Abstract
Background:
Spinal deformities are common in patients with osteogenesis imperfecta, a heritable disorder that causes bone fragility. The purpose of this study was to describe the behavior of spinal curvature during growth in patients with osteogenesis imperfecta and establish its relationship to disease severity and medical treatment with bisphosphonates.
Methods:
The medical records and radiographs of 316 patients with osteogenesis imperfecta were retrospectively reviewed. The severity of osteogenesis imperfecta was classified with the modified Sillence classification. Serial curve measurements were recorded throughout the follow-up period for each patient with scoliosis. Regression analysis was used to determine the effect of disease severity (Sillence type), patient age, and bisphosphonate treatment on the progression of scoliosis as measured with the Cobb method.
Results:
Of the 316 patients with osteogenesis imperfecta, 157 had associated scoliosis, a prevalence of 50%. Scoliosis prevalence (68%) and mean progression rate (6° per year) were the highest in the group of patients with the most severe osteogenesis imperfecta (modified Sillence type III). A group with intermediate osteogenesis imperfecta severity, modified Sillence type IV, demonstrated intermediate scoliosis values (54%, 4° per year). The patient group with the mildest form of osteogenesis imperfecta, modified Sillence type I, had the lowest scoliosis prevalence (39%) and rate of progression (1° per year). Early treatment—before the patient reached the age of six years—of type-III osteogenesis imperfecta with bisphosphonate therapy decreased the curve progression rate by 3.8° per year, which was a significant decrease. Bisphosphonate treatment had no demonstrated beneficial effect on curve behavior in patients with other types of osteogenesis imperfecta or in patients of older age.
Conclusions:
The prevalence of scoliosis in association with osteogenesis imperfecta is high. Progression rates of scoliosis in children with osteogenesis imperfecta are variable, depending on the Sillence type of osteogenesis imperfecta. High rates of scoliosis progression in type-III and type-IV osteogenesis imperfecta contrast with a benign course in type I. Bisphosphonate therapy initiated before the patient reaches the age of six years can modulate curve progression in type-III osteogenesis imperfecta.
Level of Evidence:
Prognostic Level III. See Instructions for Authors for a complete description of levels of evidence.
Peer Review:
This article was reviewed by the Editor-in-Chief and one Deputy Editor, and it underwent blinded review by two or more outside experts. The Deputy Editor reviewed each revision of the article, and it underwent a final review by the Editor-in-Chief prior to publication. Final corrections and clarifications occurred during one or more exchanges between the author(s) and copyeditors.
Osteogenesis imperfecta is a heritable disorder of type-I-collagen synthesis or modification, with a broad range of causative mutations and clinical severity. Individuals with osteogenesis imperfecta present with varying degrees of bone fragility, ligamentous laxity, short stature, and deformities of the extremities. The diagnosis of osteogenesis imperfecta is clinically based. The use of skin biopsy and DNA sequencing can supplement the clinically based classification but is not needed for diagnosis confirmation. The modified Sillence classification is the most commonly used system to identify subtypes of severity1. More than 90% of individuals with osteogenesis imperfecta demonstrate a dominant mutation in the COL1A1 or COL1A2 genes and are classified as having mild (type-I), intermediate (type-IV), or severe (type-III) disease. Rare types of both recessive and dominantly inherited osteogenesis imperfecta caused by mutations in at least eight different genes have been identified with use of recent genetic advances. Spinal deformities, including scoliosis and kyphosis in the thoracolumbar spine, spondylolisthesis in the lumbosacral spine, and basilar invagination at the craniocervical junction2, are frequently seen in patients with osteogenesis imperfecta.
The reported prevalence of scoliosis in patients with osteogenesis imperfecta is high, ranging between 39% and 88%3-7. Factors contributing to the development of scoliosis include ligamentous laxity, muscle weakness, and vertebral fracture and deformity8. Whereas it has been reported that individuals with more severe osteogenesis imperfecta have a higher prevalence and severity of scoliosis9, little is known about the behavior of scoliosis during growth in this population. To our knowledge, there are only incidental reports describing continued progression of spinal deformities into adulthood7,10.
