Abstract
Background:
High failure rates of metal-on-metal (MoM) hip implants prompted regulatory authorities to issue worldwide safety alerts. Circulating cobalt from these implants causes rare but fatal autopsy-diagnosed cardiotoxicity. There is concern that milder cardiotoxicity may be common and underrecognized. Although blood metal ion levels are easily measured and can be used to track local toxicity, there are no noninvasive tests for organ deposition. We sought to detect correlation between blood metal ions and a comprehensive panel of established markers of early cardiotoxicity.
Methods:
Ninety patients were recruited into this prospective single-center blinded study. Patients were divided into 3 age and sex-matched groups according to implant type and whole-blood metal ion levels. Group-A patients had a ceramic-on-ceramic [CoC] bearing; Group B, an MoM bearing and low blood metal ion levels; and Group C, an MoM bearing and high blood metal-ion levels. All patients underwent detailed cardiovascular phenotyping using cardiac magnetic resonance imaging (CMR) with T2*, T1, and extracellular volume mapping; echocardiography; and cardiac blood biomarker sampling. T2* is a novel CMR biomarker of tissue metal loading.
Results:
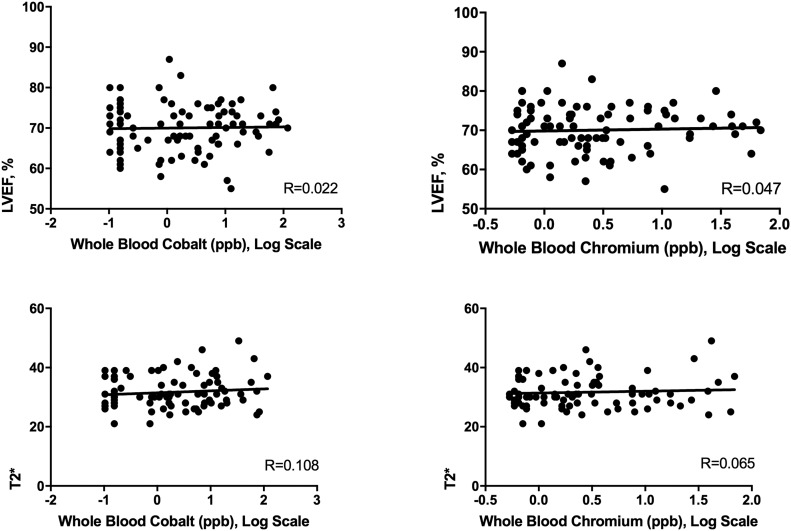
Blood cobalt levels differed significantly among groups A, B, and C (mean and standard deviation [SD], 0.17 ± 0.08, 2.47 ± 1.81, and 30.0 ± 29.1 ppb, respectively) and between group A and groups B and C combined. No significant between-group differences were found in the left atrial or ventricle size, ejection fraction (on CMR or echocardiography), T1 or T2* values, extracellular volume, B-type natriuretic peptide level, or troponin level, and all values were within normal ranges. There was no relationship between cobalt levels and ejection fraction (R = 0.022, 95% confidence interval [CI] = −0.185 to 0.229) or T2* values (R = 0.108, 95% CI = −0.105 to 0.312).
Conclusions:
Using the best available technologies, we did not find that high (but not extreme) blood cobalt and chromium levels had any significant cardiotoxic effect on patients with an MoM hip implant. There were negligible-to-weak correlations between elevated blood metal ion levels and ejection fraction even at the extremes of the 95% CI, which excludes any clinically important association.
Level of Evidence:
Therapeutic Level II. See Instructions for Authors for a complete description of levels of evidence.
Over 1 million patients worldwide have implanted metal-on-metal (MoM) hip prostheses, predominantly composed of cobalt-chromium (CoCr) alloy1. Release of nano-particulate debris and metal ions from implants has been reported to cause harm2. Local soft-tissue deposition adjacent to the joint3-5 results in high failure rates of MoM hip implants. This led regulatory agencies to issue safety alerts against their use6-8, necessitating surveillance that includes serial measurement of blood metal ion levels and cross-sectional imaging to determine whether revision surgery is warranted9.
The relationship between MoM hip implants and systemic toxicity is less clear. Elevated levels of circulating cobalt have been linked with cardiac, thyroid, and neuro-ocular abnormalities in case reports, but causation and prevalence remain to be established10-18. Diagnosis of cardiotoxicity requires invasive myocardial biopsy or postmortem examination, with no available noninvasive methods for measuring metal deposition. Heart failure is common in the patient population most likely to undergo hip replacement19, so there is concern that an association between MoM hip prostheses and heart failure may be missed. One echocardiography (EKG) study showed a 7% reduction in left ventricular ejection fraction in MoM-implant recipients20 (although all values were within the normal range). More recently, an epidemiological study that compared 121 subjects who had 1 type of MoM hip prosthesis with 3,546 subjects who had a metal-on-plastic (MoP) prosthesis showed the age-adjusted rate of hospitalization for heart failure to be equivalent to 1 such hospitalization for every 11 patients21. The Therapeutic Goods Administration (Australian Department of Health) consequently published a safety alert pending further investigation22.
Cardiac magnetic resonance imaging (CMR) is the gold-standard technique for measuring cardiac volumes and function. In addition, the CMR T2* method for assessing cardiac iron has been histologically validated and has become a routine measure, transforming the management of patients at risk for iron cardiomyopathy23,24. As cobalt exhibits a magnetic property similar to that of iron, T2* mapping has the potential to noninvasively detect tissue deposition of cobalt in patients with a failing MoM hip prosthesis, as recently demonstrated by our group25.
