Abstract
Background:
Despite recent advances, infection remains the most common etiology of arthroplasty failure. Recent work suggests that 25-hydroxyvitamin D (25D) deficiency correlates with the frequency of periprosthetic joint infection (PJI). We endeavored to examine whether 25D3 deficiency leads to increased bacterial burden in vivo in an established mouse model of PJI and, if so, whether this effect can be reversed by preoperative 25D3 supplementation.
Methods:
Mice (lys-EGFP) possessing fluorescent neutrophils were fed a vitamin D3-sufficient (n = 20) or deficient (n = 40) diet for 6 weeks. A group of 25D3-deficient mice (n = 20) were “rescued” with 1 intraperitoneal dose of 25D3 at 3 days before surgery. A stainless steel implant was inserted into the knee joint and the joint space was inoculated with bioluminescent Staphylococcus aureus (1 × 103 colony forming units [CFUs]). In vivo imaging was used to monitor bacterial burden and neutrophil infiltration. Blood was drawn to confirm 25D3 levels 3 days before surgery and on postoperative days (PODs) 0 and 14. Mice were killed at POD 21, and CFUs were quantified after culture. Myeloperoxidase (MPO) and β-N-acetylglucosaminidase (NAG) were assayed to look at neutrophil infiltration and activated tissue macrophage recruitment, respectively.
Results:
Serum values confirmed 25D3 deficiency and repletion of the 25D3-rescued group. Bacterial bioluminescence and neutrophil fluorescence were significantly greater (p < 0.05) in the 25D3-deficient group. CFU counts from the joint tissue and implant were also significantly greater in this group (p < 0.05). Rescue treatment significantly decreased bacterial burden and neutrophil infiltration (p < 0.05). Compared with the 25D3-sufficient and 25D3-rescued groups, MPO activity was higher (p < 0.02) and NAG activity was lower (p < 0.03) in the 25D3-deficient group.
Conclusions:
This study demonstrated in vivo in a mouse model of PJI that (1) 25D3 deficiency results in increased bacterial burden and neutrophil infiltration, and (2) this effect can be reversed with preoperative repletion of 25D3.
Clinical Relevance:
Considering that >65% of patients undergoing arthroplasty have insufficient or low levels of total 25D and that 25D levels can be replenished with ease using a U.S. Food and Drug Administration (FDA)-approved, oral 25D3 product, 25D deficiency may be an important modifiable risk factor in humans undergoing joint replacement.
The number of arthroplasties performed in the U.S. is expected to exceed 3.8 million by 2030, and the annual number of periprosthetic joint infection (PJI) cases is estimated to increase from 17,000 in 2005 to >266,0001. Despite advances in antiseptic protocols, surgical technique, and operating-room sterility, PJI remains the most common etiology of arthroplasty failure2. PJIs are devastating, resulting in reoperations, prolonged antibiotic therapy, and extended disability and rehabilitation, with up to 7% of patients dying between the first and second stages of exchange arthroplasty3,4. Medical costs are enormous, averaging $145,000 per patient, resulting in a proposed projected annual U.S. health-care burden of $8.6 billion by 20305,6.
While effort has been focused on perioperative antimicrobial therapies and modification of health-care systems protocols, host factors governing susceptibility to PJI have been understudied. Although smoking, obesity, and diabetes mellitus (among others) are known risk factors, mechanistic links have been difficult to ascribe, and efforts at modifying these factors have been challenging7-11.
25-hydroxyvitamin D3 (25D3) holds promise as a risk modifier for 3 important reasons. First, epidemiologic data demonstrate that >65% of patients undergoing arthroplasty have an insufficient or low level of total 25-hydroxyvitamin D (25D; accounts for both 25D2 and 25D3 in the serum)12. Second, recent epidemiologic work has suggested that vitamin-D deficiency is directly correlated with the frequency of PJI13. This coincides with literature highlighting the importance of the prohormone 25D as a locally active immune modulator for antigen-activated inflammatory cells. This is mediated through the intracellular enzymatic conversion of available 25D to 1,25-dihydroxyvitamin D (1,25D) via the CYP27B1-hydroxylase, which is coupled to the expression of the vitamin-D receptor. The vitamin-D receptor regulates the expression of genes critical to both the innate and adaptive immune responses in humans14-20. Finally, 25D levels can be returned to normal with ease, rapidity, efficiency, and low cost using an available, U.S. Food and Drug Administration (FDA)-approved, orally administered 25D3 product21. Taken together, 25D deficiency is prevalent in the population receiving total joint arthroplasty, appears to be correlated with PJI clinically and linked to the function of the innate immune response mechanistically, and can be easily improved, highlighting its promise as a modifiable risk factor.