The response of scoliotic curves to medical treatment in patients with osteogenesis imperfecta is also unknown. Cyclical intravenous bisphosphonate treatment can increase vertebral bone mineral density and improve vertebral height in patients with osteogenesis imperfecta11,12. Vertebral fractures are thought to be a major cause of scoliosis in patients with osteogenesis imperfecta, as a result of vertebral deformity and injury to the vertebral growth plates13. Treatment with bisphosphonates potentially could be beneficial, especially during the first six years of life—when the majority of vertebral development takes place14.
The purpose of our study was to define the behavior of scoliosis during growth in individuals with osteogenesis imperfecta. The first hypothesis was that prevalence, age at diagnosis of the scoliosis, and curve progression would differ significantly among different Sillence types of osteogenesis imperfecta. The second hypothesis was that early administration of bisphosphonate therapy would reduce the rate of curve progression.
Materials and Methods
Participants and Sillence Types
This retrospective, longitudinal review study was approved by the Rush University Medical Center institutional review board and was conducted in a manner that conformed to the approved protocol. The medical records of all 316 patients diagnosed with osteogenesis imperfecta at our institution between 1982 and 2012 were reviewed.
Using the modified Sillence classification, the clinic director (P.A.S.) assigned an osteogenesis imperfecta type based on features documented during the clinical encounter and a review of patient records, including radiographs, photographs, and laboratory results. The diagnosis of osteogenesis imperfecta was made clinically but, in the majority of cases, was confirmed with genetic testing. Blood or skin analysis was used when possible to supplement the assignment of an osteogenesis imperfecta type, either by comparing the identified mutation with an international database or by performing qualitative or quantitative collagen analysis.
Scoliosis was defined as a Cobb angle of >10°. All radiographs were made with the patient in the standing position when possible; if this was not possible, the patients were in the sitting position when the radiographs were made. Of the 316 participants, 157 were identified from their medical record as having scoliosis, which was confirmed by a review of standard anteroposterior radiographs. The magnitude of the deformity, curve pattern (single, double, or triple curve), and direction (left or right convexity) were measured and recorded. In the case of a double or triple curve, the major curve was used for analysis.
Eighty-seven of the 157 patients with scoliosis had confirmatory genetic testing of the osteogenesis imperfecta (fifty-three had blood tests and thirty-four, skin biopsies). Eight had rare types of osteogenesis imperfecta (six type V, one type II, and one type VIII) and were included in the demographic data but not in the analysis of progression rates.
Twenty of the 157 patients with scoliosis and osteogenesis imperfecta were treated at some point and for varying lengths of time with a spinal orthosis, either for improvement of sitting posture or management of back pain. However, we had no accurate method of determining the duration or effectiveness of treatment of the scoliosis or patient compliance with use of the orthosis and were unable to analyze the effect of bracing.
Of the 149 patients with type-I, type-III, or type-IV osteogenesis imperfecta, thirty-nine had only one radiograph and recording of curve magnitude. For evaluation of the rate of progression of scoliosis, we used the data from 110 children for whom multiple Cobb angle measurements had been performed over time. We excluded eight children from the initial group when analyzing the effect of bisphosphonates on the scoliosis progression rate because of a lack of a complete medication history. This left 102 patients with a total of 546 visits (two to fourteen visits per person) and 444 time periods analyzed.
Individuals with scoliosis were separated into two groups: those who received bisphosphonate treatment and those who did not. Sixty patients received intravenous or oral bisphosphonate therapy. To determine whether early administration of bisphosphonate therapy had an effect on curve progression, the treatment group was split into two subgroups: patients who started bisphosphonate therapy before the age of six years and those who started it later.
