual hyaluronic acid binding on the tendon surface. The tendon surface was well covered with a thin layer of cd-HA-gelatin even after 1000 cycles of motion. An area that was well coated with cd-HA-gelatin (arrows) was identified on the surface of the transversely sectioned cd-HA-gelatin-treated tendon (scale bar = 100 μm).
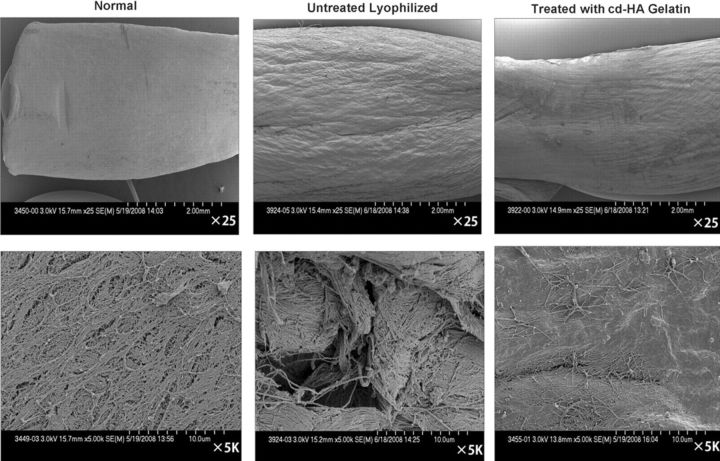
Fig. 6.
Selected scanning electron microscopic images, made after 1000 cycles of tendon excursion, showing normal flexor digitorum profundus tendon, untreated lyophilized tendon, and tendon treated with cd-HA-gelatin. The top row of images was made at low magnification (×25), and the bottom row of images was made at high magnification (×5000). The surface of the lyophilized tendons appeared to be rough, whereas the surface of the lyophilized tendon that had been treated with cd-HA-gelatin appeared to be smoother.
Discussion
Because of its smooth surface, low friction, and durability, an intrasynovial tendon is the ideal graft tendon for the restoration of finger function27,45,46. However, because of limitations in the availability of suitable intrasynovial autograft, an alternative is needed. One possibility is intrasynovial allograft, but, to eliminate allograft immunogenicity, the donor graft cells must be destroyed. We used a freeze-thaw protocol because it is commonly used to prepare allografts when cell viability is not desired as well as to reduce immunogenicity. For the purpose of long-term preservation, lyophilization is often used18,21-25 because storage does not require refrigeration and the tissue remains useful for as long as ten years18.
In the present study, we found that lyophilization changed tendon surface morphology and increased the frictional force, which may hinder tendon gliding when such tendons are used clinically. Although the reason for the tendon surface changes after lyophilization is not clear, it may be related to changes in collagen cross-linking or other surface macromolecules due to water loss. Even after rehydration, the frictional force in the lyophilized tendon remained significantly increased.
The modification of hyaluronic acid with use of the carbodiimide reaction to create new biopolymers by means of cross-linking with other biological molecules such as collagen has been widely investigated for applications such as anti-adhesion membranes47-51, biodegradable scaffolds for tissue regeneration52-55, or timed-release drug delivery vehicles56,57. The improvement in lyophilized tendon gliding ability with a surface modification with use of cd-HA-gelatin is another such potential application. Our results show that treatment with cd-HA-gelatin restores a smooth surface to the lyophilized tendon and restores the frictional force to control levels. Similar results have been seen with this engineered surface in extrasynovial and intrasynovial tendon that has not been lyophilized, as well as after tendon repair, both in vitro and in vivo28-32,58,59.
The present study had some limitations. First, it was an in vitro investigation. The effect of this intervention on tendon allograft function in vivo is not known. Second, hyaluronic acid binding protein was used to qualitatively identify residual hyaluronic acid on the tendon surface. No quantitative evaluation was used. Third, although Potenza and Melone reported no difficulty in association with the use of lyophilized tendons that had been stored for as long as ten years18, we have been unable to identify any other studies that have described the effect of storage on lyophilized tendon. In the present study, we chose two weeks as the storage period, and, therefore, we do not know the effect of longer-term storage. Finally, in theory, the repetitive motion may alter the viscoelasticity of the tendon and may in turn affect the frictional force. However, we only measured frictional force. Other tendon mechanical properties, such as tensile or compressive moduli, were not assessed.