The present study of patients with an MoM hip prosthesis was designed to assess the effect of elevated whole-blood metal ion levels on cardiac function as well as to seek evidence of cardiac metal ion deposition with gold-standard multimodality imaging and measurement of serum biomarkers to minimize potential bias.
Materials and Methods
Study Design
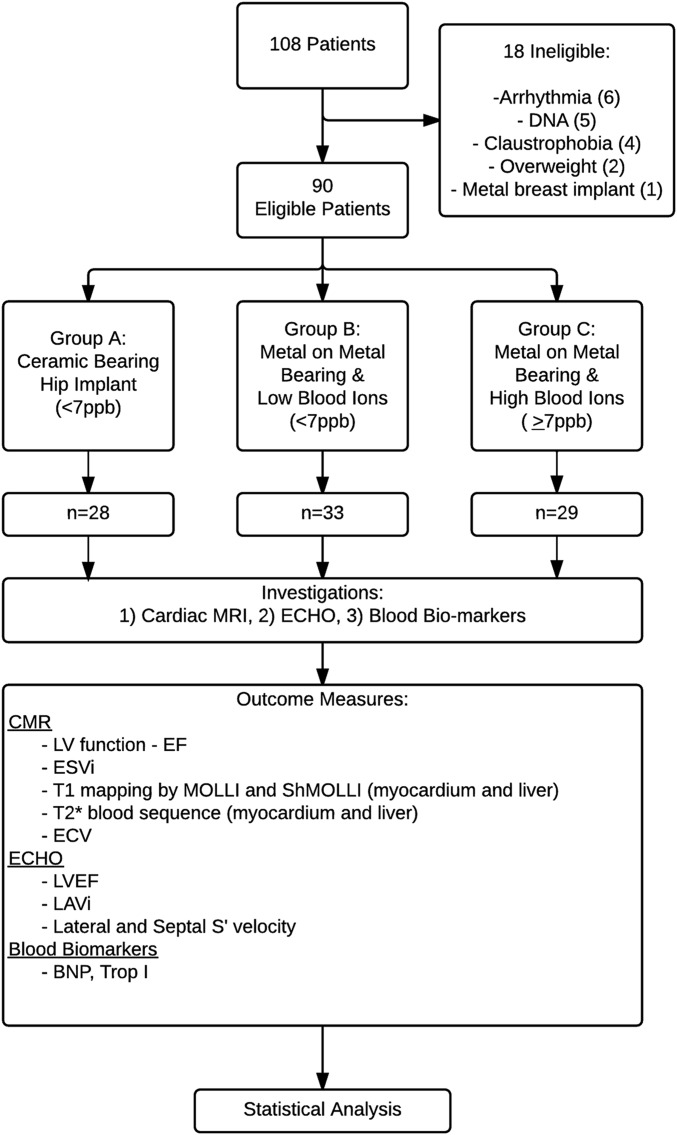
In this single-center blinded cross-sectional study, all patients underwent CMR, transthoracic EKG, and blood sampling during a single visit (Fig. 1). The research was approved by the institutional review board and ethics committee (reference: 14/LO/1722) and was registered in ClinicalTrials.gov (NCT02331264). The primary outcomes were prespecified on trial registration.
Fig. 1.
Flow diagram of the study methods. ECHO = echocardiogram, LV = left ventricular, EF = ejection fraction, ESVi = end systolic volume index, ECV = extracellular volume, LAVi = left atrial volume index, BNB = B-type natriuretic peptide, and Trop I = troponin I.
Setting
Between October 2014 and November 2015, 108 patients were recruited from specialist outpatient clinics held at the Royal National Orthopaedic Hospital and cardiac assessment was performed at the Heart Hospital (both University College London hospitals, U.K.).
Patient Groups
Patients 18 years of age or older with an MoM or ceramic-on-ceramic (CoC) hip implant in situ for >12 months were recruited. They were divided into 3 age and sex-matched groups on the basis of prosthesis type and blood metal ion levels. Group A consisted of patients with a CoC-bearing implant and normal whole-blood metal ion levels; Group B, patients with an MoM implant and low whole-blood metal ion levels (<7 ppb); and Group C, patients with an MoM implant and raised whole-blood metal ion levels (≥7 ppb). CoC was chosen over MoP couplings in Group A because of concerns regarding metal debris arising through trunnionosis in MoP implants, and 7 ppb was used as a cutoff point between Groups B and C as this represents the U.K. Medicines & Healthcare products Regulatory Agency (MHRA) recommended threshold above which there may be heightened concern6.
Exclusion criteria were prior hip revision surgery, known atrial fibrillation or impaired renal function (estimated glomerular filtration rate of <30 mL/min), and standard contraindications to magnetic resonance imaging (MRI) (e.g., a pacemaker). Six patients—4 of whom would have been assigned to group A (CoC) and 1 each of whom, to groups B and C—were excluded because of arrhythmias (e.g., atrial fibrillation).
All participants gave written informed consent conforming to the Declaration of Helsinki (5th revision, 2000). All tests were performed at a single visit. Study data were collected and managed using REDCap (Research Electronic Data Capture software, version 5.9.6, http://www.project-redcap.org/), with the cardiac investigators blinded to the study groups during data acquisition and analysis until study completion.