Rate of Curve Progression
To determine the rate of curve progression (ri, j), Cobb angles (Y) were longitudinally obtained for each participant (i). A change in Cobb angle was calculated by subtracting the measurement at a previous visit (j − 1) from the angle at the current visit (j). The change in the Cobb angle was then divided by the number obtained from subtracting the age at the previous visit (j − 1) from the participant’s age (A) at the current visit (j).
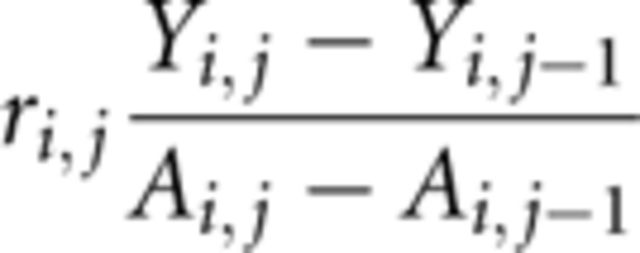 |
Participants were included in the study of curve progression rate for the duration of their care at the study institution or—for the twenty-two patients who ultimately underwent posterior spinal instrumentation and fusion for scoliosis—until spinal surgery was performed. The criteria for surgery were progressive curves in patients with active or impending functional problems (problems sitting, back pain), patient health adequate to tolerate surgery, and sufficient bone quality to support instrumentation.
To determine the effect of age at diagnosis of the scoliosis on the rate of curve progression, participants were assigned to an age group at each clinical encounter. In accordance with the description of spine growth by Dimeglio et al.14, participants were placed into one of four age groups at each of the encounters: (1) birth to five years old, (2) six to ten years old, (3) eleven to fifteen years old, and (4) sixteen years old and older.
Data Analysis
The frequencies of curve type (left or right convexity; single, double, or triple curves) and the number of participants who ultimately underwent spinal surgery were compared among the osteogenesis imperfecta types with use of chi-square analysis.
Linear regression was used in two separate investigations to model the curve progression rate over time. In the first investigation, we studied the significance of differences in Cobb angle progression rates among different combinations of age groups and osteogenesis imperfecta types. In the second, we analyzed the effects of osteogenesis imperfecta type, age at diagnosis of the scoliosis, age group, sex, and initiation of bisphosphonate therapy before or after the patient reached the age of six years as predictors of curve progression rate.
Additionally, all associated interaction effects, including osteogenesis imperfecta type with age at initiation of bisphosphonate therapy (before or after the age of six years), were included in the analysis. To account for possible correlation of within-subject measurements, we used the generalized estimating equations with the identity link function and an exchangeable working correlation matrix. The generalized estimating equations modeling corresponds to the weighted least-squares analysis with an assumed compound symmetry structure of the correlation matrix. The sandwich estimator was used to obtain consistent estimates of standard errors. A probability value of p < 0.05 was chosen to indicate a significant difference (see Appendix).
Source of Funding
Support was received under Grant UL1RR031973 from the Clinical and Translational Science Award program of the National Center for Research Resources and the National Center for Advancing Translational Sciences. The contents of the current study are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Results
Demographic Data
Of the 316 children with osteogenesis imperfecta who were studied, 157 had scoliosis, a prevalence of 50%. There were seventy-four male and eighty-three female patients, with a mean age at curve identification of seven years (range, birth to 18.8 years). The mean duration of follow-up was eight years (range, one to 20.5 years) (see Appendix). Sixty-two patients were classified as having modified Sillence type-I osteogenesis imperfecta; fifty-five, type III; thirty-two, type IV; and eight, other types (Table I). The scoliosis prevalence was 68% for patients with type III, which was significantly higher than that for the patients with type IV (54%, p < 0.01) or type I (39%, p < 0.01).
TABLE I.