In conclusion, serial freeze-thaw cycles followed by lyophilization alter tendon surface morphology and increase tendon frictional force. Surface modification with cd-HA-gelatin mitigated these adverse effects, restoring the graft to its control frictional force level and making a smoother tendon surface. We believe that the findings from the current study have important clinical implications. They suggest the possibility of improved outcomes after tendon grafting by providing an engineered, lubricated, off-the-shelf alternative to conventional extrasynovial autografts. Additional in vivo study is necessary to confirm that this surface modification can improve the outcome of lyophilized tendon allograft in vivo.
Footnotes
Investigation performed at the Orthopedic Biomechanics Laboratory, Division of Orthopedic Research, Mayo Clinic, Rochester, Minnesota
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the Musculoskeletal Transplant Foundation (MTF). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Kleinert HE Schepel S Gill T. Flexor tendon injuries. Surg Clin North Am. 1981;61:267-86. [DOI] [PubMed] [Google Scholar]
- 2.Lister GD Kleinert HE Kutz JE Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg Am. 1977;2:441-51. [DOI] [PubMed] [Google Scholar]
- 3.Chow JA Thomes LJ Dovelle S Milnor WH Seyfer AE Smith AC. A combined regimen of controlled motion following flexor tendon repair in "no man's land". Plast Reconstr Surg. 1987;79:447-55. [DOI] [PubMed] [Google Scholar]
- 4.Small JO Brennen MD Colville J. Early active mobilisation following flexor tendon repair in zone 2. J Hand Surg Br. 1989;14:383-91. [DOI] [PubMed] [Google Scholar]
- 5.Strickland JW. Flexor tendon surgery. Part 1: primary flexor tendon repair. J Hand Surg Br. 1989;14:261-72. [DOI] [PubMed] [Google Scholar]
- 6.Coenen L Boeckx W Gruwez JA. The treatment of flexor tendon lesions of the fingers. Acta Chir Belg. 1981;80:195-204. [PubMed] [Google Scholar]
- 7.Wehbé MA Mawr B Hunter JM Schneider LH Goodwyn BL. Two-stage flexor-tendon reconstruction. Ten-year experience. J Bone Joint Surg Am. 1986;68:752-63. [PubMed] [Google Scholar]
- 8.Amadio PC Wood MB Cooney WP 3rd Bogard SD. Staged flexor tendon reconstruction in the fingers and hand. J Hand Surg Am. 1988;13:559-62. [DOI] [PubMed] [Google Scholar]
- 9.Singer M Maloon S. Flexor tendon injuries: the results of primary repair. J Hand Surg Br. 1988;13:269-72. [DOI] [PubMed] [Google Scholar]
- 10.Pribaz JJ Morrison WA Macleod AM. Primary repair of flexor tendons in no-man's land using the Becker repair. J Hand Surg Br. 1989;14:400-5. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsson SO Gelberman RH Amiel D Winterton P Harwood F. Autogenous flexor tendon grafts: fibroblast activity and matrix remodeling in dogs. J Orthop Res. 1995;13:58-66. [DOI] [PubMed] [Google Scholar]
- 12.Duffy FJ Seiler JG Hergrueter CA Kandel J Gelberman RH. Intrinsic mitogenic potential of canine flexor tendons. J Hand Surg Br. 1992;17:275-7. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi M Seiler JG 3rd Boardman ND 3rd Tramaglini DM Gelberman RH Woo SL. Tensile properties of canine intrasynovial and extrasynovial flexor tendon autografts. J Hand Surg Am. 1997;22:457-63. [DOI] [PubMed] [Google Scholar]
- 14.White WL. Tendon grafts: a consideration of their source, procurement and suitability. Surg Clin North Am. 1960;40:403-13. [DOI] [PubMed] [Google Scholar]
- 15.Wehbé MA. Tendon graft donor sites. J Hand Surg Am. 1992;17:1130-2. [DOI] [PubMed] [Google Scholar]
- 16.Carlson GD Botte MJ Josephs MS Newton PO Davis JL Woo SL. Morphologic and biomechanical comparison of tendons used as free grafts. J Hand Surg Am. 1993;18:76-82. [DOI] [PubMed] [Google Scholar]
- 17.Silfverskiöld KL May EJ. Early active mobilization of tendon grafts using mesh reinforced suture techniques. J Hand Surg Br. 1995;20:301-7. [DOI] [PubMed] [Google Scholar]
- 18.Potenza AD Melone C. Evaluation of freeze-dried flexor tendon grafts in the dog. J Hand Surg Am. 1978;3:157-62. [DOI] [PubMed] [Google Scholar]