Transthoracic EKG
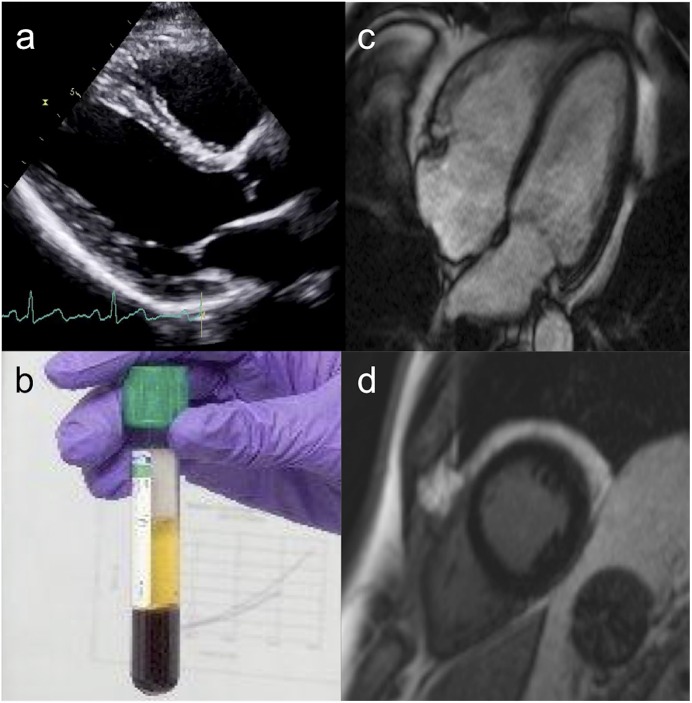
EKG was performed using a Vivid E9 ultrasound machine (GE Healthcare). Measurements were made according to the British Society of Echocardiography standard protocol26, including acquisition of standard 2-dimensional views, M-mode, spectral tissue Doppler imaging, and blood flow measurements (Fig. 2-A).
Fig. 2.
Comprehensive cardiac assessment included transthoracic EKG (Fig. 2-A), measurement of blood biomarker levels (BNP and troponin I) (Fig. 2-B), CMR (Fig. 2-C), and late gadolinium-enhancement scar imaging (Fig. 2-D).
CMR Acquisition
Patients underwent CMR at 1.5 T (MAGNETOM Avanto; Siemens Medical). Cardiac volumes and ejection fractions were calculated conventionally from short-axis cine images.
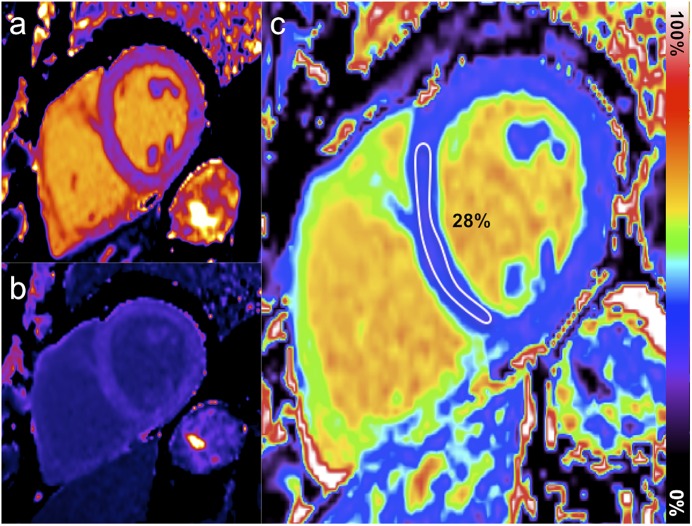
Bright-blood T2* and precontrast T1 mapping of the myocardium and liver were acquired on a mid-left ventricular short axis slice and an axial mid-hepatic slice, respectively (Figs. 2-C and 2-D). As T1 mapping is not fully standardized, we used 2 implementations: modified look-locker (MOLLI) and shortened MOLLI (ShMOLLI) sequences (MyoMaps; Siemens). Late gadolinium-enhancement images were acquired using a motion-corrected phase sensitive inversion recovery sequence to identify focal myocardial fibrosis after administering 0.1 mmol/kg of gadolinium-based contrast medium (gadoterate meglumine [Dotarem; Guerbet]). Fifteen minutes after the injection of the contrast medium, T1 sequences were repeated for extracellular volume quantification (Figs. 3-A, 3-B, and 3-C).
Fig. 3.
Precontrast (Fig. 3-A) and postcontrast (Fig. 3-B) T1 mapping combined for extracellular volume mapping (Fig. 3-C).
Blood Biomarkers
Blood sampling to measure B-type natriuretic peptide (BNP), troponin-I, and whole-blood cobalt and chromium levels (Fig. 2-B) was performed before the patients underwent CMR. BNP and high-sensitivity troponin are the most commonly used biomarkers in cardiology. They cover a range of cardiac processes, with BNP measurements used to evaluate myocyte strain and troponin measurements indicating myocyte death. Both have prognostic importance.
Whole-blood cobalt and chromium levels were measured using inductively coupled plasma mass spectrometry in the same U.K. reference laboratory to eliminate interlaboratory variation. The MHRA advises that measurements of cobalt and chromium ions be carried out by laboratories participating in the Trace Elements External Quality Assessment Scheme (TEQAS). The accuracy and reliability of these measurements are regularly audited and have demonstrated excellent agreement (96.4%; standard deviation [SD] = 2.23%, coefficient of variation = 2.3%) across the laboratories27.