The objective of the current study was to examine whether the immune system is suppressed in the 25D-deficient state, increasing the severity of a PJI. We aimed to test 2 primary hypotheses. First, does 25D3 deficiency lead to an increased bacterial burden and decreased inflammatory response in vivo in an established mouse model of PJI? Second, if that is the case, does “rescue” with preoperative 25D3 supplementation reverse this effect? If successful, such findings could pave the way for establishing a low serum 25D level as an easily modifiable host factor in humans to reduce the risk of PJI.
Materials and Methods
All animals were handled according to good animal practice as defined in the federal regulations set forth in the Animal Welfare Act, the 1996 Guide for the Care and Use of Laboratory Animals, and the Public Health Service Policy for the Humane Care and Use of Laboratory Animals as well as the policies and procedures of the University of California, Los Angeles (UCLA). All animal experiments were approved by the UCLA chancellor’s Animal Research Committee.
Staphylococcus aureus Bioluminescent Strain
Staphylococcus aureus Xen36 (PerkinElmer) is a bioluminescent derivative of the S. aureus ATCC 49525 (Wright) strain, derived from a patient with S. aureus bacteremia. Xen36 has a gram-positive optimized luxABCDE operon stably integrated into a large native plasmid22. This strain emits a blue-green light with a maximal emission wavelength of approximately 490 nm from live, actively metabolizing bacteria and was previously shown to be optimal for use because of the strength and consistency of its signal6.
Preparation of S. aureus for Inoculation
Xen36 possesses a kanamycin-resistance selection marker, enabling isolation from contaminating background strains during culture. Thus, 200 μg/mL of kanamycin (Sigma-Aldrich) was added to all culture samples. Xen 36 was streaked onto tryptic soy agar plates (tryptic soy broth [TSB] plus 1.5% Bacto agar; BD Biosciences) and grown at 37°C overnight. Single colonies were cultured in TSB and grown overnight at 37°C in a shaking incubator (240 rpm) (MaxQ 4450; ThermoFisher Scientific). Midlogarithmic-phase bacteria were obtained after a 2-hour subculture of a 1:50 dilution of the overnight culture. Cells were pelleted, resuspended, and washed 3 times in phosphate buffered saline (PBS) solution. Bacterial concentrations were estimated by measuring the absorbance at 600 nm (BioMate 3; ThermoFisher Scientific). Colony forming units (CFUs) were verified after overnight culture of plates.
Mice
We used 4-week-old male lys-EGFP mice, a genetically engineered mouse line on a C57BL/6 background possessing green-fluorescent myeloid cells (mostly neutrophils) as a consequence of “knock-in” of enhanced green fluorescent protein (EGFP) into the lysozyme M gene23,24. Animals were kept 3 mice per cage and fed either a standard diet (4,200 IU vitamin D3 per kg of feed) or a vitamin D3-deficient diet (0 IU vitamin D3 added; Research Diets) with access to bottled water. Veterinary staff carried out daily assessments.
Vitamin-D Protocol
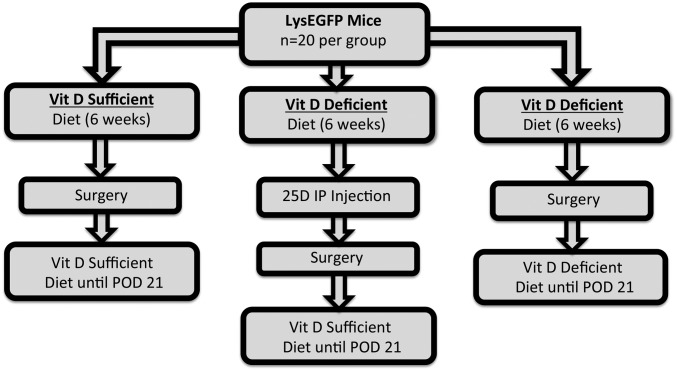
The mice were randomized to receive either a vitamin D3-sufficient (n = 20) or vitamin D3-deficient (n = 40) diet. On the basis of prior experiments, mice were fed the chosen diet for 6 weeks before surgery to ensure 25D3 sufficiency or deficiency25. Three days before surgery, a “rescued” group (n = 20) was created from the mice being fed a deficient diet by injecting 80 ng of 25D3 (Enzo Life Sciences) intraperitoneally, as previously described26; these mice were also switched over to a vitamin D3-sufficient diet (Fig. 1). To confirm 25D3 levels prior to rescue, prior to surgery, and in the postoperative period, retro-orbital blood draws were performed 3 days prior to surgery and on postoperative days (PODs) 0 and 14. Serum was separated via microcentrifuge. Individual samples (n = 10) from each group were pooled on PODs −3, 0, and 14 to ensure adequate content of 1,25D3 for radioimmunoassay and 25D3 for liquid chromatography-mass spectrometry (Heartland Assays).
Fig. 1.