- 19.Peacock EE Jr Madden JW.. Human composite flexor tendon allografts. Ann Surg. 1967;166:624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster DA Werner FW. Mechanical and functional properties of implanted freeze-dried flexor tendons. Clin Orthop Relat Res. 1983;180:301-9. [PubMed] [Google Scholar]
- 21.King GJ Edwards P Brant RF Shrive NG Frank CB. Freezing influences the healing of rabbit medial collateral ligament autografts. Clin Orthop Relat Res. 1995;316:244-53. [PubMed] [Google Scholar]
- 22.Shelton WR Treacy SH Dukes AD Bomboy AL. Use of allografts in knee reconstruction: II. Surgical considerations. J Am Acad Orthop Surg. 1998;6:169-75. [DOI] [PubMed] [Google Scholar]
- 23.Barbour SA King W. The safe and effective use of allograft tissue—an update. Am J Sports Med. 2003;31:791-7. [DOI] [PubMed] [Google Scholar]
- 24.Katsuragi R Yasuda K Tsujino J Keira M Kaneda K. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28:47-56. [DOI] [PubMed] [Google Scholar]
- 25.Cameron RR Conrad RN Sell KW Latham WD. Freeze-dried composite tendon allografts: an experimental study. Plast Reconstr Surg. 1971;47:39-46. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C Amadio PC Momose T Couvreur P Zobitz ME An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51:917-21. [DOI] [PubMed] [Google Scholar]
- 27.Uchiyama S Amadio PC Coert JH Berglund LJ An KN. Gliding resistance of extrasynovial and intrasynovial tendons through the A2 pulley. J Bone Joint Surg Am. 1997;79:219-24. [DOI] [PubMed] [Google Scholar]
- 28.Sun YL Yang C Amadio PC Zhao C Zobitz ME An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984-9. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T Sun YL Zhao C Zobitz ME An KN Amadio PC. Optimization of surface modifications of extrasynovial tendon to improve its gliding ability in a canine model in vitro. J Orthop Res. 2006;24:1555-61. [DOI] [PubMed] [Google Scholar]
- 30.Taguchi M Sun YL Zhao C Zobitz ME Cha CJ Jay GD An KN Amadio PC. Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. J Bone Joint Surg Am. 2008;90:129-35. [DOI] [PubMed] [Google Scholar]
- 31.Zhao C Sun YL Amadio PC Tanaka T Ettema AM An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88:2181-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taguchi M Sun YL Zhao C Zobitz ME Cha CJ Jay GD An KN Amadio PC. Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J Orthop Res. 2009;27:257-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graf BK Fujisaki K Vanderby R Jr Vailas AC.. The effect of in situ freezing on rabbit patellar tendon. A histologic, biochemical, and biomechanical analysis. Am J Sports Med. 1992;20:401-5. [DOI] [PubMed] [Google Scholar]
- 34.Ju YJ Tohyama H Kondo E Yoshikawa T Muneta T Shinomiya K Yasuda K. Effects of local administration of vascular endothelial growth factor on properties of the in situ frozen-thawed anterior cruciate ligament in rabbits. Am J Sports Med. 2006;34:84-91. [DOI] [PubMed] [Google Scholar]
- 35.Ferrier BM Branda LA. Synthesis and some biological properties of 1-deamino-4-glu-oxytocin (1-beta-mercaptopropionic acid-4-glutamic acid-oxytocin) and its use in preparing a hormone-agarose complex. Can J Biochem. 1975;53:21-7. [DOI] [PubMed] [Google Scholar]
- 36.Staros JV Wright RW Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220-2. [DOI] [PubMed] [Google Scholar]
- 37.Kuo JW Swann DA Prestwich GD. Chemical modification of hyaluronic acid by carbodiimides. Bioconjug Chem. 1991;2:232-41. [DOI] [PubMed] [Google Scholar]
- 38.Bulpitt P Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152-69. [DOI] [PubMed] [Google Scholar]
- 39.Hanthamrongwit M Reid WH Grant MH. Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: the effect of cross-linking agents and diamines. Biomaterials. 1996;17:775-80. [DOI] [PubMed] [Google Scholar]
- 40.An KN Berglund L Uchiyama S Coert JH. Measurement of friction between pulley and flexor tendon. Biomed Sci Instrum. 1993;29:1-7. [PubMed] [Google Scholar]