Image Analysis
All image acquisition and analyses were performed by observers blinded to the study groups. Unblinding was performed by an independent statistician once all data had been acquired and analyzed, with the data set locked.
Left ventricular volumes, ejection fraction, and mass were calculated from CMR data using standard techniques and dedicated software (CMRtools; Cardiovascular Imaging Solutions). Thresholding methods were used, and papillary muscles were considered part of the left ventricular myocardium. Volumes were subsequently indexed to body surface area. Two of the authors determined the presence of late gadolinium enhancement through visual assessment.
For T2* and T1 measurements, a region of interest was manually drawn on the interventricular septum on each image, with care taken to avoid the endocardial and epicardial contours to minimize partial voluming effect. Extracellular volume was calculated using MOLLI sequences via a fully automated method that calculates pixel-wise extracellular volume parametric maps28, based on the standard formula:
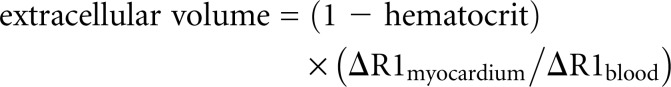 |
where ΔR1 is the change in R1 from the postcontrast to the precontrast image, and R1 = 1/T1.
Echocardiographic data were digitally stored for offline analysis with EchoPAC dimension software (GE Healthcare). Conventional analysis of left ventricular structure and systolic and diastolic function including spectral tissue Doppler parameters were performed according to guidelines, with values averaged over 2 cardiac cycles. Tissue Doppler parameters for lateral and septal walls were averaged to produce a single marker of systolic longitudinal function.
Study Size
Sample size calculations were based on previously published data20, which indicated an absolute difference in ejection fraction of 5% (with a pooled SD of approximately 8%) between patients with an MoM hip prosthesis and those with a conventional hip prosthesis. Using an alpha value of 0.05 and a 2:1 sample size ratio between MoM and CoC prostheses, we needed 62 patients with an MoM implant and 31 with a CoC implant to have a power of 80% to detect a 5% difference (Cohen delta = 0.63, classified as a medium to large effect). The MoM group was divided according to blood metal levels using 7 ppb as a cutoff6 (yielding approximately a 1:1:1 ratio overall).
Statistical Analysis
The prespecified primary end points were ejection fraction (determined with CMR) and T2* measurement. The secondary end points included left ventricular end systolic volume index, T1, and extracellular volume measurements with CMR; ejection fraction and left atrial volume index and left ventricular long axis function (mean of the lateral and septal S' velocities measured with EKG); and blood biomarkers.
The 3 groups were assessed for matching using chi-square tests (or Fisher exact tests when the expected counts were <5) for binary variables and analysis of variance (ANOVA) for continuous variables. The distribution of the metal ions was not normal, and the group variances were highly heterogeneous; hence, nonparametric tests on medians were used to compare ion levels between groups. We used parametric and nonparametric test results to establish whether there was a significant difference in the mean and/or median effect on cardiac function between the groups. The distributions of the cardiac exposure variables in each group appeared normal for outcome variables of interest, with the variance usually constant (according to the Bartlett test); thus, ANOVA (F test) was conducted for each marker. As the sample sizes were relatively small, a formal test for normality was not conducted. However, sensitivity analyses with nonparametric tests (comparing the median across groups) were performed to relax the parametric assumption, and they always confirmed the conclusions obtained for the mean (results not shown). The presence of significant results at a 5% significance level was further assessed by dividing the threshold by the number of tests conducted (Bonferroni correction).
Correlations between whole-blood metal ion levels and ejection fraction and T2* values were calculated using the Pearson correlation coefficient (with metal ions log-transformed) and Spearman correlation coefficient (with metal ions on the original scale).
The minimum detectable effect size was calculated using Cohen’s d29.
All calculations were performed using Stata 14 (StataCorp).
Results
Patients
Ninety patients completed the study. Baseline patient characteristics are shown in Table I. Patients were matched for age and sex. Group A (CoC; n = 28) had a mean age (and SD) of 65.3 ± 8.8 years, and 75% of the patients were female. Group B (MoM with a low metal-ion level; n = 33) had a mean age of 61.9 ± 11.9 years, and 64% were female. Group C (MoM with a high metal-ion level; n = 29) had a mean age of 67.6 ± 10.8 years, and 62% were female. The mean cobalt levels in groups A, B, and C were 0.17 ± 0.08, 2.47 ± 1.81, and 30.0 ± 29.1 ppb, respectively.
TABLE I.