Experimental protocol for 25-hydroxyvitamin D (25D3) supplementation. Four-week old male lys-EGFP mice were randomly fed either a vitamin D3-sufficient (n = 20) or D3-deficient (n = 40) diet. A group of 25D3-deficient mice (n = 20) were given 25D3 by intraperitoneal (IP) injection 3 days prior to surgery and switched over to a vitamin D3-sufficient diet for the remainder of the study to act as a “rescued” group. The remainder of the mice in the 25D3-sufficient and deficient groups were fed their respective diets for the remainder of the study. POD = postoperative day.
Surgical Procedure
Mice were anesthetized via inhalation of isoflurane (2%). The surgical procedure was previously described6,27. A skin incision was made over the right knee. The distal part of the femur was accessed through a medial parapatellar arthrotomy. The femoral medullary canal was manually reamed with a 25-gauge needle. An orthopaedic-grade stainless steel Kirschner wire (0.6 mm in diameter; DePuy Synthes) was placed in a retrograde fashion and cut with 1 mm protruding into the joint space. An inoculum of 1 × 103 CFUs of Xen36 in 2 μL of normal saline solution was pipetted into the joint space. The surgical site was closed with polyglycolic acid 5-0 sutures. Buprenorphine (0.1 mg/kg) was administered subcutaneously every 12 hours as an analgesic for the duration of the experiment. No antibiotics were given throughout the study period.
Quantification of Bacterial Burden Using Bioluminescence Imaging in Vivo and CFUs
Mice were anesthetized via inhalation of isoflurane (2%), and in vivo bioluminescence imaging was performed by using an in vivo imaging system (IVIS Lumina II; PerkinElmer)28. Images were obtained on PODs 0, 1, 3, 5, 7, 10, 14, 18, and 21. Data were presented on a color scale overlaid on a grayscale photograph of the mouse and quantified as maximum flux (photons per second per cm2 per steradian [photons/s/cm2/sr]) within a circular region of interest (16,103 pixels) by using Living Image software (PerkinElmer).
To confirm that the bioluminescence signals corresponded to the bacterial burden in vivo, bacteria were quantified. On POD 21, bacteria were detached from the implant by sonication in 1 mL of 0.3% polysorbate 80 in TSB for 10 minutes followed by vortexing for 5 minutes, as previously described6. In addition, bacteria in the surrounding joint tissue were measured by homogenizing bone and joint tissue (Pro200H Series homogenizer; PRO Scientific). The number of bacterial CFUs that were adherent to the implant and in the joint tissue was determined by counting CFUs after overnight culture of plates and was expressed as total CFUs harvested from the implant and joint tissue.
Quantification of Neutrophil Infiltration with Fluorescence Imaging in Vivo
Fluorescence imaging in vivo was performed using the IVIS Lumina system. EGFP-expressing neutrophils within the postoperative site were visualized by using the GFP filter for excitation (445 to 490 nm) and emission (515 to 575 nm) at an exposure time of 0.5 seconds6,28. The observer was not blinded to the treatment group. Data were presented on a color scale overlaid on a grayscale photograph of the mouse and quantified as maximum radiant efficiency ([photons/s]/[μW/cm2]) within a circular region of interest (16,103 pixels) using Living Image software.
Quantification of Neutrophil Infiltration with Myeloperoxidase Activity and Macrophage Recruitment with β-N-Acetylglucosaminidase Activity
At the conclusion of the experiment, 3 mice per group were killed and joint-tissue specimens were homogenized. The homogenate was assayed for myeloperoxidase (MPO) activity levels, a surrogate for neutrophil infiltration, using the Myeloperoxidase Colorimetric Activity Assay Kit (Sigma-Aldrich) according to the manufacturer’s instructions and expressed as milliunits/mL29. The tissue homogenate was also assayed for β-N-acetylglucosaminidase (NAG) activity levels, a surrogate for recruited tissue macrophage activity, using the β-N-Acetylglucosaminidase Assay Kit (Sigma-Aldrich) according to the manufacturer’s instructions and expressed as milliunits/mL30.
Statistical Analysis
Each experimental group had 20 mice; in previous studies, our group determined that 20 animals per group were necessary to demonstrate significance at the p < 0.05 level6,29. Data were compared using a Student t test (1 or 2-tailed where indicated). All data were expressed as the mean and the standard error of the mean (SEM). Values of p < 0.05 were considered significant.
Results
Serum Vitamin-D Metabolite Levels
The 1,25D3 and 25D3 levels were higher in the mice that were fed a vitamin D3-sufficient diet compared with those on a vitamin D3-deficient diet for all 3 time points (PODs −3, 0, and 14) (Table I). Serum 25D3 and 1,25D3 levels were comparable between the 25D3-deficient and rescued mice at POD −3; the serum 25D3 and 1,25D3 in the rescued group increased to levels that were comparable with those in the 25D3-sufficient group at PODs 0 and 14, confirming successful repletion.