Prevalence of Scoliosis in Osteogenesis Imperfecta by Modified Sillence Type
| Osteogenesis Imperfecta Type | No. of Patients | Prevalence of Scoliosis (No. [%]) |
| I | 159 | 62 (39) |
| III | 81 | 55 (68) |
| IV | 59 | 32 (54) |
| Other types (II, V, VIII) | 17 | 8 (47) |
| Overall | 316 | 157 (50) |
Curve characteristics across the entire sample showed no difference in direction, with right convex curves found in seventy-three (49%) of the 149 patients for whom data were available. Curve location was significant, however, with thoracic curves identified as the major curve in 124 (83%) (p < 0.01) of the 149 patients for whom data were available. When stratified by osteogenesis imperfecta type, left convex curves were more frequent, but not significantly so, in patients with type IV (63%, p = 0.25). One hundred and eighteen patients (79%) had a single curve. Single thoracic curves were significantly more common in patients with type-I osteogenesis imperfecta (97%) compared with those with type III (58%, p < 0.01) or type IV (81%, p < 0.01). In contrast, double curves were more common in type III (36%) than in type I (3%, p < 0.01) or type IV (19%, p < 0.01). Three patients (5%) with type III had a triple curve (Table II).
TABLE II.
Curve Characteristics Among the Osteogenesis Imperfecta Types
| No. (%) of Patients |
||||
| Curve Characteristic | Type I (N = 62) | Type III (N = 55) | Type IV (N = 32) | All (N = 149) |
| Convexity to right | 31 (50) | 30 (55) | 12 (38) | 73 (49) |
| Single curve | 60 (97) | 32 (58) | 26 (81) | 118 (79) |
| Double curve | 2 (3) | 20 (36) | 6 (19) | 28 (19) |
| Triple curve | 0 (0) | 3 (5) | 0 (0) | 3 (2) |
| Spine fusion and instrumentation | 1 (2) | 13 (24) | 8 (25) | 22 (15) |
Among the 102 patients with multiple Cobb angle measurements and a complete medication history, fifty-two (twenty with type-I, seventeen with type-III, and fifteen with type-IV osteogenesis imperfecta) had received bisphosphonate therapy at some time during the study period (see Appendix). Among the seventeen patients with type-III osteogenesis imperfecta, sixteen received cyclic infusions of pamidronate and one received oral bisphosphonate therapy (see Appendix).
Twenty-two patients (15%) underwent spinal instrumentation and fusion during the study period. Four patients had combined anterior and posterior procedures while eighteen had spinal fusions with posterior instrumentation of varying lengths. Significantly more patients with type-III (thirteen of fifty-five; 24%) (p < 0.01) or type-IV osteogenesis imperfecta (eight of thirty-two; 25%) (p < 0.01) had spinal fusion compared with one (2%) of sixty-two patients with type I.
Outcome Data
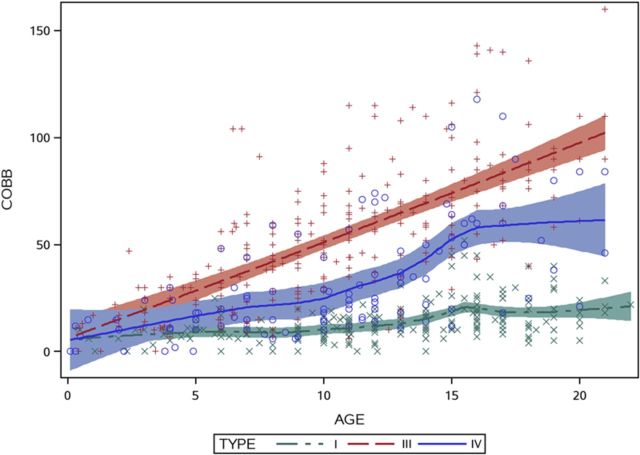
In order to provide a graphical representation of the data, Cobb angles for each radiographic measurement were plotted as a function of age for each of the three major modified Sillence types of osteogenesis imperfecta. A 95% confidence interval was used to display the best fit without assuming that the data were linear or parametric. The graph demonstrated different behavior over time among the three most common types of osteogenesis imperfecta (Fig. 1).
Fig. 1.