Patient Demographics by Study Group
| Demographic | All Patients | Group A | Group B | Group C | P Value |
| Sample (no.) | 90 | 28 | 33 | 29 | |
| Age* (yr) | 64.9 ± 10.5 | 65.3 ± 8.80 | 61.9 ± 11.9 | 67.6 ± 10.8 | 0.115 |
| Sex (F/M) (no.) | 60/30 | 21/7 | 21/12 | 18/11 | 0.525 |
| Time since implantation* (yr) | 8.76 ± 2.34 | 7.86 ± 2.62 | 9.03 ± 1.87 | 9.39 ± 2.52 | 0.041 |
| BMI† (kg/m2) | 28 | 28 (18-38) | 28 (20-45) | 27 (20-48) | 0.929 |
| Diabetes (no.; %) | 3; 3 | 1; 4 | 1; 3 | 1; 3 | 0.982 |
| Hypertension (no.; %) | 32; 36 | 11; 39 | 12; 36 | 9; 31 | 0.715 |
| Hypercholesterolemia (no.; %) | 20; 22 | 9; 32 | 4; 12 | 7; 24 | 0.105 |
| Medications (no.; %) | |||||
| β blocker | 5; 6 | 2; 7 | 0 | 3; 10 | 0.181 |
| ACE inhibiter‡ | 10; 11 | 5; 18 | 3; 9 | 2; 7 | 0.326 |
| Thiazide diuretic | 8; 9 | 2; 7 | 4; 12 | 2; 7 | 0.741 |
| Calcium channel blocker | 15; 17 | 7; 25 | 4; 12 | 4; 14 | 0.289 |
| Aspirin | 3; 3 | 1; 4 | 0 | 2; 7 | 0.317 |
| Statin | 20; 22 | 9; 32 | 4; 12 | 7; 24 | 0.121 |
| Proton pump inhibitor | 18; 20 | 8; 29 | 3; 9 | 7; 24 | 0.101 |
| Thyroxine | 8; 9 | 4; 14 | 4; 12 | 0 | 0.108 |
| Hemoglobin level† (g/dL) | 14 | 14.1 | 14.2 | 13.6 | 0.134 |
| Cobalt level† (ppb) | 10.9 | 0.17 (0.10-0.47) | 2.47 (0.73-6.97) | 30.01 (7.54-118) | <0.0001 |
| Chromium level† (ppb) | 7.7 | 0.74 (0.53-1.42) | 2.84 (0.94-10.5) | 19.6 (1.71-69.0) | <0.0001 |
The values are given as the mean and SD.
The values are given as the mean with or without the range in parentheses.
ACE = angiotensin-converting enzyme.
The overall mean time from implantation of the prosthesis to the date of the scan was slightly shorter in group A than in group B or C (7.9 ± 2.6 versus 9.0 ± 1.9 and 9.4 ± 2.5 years, F = 3.5, p = 0.041).
Although the groups were not matched for body mass index (BMI), cardiovascular risk factors, or medications, there were no significant differences between them with regard to those factors.
CMR
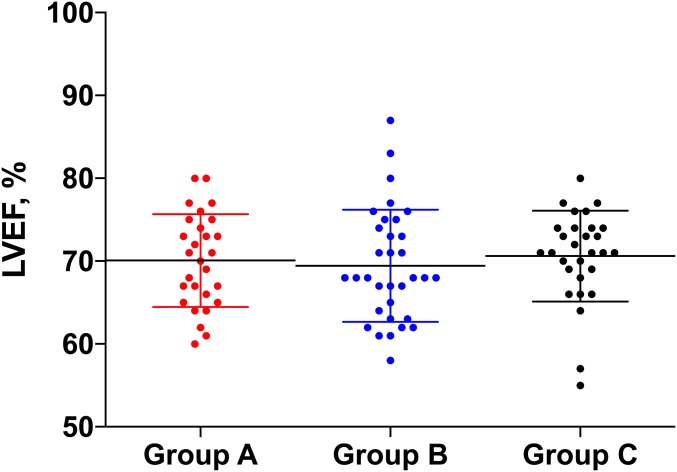
The 3 groups did not differ significantly with regard to the mean left ventricular ejection fraction (70.1% ± 6%, 69.5% ± 7%, and 70.6% ± 5%, p = 0.75; Table II and Fig. 4) or the left ventricular end systolic volume index (p = 0.86). Additionally, we found no differences in the left ventricular ejection fraction or end systolic volume index when we combined groups B and C and compared the combined group with group A (Table II).
Fig. 4.
Scatterplot demonstrating the mean left ventricular ejection fraction (LVEF), and the SD, for the 3 groups.
TABLE II.
CMR, EKG, and Blood Biomarker Results
| P Value |
|||||
| Measurement† | Group A (N = 28)* | Group B (N = 33)* | Group C (N = 29)* | A vs. B vs. C | A vs. B and C |
| CMR | |||||
| Left ventricular ejection fraction (%) | 70 ± 6 | 69 ± 7 | 71 ± 5 | 0.75 | 0.96 |
| End systolic volume index (mL/m2) | 21 ± 6 | 21 ± 8 | 20 ± 5 | 0.86 | 0.99 |
| T1 MOLLI: heart (NR = 949-1101 ms) (ms) | 1,030 ± 42 | 1,014 ± 33 | 1,022 ± 37 | 0.64 | 0.15 |
| T1 ShMOLLI: heart (NR = 900-1,020 ms) (ms) | 961 ± 31 | 957 ± 30 | 956 ± 44 | 0.82 | 0.54 |
| T2*: heart (normal = >20 ms) (ms) | 31 ± 5 | 31 ± 6 | 32 ± 6 | 0.69 | 0.85 |
| Extracellular volume: heart | 0.28 ± 0.03 | 0.27 ± 0.03 | 0.29 ± 0.04 | 0.28 | 0.62 |
| T1 mapping: liver (ms) | 616 ± 72 | 596 ± 78 | 584 ± 49 | 0.21 | 0.11 |
| T2* mapping: liver (normal = >6.3 ms) (ms) | 25 ± 4 | 26 ± 7 | 25 ± 5 | 0.67 | 0.92 |
| Extracellular volume: liver | 0.29 ± 0.04 | 0.30 ± 0.03 | 0.30 ± 0.04 | 0.62 | 0.36 |
| EKG | |||||
| Left ventricular end diastolic diameter (mm) | 43 ± 9 | 45 ± 5 | 45 ± 6 | 0.26 | 0.10 |
| Left ventricular end systolic diameter (mm) | 28 ± 6 | 30 ± 4 | 30 ± 5 | 0.18 | 0.06 |
| Left ventricular ejection fraction (%) | 65 ± 6 | 62 ± 7 | 63 ± 6 | 0.28 | 0.12 |
| Left ventricular lateral wall tissue Doppler imaging (m/s) | 0.09 ± 0.02 | 0.08 ± 0.02 | 0.09 ± 0.02 | 0.24 | 0.16 |
| Left ventricular septal wall tissue Doppler imaging (m/s) | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.08 ± 0.01 | 0.16 | 0.12 |
| Left atrial volume index (mL/m2) | 27 ± 6 | 28 ± 7 | 31 ± 8 | 0.19 | 0.20 |
| Blood biomarker sampling | |||||
| BNP (normal = <47 pmol/L) (pmol/L) | 11 ± 10 | 11 ± 8 | 25 ± 61 | 0.32 | 0.52 |
| Troponin (NR = 0-14 ng/L) (ng/L) | 7.38 ± 6.9 | 7.16 ± 5.0 | 8.62 ± 10.2 | 0.77 | 0.78 |
Data are presented as the mean and SD.