- 41.Uchiyama S Coert JH Berglund L Amadio PC An KN. Method for the measurement of friction between tendon and pulley. J Orthop Res. 1995;13:83-9. [DOI] [PubMed] [Google Scholar]
- 42.Coert JH Uchiyama S Amadio PC Berglund LJ An KN. Flexor tendon-pulley interaction after tendon repair. A biomechanical study. J Hand Surg Br. 1995;20:573-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhao C Amadio PC Zobitz ME Momose T Couvreur P An KN. Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clin Orthop Relat Res. 2002;396:223-30. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C Amadio PC Zobitz ME An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19:580-6. [DOI] [PubMed] [Google Scholar]
- 45.Zhao C Amadio PC Momose T Zobitz ME Couvreur P An KN. Remodeling of the gliding surface after flexor tendon repair in a canine model in vivo. J Orthop Res. 2002;20:857-62. [DOI] [PubMed] [Google Scholar]
- 46.Gelberman RH Chu CR Williams CS Seiler JG 3rd Amiel D. Angiogenesis in healing autogenous flexor-tendon grafts. J Bone Joint Surg Am. 1992;74:1207-16. [PubMed] [Google Scholar]
- 47.Burns JW Skinner K Colt J Sheidlin A Bronson R Yaacobi Y Goldberg EP. Prevention of tissue injury and postsurgical adhesions by precoating tissues with hyaluronic acid solutions. J Surg Res. 1995;59:644-52. [DOI] [PubMed] [Google Scholar]
- 48.Burns JW Skinner K Colt MJ Burgess L Rose R Diamond MP. A hyaluronate based gel for the prevention of postsurgical adhesions: evaluation in two animal species. Fertil Steril. 1996;66:814-21. [PubMed] [Google Scholar]
- 49.Becker JM Dayton MT Fazio VW Beck DE Stryker SJ Wexner SD Wolff BG Roberts PL Smith LE Sweeney SA Moore M. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297-306. [PubMed] [Google Scholar]
- 50.Tzianabos AO Cisneros RL Gershkovich J Johnson J Miller RJ Burns JW Onderdonk AB. Effect of surgical adhesion reduction devices on the propagation of experimental intra-abdominal infection. Arch Surg. 1999;134:1254-9. [DOI] [PubMed] [Google Scholar]
- 51.Shih HN Fang JF Chen JH Yang CL Chen YH Sung TH Shih LY. Reduction in experimental peridural adhesion with the use of a crosslinked hyaluronate/collagen membrane. J Biomed Mater Res B Appl Biomater. 2004;71:421-8. [DOI] [PubMed] [Google Scholar]
- 52.Allemann F Mizuno S Eid K Yates KE Zaleske D Glowacki J. Effects of hyaluronan on engineered articular cartilage extracellular matrix gene expression in 3-dimensional collagen scaffolds. J Biomed Mater Res. 2001;55:13-9. [DOI] [PubMed] [Google Scholar]
- 53.Galassi G Brun P Radice M Cortivo R Zanon GF Genovese P Abatangelo G. In vitro reconstructed dermis implanted in human wounds: degradation studies of the HA-based supporting scaffold. Biomaterials. 2000;21:2183-91. [DOI] [PubMed] [Google Scholar]
- 54.Park SN Park JC Kim HO Song MJ Suh H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide cross-linking. Biomaterials. 2002;23:1205-12. [DOI] [PubMed] [Google Scholar]
- 55.Mao JS Liu HF Yin YJ Yao KD. The properties of chitosan-gelatin membranes and scaffolds modified with hyaluronic acid by different methods. Biomaterials. 2003;24:1621-9. [DOI] [PubMed] [Google Scholar]
- 56.Cashman J Burt HM Springate C Gleave J Jackson JK. Camptothecin-loaded films for the prevention of postsurgical adhesions. Inflamm Res. 2004;53:355-62. [DOI] [PubMed] [Google Scholar]
- 57.Park SN Kim JK Suh H. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials. 2004;25:3689-98. [DOI] [PubMed] [Google Scholar]
- 58.Yang C Amadio PC Sun YL Zhao C Zobitz ME An KN. Tendon surface modification by chemically modified HA coating after flexor digitorum profundus tendon repair. J Biomed Mater Res B Appl Biomater. 2004;68:15-20. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka T Zhao C Sun YL Zobitz ME An KN Amadio PC. The effect of carbodiimide-derivatized hyaluronic acid and gelatin surface modification on peroneus longus tendon graft in a short-term canine model in vivo. J Hand Surg Am. 2007;32:876-81. [DOI] [PubMed] [Google Scholar]