Graphic representation of patient age versus Cobb angle measurement for patients with osteogenesis imperfecta type I (green), type III (red), and type IV (blue).
The overall progression rate of scoliosis by type is displayed in Table III. Curve progression was 6° per year in patients with type-III osteogenesis imperfecta, 4° per year in those with type IV, and 1° per year in those with type I. The differences among types were significant, as shown by the generalized linear mixed-model analysis (p < 0.01).
TABLE III.
Progression Rate of Scoliosis for Each of Three Types of Osteogenesis Imperfecta
| Osteogenesis Imperfecta Type | Progression Rate (deg/yr) | 95% Confidence Interval* | P Value† |
| I | 1 | 0.35-2.00 | 0.01 |
| III | 6 | 4.29-7.18 | <0.01 |
| IV | 4 | 2.28-6.43 | <0.01 |
The 95% confidence intervals indicate that the differences are significant among osteogenesis imperfecta types.
The p values indicate a significant difference compared with no curve progression.
The effect of age group for each osteogenesis imperfecta type on Cobb angle progression rates is presented in the Appendix. Patients with type-III osteogenesis imperfecta, in all age groups, showed significant progression, with the greatest being 8° per year between the ages of eleven and fifteen years (see Appendix). Patients with type IV demonstrated significant progression rates between six and ten years of age (p < 0.01) and between eleven and fifteen years of age (p = 0.01), and patients with type I showed significant progression rates between eleven and fifteen years of age (p < 0.01).
The generalized estimating equations model was used to determine the effect of bisphosphonate therapy on progression rates. Specifically, the strongest effect of bisphosphonate treatment was on patients with type-III osteogenesis imperfecta who had started treatment before six years of age (Table IV). The expected progression rate was 0.8° per year for a patient with type-I osteogenesis imperfecta who did not have bisphosphonate treatment. A patient with type-III osteogenesis imperfecta who did not receive bisphosphonates was expected to have a progression rate of 6.1° per year. The expected progression rate was estimated to be significantly lower (2.3° per year) if a patient with type-III osteogenesis imperfecta received bisphosphonate therapy before six years of age (p = 0.005). This is the only posttest that showed a significant effect. The effect of bisphosphonate treatment before the age of six years was not significant in patients with osteogenesis imperfecta type I or IV, and bisphosphonate therapy initiated after the age of six years had no significant effect in patients with any type of osteogenesis imperfecta.
TABLE IV.
Effect of Bisphosphonate Therapy on Progression Rate for Each Osteogenesis Imperfecta Type
| Osteogenesis Imperfecta Type | Age at Initiation of Treatment (yr) | Progression Rate (deg/yr, rounded) | 95% Confidence Interval | P Value of Treatment Effect |
| I | Never | 0.8 | −0.63-2.29 | |
| I | <6 | 2.3 | 0.68-3.81 | 0.19 |
| I | ≥6 | 1.7 | 0.70-2.83 | 0.33 |
| III | Never | 6.1 | 4.76-7.39 | |
| III | <6 | 2.3 | −1.49-5.96 | 0.01 |
| III | ≥6 | 7.5 | 1.96-8.64 | 0.46 |
| IV | Never | 3.5 | −2.43-9.45 | |
| IV | <6 | 3.1 | 0.96-5.24 | 0.91 |
| IV | ≥6 | 5.9 | 2.86-9.22 | 0.50 |
Discussion
Spinal deformities are frequently seen in patients with osteogenesis imperfecta, but little is known about their natural history. In this study, we reviewed the behavior of scoliosis during growth in a large population of children with osteogenesis imperfecta and demonstrated that curve prevalence and progression rates differ significantly among the three most common types of osteogenesis imperfecta and are higher in the more severe types. We showed that children with severe osteogenesis imperfecta had reduced scoliosis progression rates when they received bisphosphonates prior to the age of six years.