TABLE I.
Pooled Serum 25-Hydroxyvitamin D3 (25D3) and 1,25-Dihydroxyvitamin D3 (1,25D3) Values from 25D3-Deficient, Sufficient, and Rescued Mice*
| 25D3
(ng/mL) |
1,25D3
(pg/mL) |
|||||
| POD −3 | POD 0 | POD 14 | POD −3 | POD 0 | POD 14 | |
| Sufficient | 15.6 | 17.6 | 20.8 | 64.3 | 69.5 | 52.3 |
| Deficient | 2.1 | 1.8 | 1.5 | 25.7 | 34 | 21.5 |
| Rescued | 2.1 | 28.8 | 26.2 | 26.3 | 62.6 | 52.7 |
N = 10 at each time point. POD = postoperative day.
Bacterial Burden Using Bioluminescence Imaging in Vivo and CFUs
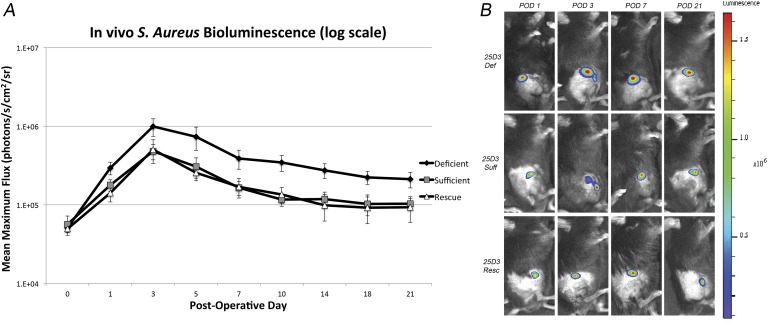
The 25D3-sufficient, deficient, and rescued groups had similar bioluminescent signals at POD 0 (5.64 × 104, 5.08 × 104, and 4.94 × 104 photons/s/cm2/sr, respectively) (Fig. 2). The bioluminescent signal for all 3 groups peaked at POD 3. From PODs 1 through 21, the 25D3-deficient group had a significantly higher bioluminescent signal than did the 25D3-sufficient group (p < 0.05 for all time points). In addition, from PODs 1 through 21, the rescued group had a bioluminescent signal that was comparable to that of the 25D3-sufficient group (p > 0.05 for all time points) and a significantly lower bioluminescent signal compared with the 25D3-deficient group (p < 0.05 for all time points).
Fig. 2.
Figs. 2-A and 2-B Measurement of bacterial burden in vivo using live-animal bioluminescence in 25D3-deficient (def), sufficient (suff), and rescued (resc) mice. A stainless steel implant was inserted into the right knee joint of the mice (n = 20 per group), and the joint space was inoculated with Xen36 Staphylococcus aureus (1 × 103 colony-forming units) possessing the bioluminescent construct in a stable plasmid. Fig. 2-A Bacterial counts as measured by S. aureus bioluminescence in vivo (mean maximum flux and standard error of the mean [logarithmic scale]). Fig. 2-B Representative in vivo S. aureus bioluminescence on a color scale overlaid on a grayscale image of the mouse. POD = postoperative day.
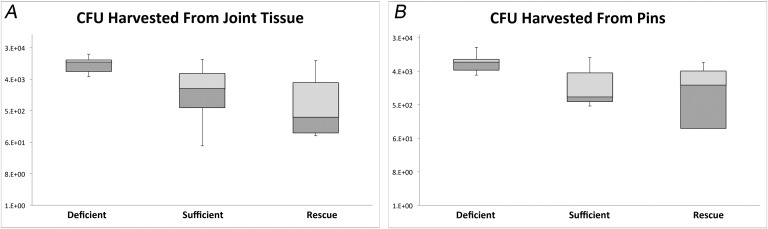
This finding was confirmed by CFU counts from the implant and surrounding joint tissue (Fig. 3). The average CFU count from the implant was 8.3 × 103 for the 25D3-deficient group compared with 2.4 × 103 for the 25D3-sufficient group (p < 0.05). The implant CFU count was 2.5 × 103 for the 25D3-rescued group, which was equivalent to that of the 25D3-sufficient group (p > 0.05) and significantly lower than that of the 25D3-deficient group (p < 0.04). The average CFU count from the surrounding joint tissue was 1.2 × 104 for the 25D3-deficient group compared with 5.4 × 103 for the sufficient group (p < 0.05). The CFU count from the surrounding joint tissue for the rescued group was 4.7 × 103, which was also similar to that of the 25D3-sufficient group (p > 0.05) and significantly lower than that of the 25D3-deficient group (p < 0.05).