NR = normal range.
The mean cardiac T2* values were normal in the 3 groups and did not differ significantly among them (p = 0.69) or between the combined MoM group (B and C) and group A (p = 0.85). All patients with high blood metal levels (Group C) had normal T2* values (>20 ms). The T1 values measured with both the MOLLI and the ShMOLLI techniques were normal in the 3 groups and did not differ significantly among the groups (MOLLI: p = 0.64; ShMOLLI: p = 0.82). The extracellular volume was normal in, and did not differ significantly among, the 3 groups (p = 0.28), with no outliers.
The liver T2* values were normal in all patients. No differences were identified among the 3 groups (p = 0.67) or between the combined MoM group (B and C) and group A (p = 0.92). The liver T1 value and extracellular volume also did not differ significantly between or among groups (Table II).
Transthoracic EKG
There was no significant difference in the left ventricular ejection fraction among groups A, B, and C (65% ± 6%, 62% ± 7%, and 63% ± 6%, respectively; p = 0.28). Similarly, the left atrial volume index was similar among the 3 groups (p = 0.19) with no differences between the combined MoM group (B and C) and group A (p = 0.20). There were also no between-group differences in the mean lateral and septal S' velocities (tissue Doppler imaging) (p = 0.24 and 0.16, respectively).
Blood Biomarkers
There were no significant differences in the BNP level among groups A, B, and C (11, 11, and 25 pg/mL, respectively). Although the mean BNP level was highest in group C, the difference failed to reach significance (F = 1.17, p = 0.32). The troponin levels also did not differ significantly among the groups (p = 0.77).
EKG
All study participants had a resting 12-lead EKG. In 5 participants (4 in group B and 1 in group C), the EKG demonstrated left bundle branch block morphology (QRS duration of >120 ms). There were no ST or T-wave abnormalities, or changes reflecting previous cardiac events such as myocardial infarction.
Metal Ions and Cardiac Function: Dose Response
A dose-response linear correlation analysis was conducted using the Pearson coefficient to determine whether there was a relationship across groups between blood cobalt and chromium levels and left ventricular ejection fraction and T2* values. There was no significant linear correlation between the blood cobalt level (log scale) and the left ventricular ejection fraction (R = 0.022, 95% confidence interval [CI] = −0.185 to 0.229; p = 0.83) or the T2* value (R = 0.108, 95% CI = −0.105 to 0.312; p = 0.32). Similarly, no significant correlation was found between the blood chromium level and the left ventricular ejection fraction (R = 0.047, 95% CI = −0.162 to 0.251; p = 0.66) or the T2* value (R = 0.065, 95% CI = −0.148 to 0.272; p = 0.55) (Fig. 5).
Fig. 5.
Correlation plots with line of best fit. Whole-blood cobalt and chromium levels are compared with the left ventricular ejection fraction (LVEF; top row) and T2* values (bottom row). Pearson correlation coefficients (R values) are provided for each.
Whole-blood metal levels were non-normally distributed, unlike the left ventricular ejection fraction and the T2* value, and log-transforms attenuated without circumventing the lack of normality. Hence, to avoid an assumption of normality, the Spearman correlation coefficient was also calculated, and it showed no significant correlation between the cobalt level and the left ventricular ejection fraction (rho = 0.038, 95% CI = −0.170 to 0.243; p = 0.72), between the cobalt level and the T2* value (rho = 0.074, 95% CI = −0.139 to 0.280; p = 0.50), between the chromium level and the left ventricular ejection fraction (rho = 0.058, 95% CI = −0.151 to 0.262; p = 0.59), or between the chromium level and the T2* value (rho = 0.012, 95% CI = −0.200 to 0.222; p = 0.92).
Discussion
There is increasing concern regarding the effects of systemic cobalt and chromium toxicity in patients with MoM hip implants. Although the evidence for toxicity is limited, patient and surgeon anxiety is fueled by increasing numbers of case reports of end-organ damage and mortality10. In this study, we investigated the effects of metal ions on cardiac function in 3 distinct groups of patients defined by the type of hip implant and the level of circulating blood cobalt. Using 2 independent cardiac imaging techniques (including the gold standard, CMR), we failed to find a significant change in cardiac function in patients exposed to elevated metal ion levels. There was no significant correlation between elevated blood metal ion levels and cardiac function; even at the extremes of the 95% CI around the correlation coefficients, the correlations were negligible to weak.