Our results corroborate previously reported findings of a higher prevalence of spinal deformities in patients with more severe types of osteogenesis imperfecta. Beighton et al. reported a 9% prevalence of mild scoliosis in patients with type-I osteogenesis imperfecta, whereas all of their twenty-one patients who had type-III osteogenesis imperfecta had a spinal deformity15. Hanscom et al. proposed a radiographically based classification system for osteogenesis imperfecta and correlated higher disease severity with increased curve severity16. Although there is agreement about the prevalence of scoliosis in patients with osteogenesis imperfecta, to our knowledge no one has previously analyzed the rate of scoliosis progression during periods of growth in children with osteogenesis imperfecta or compared the rates among the modified Sillence types.
Individuals with the most common form of osteogenesis imperfecta, modified Sillence type I, have normal to moderately reduced stature, blue sclerae, low bone density, and varying rates of fractures but are generally able to walk independently (see Appendix). Although the patients with this type in our study had a high prevalence of scoliosis (39%), the natural history of the scoliosis over time was benign. We found a low progression rate of 1° per year overall in patients with type I, and the only rate that differed significantly from this 1° rate was the 0° rate during adolescence (age eleven to fifteen years) in this group. The benign progression of scoliosis in this population is better than the rates in the patients with the other, more severe types of osteogenesis imperfecta. In those groups, the scoliosis progressed during growth, starting from birth to five years of age in children with type-III osteogenesis imperfecta and from six to ten years of age in children with type IV. Only six (13%) of the forty-seven patients with type-I osteogenesis imperfecta had a curve that progressed to >30°, and only one patient (2%) had a curve that progressed beyond 50°.
Type-III osteogenesis imperfecta is a severe form of the disorder and presents with long-bone bowing, markedly short stature, and frequent fractures (see Appendix). Most individuals with this type of osteogenesis imperfecta require assistive devices to walk. The 68% prevalence of scoliosis in patients with type-III osteogenesis imperfecta in the present study corroborates the 72% prevalence reported by Engelbert et al. in a study of ninety-six children8. Overall, the progression rate was 6° per year in our study. Scoliosis in type-III osteogenesis imperfecta follows a severely progressive course with early onset and insidious progression.
Type-IV osteogenesis imperfecta is a moderately severe form, with patients demonstrating white sclerae and reduced stature and often relying on an assistive device to walk independently (see Appendix). There is a large heterogeneity of genotypes and phenotypes included within the type-IV group. The 54% prevalence of scoliosis in type-IV osteogenesis imperfecta in this series is similar to the 61% prevalence reported by Engelbert et al.8. Eight (24%) of their thirty-three patients developed a curve of >50°. Patients with type-IV osteogenesis imperfecta have more progressive curves than those with type I but less progressive curves than those with type III.
The results of our study showed that bisphosphonate therapy decreases the rate of progression of scoliosis in type-III osteogenesis imperfecta if started before the patient reaches the age of six years. The benefits of bisphosphonate therapy may be due to improved vertebral bone quality because vertebral fragility fractures play a role in scoliosis development in osteogenesis imperfecta13. The use of cyclical intravenous pamidronate increases vertebral bone mineral density and improves vertebral height11,12,17. Semler et al. demonstrated a significant increase in vertebral volume and height after one year of treatment with pamidronate or neridronate in twenty-eight patients with type-III or type-IV osteogenesis imperfecta18. In a prospective study by Aström et al., eleven infants with severe osteogenesis imperfecta received pamidronate therapy19. After three to six years of treatment, vertebral bone density and remodeling of previously compressed vertebrae resulting in increased height, compared with that in a historical control group, were demonstrated. No child developed scoliosis or kyphosis.
Bisphosphonate therapy that was initiated after the patient reached six years of age did not appear to have any effect on scoliosis progression. Many children with severe types of osteogenesis imperfecta already demonstrated large curves at that age. Undoubtedly, medical treatment at this age has less impact on vertebral growth than if it had been initiated earlier. However, late initiation of bisphosphonate therapy may have other beneficial effects, such as minimizing vertebral compression fractures and preserving vertebral morphology. Although vertebral fragility fractures are a major factor in the development of scoliosis, other factors unaffected by bisphosphonates, such as ligamentous laxity and muscular weakness, may still contribute to spinal deformity. We cannot say with certainty that bisphosphonates change the prevalence of scoliosis in patients with osteogenesis imperfecta.