Fig. 3.
Figs. 3-A and 3-B Confirmation of bacterial burden using colony-forming unit (CFU) counts. At postoperative day 21, mice were killed and bacteria from the implant and surrounding joint tissue were processed for culture. Fig. 3-A Box-and-whisker plots (logarithmic scale) of bacterial counts as measured by CFUs in the surrounding joint tissue of the right knee (p < 0.05 for the 25D3-deficient mice compared with both 25D3-sufficient and rescued mice). Fig. 3-B Box-and-whisker plots (logarithmic scale) of bacterial counts as measured by CFUs adherent to the implant (p < 0.04 for the 25D3-deficient mice compared with both 25D3-sufficient and rescued mice). The horizontal line within the boxes indicates the median, the outside borders indicate the medians of the upper half and lower half of the data, and the whiskers indicate the minimum and maximum values of the data set.
Neutrophil EGFP Fluorescence in Vivo
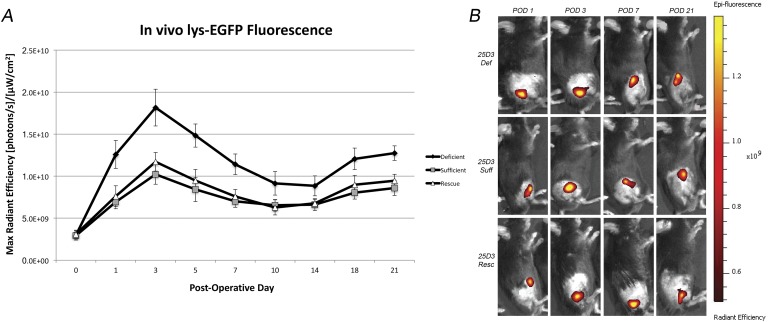
All 3 groups started with similar fluorescent signal on POD 0 (2.9 × 109, 2.9 × 109, and 3.1 × 109 [photons/s]/[μW/cm2] in the 25D3-deficient, sufficient, and rescued groups, respectively), and the signal peaked at POD 3 for all groups (Fig. 4). Interestingly, from PODs 1 through 21, the 25D3-deficient group had significantly greater fluorescent signal compared with the sufficient group (p < 0.05 for all time points). The 25D3-rescued group had fluorescent signal that was similar to that of the sufficient group (p > 0.05 for all time points) and significantly less fluorescent signal compared with the deficient group (p < 0.05 for all time points).
Fig. 4.
Figs. 4-A and 4-B In vivo neutrophil EGFP fluorescence induced by the Xen36 Staphylococcus aureus strain in 25D3-deficient (def), sufficient (suff), and rescued (resc) mice. A stainless steel implant was inserted into the right knee joint of the mice (n = 20 per group), and the joint space was inoculated with Xen36 S. aureus (1 × 103 colony-forming units) possessing the bioluminescent construct in a stable plasmid. Fig. 4-A Neutrophil infiltration (neutrophil EGFP fluorescence) as measured by fluorescence in vivo (mean maximum radiant efficiency and standard error of the mean). Fig. 4-B Representative in vivo neutrophil EGFP fluorescence on a color scale overlaid on a grayscale image of the mouse. POD = postoperative day.
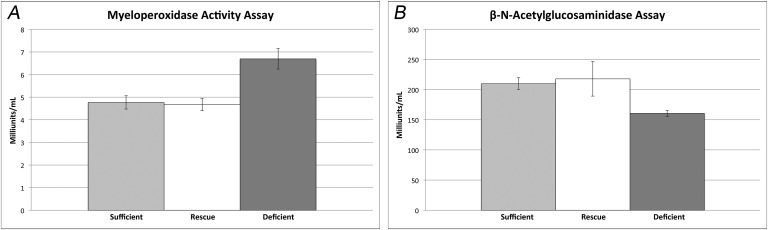
Quantification of Neutrophil Infiltration (MPO Activity) and Activated Tissue Macrophage Recruitment (NAG Activity)
Mice in the 25D3-deficient group had significantly higher MPO activity, at 6.7 milliunits/mL (p < 0.02), than did mice in the 25D3-sufficient and rescued groups, which were comparable at 4.8 and 4.7 milliunits/mL, respectively (p > 0.05) (Fig. 5). Mice in the 25D3-deficient group had significantly less NAG activity, at 160 milliunits/mL (p < 0.03), than did mice in the 25D3-sufficient and rescued groups, which were similar at 210 and 218 milliunits/mL, respectively (p > 0.05) (Fig. 5).
Fig. 5.