Our confidence in these results is based on the strengths of our study methodology, which included the use of both CMR and EKG as well as a meticulous trial design. We ensured a prespecified recruitment number, data acquisition in a dedicated cardiac imaging center separate from the recruiting center, and a cardiac care team completely blinded to the study groups (during both acquisition and analysis) until the independent statistician returned the results.
Tissue-mapping techniques also failed to identify any demonstrable metal deposition from circulating cobalt ions in the cardiac or liver tissues. Although we recently demonstrated25 that T2* mapping can detect biopsy-proven CoCr, the lack of demonstrable tissue deposition in the present study may be due to differences in the physical chemistry of cobalt ions compared with that of cobalt and chromium in combination. However, the fact that iron can be detected in the heart and liver using T2* mapping, and we previously detected CoCr (colocalized) in the liver25, indicates that the reason why the T2* findings were negative in all cases in the present study was either that the test is too insensitive or no deposition was occurring. T2* should be considered a candidate biomarker, which, if it had differed between the groups, would have indicated the biological process of deposition. The lack of functional consequences (demonstrated by complementary and independent imaging modalities [CMR and EKG] and 2 blood biomarkers) and the lack of positive T2* findings, although not definitive, are reassuring.
A potential for cardiotoxicity from metal implants, as demonstrated by an inferior left ventricular ejection fraction, has been previously reported. However, these reports are limited to individual case reports10-18 and to studies20 with less rigorous methodology. For example, Prentice et al.20 used EKG, which has a higher interobserver variability than CMR, and that may account for the difference between their and our findings. We conclude that individual case reports of heart failure (mainly in patients with extreme ion levels) are not likely to be “the tip of the iceberg” or to indicate an unrecognized epidemic of occult MoM-related heart failure.
The pathophysiology behind a possible link between elevated blood cobalt levels and cardiac toxicity is unclear. Suggested theories include cobalt interference with cardiac myocyte oxygen uptake and transmembrane transport system disruption30. Histopathological findings in cobalt-related cardiac toxicity include myofibrillar hypertrophy, interstitial fibrosis, and muscle fiber degeneration31. However, calcified fibrils or other deposits within myofibrils are often absent, differentiating cobalt-induced cardiomyopathy from other etiologies13. It has been postulated that cobalt-related cardiac toxicity is due to additional predisposing factors, including poor nutrition and excessive alcohol intake as was seen in the Quebec beer-drinkers cobalt cardiomyopathy epidemic (in which foam stabilizer contained 10 times the usual quantity of cobalt)31. Recent case reports of cardiac toxicity in association with MoM hip implants mainly involved patients with extremely elevated blood cobalt levels (>100 ppb)10. Because of current national surveillance programs, such patients are now likely to have undergone revision surgery, with presumed reduction in their risk of systemic toxicity.
We believe that these findings offer reassurance to surgeons and will help them counsel the >1 million patients worldwide with MoM hip implants. Additional work is needed, particularly from large-volume linkage studies currently under way internationally.
Investigation performed at the Royal National Orthopaedic Hospital, Stanmore, London, United Kingdom, and the Institute of Cardiovascular Science, University College London, London, United Kingdom
Disclosure: This study was sponsored by the Gwen Fish Orthopaedic Charitable Trust, which played no role in the study design, patient recruitment, collection or analysis of data, writing the report, or the decision to submit the paper for publication. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJS/E371).
Reshid Berber, MRCS, and Amna Abdel-Gadir, MRCP, contributed equally to the writing of this article.
References
- 1.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009. July;91(7):1614-20. [DOI] [PubMed] [Google Scholar]
- 2.Hart AJ, Sabah S, Henckel J, Lewis A, Cobb J, Sampson B, Mitchell A, Skinner JA. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br. 2009. June;91(6):738-44. [DOI] [PubMed] [Google Scholar]
- 3.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008. July;90(7):847-51. [DOI] [PubMed] [Google Scholar]
- 4.Schaffer AW, Pilger A, Engelhardt C, Zweymueller K, Ruediger HW. Increased blood cobalt and chromium after total hip replacement. J Toxicol Clin Toxicol. 1999;37(7):839-44. [DOI] [PubMed] [Google Scholar]
- 5.Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, Solomon L. Widespread dissemination of metal debris from implants. J Bone Joint Surg Br. 1994. September;76(5):701-12. [PubMed] [Google Scholar]
- 6.Medicines & Healthcare products Regulatory Agency. All metal-on-metal (MoM) hip replacements. https://www.gov.uk/drug-device-alerts?keywords=metal-on-metal+hip+replacements&issued_date%5Bfrom%5D=&issued_date%5Bto%5D=. Accessed 2017 May 10.
- 7.Food and Drug Administration. USA - metal-on-metal hip implants: recalls. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm. Accessed 2017 May 10.
- 8.Scientific Committee on Emerging and Newly Identified Health Risks. The safety of metal-on-metal joint replacements with a particular focus on hip implants. 2014. http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_042.pdf. Accessed 2017 May 10.
- 9.Food and Drug Administration. USA - metal-on-metal hip implants: advice for surgeons. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241667.htm. Accessed 2017 May 10.