A number of factors limit this investigation. Scoliosis was detected by physical examination, so some curves may have been missed, and the true prevalence of spinal curves may be higher. We did not study sagittal plane spine deformity, which is commonly associated with scoliosis in osteogenesis imperfecta, so we do not know whether our findings apply to those deformities as well. Many patients presented with an existing curvature. Therefore, the date of onset of scoliosis is undoubtedly younger than the age at which it was detected, which may explain why we failed to demonstrate a significant difference in patient age at scoliosis diagnosis among the types of osteogenesis imperfecta. The marked variability of osteogenesis imperfecta poses a challenge with regard to presenting generalized findings, and it was not possible to include rarer types of osteogenesis imperfecta in our analysis of curve progression. We chose to analyze patients with type-IV and type-III osteogenesis imperfecta separately because they could be distinguished from one another clinically, mostly on the basis of physical function and stature. However, there is undoubtedly overlap, with a spectrum of genotypes and phenotypes within each of the modified Sillence types.
We did not take into consideration whether the participants had used a spinal orthosis. Twenty of the participants included in the current study wore a brace at some point during care. Historically, studies have shown that bracing is ineffective and may cause rib deformity in patients with osteogenesis imperfecta3,10,20,21, but with the advent of bisphosphonate therapy, bracing may be a viable option. In conclusion, children with osteogenesis imperfecta have a higher prevalence of scoliosis than the 0.47% to 5.2% prevalence of idiopathic deformity in the current literature22. The modified Sillence type of osteogenesis imperfecta is associated with the prevalence and progression rate of the scoliosis, with patients with type I having minimal progression and those with type III developing rapidly progressive curves. In type-III osteogenesis imperfecta, initiation of bisphosphonate therapy before the patient reaches the age of six years appears to slow the progression of deformity.
Appendix
Figures showing radiographs of patients with osteogenesis imperfecta as well as tables presenting demographic data, and information on bisphosphonate treatment among the osteogenesis imperfecta types, Cobb angle progression rates for different age and osteogenesis imperfecta groups, and regression coefficients for model (1) are available with the online version of this article as a data supplement at jbjs.org.
Acknowledgments
Note: The authors thank Jessica Day, BS, for her retrospective chart review and data collection.
Investigation performed at Shriners Hospital, Chicago, Illinois
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. None of the authors, or their institution(s), have had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, no author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Sillence DO Senn A Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammerberg K Anissipour A Sugrue P. Spinal deformities in osteogenesis imperfecta. In: Smith P. Osteogenesis imperfecta. (Vol. 1). Chicago, IL: Shriners Press; 2012. [Google Scholar]
- 3.Benson DR Newman DC. The spine and surgical treatment in osteogenesis imperfecta. Clin Orthop Relat Res. 1981 Sep;(159):147-53. [PubMed] [Google Scholar]
- 4.Falvo KA Root L Bullough PG. Osteogenesis imperfecta: clinical evaluation and management. J Bone Joint Surg Am. 1974 Jun;56(4):783-93. [PubMed] [Google Scholar]