Figs. 5-A and 5-B Myeloperoxidase (MPO) and β-N-acetylglucosaminidase (NAG) activity induced by the Xen36 Staphylococcus aureus strain in 25D3-deficient, sufficient, and rescued mice. Fig. 5-A Mean MPO activity of the infected joint-tissue specimens (and standard error of the mean [SEM]) (n = 3 mice per group; p < 0.02 for the 25D3-deficient mice compared with both 25D3-sufficient and rescued mice). Fig. 5-B Mean NAG activity of the infected joint tissue specimens (and SEM) (n = 3 mice per group; p < 0.03 for the 25D3-deficient mice compared with both 25D3-sufficient and rescued mice).
Discussion
Despite substantial research into its prevention, PJI remains the most common cause of failed total joint arthroplasty2. Although advances have been made in antimicrobial therapies and modification of health-care systems protocols, host factors have been often neglected because of the perceived difficulty in modifying them7-11,31-34. Recent work on circulating levels of 25D, the metabolite used to distinguish vitamin-D sufficiency of the host, has highlighted its importance as a host factor related to the risk of infection13,15. When considering the ease with which it can be replenished, a deficient serum 25D level has the potential to be an easily modifiable risk factor in the prevention of PJI21. With this in mind, we sought to use our previously established in vivo mouse model of PJI to (1) evaluate the differences in bacterial burden and host immune response between 25D3-sufficient and deficient mice, and (2), test if this difference can be reversed with a single, preoperative dose of 25D3.
In recent years, the physiological and pathophysiological roles ascribed to normal versus deficient vitamin-D balance have been broadened35. Recent work has demonstrated a causal link between the prohormone 25D and the normal human innate immune response15-20. In pathogen-associated molecular pattern (PAMP)-activated macrophages, characterized by co-upregulation of the expression of the 25D-activating enzyme gene (CYP27B1) and the vitamin-D receptor, 25D is converted to the active metabolite 1,25D intracellularly, which then binds to the vitamin-D receptor and turns on (1) antimicrobial peptide (AMP)-generating, (2) autophagy-stimulating, and (3) inflammasome-activating genes to combat infection, especially in the presence of S. aureus15,36. This pathway is inoperable if extracellular levels of substrate 25D are inadequate. Clinical work by Maier et al. also demonstrated that there was a significant difference (p < 0.001) in serum 25D levels between patients who underwent primary total arthroplasty without PJI and those who developed a PJI13. In addition, 64% of the patients who underwent primary arthroplasty had low levels of 25D. Taken together, these recent findings highlight the potentially important role for 25D repletion in the prevention of PJI.
When comparing the 25D3-sufficient and deficient mice, it was clear that the bioluminescent signal was greater in the deficient mice, indicating increased bacterial burden on the implant and surrounding tissues. This was also confirmed by CFU counts on POD 21. When examining the host immune response, the 25D3-deficient mice had increased fluorescent signal, indicating greater neutrophil infiltration. This was confirmed by measuring MPO activity at the conclusion of the study. This is in contrast to decreased NAG activity, indicating diminished activated tissue macrophage recruitment. It is possible that inadequate host macrophage immune response leads to increased bacterial burden and this, consequently, leads to increased neutrophil infiltration in an attempt to more adequately combat the ongoing infection. Nonetheless, the data demonstrate that 25D3 deficiency increases the severity of PJI. Moreover, the 25D3-deficient mice that were replenished preoperatively with 25D3 were “rescued” from this state and had a host response to induced infection that was similar to that of the mice that were 25D3-sufficient. This finding opens the door to considerable further study of a low total 25D level as a risk factor that can be modified to help “prime” the immune system prior to arthroplasty.
There were several limitations to this study. One limitation was that these results were of a mouse model and thus may not be directly applicable to humans. Although this model is advantageous for the study of PJI, the size of the mice limited the blood available for testing of biomarkers and necessitated the pooling of serum for analysis. Ideally, serum values of vitamin 25D3 and 1,25D3 would have been measured for each mouse at a variety of time points and correlated with the level of infection for each mouse. While this would have provided a more robust analysis of the role of 25D3 and 1,25D3 deficiency in PJI, the aggregate data presented here establish a foundation on which more detailed work on larger animals can be conducted. In addition, an extreme state of 25D3 deficiency was tested, which may not be as clinically pertinent a scenario in humans, who would likely present with a lesser degree of deficiency. Furthermore, observers were not blinded to the treatment group when imaging the mice, leading to the potential for detection bias. To combat this, a predetermined region of interest in size and shape was used for both bioluminescence and fluorescence imaging.