- 10.Bradberry SM, Wilkinson JM, Ferner RE. Systemic toxicity related to metal hip prostheses. Clin Toxicol (Phila). 2014. Sep-Oct;52(8):837-47. Epub 2014 Aug 16. [DOI] [PubMed] [Google Scholar]
- 11.Machado C, Appelbe A, Wood R. Arthroprosthetic cobaltism and cardiomyopathy. Heart Lung Circ. 2012. November;21(11):759-60. Epub 2012 Apr 18. [DOI] [PubMed] [Google Scholar]
- 12.Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013. January;95-B(1):31-7. [DOI] [PubMed] [Google Scholar]
- 13.Cheung AC, Banerjee S, Cherian JJ, Wong F, Butany J, Gilbert C, Overgaard C, Syed K, Zywiel MG, Jacobs JJ, Mont MA. Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition Part 1 - history, mechanism, measurements, and pathophysiology. Bone Joint J. 2016. January;98-B(1):6-13. [DOI] [PubMed] [Google Scholar]
- 14.Samar HY, Doyle M, Williams RB, Yamrozik JA, Bunker M, Biederman RW, Shah MB. Novel use of cardiac magnetic resonance imaging for the diagnosis of cobalt cardiomyopathy. JACC Cardiovasc Imaging. 2015. October;8(10):1231-2. Epub 2015 Mar 18. [DOI] [PubMed] [Google Scholar]
- 15.Zywiel MG, Cherian JJ, Banerjee S, Cheung AC, Wong F, Butany J, Gilbert C, Overgaard C, Syed K, Jacobs JJ, Mont MA. Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition Part 2. measurement, risk factors, and step-wise approach to treatment. Bone Joint J. 2016. January;98-B(1):14-20. [DOI] [PubMed] [Google Scholar]
- 16.Khan AH, Verma R, Bajpai A, Mackey-Bojack S. Unusual case of congestive heart failure: cardiac magnetic resonance imaging and histopathologic findings in cobalt cardiomyopathy. Circ Cardiovasc Imaging. 2015. June;8(6):e003352. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert CJ, Cheung A, Butany J, Zywiel MG, Syed K, McDonald M, Wong F, Overgaard C. Hip pain and heart failure: the missing link. Can J Cardiol. 2013. May;29(5):639.e1-2. Epub 2013 Jan 9. [DOI] [PubMed] [Google Scholar]
- 18.Rizzetti MC, Liberini P, Zarattini G, Catalani S, Pazzaglia U, Apostoli P, Padovani A. Loss of sight and sound. Could it be the hip? Lancet. 2009. March 21;373(9668):1052. [DOI] [PubMed] [Google Scholar]
- 19.NICOR. National heart failure audit 2016. https://www.ucl.ac.uk/nicor/audits/heartfailure. Accessed 2017 May 10.
- 20.Prentice JR, Clark MJ, Hoggard N, Morton AC, Tooth C, Paley MN, Stockley I, Hadjivassiliou M, Wilkinson JM. Metal-on-metal hip prostheses and systemic health: a cross-sectional association study 8 years after implantation. PLoS One. 2013. June 10;8(6):e66186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillam MH, Pratt NL, Inacio MC, Roughead EE, Shakib S, Nicholls SJ, Graves SE. Heart failure after conventional metal-on-metal hip replacements. Acta Orthop. 2017. February;88(1):2-9. Epub 2016 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Australian Government Department of Health, Therapeutic Goods Administration. Alert - TGA advice regarding potential association with heart failure. 2016. https://www.tga.gov.au/alert/asr-xl-total-hip-replacements. Accessed 2017 May 10.
- 23.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001. December;22(23):2171-9. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, Sheppard MN, Porter JB, Walker JM, Wood JC, Galanello R, Forni G, Catani G, Matta G, Fucharoen S, Fleming A, House MJ, Black G, Firmin DN, St Pierre TG, Pennell DJ. On T2* magnetic resonance and cardiac iron. Circulation. 2011. April 12;123(14):1519-28. Epub 2011 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel-Gadir A, Berber R, Porter JB, Quinn PD, Suri D, Kellman P, Hart AJ, Moon JC, Manisty C, Skinner JA. Detection of metallic cobalt and chromium liver deposition following failed hip replacement using T2* and R2 magnetic resonance. J Cardiovasc Magn Reson. 2016. May 6;18(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wharton G, Steeds R, Allen J, Phillips H, Jones R, Kanagala P, Lloyd G, Masani N, Mathew T, Oxborough D, Rana B, Sandoval J, Wheeler R, O’Gallagher K, Sharma V. A minimum dataset for a standard adult transthoracic echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2015. March 1;2(1):G9-24. Epub 2015 Feb 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington CF, Taylor A. Metal-on-metal hip implants. UK quality assurance of blood cobalt and chromium after hip implants. BMJ. 2012. June 12;344:e4017. [DOI] [PubMed] [Google Scholar]
- 28.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012. September 10;14:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Routledge; 1988. [Google Scholar]
- 30.Barceloux DG. Cobalt. J Toxicol Clin Toxicol. 1999;37(2):201-6. [DOI] [PubMed] [Google Scholar]
- 31.Alexander CS. Cobalt-beer cardiomyopathy. A clinical and pathologic study of twenty-eight cases. Am J Med. 1972. October;53(4):395-417. [DOI] [PubMed] [Google Scholar]