- 5.Moorefield WG Jr Miller GR. Aftermath of osteogenesis imperfecta: the disease in adulthood. J Bone Joint Surg Am. 1980 Jan;62(1):113-9. [PubMed] [Google Scholar]
- 6.Ishikawa S Kumar SJ Takahashi HE Homma M. Vertebral body shape as a predictor of spinal deformity in osteogenesis imperfecta. J Bone Joint Surg Am. 1996 Feb;78(2):212-9. [DOI] [PubMed] [Google Scholar]
- 7.Renshaw TS Cook RS Albright JA. Scoliosis in osteogenesis imperfecta. Clin Orthop Relat Res. 1979 Nov-Dec;(145):163-7. [PubMed] [Google Scholar]
- 8.Engelbert RH Uiterwaal CS van der Hulst A Witjes B Helders PJ Pruijs HE. Scoliosis in children with osteogenesis imperfecta: influence of severity of disease and age of reaching motor milestones. Eur Spine J 2003 Apr;12(2):130-4. Epub 2002 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelbert RH Gerver WJ Breslau-Siderius LJ van der Graaf Y Pruijs HE van Doorne JM Beemer FA Helders PJ. Spinal complications in osteogenesis imperfecta: 47 patients 1-16 years of age. Acta Orthop Scand. 1998 Jun;69(3):283-6. [DOI] [PubMed] [Google Scholar]
- 10.Cristofaro RL Hoek KJ Bonnett CA Brown JC. Operative treatment of spine deformity in osteogenesis imperfecta. Clin Orthop Relat Res. 1979 Mar-Apr;(139):40-8. [PubMed] [Google Scholar]
- 11.Land C Rauch F Munns CF Sahebjam S Glorieux FH. Vertebral morphometry in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate treatment. Bone. 2006 Oct;39(4):901-6. Epub 2006 May 26. [DOI] [PubMed] [Google Scholar]
- 12.Letocha AD Cintas HL Troendle JF Reynolds JC Cann CE Chernoff EJ Hill SC Gerber LH Marini JC Marini JC. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res. 2005 Jun;20(6):977-86. Epub 2005 Jan 18. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe G Kawaguchi S Matsuyama T Yamashita T. Correlation of scoliotic curvature with Z-score bone mineral density and body mass index in patients with osteogenesis imperfecta. Spine (Phila Pa 1976). 2007 Aug 1;32(17):E488-94. [DOI] [PubMed] [Google Scholar]
- 14.Dimeglio A Bonnel F Canavese F. Normal growth of the spine and thorax. In: Akbarnia BA Yazici M Thompson GH. The growing spine: Management of spinal disorders in young children. Heidelberg: Springer; 2011. [Google Scholar]
- 15.Beighton P Spranger J Versveld G. Skeletal complications in osteogenesis imperfecta. A review of 153 South African patients. S Afr Med J. 1983 Oct 1;64(15):565-8. [PubMed] [Google Scholar]
- 16.Hanscom DA Winter RB Lutter L Lonstein JE Bloom BA Bradford DS. Osteogenesis imperfecta. Radiographic classification, natural history, and treatment of spinal deformities. J Bone Joint Surg Am. 1992 Apr;74(4):598-616. [PubMed] [Google Scholar]
- 17.Rauch F Glorieux FH. Osteogenesis imperfecta, current and future medical treatment. Am J Med Genet C Semin Med Genet. 2005 Nov 15;139C(1):31-7. [DOI] [PubMed] [Google Scholar]
- 18.Semler O Beccard R Palmisano D Demant A Fricke O Schoenau E Koerber F. Reshaping of vertebrae during treatment with neridronate or pamidronate in children with osteogenesis imperfecta. Horm Res Paediatr. 2011;76(5):321-7. Epub 2011 Sep 27. [DOI] [PubMed] [Google Scholar]
- 19.Aström E Jorulf H Söderhäll S. Intravenous pamidronate treatment of infants with severe osteogenesis imperfecta. Arch Dis Child. 2007 Apr;92(4):332-8. Epub 2006 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yong-Hing K MacEwen GD. Scoliosis associated with osteogenesis imperfecta. J Bone Joint Surg Br. 1982;64(1):36-43. [DOI] [PubMed] [Google Scholar]
- 21.Hanscom DA Bloom BA. The spine in osteogenesis imperfecta. Orthop Clin North Am. 1988 Apr;19(2):449-58. [PubMed] [Google Scholar]
- 22.Konieczny M Senyurt H Krauspe R. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop. 2013 Feb;7(1):3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]