A final limitation was the use of lys-EGFP mice containing fluorescent neutrophils instead of MacGreen mice, which contain fluorescent macrophages37. As the macrophage is the primary cell responsible for initiating the “foreign-body reaction” to implanted hardware, it plays an important role in protecting the implant from contaminant bacteria38. When the 25D level in the serum of the host is too low, this results in the failure of the macrophage to mount effective autophagy (killing) of bacteria, thus blunting the innate immune response39. Longitudinal visualization of the difference in macrophage response might have yielded more direct information about the effect that 25D deficiency has on the innate immune response. Nevertheless, the lys-EGFP species was previously validated in our model28. As such, it was thought to be more likely to produce a consistent, predictable result than a strain with a potentially different immunology. In addition, activated tissue macrophage recruitment was examined at the end of the study and demonstrated decreased activity in the 25D3-deficient mice. Future study using MacGreen mice may be able to more fully evaluate the difference in macrophage recruitment over the course of a PJI.
Despite these weaknesses, we believe that this study successfully provides in vivo evidence that the 25D-deficient state of the host increases bacterial burden and neutrophil infiltration in the setting of PJI. Moreover, this effect can be reversed by preoperative administration of 25D3. With 25D deficiency established as an important and easily modifiable risk factor in vivo, additional studies should (1) examine whether this single-dose “rescue” effect with 25D3 administration also exists for humans, and (2) elucidate the exact mechanism behind the blunted immune response in the 25D-deficient population. In the future, a “personalized” approach to 25D3 supplementation may be used for 25D-deficient patients prior to arthroplasty to help “prime” the immune system and prevent PJI.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, David Geffen School of Medicine at the University of California, Los Angeles, Los Angeles, California
A commentary by Andre J. van Wijnen, PhD, and Matthew P. Abdel, MD, is linked to the online version of this article at jbjs.org.
Disclosure: This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (award number 5K08AR069112-01). On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJS/E358).
Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007. April;89(4):780-5. [DOI] [PubMed] [Google Scholar]
- 2.Rezapoor M, Parvizi J. Prevention of periprosthetic joint infection. J Arthroplasty. 2015. June;30(6):902-7. Epub 2015 Mar 18. [DOI] [PubMed] [Google Scholar]
- 3.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004. April 1;350(14):1422-9. [DOI] [PubMed] [Google Scholar]
- 4.Berend KR, Lombardi AV, Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013. February;471(2):510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006. April;27(11):2331-9. Epub 2005 Dec 20. [DOI] [PubMed] [Google Scholar]
- 6.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, Miller LS. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 2010. September 7;5(9):e12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in Medicare patients undergoing TKA. Clin Orthop Relat Res. 2012. January;470(1):130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris AHS, Reeder R, Ellerbe L, Bradley KA, Rubinsky AD, Giori NJ. Preoperative alcohol screening scores: association with complications in men undergoing total joint arthroplasty. J Bone Joint Surg Am. 2011. February 16;93(4):321-7. [DOI] [PubMed] [Google Scholar]
- 9.Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009. September;24(6)(Suppl):84-8. Epub 2009 Jul 15. [DOI] [PubMed] [Google Scholar]
- 10.Jämsen E, Nevalainen P, Kalliovalkama J, Moilanen T. Preoperative hyperglycemia predicts infected total knee replacement. Eur J Intern Med. 2010. June;21(3):196-201. Epub 2010 Mar 15. [DOI] [PubMed] [Google Scholar]
- 11.Parvizi J, Sullivan TA, Pagnano MW, Trousdale RT, Bolander ME. Total joint arthroplasty in human immunodeficiency virus-positive patients: an alarming rate of early failure. J Arthroplasty. 2003. April;18(3):259-64. [DOI] [PubMed] [Google Scholar]
- 12.Lavernia CJ, Villa JM, Iacobelli DA, Rossi MD. Vitamin D insufficiency in patients with THA: prevalence and effects on outcome. Clin Orthop Relat Res. 2014. February;472(2):681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier GS, Horas K, Seeger JB, Roth KE, Kurth AA, Maus U. Is there an association between periprosthetic joint infection and low vitamin D levels? Int Orthop. 2014. July;38(7):1499-504. Epub 2014 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams JS, Ramin J, Rafison B, Windon C, Windon A, Liu PT. Redefining human vitamin D sufficiency: back to the basics. Bone Res. 2013;1(1):2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006. March 24;311(5768):1770-3. Epub 2006 Feb 23. [DOI] [PubMed] [Google Scholar]
- 16.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl). 2010. May;88(5):441-50. Epub 2010 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in defense of the human immune response. Ann N Y Acad Sci. 2007. November;1117:94-105. Epub 2007 Jul 26. [DOI] [PubMed] [Google Scholar]
- 18.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, Teles R, Montoya D, Iyer SS, Bruns H, Lewinsohn DM, Hollis BW, Hewison M, Adams JS, Steinmeyer A, Zügel U, Cheng G, Jo EK, Bloom BR, Modlin RL. Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011. October 12;3(104):104ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C, Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010. August;10(4):482-96. Epub 2010 Apr 27. [DOI] [PubMed] [Google Scholar]
- 20.Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zügel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007. March;117(3):803-11. Epub 2007 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jetter A, Egli A, Dawson-Hughes B, Staehelin HB, Stöcklin E, Gössl R, Henschkowski J, Bischoff-Ferrari HA. Pharmacokinetics of oral vitamin D3 and calcifediol. Bone. 2013. October 24;59:14-9. Epub 2013 Oct 24. [PubMed] [Google Scholar]
- 22.Francis KP, Joh D, Bellinger-Kawahara C, Hawkinson MJ, Purchio TF, Contag PR. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun. 2000. June;68(6):3594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MH, Curry FRE, Simon SI. Dynamics of neutrophil extravasation and vascular permeability are uncoupled during aseptic cutaneous wounding. Am J Physiol Cell Physiol. 2009. April;296(4):C848-56. Epub 2009 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MH, Liu W, Borjesson DL, Curry FR, Miller LS, Cheung AL, Liu FT, Isseroff RR, Simon SI. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol. 2008. July;128(7):1812-20. Epub 2008 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010. June;151(6):2423-32. Epub 2010 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albert DM, Marcus DM, Gallo JP, O’Brien JM. The antineoplastic effect of vitamin D in transgenic mice with retinoblastoma. Invest Ophthalmol Vis Sci. 1992. July;33(8):2354-64. [PubMed] [Google Scholar]
- 27.Pribaz JR, Bernthal NM, Billi F, Cho JS, Ramos RI, Guo Y, Cheung AL, Francis KP, Miller LS. Mouse model of chronic post-arthroplasty infection: noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study. J Orthop Res. 2012. March;30(3):335-40. Epub 2011 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niska JA, Meganck JA, Pribaz JR, Shahbazian JH, Lim E, Zhang N, Rice BW, Akin A, Ramos RI, Bernthal NM, Francis KP, Miller LS. Monitoring bacterial burden, inflammation and bone damage longitudinally using optical and μCT imaging in an orthopaedic implant infection in mice. PLoS One. 2012;7(10):e47397 Epub 2012 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI, Francis KP, Iwakura Y, Miller LS. Protective role of IL-1β against post-arthroplasty Staphylococcus aureus infection. J Orthop Res. 2011. October;29(10):1621-6. Epub 2011 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiner RG, Tanner AR, Keyhani AH, Wright R. A comparative study of lysosomal enzyme activity in monocytes and Kupffer cells isolated simultaneously in a rat model of liver injury. Clin Exp Immunol. 1981. February;43(2):376-80. [PMC free article] [PubMed] [Google Scholar]
- 31.DiPiro JT, Vallner JJ, Bowden TA, Jr, Clark BA, Sisley JF. Intraoperative serum and tissue activity of cefazolin and cefoxitin. Arch Surg. 1985. July;120(7):829-32. [DOI] [PubMed] [Google Scholar]
- 32.Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992. January 30;326(5):281-6. [DOI] [PubMed] [Google Scholar]
- 33.Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD, Gambir A, Huang R, Kissin Y, Kobayashi H, Kobayashi N, Krenn V, Drago L, Marston SB, Meermans G, Perez J, Ploegmakers JJ, Rosenberg A, Simpendorfer C, Thomas P, Tohtz S, Villafuerte JA, Wahl P, Wagenaar FC, Witzo E. Diagnosis of periprosthetic joint infection. J Arthroplasty. 2014. February;29(2)(Suppl):77-83. Epub 2013 Dec 15. [DOI] [PubMed] [Google Scholar]
- 34.Alijanipour P, Karam J, Llinás A, Vince KG, Zalavras C, Austin M, Garrigues G, Heller S, Huddleston J, Klatt B, Krebs V, Lohmann C, McPherson EJ, Molloy R, Oliashirazi A, Schwaber M, Sheehan E, Smith E, Sterling R, Stocks G, Vaidya S. Operative environment. J Arthroplasty. 2014. February;29(2)(Suppl):49-64. Epub 2013 Dec 15. [DOI] [PubMed] [Google Scholar]
- 35.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012. June;33(3):456-92. Epub 2012 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, Latif H, Kim JN, Lockhart A, Artis K, David G, Taylor P, Streib J, Dorrestein PC, Grier A, Gill SR, Zengler K, Hata TR, Leung DY, Gallo RL. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017. February 22;9(378):eaah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasmono RT, Williams E. Generation and characterization of MacGreen mice, the Cfs1r-EGFP transgenic mice. Methods Mol Biol. 2012;844:157-76. [DOI] [PubMed] [Google Scholar]
- 38.Higgins DM, Basaraba RJ, Hohnbaum AC, Lee EJ, Grainger DW, Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Am J Pathol. 2009. July;175(1):161-70. Epub 2009 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009. April 1;182(7):4289-95. [DOI] [PMC free article] [PubMed] [Google Scholar]